Abstract
Background
The diagnosis of cholangiocarcinoma (CCA) in patients with primary sclerosing cholangitis (PSC) is particularly difficult. The role of volatile organic compounds (VOCs) for diagnosis of CCA in PSC patients is not known.
Aim
Our aim was to identify potential VOCs in the headspaces (gas above the sample) in bile which may predict CCA in PSC patients.
Design
Prospective cross-sectional study
Setting
Referral center
Patients
Prospective study in which bile was aspirated in 32 patients undergoing ERCP for PSC and CCA complicating PSC.
Main Outcome Measurements
Selected ion flow tube mass spectrometry was used to analyze the concentration of 22 prevalent VOCs in bile samples. Logistic regression analysis was performed to build a predictive model for diagnosis of CCA.
Results
Several compounds (ethanol, acrylonitrile, acetonitrile, acetaldehyde, benzene, carbon disulfide, dimethyl sulfide, 2-propanalol) were significantly different in patients with CCA complicating PSC compared with PSC. (P<.05) Using receiver operating characteristic curve analysis, we developed a model for the diagnosis of CCA adjusted for age and gender based on VOC levels of acrylonitrile, 3-methylhexane and benzene. The model [2.3239* log (acrylonitrile) + 0.9871*log (3-methylhexane) + 0.8448*log (benzene)] < −0.12 identified the patients with CCA [area under the curve (AUC=0.89)], with 90.5% sensitivity and 72.7% specificity. (p=0.02)
Limitations
Sample size
Conclusions
The measurement of VOCs in biliary fluid may be useful to diagnose CCA in PSC patients. A larger study with a longitudinal study design is required to confirm our pilot observations to diagnose CCA early in patients with PSC. (NCT01565460)
Keywords: Endoscopic retrograde cholangiopancreatography, volatile organic compounds, bile, cholangiocarcinoma, primary sclerosing cholangitis
INTRODUCTION
Cholangiocarcinoma (CCA) is a primary malignancy of the biliary system which presents clinically as biliary strictures.1 CCA involves the confluence of the right and left hepatic ducts (perihilar carcinomas) in 50% of cases, whereas the remaining CCAs arise from the intrahepatic ducts or more distally.2 These strictures are difficult to diagnose because there is often no mass evident on cross-sectional and endoscopic imaging.
Primary sclerosing cholangitis (PSC) is one of the strongest independent risk factors for CCA.3 However, the diagnosis of CCA is particularly difficult in PSC because fibrotic changes may decrease the yield of brush cytology and other tissue acquisition methods. Timely diagnosis is necessary because liver transplantation is the only potential cure, and may only be offered if CCA is diagnosed in an early stage. Currently, there is no acceptably sensitive or specific diagnostic marker to detect CCA in patients with PSC.
ERCP with bile duct brushings for cytology with or without fluorescence in situ hybridization (FISH) is typically performed to diagnose CCA. However, the sensitivity of brushings for cytology in diagnosing CCA is low; 4,5 and the use of FISH only moderately increases the sensitivity.6,7 Endoscopic ultrasound with fine needle aspiration (EUS-FNA) may be done if ERCP brushings are negative or inconclusive and if there is localized bile duct wall thickening, but potential tumor seeding remains a concern, and their routine use is questionable in hilar strictures. 8
Volatile organic compounds (VOCs) can be detected in the headspace of bile. “Headspace” is the gas space above the sample. Volatile sample components diffuse into the gas phase, forming the headspace gas. Headspace analysis is the analysis of the components present in that gas. We have previously described the utility of measuring VOCs in bile in the diagnosis of pancreas cancer and differentiating it from chronic pancreatitis. 9 PSC patients are unique and different from patients with other benign conditions and pancreatic cancer. The main challenge in PSC patients is that, once we see a dominant stricture, the diagnosis of CCA in these patients is particularly challenging. In the currently study, we sought to specifically study PSC patients and extend these observations to determine the role of VOCs in the diagnosis of CCA. The aim of our pilot study was to identify potential VOCs in the bile that could be used to distinguish PSC-CCA from PSC without CCA.
