Abstract
Chemotherapy with docetaxel (Doc) is a standard treatment for metastatic and castration-resistant prostate cancer. However, chemoresistance and side effects of Doc limit its clinical success. We investigated whether natural products green tea (GT) and quercetin (Q), a flavonoid from apples and onions, will enhance the efficacy of Doc in androgen-independent (AI) prostate cancer cells. Two cell lines including LAPC-4-AI and PC-3 were treated in vitro with 40μM of (−)-epigallocatechin gallate (EGCG), 5μM of Q, 2nM or 5nM of Doc alone or in combination. The mixture of EGCG+Q+Doc increased the anti-proliferative effect by 3-fold in LAPC-4-AI cells and 8-fold in PC-3 cells compared to Doc alone. EGCG, Q and Doc in combination significantly enhanced cell cycle arrest at G2/M phase and increased apoptosis in both LAPC-4-AI and PC-3 cells compared to Doc alone. The mixture increased the inhibition of PI3K/Akt and the signal transducer and activator of transcription (Stat) 3 signaling pathways compared to Doc alone, and decreased the protein expression of multidrug resistance-related protein (MRP1). In addition, the combination with EGCG and Q increased the inhibition of tumor cell invasion and colony formation in both LAPC-4-AI and PC-3 cells compared to Doc alone, and decreased the percentage of CD44+/CD24− stem-like LAPC-4-AI cells. In summary, GT and Q enhanced the therapeutic effect of Doc in castration-resistant prostate cancer cells through multiple mechanisms including the downregulation of chemoresistance-related proteins. This study provides a novel therapeutic modality to enhance the efficacy of Doc in a non-toxic manner.
Keywords: Green tea polyphenol, quercetin, docetaxel, prostate cancer, combination
1. Introduction
Prostate cancer is the most commonly diagnosed male malignancy and the second-leading cause of cancer death among men in the United States [1]. Androgen deprivation therapy (ADT) remains the main treatment for advanced and metastatic prostate cancer [2]. However, despite initial response nearly all patients on ADT progress to castration-resistant prostate cancer (CRPC) in 18–24 months and no curative treatments currently exist for CRPC [3]. Chemotherapy with docetaxel (Doc) is currently a standard treatment for metastatic and castration-resistant prostate cancer, and remains a backbone in current drug development [4]. Doc, a member of the family of taxanes, is an analogue of paclitaxel, a naturally occurring mitotic inhibitor isolated from the bark of the Pacific yew tree Taxus Brevifolia. Doc binds to microtubules causing mitotic arrest and ultimately cell apoptosis [5]. However, the development of chemoresistance to Doc is observed in most patients and limits its clinical success [5]. The upregulation of multidrug resistance (MDR) phenotypes including p-glycoprotein and multidrug resistance-associated proteins (MRPs) may be one of the mechanisms of Doc resistance [5]. In addition, the alterations in signaling pathways may cause resistance to Doc-induced apoptosis [5]. For example, the overexpression of anti-apoptotic gene Bcl-2 and activation of nuclear factor-kappa B (NFκB) and Akt activity are commonly observed in CRPC patients undergoing Doc treatment [4, 6]. The median progression-free survival with Doc treatment remains around 6 months and overall survival less than 2 years [4]. In addition, some severe side effects are associated with Doc treatment including the suppression of bone marrow function leading to immunodysfunction and anemia [7]. Clearly, it is of high clinical significance to enhance the efficacy of Doc at lower doses in a less-toxic manner and to reduce its side effects.
Green tea (GT) is produced from the leaves of the plant Camellia sinensis. The major bioactive components of GT are GT polyphenols (GTPs), mainly including (−)-epigallocatechin, (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin, and (−)-epicatechin-3-gallate, with EGCG as the most abundant and most bioactive component [8]. The anti-cancer activities of GTPs have been demonstrated in several cancers including prostate, mammary gland, colon, pancreas, liver, esophagus and liver cancer [8, 9]. GTPs target multiple signaling pathways in anti-carcinogenesis including the inhibition of NFκB and PI3K/Akt pathways, the induction of apoptosis and cell cycle arrest [9–12]. Oral infusion of GTPs equivalent to a realistic dose for human consumption (4–6 cups of tea daily for an average adult human) significantly inhibited prostate cancer development and distant site metastasis in transgenic adenocarcinoma in mouse prostate (TRAMP) mouse models and increased their overall survival when GT was administered during tumor initiation [13]. A one-year GT extract intervention in men with high-grade prostate intraepithelial neoplasia (PIN) showed a lower prostate cancer incidence of 3% in the tea group consuming 600 mg/d GT extracts versus 30% in the placebo group [14]. Likewise, in a pre-prostatectomy trial of a GT supplement, McLarty et al. demonstrated a decrease in serum prostate-specific antigen (PSA) levels and decreased prostate tissue vascular endothelial growth factor (VEGF) and hepatocyte growth factor concentrations [15]. Nevertheless, the low bioavailability and extensive methylation of GTPs in vivo to less active metabolites limit the anti-cancer activity of GT [9, 16]. We were able to demonstrate that the combined use of quercetin (Q) with GT significantly increased the bioavailability and cellular uptake of GTPs and decreased their methylation in vitro and in vivo, possibly through the inhibition of multidrug resistance-associated proteins (MRPs) and catechol-O-methyltransferase (COMT), leading to a synergistically enhanced inhibition of xenograft prostate tumor growth in severe combined immunodeficiency (SCID) mice [17–19]. Q is a flavonoid found in most edible vegetables and fruits particularly in onions, apples, and red wine. The inhibitory effects of Q on MRPs, p-glycoprotein, and COMT have been well documented [20–23]. Q itself has exhibited chemopreventive activities especially in prostate cancer through multiple mechanisms including the induction of apoptosis and the inhibition of proliferation and insulin-like growth factor (IGF)-1 pathway [24–27].
