Abstract
Background
People with low back pain (LBP) exhibit impaired anticipatory postural adjustment (APAs).
Objective
To evaluate whether current motor retraining treatments address LBP-associated changes in movement coordination during tasks that do and do not require APAs.
Design
Prospectively registered, randomized controlled trial with a blinded assessor.
Setting
Outcome evaluations occurred in a university laboratory; treatments, in outpatient physical therapy clinics.
Patients
Fifteen subjects without LBP and 33 subjects with chronic, recurrent, non-specific LBP.
Intervention
Twelve subjects with LBP received stabilization treatment, 21 received Movement System Impairment (MSI)-based treatment, over 6 weekly 1-hour sessions plus home exercises.
Measurements
Pre- and post- treatment, surface electromyography (EMG) was recorded bilaterally from trunk and leg muscles during unsupported and supported leg-lifting tasks, which did and did not require an APA, respectively. Vertical reaction forces under the contralateral leg were recorded to characterize the APA. Oswestry disability scores and numeric pain ratings were also recorded.
Results
Persons with LBP demonstrated an impaired APA compared to persons without LBP, characterized by increased pre-movement contralateral force application and increased post-movement trunk EMG amplitude, regardless of the task. Following treatments, both groups similarly improved in disability and function; however, APA characteristics did not change (i.e. force application or EMG amplitude) in either task.
Limitations
Treating clinicians were not blinded to treatment allocation, only short-term outcomes were assessed, and main effects of treatment do not rule out non-specific effects of time or repeated exposure.
Conclusions
Movement impairments in persons with LBP are not limited to tasks requiring an APA. Stabilization and MSI-based treatments for LBP do not ameliorate, and may exacerbate, APA impairments (i.e., excessive force application and increased post-movement trunk muscle activation).
Keywords: Low back pain (LBP), anticipatory postural adjustment (APA), aberrant movement patterns, stabilization approach, movement systems impairment (MSI) approach
INTRODUCTION
Low back pain (LBP) affects up to 80% of people at some point in their lifetime, with an annual incidence in the U.S. population of 15-45%. [1] Injuries to the lower back are a frequent cause of limited activity [2] and the proportion of the adult population affected by chronic back pain and the medical and social costs associated with it are rising. [3,4] With as many as 85% of persons experiencing LBP also experiencing recurring symptoms [1], LBP represents a significant socioeconomic and public health concern without an effective long-term treatment strategy.
LBP is difficult to treat because understanding of its etiology is limited. Despite numerous differential diagnoses, the pathoanatomical basis of LBP is rarely identified. [5] Several authors have suggested altered neural control of movement, or aberrant movements, may represent one possible mechanism for the development and recurrence of LBP. [6-9] One aspect of postural coordination where aberrant movement patterns arise in LBP is the anticipatory postural adjustment (APA). APAs represent muscle activations within supporting body segments to stabilize and facilitate a voluntary limb movement against the anticipated forces resulting from the limb movement. [10,11] The role of APAs is to minimize negative consequences of a predicted postural perturbation, [11] but it also provides insight into the central control of posture. [11] Delayed APAs in contralateral abdominal muscles during rapid arm-raising tasks [7,10,12,13] therefore provides evidence that LBP associates with altered central neural control of movement. Mok et al. [14] also demonstrated decreased anticipatory lumbopelvic movement in preparation for arm raises in persons with LBP, which corresponded to increased spinal displacement. In contrast to this evidence, the delayed APA is not a consistent finding in LBP, with studies finding delays in subgroups [15], side-specific delays [16] or no delays at all. [17-19]
Despite reported inconsistencies in delayed APAs, treatment approaches aimed at correcting impairments in APAs have been developed. One treatment is based upon the Movement System Impairment (MSI) approach, [20,21] which assumes that altered spinal movement precision may result in specific changes in the neuro-musculoskeletal system, such as altered movement patterns, and focuses on promoting pain-free movement patterns. Another is the trunk stabilization (STB) approach that focuses on improving spinal stability through motor control of deep trunk muscles (transversus abdominis, internal oblique and multifidus) [22-24]; strengthening of the flexor, extensor, and oblique trunk muscles [22]; and (3) incorporating trunk muscle control into activities of daily living. Both approaches aim to improve the patient's ability to control the trunk and stabilize the spine during activities of daily living, isolated trunk movements, or trunk movements induced by limb movement, thereby decreasing aberrant trunk movement patterns during voluntary movements. However, the MSI, compared to the STB exercise, approach emphasizes the precision of movement patterns, which may be more critical to remediation of postural impairments.