METHODS
Bile samples used in this study were obtained from our endoscopic bile repository. Our earlier publications have discussed this prospectively maintained database of bile obtained during ERCP and the inclusion and exclusion criteria of patients recruited. 9–11 The database was established in 2012 and includes bile samples linked to demographic, clinical, and cholangiographic information. The study was approved by the Cleveland Clinic Institutional Review Board and registered with the National Institute of Health (NIH) clinical trial registry. (NCT01565460) The bile samples were obtained from March 2012 to December 2012.
The diagnosis or PSC without CCA and PSC with CCA was defined based on cytology results, surgical pathology, and careful clinical follow-up with subsequent imaging tests. All patients had clinical follow-up of atleast 1 year to December 2013. The diagnosis of PSC with CCA was based on tissue diagnosis either at surgery or on brush or fine needle aspiration cytology on initial or subsequent ERCP or EUS during follow-up. The diagnosis of PSC without CCA was based on ERCP findings or magnetic resonance cholangiopancreatography at our institution. In patients with PSC alone, the cross-sectional imaging showed no mass, the patients did well clinically and/or liver transplantation explants did not show cancer. So, all patients included in this study had PSC.
Biliary Fluid Sampling Procedure
At the time of ERCP, bile was obtained once cannulation of the common bile duct was accomplished before injection of contrast. Approximately 1 to 5 mL of bile was aspirated through the sphincterotome. The bile samples were transported to the laboratory on ice, and frozen at −80°C until use.
VOCs Measurement in Bile
Approximately 1ml of bile from the samples was extracted and centrifuged. Bile samples were centrifuged for 8 minutes at 150g and 4°C. Subsequently, 200 microliters was extracted and put into a 20 ml headspace vial and the vial was sealed. The samples were heated to 40°C to allow the VOCs in the headspace to equilibrate with the samples. 20ml of headspace gas was removed with a gas syringe and analyzed with a selected ion flow tube mass spectrometry instrument (VOICE200® SIFT-MS, Syft Technologies Ltd, Christchurch, New Zealand). The mass spectrometry assay was comprised of the following 22 common analytes: 2-propanol, acetaldehyde, acetone, acetonitrile, acrylonitrile, benzene, carbon disulfide, dimethyl sulfide, ethanol, isoprene, pentane, 1-decene, 1-heptene, 1-nonene, 1-octene, 3-methylhexane, 2-nonene, ammonia, ethane, hydrogen sulfide, triethyl amine, and trimethyl amine (TMA).
The measurements were made in duplicate at the time of the analysis (1 sample with 1 20 mL aspiration that was run twice). The coefficient of variation for each VOC was within 5%.
Statistical analysis
Descriptive statistics were computed for all factors. These include medians, 25th and 75th percentiles, range or mean and standard deviation for quantitative variables and frequencies and percentages for categorical factors. The 2 diagnosis groups of CCA and PSC were compared with respect to bile VOC’s using the Wilcoxon rank sum test for pairwise group comparisons. Logistic regression analysis was performed to build predictive models for CCA, with the different compounds were considered for inclusion. A model was created for prediction of CCA vs. PSC. The bile VOC’s included as independent variables for the logistic regression models were selected in a stepwise fashion based on greatest improvement among candidate predictors in Akaike’s information criterion. Receiver Operating Characteristics (ROC) analysis was performed and the area under the ROC curves (AUC) and corresponding 95% confidence intervals were calculated. R software version 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria) was used to perform all analyses.
RESULTS
The headspaces from 32 bile samples were analyzed (21 with PSC and no CCA and 11 with PSC and CCA). Table 1 highlights the characteristics of the entire study cohort with comparative laboratory features at the time of ERCP.
Table 1.
