Abstract
Background & Aims
The lymphatic chemokine CCL21 is required for dendritic cell (DC) migration from tissues to lymph nodes, which helps establish tolerance to foreign yet harmless antigens. We demonstrate that CCL21 is almost completely absent from SAMP1/YitFc (SAMP) mice, which spontaneously develop chronic ileitis that resembles Crohn’s disease, and that DC migration is severely impaired in these mice compared with AKR mice (controls). Toll-like receptor (TLR) agonists like the TLR7 agonist R848 induce DC maturation and mobilization.
Methods
We collected intestinal and other tissues and mesenteric lymph nodes (MLN) from SAMP mice. Expression of CCL21 was measured by quantitative PCR and immunofluorescence analyses; spontaneous and induced migration of DCs were assessed by flow cytometry. We analyzed production of retinoic acid by DCs and their ability to induce development of T-regulatory (Treg) cells. Mice were fed R848 to determine its effects on migration of DCs and development of ileitis in SAMP mice.
Results
SAMP mice expressed almost no CCL21 in any tissue tested. Their CD11b+CD103+ DCs were defective in migration from the ileal lamina propria to the MLN. DCs from SAMP mice also had a greatly reduced ability to produce retinoic acid and induce development of Treg cells, compared with control mice. Young SAMP mice had reduced CCL21 expression and decreased DC migration before developing ileitis. Administration of R848 to these mice increased migration of DC to the MLN and development of Treg cells there, and reduced the severity of ileitis.
Conclusions
Loss of CCL21 signaling and DC migration is required for development of ileitis in SAMP mice. Reagents such as R848, which activate DC migration to the MLN, might be developed as treatments for patients with Crohn’s disease.
Keywords: immune regulation, oral tolerance, CD, small intestine
Introduction
Crohn’s disease is thought to be caused by an abnormal immune response to commensal bacteria in a genetically susceptible host1. Although the adaptive immune system plays an important role, defects in the macrophage and dendritic cell (DC) compartment have been reported, and a possible pathogenic role is supported by clinical data2. Migratory DCs are crucial in establishing tolerance towards foreign yet harmless antigens3. Crohn’s patients are unable to establish tolerance to orally delivered experimental antigens4, consistent with a migratory DC defect.
SAMP1/YitFc (SAMP) mice develop spontaneous chronic ileitis that resembles Crohn’s disease. This mouse strain was derived from AKR mice5, which do not develop intestinal inflammation. The most severe inflammatory changes in SAMP mice appear in the terminal ileum. Inflammation is transmural and discontinuous, with presence of normal areas of gut mucosa alternating with inflamed regions6 and shares many properties with Crohn’s disease including alterations in epithelial morphology, granulomas, crypt hyperplasia, infiltration of both acute and chronic inflammatory cells, spontaneous skin lesions and in some instances perirectal fistulas6, 7.
Both Th1 and Th2 adaptive immune responses are detectable during the course of the disease8. Ileitis in SAMP mice improves upon administration of corticosteroids9 or TNF blockade6, treatments that are also effective in Crohn’s patients1.
Several genetic defects have been described but the ultimate cause of ileitis in SAMP mice is unknown5, 10. Bone marrow transfer experiments have shown that the primary disease-causing defect resides in the non-hematopoietic compartment of SAMP mice11. Increased permeability of intestinal epithelial cells has been demonstrated in SAMP mice11, 12 along with defects in Treg function13 and abnormal NOD2 responses14.
Several subsets of DCs are constitutively found in the lamina propria (LP) of the terminal ileum. LP DCs are CD64−MHCII+CX3CR1−CD11c+CD103+ or CD103− cells that can be further subdivided based on the expression of CD11b15. Migratory DCs (mDCs) that include MHCII+CD11c+CD103+CD11b+ cells have been implicated in mounting immune responses towards pathogens. Migration of DCs from the LP to the MLN is also crucial in establishing oral tolerance to harmless food and commensal antigens16. Tolerogenic properties of CD103+ DCs depend on the production of TGF-β and retinoic acid (RA). RA is involved in B cell isotype switching to IgA, in Treg generation in lymph nodes16–19 and in imprinting Tregs with gut-homing receptors20, 21.
Once mDCs acquire antigens and maturation signals in the LP, they migrate to MLN22–25. This requires the expression of the chemokine receptor CCR7 on DCs and its ligand CCL21 in the afferent lymphatics16, 26. CCL21 has a highly charged C-terminus that facilitates binding to glycosaminoglycans displayed on the surface of lymphatic endothelial cells27. Mice express at least two isoforms of CCL2128: CCL21-Ser which is expressed in lymphatic organs, including lymph nodes and spleen and CCL21-Leu which is expressed by lymphatic endothelial cells in peripheral organs29. In the terminal lymphatic vessels of mouse skin, CCL21 is organized in discrete deposits that form portals through which DC crawl into the lymphatic system30. Away from the lymphatic vessels CCL21 is arranged in a gradient that directs mDCs to terminal lymphatics31. CCL21 is produced by lymphatic endothelial cells, high endothelial venules and fibroblastic reticular cells in lymph nodes32.
In preliminary experiments, we saw that CD11c+MHCIIhiCD103+CD11b+ DCs were missing from SAMP MLN. We therefore hypothesized that a potential defect in DC migration from LP to MLN may contribute to ileitis in SAMP mice by impairing tolerogenic responses. We discovered that CCL21 is absent from SAMP lymphatic vessels. Overcoming the DC migration defect significantly diminished ileitis in SAMP mice, suggesting that defective CCL21 expression is pathogenic. Manipulating DC trafficking may open new avenues for treatment of Crohn’s disease.