The two treatment approaches differ, however, in the number of subgroups of LBP proposed and exercises prescribed. The STB treatment focuses on promoting deep trunk muscle activation primarily through an abdominal hollowing maneuver with co-contraction of the multifidus muscle and strengthening to improve the APA; whereas, the MSI approach focuses on improving the APA with exercises that modify the coordination of trunk movement. Research support for treatment programs aiming to ameliorate APA impairments in persons with LBP is lacking. [25] In fact, randomized control trials have yet to demonstrate improved APA coordination following treatment that included trunk stabilization exercises. [26,27]
The lack of improved APA coordination following STB treatment may be related to the task selected to represent APA coordination. The rapid arm raise, for example, best differentiates people with and without LBP when performed at a functionally irrelevant maximum velocity, thus failing to represent movement characteristics during most activities of daily living. Therefore, there is a need to develop clinic-friendly tools to assess APAs and understand the relationship between LBP and anticipatory postural control. One such protocol has used supported and unsupported leg raising tasks at self-selected movement velocities to demonstrate that anticipatory force application is required during an unsupported leg raise in young, healthy participants. [28] These procedures rely only on force application, not muscle activation, and thus are easy to implement in the clinic to track treatment progress. Because subjects perform both tasks at functionally relevant movement velocities, these tasks may be more appropriate for determining the effects of treatment on postural coordination.
Thus, objective 1 of this study was to measure self-rated disability, pain, muscle activation amplitude, and force application during an unsupported and supported leg-raising task (which do and do not require an APA, respectively), in persons with and without LBP. Objective 2 of this study was to assess the influence of 6 weeks of STB or MSI-directed exercise treatment on the APA of persons with chronic, recurrent LBP during the two leg-raising tasks. We hypothesized that persons with LBP would have an impaired APA, evidenced by delayed and reduced force production compared to persons without LBP. Following treatment for patients with LBP, we hypothesized that (1) self-rated disability and pain scores would decrease after both STB and MSI treatment and (2) APAs, represented by force application, would improve more for persons receiving the precision of trunk control-focused MSI treatment.
METHODS
Design Overview
This study was a prospectively registered (NCT01362049), 2-arm randomized controlled trial with a blinded assessor. The trial's primary objective was to determine if subjects matched to treatment based on particular clinical features would improve their function and decrease their symptoms more over short (7 weeks) and long term (12 months) follow-up periods compared to patients who were not matched to treatment. [29]
Primary outcome measures were changes in Modified Oswestry Disability (ODI) [30] scores and Numeric Pain Ratings (NPR) [31]. After undergoing a standard history and physical exam to determine their clinical features, subjects were randomly assigned to receive either STB or MSI-directed treatment. This study reports on a secondary objective to determine the efficacy of STB and MSI-based treatments to modify impairments in APAs associated with LBP in the short term, within one-week post-treatment completion.
Setting and Participants
Assessments, pre- and post- treatment, were conducted at the University of Vermont's Human Motion Analysis Laboratory. Treatment was conducted at one of four outpatient physical therapy clinics in the Burlington, VT area.
Subjects in this study were part of a clinical trial (NCT01362049), funded by the National Institutes of Health (R01HD040909), in which subjects with LBP (n=1022) were assessed for inclusion through phone/email contact. Subjects who were admitted to the study (1) were between 21 and 55 years old, (2) had a history of chronic LBP (≥ 12 months) with or without recurrences, (3) could stand and walk independently, (4) had a ODI score of ≥ 19%, and/or a score < 8 on at least one activity from the Patient Specific Functional Scale, [32] (5) could understand English, and (6) were currently employed or actively engaged in daily activities. Exclusion criteria included: structural spinal deformity, spinal fracture, osteoporosis, systemic disease processes, disc herniation, previous spinal surgery, pregnancy or less than 6 months post-partum or post-weaning, magnified symptom behavior, [33] and a body-mass index of greater than 30.
Fifteen subjects without LBP were tested for comparison against a sub-group of 15 persons with LBP, matched on age, sex, height, and gender, prior to treatment (objective 1). Subjects without LBP were recruited using the following inclusion criteria: an absence of neurological, psychiatric, cardiovascular or musculoskeletal disorders, as well as no severe musculoskeletal injuries. All subjects provided written informed consent, and the rights of each subject were protected as verified by approval of the Institutional Review Board at the University of Vermont.