Demographic and clinical characteristics of Study Cohort
| Factor | Cholangiocarcinoma (CCA) N=11 |
Primary Sclerosing Cholangitis (PSC) N=21 |
p- value (PSC VS CCA) |
|---|---|---|---|
| Male, N (%) | 7(63.6%) | 11(52.4%) | 0.71 |
| Caucasian, N (%) | 9(81.8%) | 17(85%) | 0.53 |
| Body mass index, Mean (SD) | 27.26(9.5) | 25.1(5.0) | 0.97 |
| Albumin median [interquartile range] (g/dL) | 3.4(2.9–4.4) | 3.5(3.0–4.1) | 0.77 |
| Bilirubin median [interquartile range] (mg/dL) | 2.2(0.8–10.3) | 1.8(0.5–3.4) | 0.41 |
| Concomitant Inflammatory bowel disease (IBD) | 2 (18.2%) | 12 (57.1%) | 0.03 |
| Current Smoking | 3 (27.3%) | 3 (14.3%) | 0.39 |
| Current alcohol use | 2 (18.2%) | 1 (4.8%) | 0.27 |
| Distribution of PSC | |||
| Intrahepatic | 0 | 4 (19%) | |
| Extrahepatic | 2 (18.2%) | 4 (19%) | 0.10 |
| Both | 9 (81.8%) | 13 (61.9%) | |
| Alkaline phosphatase median [interquartile range] (U/L) | 414(225.5–709.5) | 227.5(154.8–475.5) | 0.27 |
| Aspartate aminotransferase median [interquartile range] (U/L) | 93(26.5–127) | 75.5(32.3–101) | 0.74 |
| Alanine aminotransferase median [interquartile range] (U/L) | 89(34.5–107.5) | 58(40–113) | 0.91 |
| Carbohydrate antigen 19-9 (CA 19-9), U/L, mean (SD) | 250.2 (114.6) | 162.6 (58.1) | 0.14 |
Clinical Characteristics of the Study cohort
Among the 21 patients with PSC and no CCA, 6 had dominant strictures. Of 6 patients with dominant strictures all 6 had negative brush cytology and FISH and none developed CCA during the minimum 1 year follow-up. Five of the 21 (23.8%) patients had established PSC cirrhosis. Four patients in this group who underwent liver transplantation did not have any CCA in their explants.
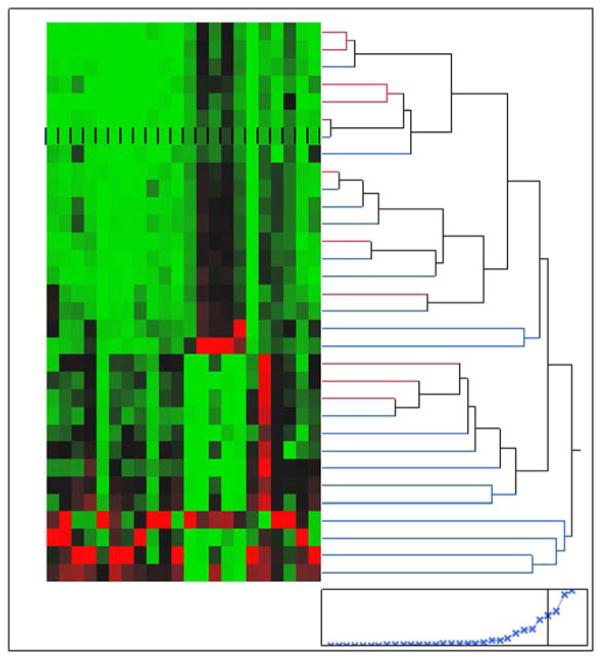
All 11 patients with CCA had underlying PSC either before or at the time of diagnosis of CCA. With regard to the distribution of CCA, 5 patients had hilar CCA, whereas the remaining 6 patients had distal CCA. Before ERCP, only 3 patients had an obvious mass lesion on cross-sectional imaging. Brushings for cytology were performed 3 times which finally revealed CCA in 3 patients, whereas 2 patients had FISH positive for polysomy after 4 non-diagnostic brushings. EUS with FNA of the lymph nodes established diagnosis in 2 patients, whereas EUS-FNA of the mass revealed diagnosis in 3 patients. One patient had a diagnosis of CCA established at the time of liver transplantation. Figure 1 demonstrates the spectrum of compounds which are seen in bile.
Figure 1.
Demonstrates the spectrum of compounds which are seen in bile. The various colors demonstrate the varying compounds.