Results
Reduced numbers of mDCs in SAMP MLN
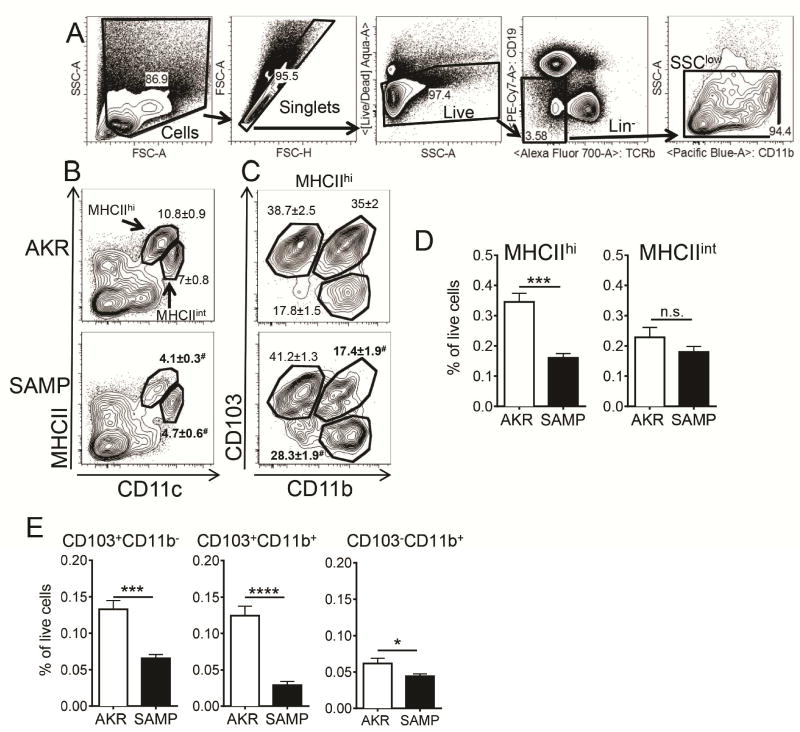
In the MLN, DCs account for less than one percent of all cells. To study DCs, we performed flow cytometry analysis of MLN and gated out B cells (by CD19), αβ T cells (by TCRβ) and granulocytes (by side scatter, Figure 1A). CD11c+ DCs in the MLN form two distinct populations33, migratory MHCIIhi and resident MHCIIint DCs (Figure 1B). MHCIIhi cell populations can be further subdivided by expression of CD11b and CD103 (22, Figure 1C). We found the MHCIIhi population reduced in SAMP mice (Figure 1, B and D), with the most severe reduction by more than 75% in CD11b+CD103+ mDCs (Figure 1E). CD103+CD11b− cells were reduced by about half, and CD103− cells were reduced by about 30% (Figure 1E). Reduction in the frequency of DCs was not due to a mere expansion of other leukocyte subsets, as total numbers of CD103+CD11b+ cells was reduced (Figure 5B). We conclude that mDCs are diminished in MLN of SAMP mice.
Figure 1. Defective MLN DC in SAMP mice.
(A) Single cell suspensions were prepared by incubating minced tissues in collagenase and DNAse I. Sequential gates were set to exclude debris, doublets, dead cells, cells of B and T cell lineage and granulocytes (SSChi). (B) Representative flow cytometry analysis of DC in AKR and SAMP MLN from 10–20 weeks old mice. Two populations of CD11c+MHCIIhi and CD11chiMHCIIint MLN DCs were detected. (C) MHCIIhi cells contained 3 major subpopulations: CD103+CD11b+, CD103+CD11b− and CD103−CD11b− cells. Mean of percentages of parent gate ± SEM are indicated on the plots, # denotes statistically significant differences between AKR and SAMP mice. (D) The fraction of MHCIIhi and MHCIIint cells and (E) subpopulations of MHCIIhiCD11c+ cells was calculated as % of all live cells. Results are the mean ± SEM of 3 experiments with n=5 in AKR and n=6 in SAMP groups. n.s – not significant, **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05 by two-sided Student’s t test
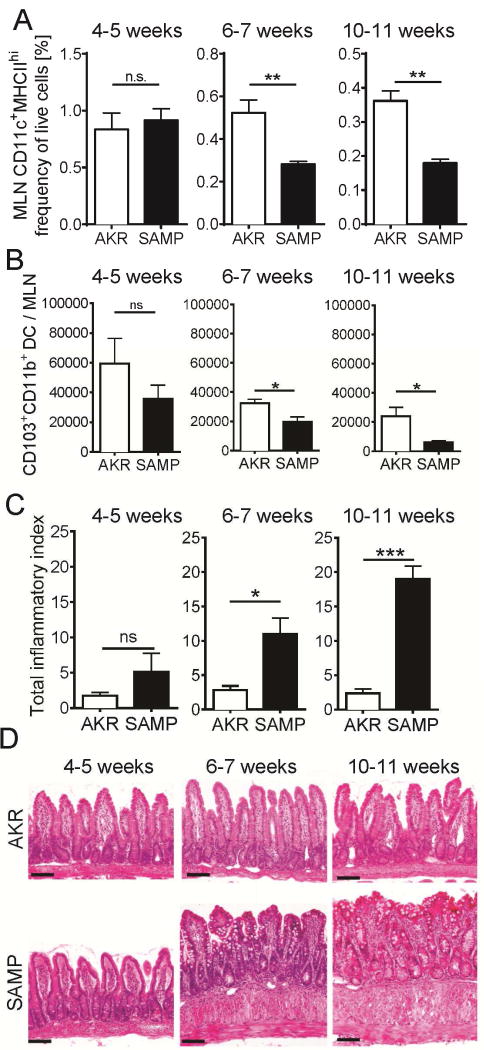
Figure 5. Loss of MHCIIhi DC in MLN of SAMP mice is concomitant with the onset of clinical disease.