In respect to the multiple targeting activities of GTPs and Q in anti-carcinogenesis particularly their activities on NFκB, PI3K/Akt pathways and MRPs, they can be ideal candidates to be combined with Doc to enhance the therapeutic effect in a non-toxic manner. In the present study we investigated the combined therapeutic effect of the mixture of EGCG, Q and Doc in androgen-independent LAPC-4-AI, and PC-3 prostate cancer cells. This study is anticipated to provide a novel modality to improve clinical practice in treatment of CRPC with enhanced drug efficacy and reduced side effects.
2. Materials and Methods
2.1. Cell line and cell culture
The androgen-independent PC-3 human prostate cancer bone metastasis cell line was purchased from American Type Culture Collection (ATCC, Chicago, IL). The localized prostate cancer LAPC-4 cell line is a gift from Dr. Charles Sawyers’ laboratory at UCLA. Androgen-independent LAPC-4-AI cells were developed by culturing androgen-dependent LAPC-4 cells in medium supplemented with androgen free charcoal-stripped fetal bovine serum (FBS). The proliferation of parental LAPC-4 cells was decreased by 20% after 96h culture in androgen free medium with fresh medium changed every two days, compared to that in regular medium. However, the growth of LAPC-4-AI cells was not reduced in androgen free medium. The LAPC-4-AI cells were used in this study in addition to PC-3 cells. Both cell lines were cultured in RPMI 1640 medium, supplemented with 10% (v:v) of FBS, 100 IU/mL of penicillin and 100 μg/mL of streptomycin at 37 °C in a 5% CO2 incubator. Normal human prostate epithelial PrEC cells were purchased from Lonza Walkersville, Inc (Walkersville, MD) and maintained in prostate epithelial cell PrEGM medium (Lonza Walkersville, Inc).
2.2. Cell proliferation assay
LAPC-4-AI and PC-3 cells were seeded into opaque-wall 96-well plates at a density of 8×103 per well. An inhibition curve was achieved for individual compound including EGCG, Q and Doc by incubation of both cell lines with multiple doses of each compound for 48h. A dose that leads to 10–30% cell growth inhibition by each compound was selected for the combination study. Cells were treated with the following: vehicle control (DMSO), 40μM EGCG (Sigma-Aldrich, St Louis, MO), 5μM Q (Sigma-Aldrich), 5nM Doc (Sigma-Aldrich), EGCG+Q, EGCG+Doc, Q+Doc, or EGCG+Q+Doc for 24 and 48h. In addition, the combined effect of the mixture was compared with a higher dose of Doc at 20nM. Cell proliferation was measured with adenosine triphosphate (ATP) assay using the CellTiter-Glo® Luminescent cell viability assay kit (Promega Corporation, Madison, WI). To minimize the effect of hydrogen peroxide (H2O2) that may be formed by autoxidation and/or dimerization of EGCG and Q in cell culture medium [28], 50 units/mL of catalase was added to the medium prior to EGCG, Q and Doc in all the experiments in the present study. There were four wells for each of the treatments and the experiment was repeated twice.
2.3. Cell cycle and apoptosis analysis
When 50–60% confluent in T25 flasks, both LAPC-4-AI and PC-3 cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc for 48h. Cells were trypsinized and monolayers attaching to the bottom were collected. The procedures for cell cycle and apoptosis analysis using a small cytometer Cellometer Vision (Nexcelom Bioscience LLC, Lawrence, MA) were described previously [29, 30] with minor modifications. Briefly, for cell cycle assay cells were centrifuged and pellet resuspended and fixed in cold methanol. The cells were centrifuged again and pellet was stained in propidium iodide (PI) solution (Nexcelom Bioscience LLC) for imaging cytometry using Cellometer Vision. For apoptosis assay, cells were centrifuged and pellets were resuspended in Annexin V binding buffer and double-stained with Annexin V-FITC (Nexcelom Bioscience LLC) and PI (Nexcelom Bioscience LLC) for Cellometer analysis. A positive control was generated by heating cells in a 45°C water bath for 10min. Non-treated cells were used as negative control. Both controls were processed with the samples. The fluorescence data generated by the Cellometer software were converted into FCS files and analyzed by De Novo FCS Express 4 software (Los Angeles, CA). The experiment was performed in triplicate.
2.4. Western blot analysis of protein biomarkers
LAPC-4-AI and PC-3 cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc for 48h. Total protein was extracted using RIPA buffer (Santa Cruz Technology, CA). The procedure for Western blot analysis was described before [31]. Briefly, 50 μg of protein was separated on a 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA). Proteins were electrotransferred to nitrocellulose membranes. Membranes were incubated with primary anti-human antibodies for the detection of Bax (sc-493), Bcl-2 (sc-509), MRP1 (sc-7773, Santa Cruz Technology), Akt (4685), p-Akt (Ser473, 4058), STAT3 (9132), and p-STAT3 (4058, Cell Signaling Technology, Danvers, MA). GAPDH protein was used as loading control. Protein was visualized and analyzed using a ChemiDoc XRS chemiluminescence detection and imaging system (Bio-Rad Laboratories, Irvine, CA).