Biliary VOCs levels in patients with cholangiocarcinoma and PSC
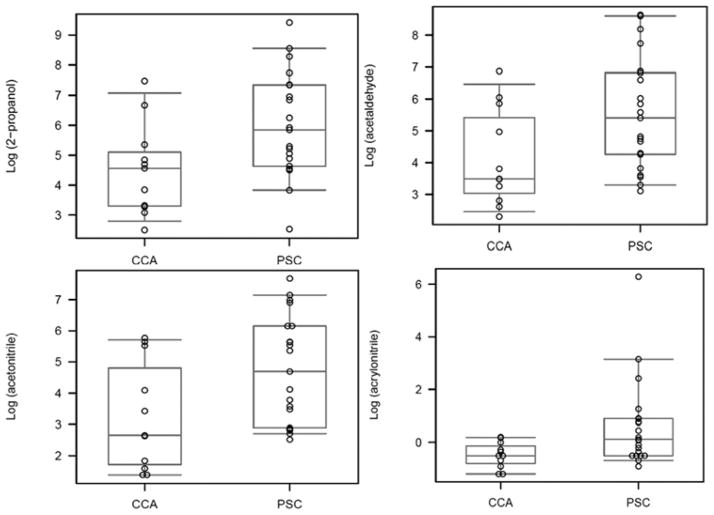
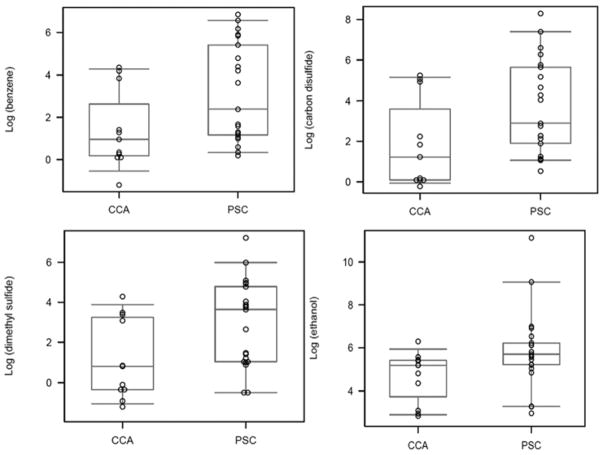
Several compounds were significantly decreased in patients with CCA in setting of PSC compared with PSC without CCA, including ethanol, acrylonitrile, acetonitrile, acetaldehyde, benzene, carbon disulfide, dimethyl sulfide, and 2-propanalol. [Table 2, Figures 2 and 3]. The levels of VOCs were adjusted for smoking and alcohol use and the results were unchanged. (data not shown)
Table 2.
Volatile Organic Compounds in our Study Cohort
| Volatile organic compounds | Cholangiocarcinoma Median (Interquartile range), ppb | Primary Sclerosing Cholangitis Median (Interquartile range), ppb | P value |
|---|---|---|---|
| 2-propanol | 95.3(27.2–169.9) | 346(102.8–1550) | 0.034 |
| Acetaldehyde | 32.8(21.3–249.4) | 223.8(71–921) | 0.037 |
| Acetone | 343.2(207.5–871.9) | 862.3(519.1–1990) | 0.07 |
| Acetonitrile | 14.3(5.6–154.8) | 109(18.1–470) | 0.025 |
| Acrylonitrile | 0.6(0.5–0.9) | 1.12(0.6–2.43) | 0.017 |
| Benzene | 2.6(1.2–25.3) | 10.9(3.2–223) | 0.02 |
| carbon disulfide | 3.4(1.1–74.8) | 18.1(6.7–282) | 0.025 |
| dimethyl sulfide | 2.2(0.7–26) | 38.1(2.8–120) | 0.016 |
| Ethanol | 177.3(50.3–224.4) | 299(183.4–501) | 0.016 |
| Isoprene | 1.7(0.9–6.0) | 5.4(1.8–18) | 0.07 |
| Pentane | 20.3(10.5–123.9) | 62.1(26.5–213) | 0.07 |
| 1-decene | 3.8(1.8–5.4) | 3.9(0.07–5.1) | 0.55 |
| 1-heptene | 9.6(3.23–13.1) | 6.3(0.09–19.3) | 0.84 |
| 1-nonene | 2.5(1.9–5.3) | 5.3(2.4–8.8) | 0.12 |
| 1-octene | 10.2(4.1–13) | 10(0.5–13.8) | 0.98 |
| 3-methylhexane | 14.8(6–23.6) | 18.9(1.7–33.1) | 0.49 |
| (E)-2-nonene | 4.6(2.1–126.3) | 55.5(5.1–1620) | 0.049 |
| Ammonia | 62.9(56.7–128.9) | 90.4(63.6–145) | 0.49 |
| Ethane | 89.5(67.6–116) | 105.5(97.9–133) | 0.08 |
| hydrogen sulfide | 0.4(0.3–0.5) | 0.3(0.3–0.5) | 0.87 |
| triethyl amine | 0.9(0.7–1.6) | 2(0.9–9.8) | 0.04 |
| trimethyl amine | 12.4(10.1–42) | 35.2(13.6–69.7) | 0.1 |
Figure 2.