(A–B) MLN MHCIIhi DCs and ileitis (C) were analyzed in 4–5, 6–7 and 10–11 weeks old mice (mean ± SEM, n≥3). (B) Diminished numbers of CD11c+MHCIIhiCD103+CD11b+ cells in the MLNs of >6 weeks old SAMP mice. (D) Sections of terminal ileum were stained with H&E. Bar = 100 μm. n.s – not significant, *** p<0.001, ** p<0.01, * p<0.05 by two-sided Student’s t test
Defective egress of CD11b+CD103+ DCs from the SAMP terminal ileum
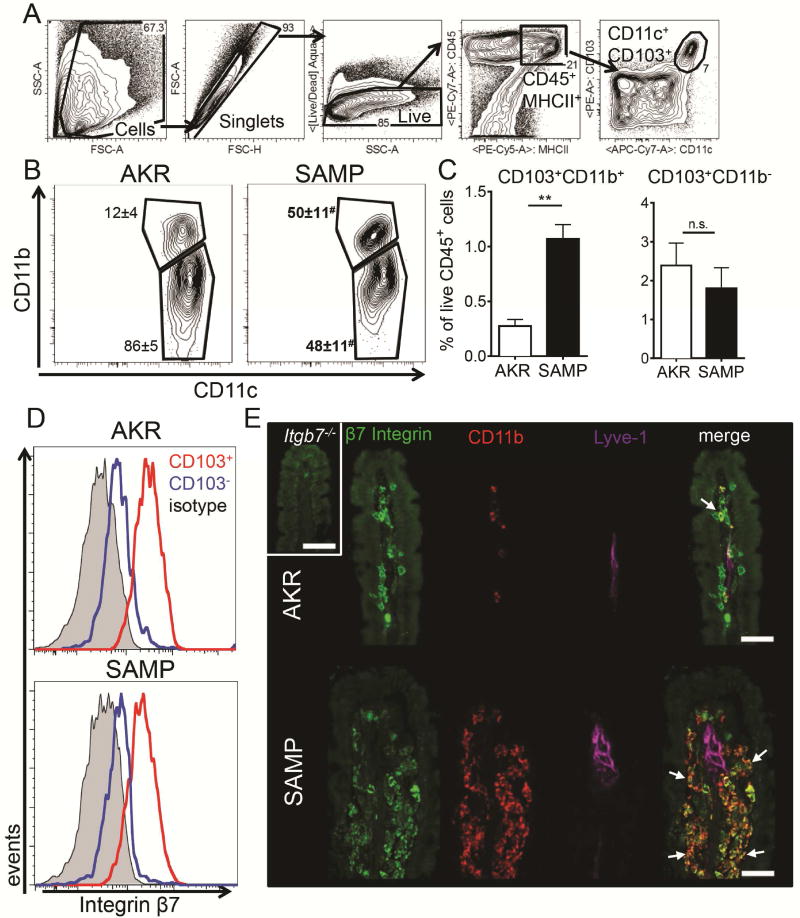
The relative decrease of CD11b+CD103+ cells in the MLN could result from a developmental or a migration defect. In the former case, CD11b+CD103+ DCs would be expected to also be reduced in the small intestinal LP, whereas in the latter case, these cells would accumulate in the LP. We analyzed DCs in the LP of young (5–7 week old) SAMP and AKR control mice (Figure 2) by gating on live CD11c+CD103+ cells (Figure 2A). In SAMP mice, there was a significant accumulation of CD11b+CD103+ DCs in the LP (Figure 2, B and C), suggesting that these mDCs were retained in the LP of SAMP mice. To facilitate immunostaining, we took advantage of the fact that CD103 pairs with β7 to form αEβ7 integrin (Figure 2D). Immunofluorescence analysis of the LP of the small intestine showed accumulation of CD11b+β7 integrin+ DCs in SAMP mice (Figure 2E). The collecting lymphatic vessels labeled with Lyve1 (Lymphatic Vessel Endothelial Receptor 1) were much wider in adult SAMP mice (Figure 2E) but had normal diameter in 10 days old mice (data not shown).
Figure 2. Accumulation of CD103+CD11b+ mDC in the terminal ileum LP of SAMP mice.
LP cells were prepared from 5–7 weeks old AKR and SAMP mice by digestion and gated (A) on live single CD45+MHCII+CD103+CD11c+ cells. (B) Representative flow cytometry plots of CD103+CD11c+CD11b+ and CD11b− population in AKR and SAMP LP. Percentages of parent gate (mean ± SEM) are indicated on the plots, # denotes significant differences between AKR and SAMP mice. (C) Frequency of gated populations among all live leukocytes. Results are the mean ± SEM of 2 experiments with n=4 for AKR and n=3 for SAMP groups. (D) Expression of β7 integrin on CD103+ (red) and CD103− (blue) MHCIIhigh MLN DCs. Isotype control (grey) was done on MHCIIhigh cells. (E) Sections of terminal ileum from 20 weeks old AKR and SAMP mice were stained with antibodies to β7 integrin (green), CD11b (red), and Lyve1 (Lymphatic Vessel Endothelial Receptor 1, magenta). Shown is maximum intensity projection of a 6 μm confocal stack. β7+CD11b+ cells (arrows) are enriched in SAMP LP. Control staining (insert) was done on tissue from β7-deficient mouse. Representative of 2 experiments with at least 2 mice per group. Bar = 20 μm. ** p<0.01, # p<0.05
Lack of CCR7 chemokine ligands in SAMP mice
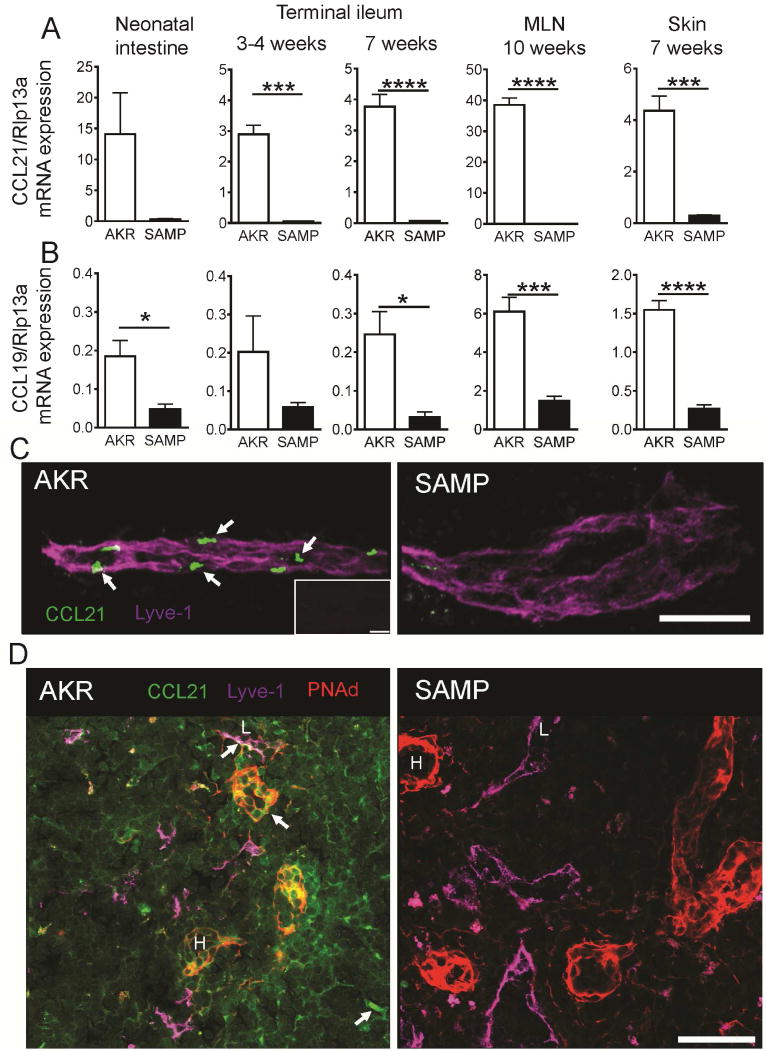
DC egress to the MLN from the LP is CCR7-dependent24, 27. Flow cytometry analysis of MLN confirmed that CD11c+MHCIIhigh DCs are greatly reduced in MLN of CCR7-deficient mice (Supplementary Figure 1). The defect was mostly due to a sharp reduction in MHCIIhi DCs and an almost complete absence of MHCIIhi CD11b+CD103+ DCs. Since the migration defect affected the same cell type that failed to accumulate in the MLN of SAMP mice, we hypothesized that CCR7 expression in SAMP mice may be defective. However, we did not find any difference in CCR7 levels between SAMP and AKR DCs (Supplementary Figure 3B). Next, we tested whether the defect was in the expression of the CCR7 ligands, CCL19 and CCL21, in peripheral and lymphatic organs. In the small intestine of neonatal (before ileitis onset) and terminal ileum of adult SAMP mice, CCL21 mRNA expression was sharply reduced (Figure 3A) and CCL19 expression was reduced 4–8 