2.5. Cell invasion assay
The ability of EGCG and Q to enhance the effect of Doc in inhibition of cell invasion was tested in LAPC-4-AI and PC-3 cells using transwell chamber assay. The chamber is 24-well plate based with an insert of 8μm pore size polyethylene terephthalate membrane (Corning Life Sciences, Tewksbury, MA). Cells were cultured until 50–60% confluency and treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc or EGCG+Q+Doc for 48h. The cells were starved in serum-free medium overnight. The transwell chamber insert was coated with 20μl of 1:6 diluted Matrigel (BD, Cambridge, MA) and incubated in 37°C for 20 min to solidify. After trypsinization 1×05 cells were collected, suspended in 200μl serum-free medium and added on the upper well. 300μl of complete growth medium was added to the bottom. After 20h incubation, cells were fixed with 5% glutaraldehyde and stained with 0.5% toluidine blue as desribed previously [32]. Cells on the upper membrane was wiped off with a cotton swab. Invaded cells on the lower membrane was counted under a microscope at ×200 magnification. Three fields were randomly selected and counted for each well. The experiment was performed in triplicate.
2.6. Tumor cell colony formation assay
LAPC-4-AI and PC-3 cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc or EGCG+Q+Doc for 48h. A 24-well plate was coated with 200μl Matrigel per well and incubated in 37°C for 20 min. After trypsinization 2×104 cells in 200μl complete medium were added to each well. Cells were incubated for 5 days. 200μl of fresh medium was added every two days. Pictures were taken from three fields of each well under a microscope at ×100 magnification. The nmber of cell colony which contained at least 10 cells were counted. The experiment was perfored in triplicate.
2.7. Flow cytometry analysis of CD44/CD24 surface markers
CD44+/CD24− prostate cancer cells have been show to possess stem cell characteristics and are more proliferative, clonogenic, tumorigenic, and metastatic than CD44−/CD24− cells [33]. We evaluated the ability of the combination treatment to modulate the cell expression of these surface markers using flow cytometry analysis. LAPC-4-AI and PC-3 cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc or EGCG+Q+Doc for 48h. Cells were trypsinized and 1×106 cells were collected and suspended in 1mL of fresh growth medium. The cells were double-stained with 20ul of each of CD24-PE and CD44-FITC conjugates (Nexcelom Bioscience LLC, Lawrence, MA), and incubated at 4 °C on a rocker for 1h. The cells were centrifuged at 2000 rpm for 5 min and re-suspended in 500ul PBS for flow cytometry analysis on a BD LSRFortessa X-20 Cytometer (BD Biosciences, San Jose, CA). The data were analyzed using FACSDiva 7.0 software (BD Biosciences). The experiment was performed in triplicate.
2.8. Statistical analysis
The statistical analyses were performed using SPSS software (Version 20.0, Chicago, IL). Data were presented as mean ± standard deviation (SD). Comparison of means was performed by one-way analysis of variance (ANOVA) with Tukey’s posttest for paired comparisons. Differences were considered significant if P<0.05.
3. Results
3.1. Enhanced anti-proliferative effect
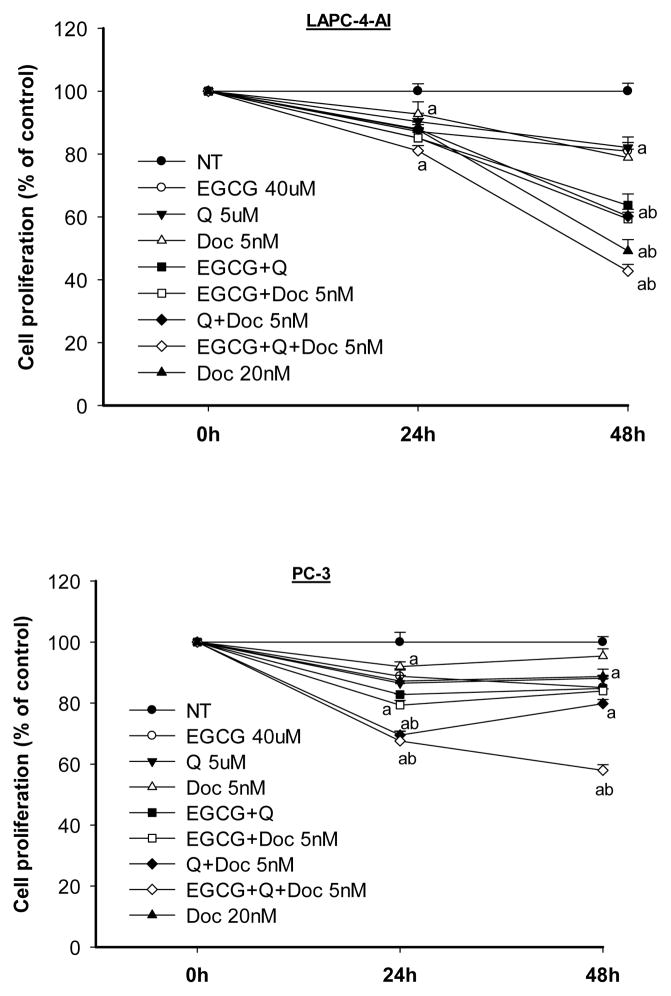
Both EGCG and Q significantly increased the anti-proliferative effect of Doc in LAPC-4-AI and PC-3. The strongest anti-proliferative effect was achieved by treatment with a mixture of the three chemicals (Fig. 1). In LAPC-4-AI cells treatment with EGCG and Q with Doc 5 nM inhibited proliferation to the same extend as Doc 20 nM alone. At 48h, the growth of LAPC-4-AI cells was inhibited by 19% (EGCG), 18% (Q), 21% (5nM Doc), 36% (EGCG+Q), 41% (EGCG+Doc), 40% (Q+Doc), and 57% (EGCG+Q+Doc). PC-3 cells were less sensitive to Doc than LAPC-4-AI but the combination treatment with EGCG, Q and Doc 5 nM exhibited a much stronger anti-proliferative effect as compared to all individual treatments. At 48h PC-3 cell growth was inhibited by 5% and 11% by Doc at 5nM and 20nM, respectively. However, the combination of 5nM Doc with EGCG and Q inhibited PC-3 cell growth by 42% (Fig. 1).