Figure demonstrates the box-plot of the various volatile organic compounds (panels A–D) in patients with cholangiocarcinoma (CCA) and primary sclerosing cholangitis (PSC)
Figure 3.
Figure demonstrates the box-plot of the various volatile organic compounds (panels A–D) in patients with cholangiocarcinoma (CCA) and primary sclerosing cholangitis (PSC)
Biliary VOCs as a Diagnostic Test
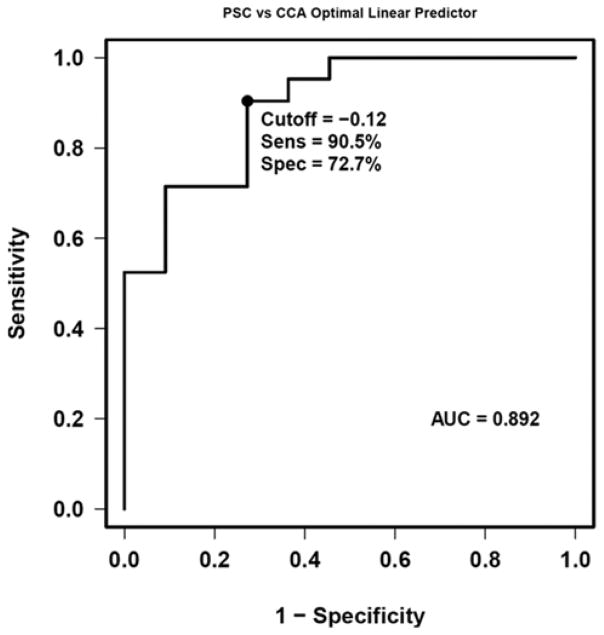
A model was developed for the diagnosis of CCA based on VOC levels of acrylonitrile, 3-methylhexane and benzene. The model [2.3239*log (acrylonitrile) +0.9871*log (3-methylhexane) + 0.8448*log (benzene)] optimized discrimination of PSC patients with and without CCA with an ROC area under the curve (AUC) of 0.89. A cutpoint of < −0.12 optimized test performance with 90.5% sensitivity and 72.7% specificity. (p=0.02) Figure 4 shows the ROC curve for the VOC model for the diagnosis of CCA. We also included another control group of patients with common bile duct stones (N=11) to compare the levels of VOCs. The levels of VOCs in PSC and common bile duct stones were no different, particularly for those which were significantly different between PSC-CCA and PSC without CCA. [Supplementary table 1]
Figure 4.
Receiver operating characteristics (ROC) curve and ROC characteristics as defined by area under the curve (AUC) for volatile organic compounds and the logistic regression model for differentiation of cholangiocarcinoma from primary sclerosing cholangitis. A value < −0.12 identified the patients with CCA [AUC=0.89], with 90.5% sensitivity and 72.7% specificity.
Adjustment for Other Co-variates
The addition of age and gender did not appear to have substantial impact on the significance of the predictive model for CCA diagnosis. We also adjusted for age, sex, current smoking and alcohol, presence of inflammatory bowel disease and positive cytology and it did not have an impact on the predictive model for CCA diagnosis. (Table 3)
Table 3.