fold when compared to AKR mice (Figure 3B). To test whether the chemokine expression defect was limited to the ileum, we measured CCL21 and CCL19 mRNA in skin, mesenteric and peripheral lymph nodes and found that expression of both chemokines was also defective there, suggesting that both CCL21 isoforms are depleted in SAMP mice (Figure 3A,B and data not shown). Abnormal expression of CCL19 and CCL21 mRNA was also seen in colonic biopsies from ulcerative colitis and Crohn’s disease patients (Supplementary Figure 4), corroborating previous reports41,43. Confocal immunofluorescence staining of intestinal whole mounts showed abundant CCL21 immunoreactivity (green) along initial lymphatic vessels in intestinal villi (labeled with Lyve1 in magenta, Figure 3C) of AKR control mice. In the lymph nodes of AKR mice CCL21 was concentrated in Lyve1− cells as well as present in the form of diffuse pool. In both analyzed tissues, CCL21 was only sporadically detectable by microscopy in SAMP mice (Figure 3C,D). CCR7 ligands also play an important role in homing of naïve T cells to secondary lymphoid organs27, and adoptively transferred T cells homed less efficiently to lymph nodes of SAMP mice (Supplementary Figure 2 and Supplementary Video 1). Taken together, these findings suggest that defective DC homing from the LP to the MLN of SAMP mice is due to defective expression of CCL21 in lymphatics or MLN.
Figure 3. Reduced CCL21 expression in SAMP mice.
Quantitative RT-PCR for CCL21 (A) and CCL19 (B) in neonatal intestine, terminal ileum, mesenteric lymph nodes and skin of AKR and SAMP mice; mean±SEM for n=3–5 mice/group. Ribosomal protein L13A (Rpl13a) mRNA was used as a reference. (C) Whole mount staining of terminal ileum with antibodies to CCL21 (green) and Lyve1 (magenta). Confocal immunofluorescence demonstrates abundant presence of CCL21 protein (arrows) in Lyve1+ lymphatic vessel of AKR, while weak expression of CCL21 can be seen only sporadically in SAMP mice; Maximum intensity projection of 15 × 1 μm Z stacks. Bar = 100 μm Representative of 3 experiments with total n=4 mice/strain. Isotype control is shown in the insert. Bar = 30 μm. (D) Sections of MLN form AKR and SAMP mice were stained with antibodies to CCL21 (green), peripheral node addressin (red) and Lyve-1 (magenta). Diffuse and concentrated (arrows) pools of CCL21 in lymphatic vessels (L) and high endothelial venules (H) are absent in SAMP lymph nodes. Bar = 50 μm. Representative of 2 experiments.
Defective functions of SAMP MLN DCs
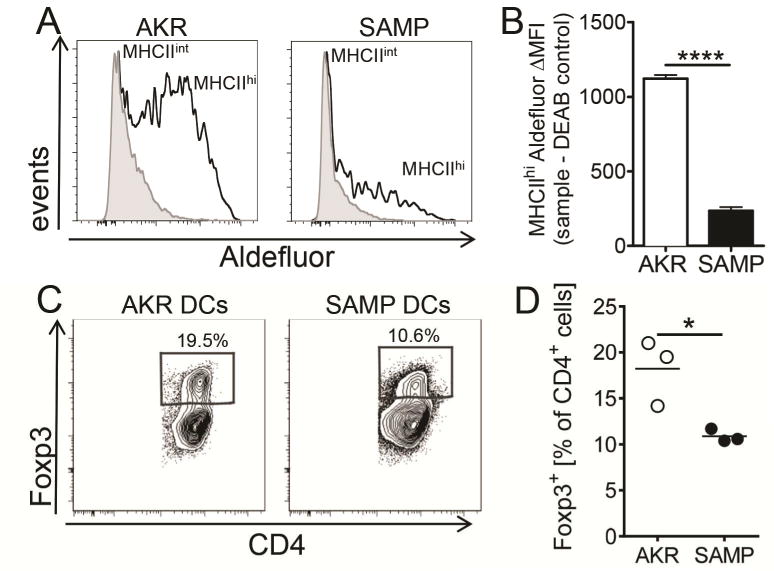
Induced regulatory CD4 T cells (iTreg), which express the transcription factor Foxp334 are found in intestinal tissue and contribute to controlling inflammation35. In intestinal tissues, iTregs are induced by so-called tolerogenic DCs, which express TGFβ and RALDH activity36. Physiologically, RALDH enzymes convert retinal to RA, which is known to promote iTreg differentiation35, 37. RALDH activity can be monitored in single cells by the fluorogenic substrate Aldefluor38. More than 60% of MHCIIhigh MLN DCs isolated from control AKR mice express Aldefluor activity, contrasting with less than 30% of MHCIIhigh SAMP MLN DCs (Figure 4A). Also, the amount of enzymatic activity per cell as detected by median fluorescence intensity (MFI) was much lower in SAMP than in AKR MHCIIhi DCs (Figure 4B). To test whether this defective RALDH expression resulted in a reduced ability to induce iTregs in vitro, we incubated naïve splenic AKR CD4+CD45RbhighCD25− T cells with CD11c+ DCs isolated from MLNs of AKR or SAMP mice in the presence of anti-CD3 and TGFβ. While AKR DCs induced 18±2% Foxp3-expressing iTregs, SAMP DCs induced only 11±0.4% (Figure 4C, D).