Fig. 1.
EGCG and Q in combination with Doc enhanced the antiproliferative effect in androgen-independent LAPC-4-AI and PC-3 cells. Cells were treated with the indicated concentrations of EGCG, Q and Doc alone or in combination for 24 and 48h. Cell proliferation was measured by ATP assay. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel. Compared to a-NT; b- EGCG, Q or low dose of Doc, P<0.05.
A combination index (CI) was calculated for the mixture of all three chemicals using the CompuSyn software (ComboSyn, Inc., Paramus, NJ) which is based on the widely accepted Chou-Talalay equation and mass-action law [34]. The value of CI less than 1 indicates a synergistic effect of a combination, equal to 1 additive, and greater than 1 antagonistic [34]. The combination of a series of concentrations of EGCG (40–60 μM) and Q (5–10 μM) with Doc (2–5 nM) achieved CIs of 0.6–0.8 in LAPC-4-AI cells, and 0.6–0.7 in PC-3 cells.
3.2. Effect on cell cycle arrest and apoptosis
The strongest effect observed on LAPC-4-AI cells by treatment with EGCG+Q was a 8-fold increase in apoptosis, whereas the addition of Doc 5nM had a stronger effect on cell cycle arrest in the G2/M phase, which was further increased by the combination treatment with EGCG, Q and Doc (Table 1). In PC-3 cells treatment with EGCG and Q did not induce apoptosis as much as in LAPC-4-AI cells but the combination treatment with all 3 compounds three-fold (Table 2).
Table 1.
Cell cycle distribution and apoptosis in LAPC-4-AI cells
| Treatment | Cell cycle distribution (%)
|
Apoptosis (%) | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| NT | 71.8 ± 1.7a | 13.1 ± 2.1a | 14.9 ± 0.9a | 2.2 ± 0.2a |
| EGCG+Q | 63.4 ± 3.4b | 16.0 ± 2.1ab | 19.9 ± 2.1b | 17.6 ± 1.3b |
| Doc 5nM | 60.0 ± 3.3b | 17.4 ± 2.6ab | 21.7 ± 2.6b | 7.6 ± 0.4c |
| EGCG+Q+Doc | 52.6 ± 2.2c | 18.4 ± 0.7b | 28.7 ± 1.4c | 15.1 ± 0.7b |
Androgen-independent LAPC-4-AI cells were cultured in T25 flasks until 50–60% confluent. Cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc for 48h. Cells were trypsinized and the monolayer attached to the bottom was collected for cell cycle and apoptosis analysis using a small cytometry system Cellometer Vision. Cells were stained with propidium iodide (PI) for cell cycle assay, double-stained with Annexin V-FITC and PI for apoptosis assay. The experiment was performed in triplicate and repeated twice with similar results. Values from one representative experiment were presented in mean ± SD. Values with different superscripts in each of the columns are significantly different. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel.
Table 2.
Cell cycle distribution and apoptosis in PC-3 cells
| Treatment | Cell cycle distribution (%)
|
Apoptosis (%) | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| NT | 72.9 ± 0.8a | 8.9 ± 1.1a | 17.9 ± 0.4a | 3.5 ± 0.2a |
| EGCG+Q | 66.3 ± 0.7b | 11.9 ± 0.2b | 21.5 ± 1.0b | 4.9 ± 0.3b |
| Doc 5nM | 71.2 ± 0.5a | 10.0 ± 1.3a | 18.0 ± 0.2a | 5.5 ± 1.1b |
| EGCG+Q+Doc | 62.6 ± 0.5c | 11.8 ± 0.6b | 24.5 ± 1.1c | 10.1 ± 0.7c |
PC-3 cells were cultured in T25 flasks until 50–60% confluent. Cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc for 48h. Cells were trypsinized and monolayer attaching to the bottom was collected for cell cycle and apoptosis analysis using a small cytometor Cellometer Vision. Cells were stained with propidium iodide (PI) for cell cycle assay, double-stained with Annexin V-FITC and PI for apoptosis assay. The experiment was performed in triplicate and repeated twice with similar results. Values from one representative experiment were presented in mean ± SD. Values with different superscripts in each of the columns are significantly different. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel.
3.3. Modulation on protein expression involved in apoptosis, proliferation and drug resistance
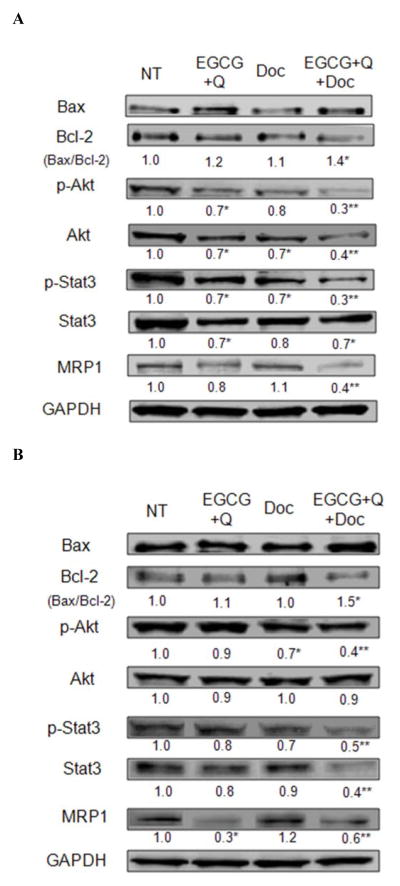
The combination treatment of EGCG, Q and Doc significantly increased the ratio of Bax/Bcl-2 protein expression compared to control in both LAPC-4-AI and PC-3 cells, mainly through decreasing the expression of Bcl-2 (Fig. 2). The three chemicals in combination significantly increased the inhibition of the phosphorylation of both Akt and the signal transducer and activator of transcription (Stat) 3 compared to EGCG+Q or Doc alone in both cell lines. The mixture also increased the inhibition of Akt and Stat3 protein expression in LAPC-4-AI cells and PC-3 cells, respectively, compared to EGCG+Q or Doc alone. In LAPC-4-AI cells only the combination of all three compounds significantly inhibited the protein expression of MRP1, while in PC-3 cells EGCG+Q had a stronger effect on MRP1 protein expression compared to the treatment with all three compounds (Fig. 2).