Linear Predictors for Differentiation in our Study Cohort
| Groups to Differentiate | Optimal Linear Predictor (based on logistic regression model) | P-value for Linear Predictor | P-value for Linear Predictor adjusted for age and gender | P-value for Linear Predictor adjusted for age, sex, smoking, alcohol, inflammatory bowel disease and positive cytology |
|---|---|---|---|---|
| PSC from CCA | 2.3239*log(acrylonitrile) + 0.9871*log(3- methylhexane) + 0.8448*log(benzene) | 0.015 | 0.033 | 0.027 |
PSC- primary sclerosing cholangitis
CCA- cholangiocarcinoma
The linear predictors are the coefficients of a logistic regression model with log-transformed volatile organic compounds (VOCs) parameters selected in a stepwise fashion using Akaike’s information criterion. Age and gender were added to the resulting model as covariates to obtain an adjusted p-value for the linear predictor. Additional adjustments for current smoking, alcohol use, inflammatory bowel disease, and positive cytology also don’t explain the statistical significance among those results that were significant without adjustment.
DISCUSSION
In PSC patients who often have multiple fibrotic strictures, the diagnosis of occult CCA can be very challenging. Our current research was to identify bio-markers through bile aspiration which could facilitate the early diagnosis of CCA in patients with PSC. We observed that VOCs of bile fluid may help differentiate CCA and PSC strictures. Our study showed that patients with CCA have a specific pattern of VOC print which are decreased compared to PSC without CCA.
Breath analyses of VOCs have been performed in gastrointestinal and liver diseases. 12–16 However, bile is readily aspirated during ERCP, and VOCs in bile may be more representative of local perturbations in the biliary tract, and are less likely to be influenced by confounding environmental or dietary factors. Several recent studies suggest that VOCs may help diagnose various forms of cancer. VOCs in breath and feces were able to identify colorectal cancers with a sensitivity of up to 85%. 12,13 Our exploration of bile VOCs in pancreatic cancer revealed a specific pattern of VOCs that could accurately identify pancreatic cancer. 9
The decrease in the concentrations of certain VOCs in CCA in PSC patients when compared to PSC patients without CCA is intriguing. For example, the concentration of benzene was lower in CCA. Benzene is an environmental pollutant produced from many sources including tobacco smoking, automobile exhausts and has been linked with alterations in gene expressions.17 Nitrogen containing VOCs like acetonitrile and acrylonitrile have also been identified in breath analysis of smokers along with benzene. 18 In our study, we found significantly decreased levels of benzene, acetonitrile and acrylonitrile in patients with CCA. This persisted even after adjusting for current smoking.
Carbon disulfide, dimethyl sulfide and mercaptopurines are formed from incomplete liver metabolism of sulfur containing amino acids, and increased levels of sulfur containing VOCs and their role in fetor hepaticus has been reported in patients with liver cirrhosis. 16,19 In our study, the levels of carbon disulfide and dimethyl sulfide were significantly elevated in PSC patients (irrespective of the presence or absence of cirrhosis) when compared to patients with CCA.
Endogenous alkanes such as ethane and pentane are formed from peroxidation of polyunsaturated fatty acids which are found in the cell membrane. 20 Inflammatory conditions lead to oxidative stress; and the role of oxidative stress in the pathogenesis of liver diseases including infectious and autoimmune etiologies has been reported. 21 In our study, the levels of ethane and pentane were higher in PSC relative to CCA, suggestive of their role in inflammatory conditions, though they were not statistically significant. Methylated hydrocarbons have also been identified as products of lipid peroxidation. 22 In our study, methyl hexane a branched chain alkane was found to be one of the optimal linear predictors of CCA, based on logistic regression analysis.
We believe that our preliminary observations of bile VOCs for CCA diagnosis are clinically significant for a number of reasons. We identified specific pattern of VOC signature that could be explored in the diagnosis of CCA. Although the use of current technology would be more expensive than currently used cytology and fluorescence in situ hybridization, as suggested previously, the development of enzyme linked immunosorbent assay would make these analyses cheaper to less than $50.
Breath testing is also an attractive option for diagnosing patients with cancer. VOCs are present in breath and are present in a gaseous state due to its high vapor pressure. 20 It would be interesting to see whether VOCs from headspace analysis in our study will translate into a similar exhaled breath print that can non-invasively diagnose CCA.