Figure 4. SAMP DCs are defective in generating Foxp3+ cells in vitro.
Capacity of SAMP and AKR MLN DCs to produce RA was measured by RALDH activity in Aldefluor assay. (A) Representative histograms of MLN MHCIIint (gray) and MHCIIhi (open) cells from AKR and SAMP mice (B) Aldefluor ΔMFI signal was calculated by subtracting median fluorescence intensity of MLN MHCIIhi cells treated with RALDH inhibitor DEAB from corresponding non-treated samples. Mean ± SEM from 3 experiments and n=4 mice for each group. (C–D) Naïve AKR CD4+CD45RbhiCD25− T cells were incubated with magnetically enriched and FACS sorted CD11c+ DC from AKR or SAMP MLN for 5 days in the presence of 2 μg/ml anti-CD3ε and 5 ng/ml TGFβ1. (C) Representative flow cytometry plots of Foxp3 expression on cultured CD4+ cells. (D) Data from 2 experiments are presented and mean is indicated. **** p<0.0001, * p<0.05 by two-sided Student’s t test
Next, we tested how defective DC migration and diminished DC RALDH expression correlated with disease activity (ileitis). The terminal ileum of AKR and SAMP mice was harvested at 4–5 (before ileitis onset), 6–7 or 10–11 weeks of age, fixed, embedded and analyzed by scoring H&E stained slides as described9. The number of CD11c+MHCIIhigh DCs in the MLN decreased over time, but the reduction was significantly more pronounced in SAMP than in AKR mice (Figure 5A). The absolute number of MLN CD103+CD11b+ DCs also decreased more in SAMP than in AKR mice, reaching statistical significance at 6–7 weeks (Figure 5B). This DC defect coincided with the appearance of inflammation as determined by lymphocyte and neutrophil infiltration (Figure 5C and D).
TLR7 agonist R848 can overcome DC recruitment defect in SAMP mice
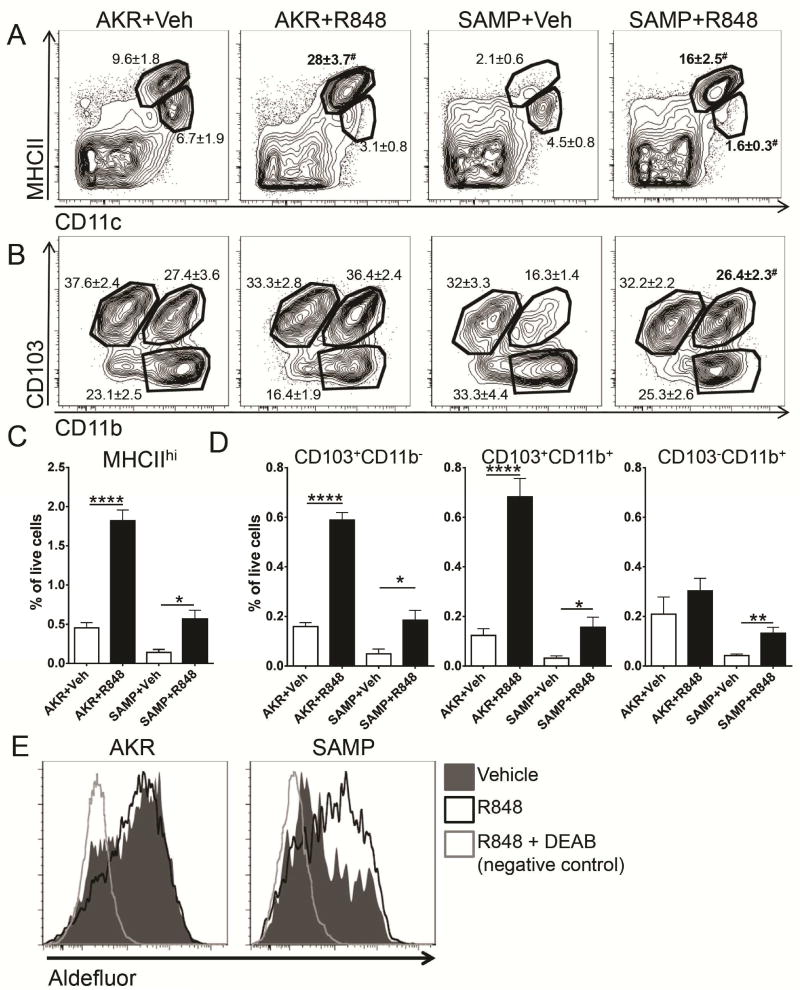
Attempts to directly correct the defective CCL21 expression in lymphatics of SAMP mice were not successful (data not shown). Since Toll-like receptor (TLR) agonists are known inducers of DC maturation and mobilize DCs in vivo39, we tested if AKR and SAMP DC can be mobilized from LP to MLN, by the TLR7 agonist R84823, 25. Eighteen hours after oral gavage, R848 augmented the percentage of MHCIIhigh MLN DCs in AKR mice 3–4 fold (Figure 6A), due to an increase in CD103+CD11b− and CD103+CD11b+, but not the CD103−CD11b+ subsets (Figure 6B and D). In SAMP mice, the frequency of DCs was sharply lower than in AKR mice, but the R848 gavage induced a similar 3–4 fold increase (Figure 6A–D). R848 treatment restored all MHCIIhigh subsets to levels similar to untreated AKR mice (Figure 6C, D). RALDH activity in CD11c+MHCIIhi cells was also increased after R848 treatment (Figure 6E).
Figure 6. TLR7 agonist R848 can overcome DC recruitment defect in SAMP mice.