Fig. 2.
Modulations on the expression and phosphorylation of proteins involved in apoptosis, proliferation and drug resistance. LAPC-4-AI (A) and PC-3 (B) cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc at same concentrations for 48h. Protein expression and phosphorylation were analyzed with Western blot. Data are presented as mean values. * Compared to NT, ** compared to NT, GT+Q or Doc group, P<0.05. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel.
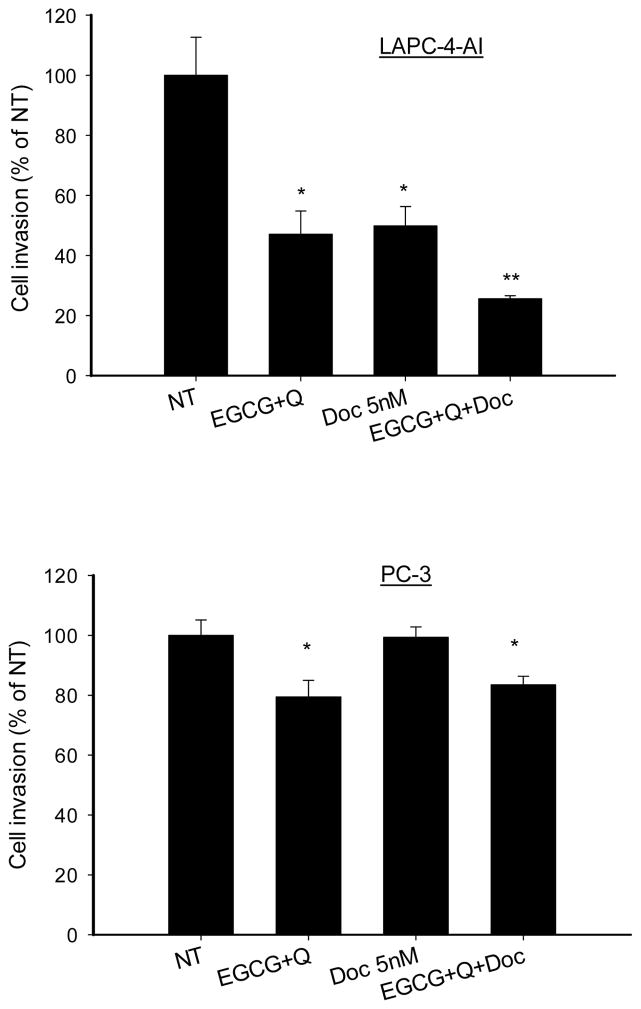
3.4. Inhibition of tumor cell invasion
The invasion of LAPC-4-AI cells through Matrigel was inhibited by 53% (EGCG+Q), 50% (Doc 5nM) and 74% (EGCG+Q+Doc) compared to control (Fig. 3). In PC-3 cells Doc alone did not inhibit the cell invasion compared to control. However, both EGCG+Q and the combination of Doc with EGCG and Q significantly inhibited PC-3 cell invasion by 20% compared to control or Doc alone (Fig. 3).
Fig. 3.
EGCG and Q in combination with Doc enhanced the inhibition of tumor cell invasion. LAPC-4-AI (A) and PC-3 (B) cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc or EGCG+Q+Doc at same concentrations for 48h. The cells were starved in serum-free medium overnight. Then the cells were seeded on the upper membrane of transwell chamber which was pre-coated with Matrigel. Complete growth medium was added to the bottom. After 20h incubation, cells on the lower membrane of chambers were stained and counted. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel. * Compared to NT or Doc treatment, P<0.05.