Randomization and Interventions
Laboratory Assessment Protocol
Prior to randomization, subjects visited the University of Vermont's Human Motion Analysis Laboratory for initial assessments. Subjects with LBP were tested during acute flare-up [34] and levels of pain and disability were recorded during each testing session, using the NPR [35] and the ODI [30], respectively (Table 1). Testing sessions occurred following 6 weeks of physical therapy treatment (at week 7). Subjects without LBP were tested only once to provide the comparison to the matched subgroup of subjects with LBP prior to their treatment.
Table 1.
Subject demographics and questionnaire data
| Week | MSI* | STB† | Within-Group p-value | Between-Group p-value | |
|---|---|---|---|---|---|
| n | 21 | 12 | |||
| Age (years) | 0 | 41.6 (10.9) | 43.1 (11.9) | 0.713 | |
| Height (cm) | 0 | 175.9 (2.5) | 171.6 (3.3) | 0.112 | |
| Weight (kg) | 0 | 76.6 (25.4) | 67.6 (26.3) | 0.042 | |
| BMI | 0 | 24.6 (2.7) | 22.7 (2.1) | 0.045 | |
| % Male | 0 | 15 | 6 | 0.125 | |
| Oswestry Disability | 0 | 19.9 (9.4) | 17.2 (7.9) | 0.0 00 | 0.229 |
| Index (%) | 7 | 11 (6.8) | 8.4 (5.7) | ||
| Numeric Pain | 0 | 3.6 (1.6) | 2.8 (1.6) | 0.000 | 0.232 |
| Rating (/10) | 7 | 1.5 (1.0) | 1.3 (1.0) |
MSI – Movement System Impairment treatment group
STB – Stabilization treatment group
Muscle activity was recorded via bipolar surface EMG, 1-cm silver, silver-chloride disc electrodes (Norotrodes with fixed 2-cm inter-electrode distance; Myotronics, WA, USA) placed over: the bilateral erector spinae at the level of the 3rd lumbar vertebrae, external oblique, internal oblique, rectus abdominis, rectus femoris (RF), long head of biceps femoris (BF) and the left tibialis anterior (TA) muscles. Trunk muscle electrode placements were standardized based on anatomical landmarks [36], and lower-limb electrodes were placed according to Hermens et al. [37] Skin impedance was maintained under 10 kΩ, while signals were sampled at 1000 Hz, pre-amplified by 1000 at the skin's surface, and then amplified further for a total amplification of 2000-10000.
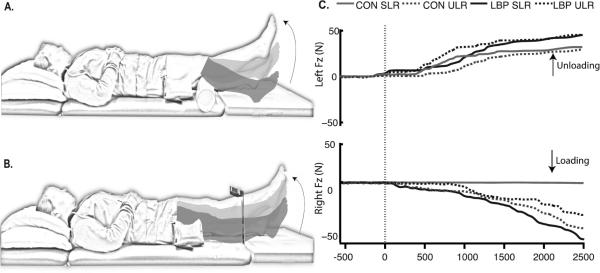
Subjects rested each foot on separate force plates (AMTI OR6-7-1000 ©, AMTI, Inc., MA, USA) while supine and performed 4 repetitions of 2 voluntary left leg movements: a supported leg raise (SLR) (Figure 1A) and an unsupported leg raise (ULR) (Figure 1B). For the SLR task, a 10-cm diameter bolster was placed under the left knee. For the ULR task, a target was fixed 20 cm above the participant's shank midway between the lateral malleolus and the patella. Subjects lifted their left legs from the force plate to the target height or to full knee extension for the ULR and SLR tasks, respectively, held the position for 2 seconds, then lowered the leg.
Figure 1.
Experimental tasks and variables. A) Supported leg raise task (SLR); B) Unsupported leg raise task (ULR), and; C) Schematic of the vertical ground reaction forces under the feet (Fz) as well as representative EMG from a subject without LBP. The graphs of representative EMG illustrate the task dependence and temporal characteristics of EMG burst activity for muscles of the leg: from top to bottom, the left long-head of biceps femoris (BFM), the left rectus femoris (RFM), the right BFM, and the right RFM. Black traces represent responses during the ULR task; gray traces represent responses during the SLR task. Time 0 represents movement onset. The dashed black vertical line indicates the movement onset.