The strengths of our study were multiple. Our lab personnel involved in the head space analysis in our study were blinded to all clinical information, and all the patients observed overnight fasting before the procedure which could have eliminated the dietary factors from confounding the results of our study. Also, we had adjusted the levels of VOCs for smoking and alcohol use. All the patients included in our analysis had good follow-up prospectively to determine if PSC patients developed CCA or not. The major limitation of our study is that ours was a prospective cross-sectional study. Designing a longitudinal study with bile aspiration prospectively in PSC patients with aggressive surveillance when there is a change in the VOC on follow-up could make it a practical and clinically useful marker in following PSC patients. The small sample size of CCA patients also limits our observations. However, our cohort was well characterized with good clinical follow-up and these patients had a diagnosis of CCA in the background of PSC.
In conclusion, we have shown that bile VOCs may help diagnose CCA in the setting of PSC based on the levels of the three compounds namely acrylonitrile, methyl hexane and benzene, with a sensitivity and specificity of 90.5% and 72.7% respectively. The pattern of VOCs observed in CCA and PSC groups is intriguing, but a definite explanation for the observed changes is beyond the scope of our current observations. Further understanding of the significance of the VOCs detected will enhance research to identify accurate bio-markers. The results observed in bile headspace may someday be translated to breath analysis for a true non-invasive diagnosis of CCA.
Supplementary Material
Acknowledgments
Grant Support: The study is supported by a research grant from the American College of Gastroenterology (ACG) Grant (to U.N), BRCP 08-049 Tech 09-003 Third Frontier Program grant from the Ohio Department of Development (ODOD) [to R.D] and in part by the National Institutes of Health, National Center for Research Resources, CTSA, UL1TR 000439-06 Cleveland, Ohio. Dr Dweik is also supported by the following grants: HL107147, HL081064, HL103453, HL109250, and RR026231 from the National Institutes of Health (NIH).
We also would like to thank the Clinical Research Unit members at the Cleveland Clinic who helped in processing the bile samples.
ACRONYMS/ABBREVIATIONS
- AUC
area under curve
- CCA
cholangiocarcinoma
- EUS
endoscopic ultrasound
- ERCP
endoscopic retrograde cholangiopancreatography
- FNA
fine needle aspiration
- FISH
fluorescence in situ hybridization
- PSC
primary sclerosing cholangitis
- TMA
trimethyl amine
- VOCs
volatile organic compounds
Footnotes
Presented as an oral presentation at the Digestive Disease Week 2014 in Chicago, Illinois
Conflict of Interest
The authors declared no financial conflict of interest pertaining to this paper.
Specific author contributions:
Study concept and design, paper preparation and revisions -U Navaneethan
Data monitoring and paper preparation– N Gutierrez, V Lourdusamy
Running the bile samples-D Grove
Statistical Analysis-J Hammel
Obtaining bile samples – U Navaneethan, A Bhatt, M Sanaka and M Parsi
Study concept, design, and critical revisions- U Navaneethan, M Parsi, J Vargo, T Stevens and R Dweik
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Victor DW, Sherman S, Karakan T, Khashab MA. Current endoscopic approach to indeterminate biliary strictures. World J Gastroenterol. 2012 Nov 21;18(43):6197–205. doi: 10.3748/wjg.v18.i43.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarnagin W, Winston C. Hilar cholangiocarcinoma: diagnosis and staging. HPB (Oxford) 2005;7:244–51. doi: 10.1080/13651820500372533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, Gentile R, Alvaro D. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol. 2010 Nov 15;2(11):407–16. doi: 10.4251/wjgo.v2.i11.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron TH, Harewood GC, Rumalla A, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol. 2004;2:214–219. doi: 10.1016/s1542-3565(04)00006-0. [DOI] [PubMed] [Google Scholar]