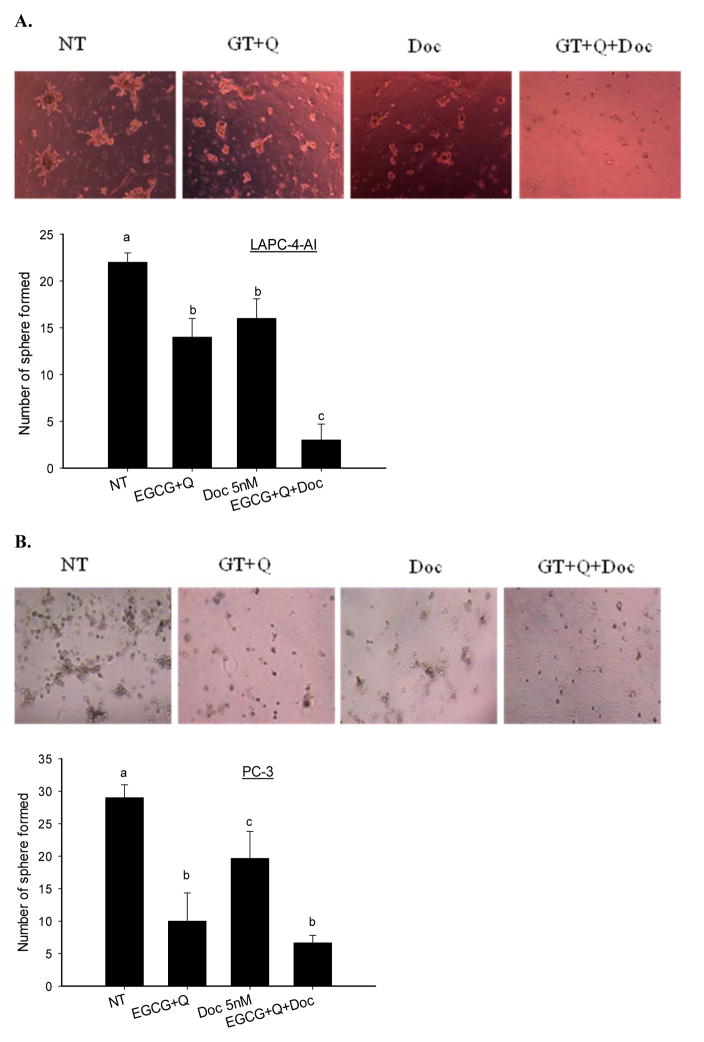
3.5. Inhibition of colony formation
The combination of EGCG, Q and Doc significantly enhanced the inhibition of tumor cell colony formation in LAPC-4-AI cells compared to EGCG+Q or Doc alone (Fig. 4). After 5 days the formation of tumor colony was inhibited by 36% (EGCG+Q), 27% (Doc 5nM) and 86% (ECGG+Q+Doc) in LAPC-4-AI cells. In PC-3 cells treatment with EGCG+Q demonstrated a 2-fold stronger effect compared to Doc in inhibition of tumor colony formation (Fig. 4). The formation of tumor colony was inhibited by 66% (GT+Q), 32% (Doc) and 77% (GT+Q+Doc) in PC-3 cells after 5-days incubation.
Fig. 4.
EGCG and Q in combination with Doc enhanced the inhibition of tumor cell colony formation. LAPC-4-AI (A) and PC-3 (B) cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc at same concentrations for 48h. Then the cells were seeded onto 24-well plate which was pre-coated with Matrigel. Fresh medium was changed every two days and the cells were allowed to grow for 5 days. The number of colonies containing at least 10 cells was counted. Data are presented as mean ± SD. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel. Compared to NT, *P<0.05; **P<0.01.
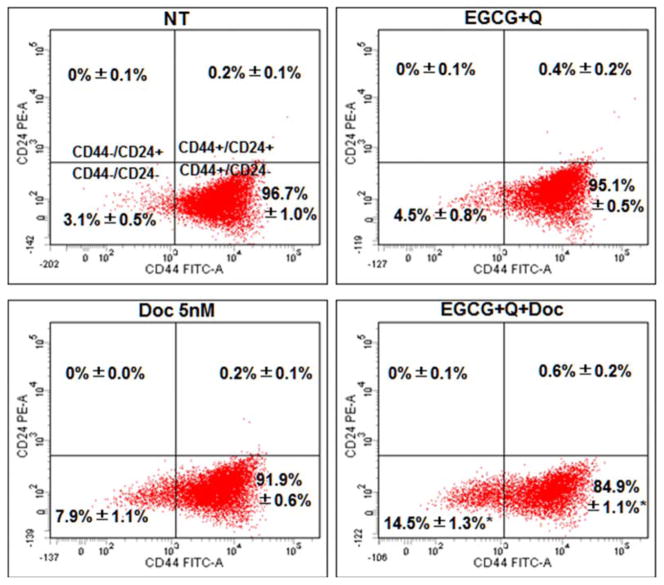
3.6. Modulation on surface marker expression
Doc alone slightly and not significantly decreased the percentage of CD44+/CD24− cells in LAPC-4-AI cells compared to control (Fig. 5). Treatment with EGCG+Q did not change the expression of these surface markers. However, the combination of EGCG and Q with Doc significantly decreased the percentage of CD44+/CD24− LAPC-4-AI cells compared to control, EGCG+Q, or Doc alone (Fig. 5). There was no effect observed in PC-3 cells with any treatment in modulation of these surface markers.
Fig. 5.
EGCG, Q and Doc in combination decreased the percentage of CD44+/CD24− LAPC-4-AI cells.
LAPC-4-AI cells were treated with vehicle control, 40μM EGCG+5μM Q, 5nM Doc, or EGCG+Q+Doc at same concentrations for 48h. The monolayer cells attaching to the bottom were collected and double-stained with CD24-PE and CD44-FITC conjugates for flow cytometry analysis using a BD LSRFortessa X-20 Cytometer. The experiment was performed in triplicate. Data are presented as mean values. NT: non-treatment, DMSO control; Q: quercetin; Doc: docetaxel. * Compared to NT, P<0.05.