Randomization
To determine the effects of treatment on impaired APAs, a secondary analysis within the clinical trial, 56 subjects participated in either a ballistic or self-paced voluntary postural coordination task; 33 subjects were assessed during self-paced supported and unsupported leg lifting tasks (reported here) and 23 participated in a ballistic task (reported separately). The remaining 68 subjects were allocated for assessment on automatic postural responses to surface translations that perturb standing balance (reported separately). Following laboratory testing, subjects (n = 33) with LBP were randomized to receive one of two treatments: STB or MSI-based exercise treatment. Computer-generated randomization with centralized allocation concealment was used to randomize subjects into each treatment. Neither the study coordinator nor the treating therapist were masked to treatment assignment; all other personnel were unaware of the treatment assignment throughout the study.
Treatment Protocols
After randomization, each subject in the LBP group was scheduled for the first treatment, usually within 3-6 days of the laboratory session. The physical therapist (PT) progressed subjects through the standardized treatment protocols, providing one 45-60 minute treatment per week for 6 weeks, combined with a home exercise program and weekly exercise log. All clinicians practiced >50% of their time in an outpatient orthopaedic setting with an average of 13.7 ± 8.4 years of experience. Each PT passed a written test to demonstrate an acceptable level of knowledge regarding exercise progression for each treatment.
The STB protocol focused on 3 components of spinal stability: (1) motor control of the deep trunk muscles [22-24]; (2) strengthening of the flexor, extensor, and oblique trunk muscles [22] by focusing on repeated submaximal efforts to mimic the function of these muscles in spine stabilization [38,39] and (3) an education booklet [40] that describes proper body mechanics of the spine during activities of daily living.
The PT tailored the MSI protocol to focus on: (1) education regarding how the subject's lumbopelvic movement patterns and postures repeated daily might accelerate lumbar-tissue stress as well as education about positions or postures to control symptoms; (2) exercises to modify the subject's specific trunk movements and postures in particular directions that were pain free; and (3) functional-activity modifications (based on their Patient Specific Functional Scale) to change the subject's trunk-movement and alignment patterns [29].
Treatment adherence was ensured using treatment audits. Two additional PTs (SW and AT), trained in both treatment protocols performed chart audits on 54 of the 124 subjects (25 STB and 29 MSI) while the subjects were still in treatment. The auditors also performed an in-person audit on 45 of these 54 subjects where individual treatment sessions were observed. Post-treatment, the lead physical therapy examiner (ROM) audited patient charts (n = 123) for documentation thoroughness and accuracy.
Outcome Measures and Follow-up
Vertical ground reaction force data (Fz) were low-pass filtered at 10 Hz using Matlab software (Matlab, Mathworks Inc., USA), and the mean of the first 500 ms of the signal was subtracted (as a baseline). Onset of unloading under the ipsilateral limb and onset of loading of the contralateral limb were defined as the point where the force signal exceeded ± 3 standard deviations greater than the mean of the initial 500 ms of the respective Fz signal (Figure 1C). Peak loading force was defined as the peak Fz amplitude under the contralateral foot. Multi-segmental postural coordination was characterized via the time of contralateral limb loading relative to movement onset, peak loading amplitude, and percentage of trials where loading occurred.
EMG signals were band-pass filtered at 35-200 Hz, baseline corrected by subtracting the mean of the first 500 ms of the signal, and full-wave rectified. The high-pass limit was set to minimize cardiac artifact [41]. EMG amplitude was assessed over 2 epochs: pre-movement (PRE) 200 ms prior to movement onset, and movement-related (MOVE) from 25 to 2025 ms post-movement onset. Raw EMG amplitude was rectified and integrated across each epoch. To facilitate group comparisons and data from different testing days, each muscle's integrated EMG amplitude was normalized to that muscle's maximum amplitude identified across all leg-lift trials. This task-generated reference contraction, rather than the typical maximum voluntary contraction, was used for normalization because subjects with LBP may be unwilling to generate a voluntary contraction to their maximum capability [42].
Sample Size Estimation
Objective 1's sample size was determined based on impaired APAs muscle activation in persons with LBP [6]. Assuming a Type I error rate of 5%, a sample size of 7 participants/group would be sufficient to detect impaired preparatory activation of 160 ms during limb movement in persons with LBP. Objective 2's sample size was determined based on improvements in numeric pain ratings during treatment of LBP [43]. Assuming a Type I error rate of 5%, a sample size of 15 participants/group would provide greater than 80% power to detect a significant group-by-time interaction similar to those reported [43]. Subjects were randomly assigned to receive treatment at the location most convenient for them; thus treatment location and PT clinician were not included as factors in the analysis.