(A) Migration of CD11c+MHCIIhi DC into MLN was measured 18h after gavage of R848 (0.5 μg/g). (B) Treatment induced migration of CD103+ CD11b+ and CD11b− cells in the AKR mice. Percentages of parent gate ± SEM are indicated on the plots, # denotes significant differences between vehicle and R848 treated mice of the same strain. (C) Frequency of migratory cells at baseline (vehicle treated mice) and after TLR induction (R848) was lower in MLN of SAMP mice. n=5–6 in each group. (E) Activity of RA-synthesizing enzyme in CD11c+MHCIIhi MLN cells following TLR7 ligand treatment was measured by flow cytometry. Filled histograms show vehicle-treated mice, black open histograms depict R848-treated mice. Control sample treated with RALDH inhibitor DEAB is shown as gray open histograms. Data are representative of 3 experiments. **** p<0.0001, ** p<0.01, * p<0.05, # p<0.05 by two-sided Student’s t test
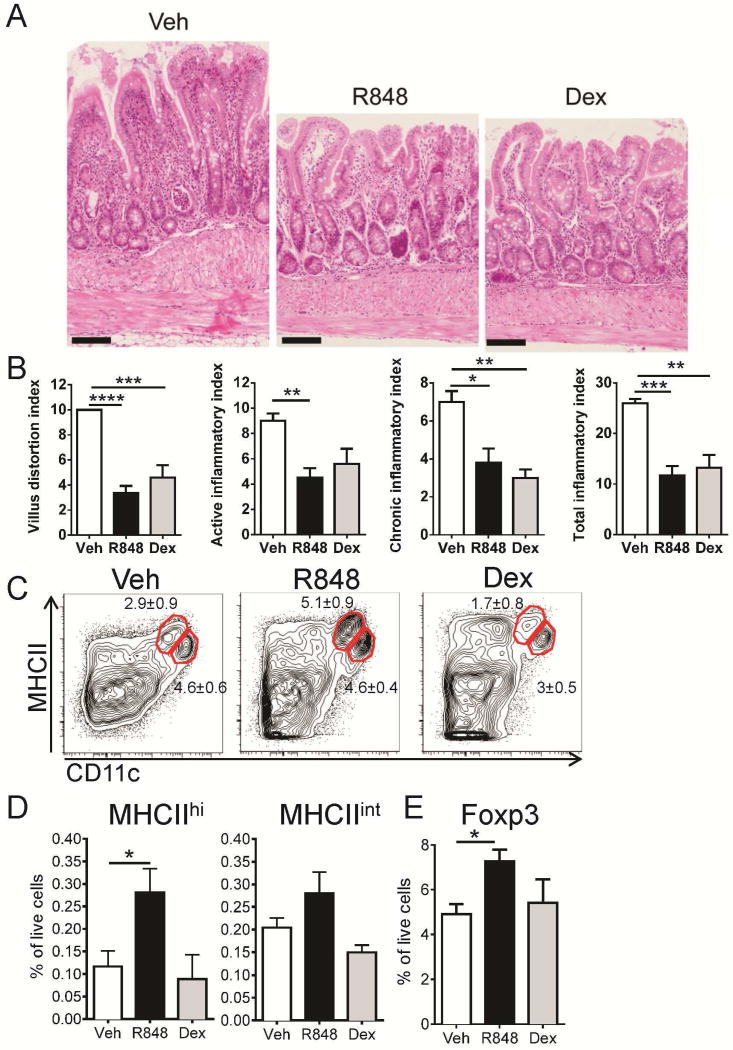
Encouraged by these results, we tested the clinical effect of 7-day treatment with R848 (0.5 μg/g, by oral gavage daily) on ileitis in 30 weeks old SAMP mice (4–6 mice/group). As a positive control we treated a group of SAMP mice with dexamethasone (Dex), which is known to reduce inflammation in SAMP mice9. Treatment with R848 resulted in an improvement in villus architecture, decreased infiltration of inflammatory cells and reduction in thickening of the gut wall (Figure 7A and B). Interestingly, R848 treatment was as effective as the positive control dexamethasone, bringing the inflammatory score down by more than half (Figure 7B). Unlike corticosteroid treatment, R848 induced a significant increase in the MHCIIhi DCs (Figure 7C, D) and a small but statistically significant increase in CD4+Foxp3+ cells in the MLN (Figure 7E).
Figure 7. Treatment with R848 reduces inflammation in the terminal ileum.
(A–B) Effect of treatment with R848 on ileitis. Outcomes of a 7 day course of R848 treatment (0.5 μg/g body mass, by oral gavage) on >30 weeks old mice. Control animals received either vehicle (H2O) by daily gavage or dexamethasone (Dex) by IP injection (100 μg every second day). (A) Ileal swiss-rolls were stained with H&E and (B) total inflammatory score was assessed. n=4–8 per group. (C–F) R848 induces mDCs and MLN Foxp3+ cells. (C) Representative flow cytometry plots and (D) frequency of DC subsets were analyzed among all live cells recovered from the lymph nodes. (E) Cells were gated to include CD4+ TCRβ+ T cells and expression of Foxp3 was determined by intracellular staining. Shown is percentage of CD4+Foxp3+ cells among all live MLN cells. Data from 2 experiments are presented as mean ± SEM, n=7–12 per group. **** p<0.0001, *** p<0.001, ** p<0.01, * p<0.05 by one-way analysis of variance followed by Dunnett’s multiple comparisons test
Taken together, our data show that SAMP mice have a defect in trafficking of DCs from LP to MLN due to sharply reduced CCL21 expression. The remaining MLN DCs isolated from SAMP mice are defective in RALDH activity and iTregs induction, suggesting that the migration defect affects tolerogenic DCs. Since the defect in trafficking of tolerogenic DCs precedes disease onset, this suggests that DCs play a major role in the SAMP model of ileitis. Consistent with this, promoting LP DCs migration into the MLN by administration of an oral TLR7 agonist dramatically reduced disease scores.
Discussion
Our data show that homeostatic DC trafficking is dramatically disturbed in the small intestine of SAMP mice. The loss of mDCs in MLN results from aberrant migration of MHCIIhiCD103+CD11b+ DCs from the LP to the MLN due to defective CCL21 expression in lymphatics. Because mDCs are missing in the MLN of SAMP mice, they cannot effectively induce Tregs. Increasing DC trafficking by treatment with the orally available TLR7 ligand R848 improved DC migration, RA production and enhanced induction of Foxp3+ cells. This data suggests that therapies aimed at improved DC trafficking might be useful in patients with Crohn’s disease.