4. Discussion
The present study demonstrates that a novel regimen by combining natural products GT and Q with Doc significantly enhanced the therapeutic effect of Doc in castration-resistant prostate cancer cells. Both GT and Q were able to increase the anti-proliferative effect of Doc, and the strongest effect was achieved by the combination of the three chemicals. Doc is currently a standard first-line treatment for CRPC usually used in combination with prednisone. An enhanced efficacy of Doc along with reduced side effects will provide significant benefits to CRPC patients to improve survival and quality of life. Several chemotherapy drugs have been tested for combination with Doc to improve CRPC treatment [35]. However, no superiority to Doc/prednisone effect has been shown in phase III trials. In addition, these drug-drug combinations increase the challenge of adverse effects [35]. The anti-cancer activities of GT and Q have been well demonstrated in many preclinical studies [8, 9]. Although results from human studies are not consistent, the majority of these studies support a preventive effect of GT in prostate cancer [9]. Both GT and Q target multiple signaling pathways involved in carcinogenesis, which may potentially provide a systemic control on cancer growth [8]. Since a cancer may have hundreds of gene mutations and dysfunctions and many pathways crosstalk with each other in tumor growth, it may not be able to control a cancer by targeting single or few signaling pathways. As a result chemoresistance may appear during treatment, which is a major reason of the failure of chemotherapy drugs [36]. Natural products like GT and Q have been shown to selectively target cancer cells, while with minimum toxicity in normal cells [37]. The present study demonstrated that GT and Q in combination with Doc did not increase the toxicity in normal prostate epithelial PrEC cells compared to individual compounds (data not shown). The enhanced anti-proliferative effect of the mixture was associated with an increased induction of apoptosis in both LAPC-4-AI and PC-3 cells compared to Doc alone. An increased ratio of Bax to Bcl-2 protein expression by Western blot analysis was associated with the observations from the fluorescence imaging of apoptosis using Cellometer. In addition, these three chemicals in combination enhanced the cell cycle arrest at G2/M phase in both LAPC-4-AI and PC-3 cells compared to EGCG+Q or Doc. These results suggest a promising non-toxic means by combination with GT and Q to enhance the efficacy of Doc.
Both GT and Q target multiple events and signaling pathways throughout the stages of tumor initiation, promotion and progression [10, 38]. The combination treatment may increase the effect on these molecular targets by a sum of the activities of individual compounds. Regarding the important role of phosphatidylinositol 3-kinases (PI3K)/Akt pathway in cancer growth and progression as well as the development of drug resistance [3], we examined the combined effect of the mixture on this pathway. Akt functions upon phosphorylation by phosphorylated PI3K and activates its substrates, one being mTOR, leading to increased cell proliferation and survival [3]. The combination of GT and Q with Doc significantly increased the inhibition of the phosphorylation of Akt in both LAPC-4-AI and PC-3 cell lines compared to GT+Q or Doc alone. We further evaluated the inhibitory effect of the combination treatment on the protein expression of MRP1, a transport protein commonly found involved in the resistance to chemotherapy drugs [5]. The results demonstrated that GT and Q significantly decreased the level of MRP1 protein in both cells lines with or without the combination with Doc. These results suggest a promise of GT and Q to inhibit the development of chemoresistance during Doc treatment.
The invasion and colony formation of tumor cells play a critical role in development of metastasis, thus they are important targets in cancer therapy [39]. The treatment with GT+Q+Doc exhibited the strongest effect on tumor cell invasion and colony formation in LAPC-4-AI cells, and a stronger effect than docetaxel alone in PC-3 cells. An increased inhibition of the STAT3 signaling pathway by the combination treatment may partly contribute to the enhanced inhibitory effect on cell invasion and colony formation in both cell lines. The transcription factor STAT3 becomes activated by phosphorylation in response to cytokines and growth factors, then it enters the nucleus to mediate the expression of various genes in regulation of cell growth, survival and motility [40]. A recent study showed that the inhibition of STAT3 by EGCG significantly inhibited cell motility, migration and invasion, and increased apoptosis in human pancreatic cancer cells [41]. In addition, we observed a decreased expression of CD44 surface protein in LAPC-4-AI cells by the combination treatment, which may also contribute to the reduced cell invasion and colony formation in LAPC-4-AI cells. CD44+/CD24− prostate cancer cells have been shown to possess stem cell-like characteristics [42]. These cells are more proliferative, clonogenic, tumorigenic, and metastatic than CD44-/CD24− cells [33]. They are responsible for tumor initiation and formation, and are predictive of poor prognosis in prostate cancer patients [42]. The ability of EGCG and Q to inhibit cell invasion and colony formation was also demonstrated by Tang, et al in stem cell-like prostate cancer cells [43], where the combination of EGCG and Q synergistically enhanced the inhibitory effect. A recent study showed that EGCG in combination with paclitaxel significantly decreased the bone metastasis of prostate tumors in SCID mice after a 2-month treatment with EGCG (228 mg/kg, i.p.) plus paclitaxel (20 mg/kg, i.p.) biweekly, and significantly increased survival [44]. Based on the present results we anticipate that a stronger combined effect will be achieved in vivo through the combination of both GT and Q with these taxanes.
In summary, the combination with natural products GT and Q significantly enhanced the therapeutic effect of Doc in androgen-independent prostate cancer cells through enhanced modulations on multiple signaling pathways and events involved in carcinogenesis and cancer therapy. Future in vivo animal studies will be important to confirm this novel therapeutic modality by combining GT and Q with Doc to enhance the efficacy of Doc in treatment of CRPC in a cost efficient and non-toxic manner.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH, NCI, NIMHD, NCATS) Grants: U54 CA143931-01, U54MD007598, UL1TR000124 (J.V. Vadgama); and NIH/National Center for Advancing Translational Sciences (NCATS) UCLA CTSI Grant KL2TR000122 (P. Wang).
Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards CA-16042 and AI-28697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA.