Statistical Analysis
Measures of participant characteristics (i.e., age, height, weight, and pre-treatment ODI and NPR) were compared using independent samples t-tests for each group, where groups were CON vs. LBP for objective 1 and STB vs. MSI treatment for objective 2. Differences in gender between groups (CON vs. LBP or TBC vs. MSI) were assessed by Chi-square.
Differences in contralateral limb loading (i.e., time of loading, percent onset of Fz, and peak Fz amplitude) were assessed via mixed model (2-group) repeated measures ANOVA to assess the effects of group (CON vs. LBP or STB vs. MSI), task (ULR vs. SLR), and, for objective 2 only, visit (0 vs. 7 weeks post-treatment). Similarly, a mixed model (2 group) repeated measures ANOVA was also used to assess effects of group (CON vs. LBP or STB vs. MSI) and task (ULR vs. SLR) on pre-treatment EMG for each muscle in each epoch (PRE vs. MOVE) (objective 1) and the pre- minus post-treatment change in EMG amplitude (∆EMG) (objective 2). BMI was used as a covariate in the model for all EMG analyses.
For each significant (P < 0.05) interaction found, the simple effects were examined in order to elucidate the nature of the differences. Statistical analyses were performed using SAS 9.2 Software (SAS Institute Inc., USA).
Role of the Funding Source
This study was funded by the National Institutes of Health, USA, (NIH2R01HD040909), awarded to SM Henry as Principal Investigator.
Results
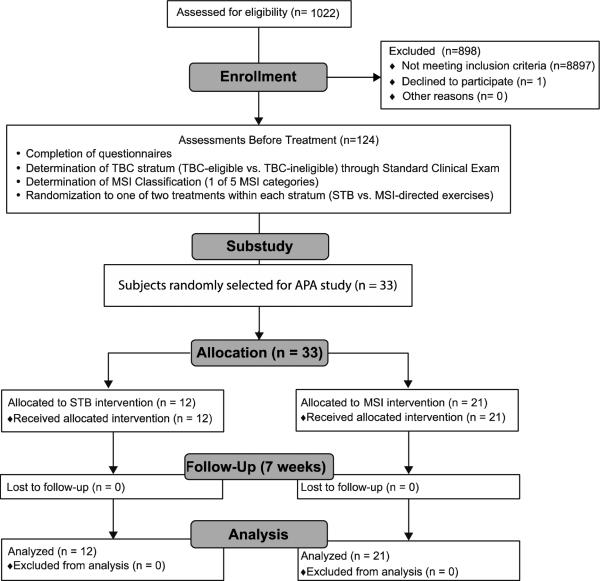
From March 2010 to September 2011, 1022 people with LBP inquired and were screened by clinical exam or interview for the study, resulting in a study cohort of 102 subjects with LBP. Of these, 33 were randomly selected and participated in this APA protocol (objective 2). The dropout rate for the APA protocol was 0%, (Figure 2). There were no adverse effects to report at any point in the treatment program. Of these 33 subjects with LBP, a subgroup of 15 were compared to 15 age-, sex-, weight-, height-, and gender-matched control (CON) subjects (Table 1) (objective 1).
Figure 2.
Flow of subjects through APA substudy.
Subject Demographics and Questionnaires
For objective 1, there were no significant differences in age, height, weight, BMI, or gender distribution between subjects with and without LBP (Table 1) (group main effects, range F = 0.05-0.06, range P = 0.11-0.97). For objective 2, there were also no significant differences in age, height, or gender distribution between subjects receiving MSI versus STB treatment (Table 1) (group main effects, range F = 0.37-1.63, range P = 0.13-0.71). However, subjects receiving MSI treatment had significantly higher weight and BMI compared to subjects who received STB treatment (group main effect, F = 2.1 and 2.2, P = 0.042 and 0.045, respectively); thus, BMI was used as a covariate in all statistical analyses.
For objective 1, subjects with LBP had significantly higher ODI and NPR scores than subjects without LBP (group main effect, t = 8.8 and 3.4, P < 0.001 and 0.002, respectively) (Table 2). For objective 2, both treatment groups demonstrated similar and significant decreases in ODI and NPR (visit main effects, F = 43.3 and F = 39.7, P < 0.001, respectively) scores post-treatment. There were no significant differences between treatment groups in ODI (group main effect, F = 1.5, P = 0.229) or NPR (group main effect, F = 1.5, P = 0.232) scores across all time points (Table 1).