The inflamed mucosa in Crohn’s patients and SAMP mice harbors increased numbers of DCs40, 41. Many of these cells express CD83 and CCR7, suggesting that they are mature DCs41. Normally, these cells would be expected to migrate to the MLN, but in Crohn’s patients they appear to be retained in the LP. This is similar to the phenotype we found in the SAMP model. In patients, increased retention of CCR7+ DCs in the inflamed LP is thought to be mediated by excessive production of CCL19 by DCs and CCL21 by fibroblastic reticular cells and lymphatic endothelial cells41. This can be reproduced in animal tissues, where providing exogenous CCL21 also resulted in DCs stranded in the parenchyma31. Transgenic overexpression of CCL21 under the H-2Kb promoter caused a decrease in DC migration to the draining lymph node in a Leishmania major infection model42. In healthy tissue CCL21 forms an orderly gradient which guides DCs into the terminal lymphatics31. Overexpression masks this gradient and prevents mature DCs from migrating toward lymphatics and leaving the inflamed LP. Dysregulation of CCL21 and CCL19 production in the ileum and MLN was also found in another mouse model of chronic ileitis (TNFΔARE)43. Aberrant expression of CCR7 ligands impairs leukocyte trafficking leading to accumulation of T cells in inflamed gut43. The same group previously identified an impaired balance between CD103+ and CD103− dendritic cells in the MLN of TNFΔARE44. In the SAMP model, the CCL21 gradient is absent, which has the same functional consequence. Taken together, our and previously published data strongly suggest that chemokine defects are tightly linked to two relevant murine models of chronic small intestinal inflammation. The accumulation of DCs in the LP of SAMP mice is remarkably similar to that found in Crohn’s patients and emphasizes the relevance of this mouse model. Our data suggest that the DC migration defect depends on the reduced expression of CCL21, as this chemokine is sufficient in guiding DC trafficking in CCL19 knockout mice26.
Histopathological evaluations of intestinal material obtained from Crohn’s patients before the wide-spread use of disease-modifying drugs showed dilation of lymphatic terminals, submucosal edema, lymphocytic thrombi within lymphatics, and aggregates of lymphocytes often containing granulomas45, 46. Here we report dilated lymphatics and edema in the SAMP model, underlining the critical involvement of the lymphatic system in both Crohn’s disease and SAMP ileitis.
Gut CD103+ mDCs have the unique potential to drive conversion of naïve CD4 T cells into iTregs in a process that depends on TGF-β and is potentiated by RA34. Our results show that defects in CD103+ migratory SAMP DCs and significant loss of RA-producing DCs in the MLN translates into less efficient conversion of naïve CD4 T cells into Foxp3+ Tregs. Recent studies showed that though mutually redundant47, 48, CD103+CD11b− and CD103+CD11b+ cells are jointly required for Treg homeostasis49. Data from our in vivo rescue experiments demonstrate that R848-induced mobilization of intestinal DCs in SAMP mice increased the frequency of Foxp3+ cells in the MLN. Extrathymically generated iTregs are critically involved in controlling mucosal inflammation35. A recent study suggested that dysfunctional Tregs may play an important role in SAMP ileitis13. In addition, we found that SAMP DCs were less efficient at inducing iTregs.
Loss of CD103+CD11b+ mediated by targeted DC-specific deletion of Notch247, 50 or the transcription factor IRF451 does not predisposes mice to spontaneous ileitis, highlighting the redundancy of regulatory mechanisms. Similarly, Crohn’s requires a combination of multiple genetic and environmental factors1. It is likely that SAMP mice combine several defects that culminate in the ileitis phenotype. Disrupted mucosal barrier integrity in SAMP mice11 might be particularly important as a mechanism which allows for increased translocation of food and bacterial antigens. This in conjunction with deficits in tolerogenic DCs and aberrant naïve T cells homing might promote ileitis. Because in SAMP mice both CCL21 and CCL19 are defective from birth, it is very likely that their absence impacts the development of mucosal immunity. Our finding that DC mobilization improves ileitis suggests that manipulation of dendritic cell trafficking might provide novel therapeutic avenues for patients affected by Crohn’s disease.
Materials and Methods
Mice and treatments
SAMP1/YitFc mice (SAMP), a substrain of the SAMP1/Yit line developed at Yakult Central Institute for Microbiological Research (Tokyo, Japan), B6.129P2(C)-Ccr7tm1Rfor/J (CCR7-deficient), C57BL/6-Itgb7tm1Cgn/J (integrin β7-deficient), C57BL/6J and AKR/J mice were obtained from established colonies at the La Jolla Institute for Allergy and Immunology (La Jolla, CA). Mice were treated with 0.5 mg/kg of R848 (Invivogen, San Diego, CA) by oral gavage. Control mice received sterile water. A group of mice received 100 μg dexamethasone (Butler Schein, Dublin, OH) intraperitoneally every second day. All animals were housed in a specific pathogen–free facility, and all experiments were approved by the institutional committee for animal use
Sample preparation
Mice were euthanized by CO2 inhalation at times required by the experimental design. Human colonic material was harvested from patients undergoing flexible sigmoidoscopy or colonoscopy for diagnostic or surveillance purposes. All studies were approved by the Internal Review Board of Case Medical Center. Detailed methods for immunohistochemical and immunofluorescence analysis, quantitative polymerase chain reaction, cell isolation, flow cytometry and cell sorting, cell cultures and short term homing are available in Supplementary Methods.
Statistical analysis
Results are expressed as mean ± SEM unless indicated otherwise. Data were analyzed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Differences between individual groups were assessed using two-sided unpaired Student t test. Multiple groups were compared with control group using a one-way analysis of variance followed by Dunnett’s multiple comparisons test. P ≤ 0.05 was considered significant.
Supplementary Material
Acknowledgments
Grant support:
This work was supported by NIH DK091222-01 to Fabio Cominelli and Crohn’s and Colitis Foundation of America Research Fellows Award to Zbigniew Mikulski.
We thank Hui Ouyang, Kathleen Lloyd and Jackie Miller for their outstanding technical help in maintaining the mouse colony; Ildefonso Vicente-Suarez for CCR7-deficient mice and members of Cheroutre and Kronenberg laboratories for their support, reagent sharing and helpful discussions.