Abbreviations
- AR
androgen receptor
- AI
androgen-independent
- ATP
adenosine triphosphate
- CRPC
castration-resistant prostate cancer
- GT
green tea
- GTPs
green tea polyphenols
- EGCG
(−)-epigallocatechin-3-gallate
- FBS
fetal bovine serum
- MRP
multidrug resistance-associated protein
- mTOR
mammalian target of rapamycin
- NFκB
nuclear factor-kappa B
- PI3K
phosphatidylinositol 3-kinases
- Q
quercetin
- SCID
severe combined immunodeficiency
- Stat
signal transducer and activator of transcription
Footnotes
Conference Presentation: Presented at the American Association for Cancer Research (AACR) Advances in Prostate Cancer Research 2014 meeting held in San Diego, CA.
Conflict of Interest Statement: None declared.
References
- 1.American Cancer Society. Cancer facts & figures 2014. American Cancer Society; Atlanta, GA: 2014. [Google Scholar]
- 2.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–8. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–49. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, Oh WK. Castration-resistant prostate cancer: new science and therapeutic prospects. Ther Adv Med Oncol. 2010;2:189–207. doi: 10.1177/1758834009359769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: molecular mechanisms and development of new generation taxanes. ChemMedChem. 2007;2:920–42. doi: 10.1002/cmdc.200600308. [DOI] [PubMed] [Google Scholar]
- 6.Codony-Servat J, Marin-Aguilera M, Visa L, Garcia-Albeniz X, Pineda E, Fernandez PL, et al. Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castration-resistant prostate cancer. Prostate. 2012 doi: 10.1002/pros.22591. [DOI] [PubMed] [Google Scholar]
- 7.van Oosterom AT, Schrijvers D, Schriivers D. Docetaxel (Taxotere), a review of preclinical and clinical experience. Part II: Clinical experience. Anticancer Drugs. 1995;6:356–68. doi: 10.1097/00001813-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:201–8. doi: 10.1016/s0027-5107(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 9.Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: green tea versus black tea. Mol Nutr Food Res. 2011;55:905–20. doi: 10.1002/mnfr.201000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. 2010;62:931–7. doi: 10.1080/01635581.2010.509536. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- 12.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–9. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 15.McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila Pa) 2009;2:673–82. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (−)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer. 2012;64:580–7. doi: 10.1080/01635581.2012.661514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Heber D, Henning SM. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012;3:635–42. doi: 10.1039/c2fo10254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Vadgama JV, Said JW, Magyar CE, Doan N, Heber D, et al. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J Nutr Biochem. 2014;25:73–80. doi: 10.1016/j.jnutbio.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, Bladeren PJ, et al. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69:699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab Dispos. 2004;32:497–504. doi: 10.1124/dmd.32.5.497. [DOI] [PubMed] [Google Scholar]
- 22.Singh A, Naidu PS, Kulkarni SK. Quercetin potentiates L-Dopa reversal of drug-induced catalepsy in rats: possible COMT/MAO inhibition. Pharmacology. 2003;68:81–8. doi: 10.1159/000069533. [DOI] [PubMed] [Google Scholar]
- 23.Kim KA, Park PW, Park JY. Short-term effect of quercetin on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein, in healthy volunteers. Eur J Clin Pharmacol. 2009;65:609–14. doi: 10.1007/s00228-009-0627-6. [DOI] [PubMed] [Google Scholar]
- 24.Vijayababu MR, Arunkumar A, Kanagaraj P, Venkataraman P, Krishnamoorthy G, Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol Cell Biochem. 2006;287:109–16. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- 25.Aalinkeel R, Bindukumar B, Reynolds JL, Sykes DE, Mahajan SD, Chadha KC, et al. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate. 2008;68:1773–89. doi: 10.1002/pros.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijayababu MR, Arunkumar A, Kanagaraj P, Arunakaran J. Effects of quercetin on insulin-like growth factors (IGFs) and their binding protein-3 (IGFBP-3) secretion and induction of apoptosis in human prostate cancer cells. J Carcinog. 2006;5:10. doi: 10.1186/1477-3163-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 28.Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, et al. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–9. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 29.Chan L, Zhong X, Qiu J, Li P, Lin B. Cellometer vision as an alternative to flow cytometry for cell cycle analysis, mitochondrial potential, and immunophenotyping. Cytometry A. 2011;79:507–17. doi: 10.1002/cyto.a.21071. [DOI] [PubMed] [Google Scholar]
- 30.Chan LL, Lai N, Wang E, Smith T, Yang X, Lin B. A rapid detection method for apoptosis and necrosis measurement using the Cellometer imaging cytometry. Apoptosis. 2011;16:1295–303. doi: 10.1007/s10495-011-0651-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila Pa) 2010;3:985–93. doi: 10.1158/1940-6207.CAPR-09-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell MJ. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–15. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- 33.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 34.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 35.Vishnu P, Tan WW. Update on options for treatment of metastatic castration-resistant prostate cancer. Onco Targets Ther. 2010;3:39–51. doi: 10.2147/ott.s5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3:328–51. [PMC free article] [PubMed] [Google Scholar]
- 37.Cross SE, Jin YS, Lu QY, Rao J, Gimzewski JK. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology. 2011;22:215101. doi: 10.1088/0957-4484/22/21/215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibellini L, Pinti M, Nasi M, Montagna JP, De Biasi S, Roat E, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;2011:591356. doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–9s. [PubMed] [Google Scholar]
- 40.Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets. 2006;6:107–21. doi: 10.2174/156800906776056491. [DOI] [PubMed] [Google Scholar]
- 41.Tang SN, Fu J, Shankar S, Srivastava RK. EGCG enhances the therapeutic potential of gemcitabine and CP690550 by inhibiting STAT3 signaling pathway in human pancreatic cancer. PLoS One. 2012;7:e31067. doi: 10.1371/journal.pone.0031067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–65. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stearns ME, Wang M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl Oncol. 2011;4:147–56. doi: 10.1593/tlo.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]