- 5.Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014 May;79(5):783–9. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006 Oct;131(4):1064–72. doi: 10.1053/j.gastro.2006.08.021. Epub 2006 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2013 Dec 19; doi: 10.1016/j.gie.2013.11.001. pii: S0016-5107(13)02532-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Levy MJ, Heimbach JK, Gores GJ. Endoscopic ultrasound staging of cholangiocarcinoma. Curr Opin Gastroenterol. 2012 May;28(3):244–52. doi: 10.1097/MOG.0b013e32835005bc. Review. [DOI] [PubMed] [Google Scholar]
- 9.Navaneethan U, Parsi MA, Gutierrez NG, et al. Volatile Organic Compounds in Bile Can Diagnose Malignant Biliary Strictures in the Setting of Pancreatic Cancer-A Preliminary Observation. Gastrointestinal Endoscopy. doi: 10.1016/j.gie.2014.04.016. (In press) [DOI] [PubMed] [Google Scholar]
- 10.Navaneethan U, Gutierrez NG, Venkatesh PG, et al. Lipidomic Profiling of Bile in Distinguishing Benign From Malignant Biliary Strictures: A Single-Blinded Pilot Study. Am J Gastroenterol. 2014 Apr 8; doi: 10.1038/ajg.2014.60. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Navaneethan U, Gutierrez NG, Jegadeesan R, et al. Vascular endothelial growth factor levels in bile distinguishes pancreatic cancer from other etiologies of biliary stricture: a pilot study. Dig Dis Sci. 2013 Oct;58(10):2986–92. doi: 10.1007/s10620-013-2764-0. [DOI] [PubMed] [Google Scholar]
- 12.Altomare DF, Di Lena M, Porcelli F, et al. Exhaled volatile organic compounds identify patients with colorectal cancer. Br J Surg. 2013 Jan;100(1):144–50. doi: 10.1002/bjs.8942. [DOI] [PubMed] [Google Scholar]
- 13.de Meij TG, Larbi IB, van der Schee MP, et al. Electronic nose can discriminate colorectal carcinoma and advanced adenomas by fecal volatile biomarker analysis: proof of principle study. Int J Cancer. 2014 Mar 1;134(5):1132–8. doi: 10.1002/ijc.28446. [DOI] [PubMed] [Google Scholar]
- 14.Pelli MA, Trovarelli G, Capodicasa E, De Medio GE, Bassotti G. Breath alkanes determination in ulcerative colitis and Crohn’s disease. Dis Colon Rectum. 1999 Jan;42(1):71–6. doi: 10.1007/BF02235186. [DOI] [PubMed] [Google Scholar]
- 15.Hanouneh IA, Zein NN, Cikach F, et al. The Breathprints in Patients with Liver Disease Identify Novel Breath Biomarkers in Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2013 Sep 10; doi: 10.1016/j.cgh.2013.08.048. pii: S1542-3565(13)01310-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Velde S, Nevens F, Van Hee P, van Steenberghe D, Quirynen M. GC-MS analysis of breath odor compounds in liver patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:344–348. doi: 10.1016/j.jchromb.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Thomas R, Hubbard AE, McHale CM, et al. Characterization of changes in gene expression and biochemical pathways at low levels of benzene exposure. PLoS One. 2014 May 1;9(5):e91828. doi: 10.1371/journal.pone.0091828. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buszewski B, Ulanowska A, Ligor T, Denderz N, Amann A. Analysis of exhaled breath from smokers, passive smokers and non-smokers by solid-phase microextraction gas chromatography/mass spectrometry. Biomed Chromatogr. 2009 May;23(5):551–6. doi: 10.1002/bmc.1141. [DOI] [PubMed] [Google Scholar]
- 19.Dadamio J, Van den Velde S, Laleman W, et al. Breath biomarkers of liver cirrhosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Sep 15;905:17–22. doi: 10.1016/j.jchromb.2012.07.025. Epub 2012 Jul 31. [DOI] [PubMed] [Google Scholar]
- 20.Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis--focus on volatile organic compounds. Clin Chim Acta. 2004 Sep;347(1–2):25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Probert CS, Ahmed I, Khalid T, Johnson E, Smith S, Ratcliffe N. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J Gastrointestin Liver Dis. 2009 Sep;18(3):337–43. [PubMed] [Google Scholar]
- 22.Phillips M, Cataneo RN, Greenberg J, Grodman R, Gunawardena R, Naidu A. Effect of oxygen on breath markers of oxidative stress. Eur Respir J. 2003 Jan;21(1):48–51. doi: 10.1183/09031936.02.00053402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.