Abbreviations
- DC
dendritic cell
- SAMP
SAMP1/YitFc
- Tregs
regulatory T cells
- MLN
mesenteric lymph nodes
- LP
lamina propria
- mDC
migratory DC
- RA
retinoic acid
- MFI
median fluorescence intensity
- iTreg
induced regulatory T cells
- TLR
Toll-like receptor
Footnotes
Disclosures:
The authors have no conflicting financial interests.
Author Contributions:
ZM, KL - study concept and design; ZM, RJ, IS, GK, WG, HN, GC - acquisition, analysis and interpretation of data; ZM, IS, GC, HN, KL - drafting of the manuscript; KL, TTP, FC - obtained funding; KL - study supervision; all authors participated in critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith AM, Rahman FZ, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J Exp Med. 2009;206:1883–97. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner HL, da Cunha AP, Quintana F, et al. Oral tolerance. Immunol Rev. 2011;241:241–59. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraus TA, Mayer L. Oral tolerance and inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:692–6. doi: 10.1097/01.mog.0000182862.88798.28. [DOI] [PubMed] [Google Scholar]
- 5.Pizarro TT, Pastorelli L, Bamias G, et al. SAMP1/YitFc mouse strain: A spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn’s disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera-Nieves J, Bamias G, Vidrich A, et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology. 2003;124:972–82. doi: 10.1053/gast.2003.50148. [DOI] [PubMed] [Google Scholar]
- 8.Bamias G, Martin C, Mishina M, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–66. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 9.Burns RC, Rivera-Nieves J, Moskaluk CA, et al. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–36. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 10.Kozaiwa K, Sugawara K, Smith MF, Jr, et al. Identification of a quantitative trait locus for ileitis in a spontaneous mouse model of Crohn’s disease: SAMP1/YitFc. Gastroenterology. 2003;125:477–90. doi: 10.1016/s0016-5085(03)00876-x. [DOI] [PubMed] [Google Scholar]
- 11.Olson TS, Reuter BK, Scott KG, et al. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–52. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107:8017–22. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa D, Okazawa A, Corridoni D, et al. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn’s disease. Mucosal Immunol. 2013;6:267–75. doi: 10.1038/mi.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corridoni D, Kodani T, Rodriguez-Palacios A, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110:16999–7004. doi: 10.1073/pnas.1311657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlitzer A, McGovern N, Teo P, et al. IRF4 Transcription Factor-Dependent CD11b(+) Dendritic Cells in Human and Mouse Control Mucosal IL-17 Cytokine Responses. Immunity. 2013;38:970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 19.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Cerovic V, Houston SA, Scott CL, et al. Intestinal CD103(−) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.53. [DOI] [PubMed] [Google Scholar]
- 23.Yrlid U, Milling SW, Miller JL, et al. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-alpha and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol. 2006;176:5205–12. doi: 10.4049/jimmunol.176.9.5205. [DOI] [PubMed] [Google Scholar]
- 24.Jang MH, Sougawa N, Tanaka T, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176:803–10. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 25.Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britschgi MR, Favre S, Luther SA. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur J Immunol. 2010;40:1266–71. doi: 10.1002/eji.200939921. [DOI] [PubMed] [Google Scholar]
- 27.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 28.Luther SA, Tang HL, Hyman PL, et al. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–9. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen SC, Vassileva G, Kinsley D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–8. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 30.Tal O, Lim HY, Gurevich I, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–53. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber M, Hauschild R, Schwarz J, et al. Interstitial Dendritic Cell Guidance by Haptotactic Chemokine Gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SN, Hosiawa-Meagher KA, Konieczny BT, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–4. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 33.Satpathy AT, Kc W, Albring JC, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 35.Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–9. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott CL, Aumeunier AM, Mowat AM. Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol. 2011;32:412–9. doi: 10.1016/j.it.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Benson MJ, Pino-Lagos K, Rosemblatt M, et al. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–77. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster R, Braun A, Worbs T. Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol. 2012;33:271–80. doi: 10.1016/j.it.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Salim SY, Silva MA, Keita AV, et al. CD83+CCR7− dendritic cells accumulate in the subepithelial dome and internalize translocated Escherichia coli HB101 in the Peyer’s patches of ileal Crohn’s disease. Am J Pathol. 2009;174:82–90. doi: 10.2353/ajpath.2009.080273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middel P, Raddatz D, Gunawan B, et al. Increased number of mature dendritic cells in Crohn’s disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–7. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unsoeld H, Mueller K, Schleicher U, et al. Abrogation of CCL21 chemokine function by transgenic over-expression impairs T cell immunity to local infections. Int Immunol. 2007;19:1281–9. doi: 10.1093/intimm/dxm098. [DOI] [PubMed] [Google Scholar]
- 43.McNamee EN, Masterson JC, Jedlicka P, et al. Ectopic lymphoid tissue alters the chemokine gradient, increases lymphocyte retention and exacerbates murine ileitis. Gut. 2013;62:53–62. doi: 10.1136/gutjnl-2011-301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins CB, Aherne CM, McNamee EN, et al. Flt3 ligand expands CD103+ dendritic cells and FoxP3+ T regulatory cells, and attenuates Crohn’s-like murine ileitis. Gut. 2011 doi: 10.1136/gutjnl-2011-300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Kruiningen HJ, Colombel JF. The forgotten role of lymphangitis in Crohn’s disease. Gut. 2008;57:1–4. doi: 10.1136/gut.2007.123166. [DOI] [PubMed] [Google Scholar]
- 46.von der Weid PY, Rehal S, Ferraz JG. Role of the lymphatic system in the pathogenesis of Crohn’s disease. Curr Opin Gastroenterol. 2011;27:335–41. doi: 10.1097/MOG.0b013e3283476e8f. [DOI] [PubMed] [Google Scholar]
- 47.Lewis KL, Caton ML, Bogunovic M, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelson BT, Kc W, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welty NE, Staley C, Ghilardi N, et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J Exp Med. 2013;210:2011–24. doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satpathy AT, Briseno CG, Lee JS, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persson EK, Uronen-Hansson H, Semmrich M, et al. IRF4 Transcription-Factor-Dependent CD103(+)CD11b(+) Dendritic Cells Drive Mucosal T Helper 17 Cell Differentiation. Immunity. 2013;38:958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.