Contralateral Force Application
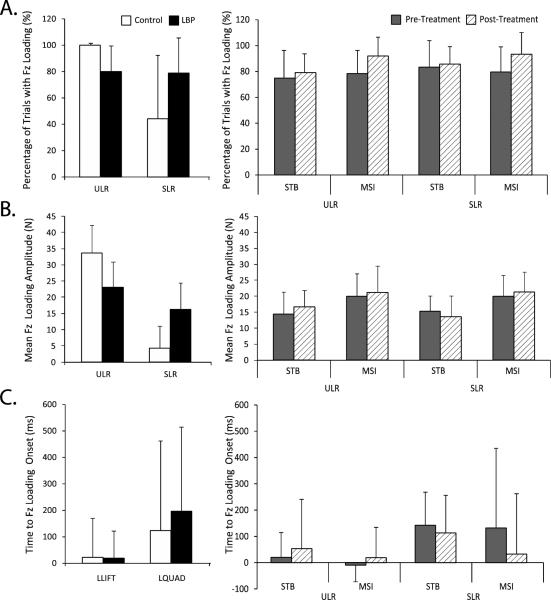
For objective 1, there were significant group-by-task interaction effects for percent trials with Fz onset (F = 13.5, P = 0.001) and Fz amplitude (F = 21.8, P < 0.001). Within-group effects indicated that subjects without LBP had a higher percentage of trials with contralateral Fz onset (F = 28.8, P < 0.001) and higher Fz amplitude (F = 75.5, P < 0.001) during the ULR task as compared to the SLR task. Subjects with LBP had no between-task differences in Fz onset percentage (F = 0.03, P = 0.861) (Figure 3). However, subjects with LBP had significantly higher Fz amplitudes (F = 4.4, P = 0.04) during the SLR task compared to the ULR task.
Figure 3.
Summary of vertical reaction force data from the contralateral limb. A) Percentage of trials where movement onset occurred during supported (SLR and unsupported (ULR) leg raises; B) Mean vertical reaction force amplitude during SLR and ULR, and; C) Time to vertical reaction force loading relative to movement onset. In the left panel, white bars represent the control group, while black represent the age-, sex-matched subgroup of persons with LBP prior to receiving treatment. In the right panel, grey and striped bars represent pre- and post-treatment values, respectively for the STB or MSI groups as indicated.
Within-task effects indicated that, in the ULR task, subjects without LBP had higher Fz amplitudes (F = 14.4, P < 0.001) than subjects with LBP, and during the SLR task, subjects with LBP had higher Fz percent onset (F = 8.7, P = 0.007) than subjects without LBP. There were no differences in timing of Fz onset between groups or tasks (F = 0.13, P = 0.718 and F = 4.0, P = 0.064) (Figure 3).
For objective 2, treatment had little effect on contralateral force application with no significant group or task differences in either post- minus pre-treatment change in Fz amplitude (F = 0.53, P = 0.476) or timing of Fz loading (F = 0.30, P = 0.593). However, there was a significant simple visit effect (F = 10.2, P = 0.003), in which Fz onset percentage increased post-treatment across treatment groups and tasks.
EMG Amplitude
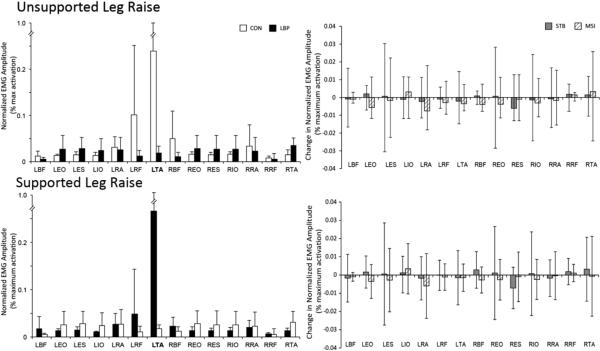
For objective 1, there were significant group-by-muscle interaction effects during both the PRE phase (F = 2.94, P < 0.001) and the MOVE phase (F = 18.5, P < 0.001), (Figures 4 and 5, respectively). During the PRE phase, subjects with LBP demonstrated lower EMG amplitude than subjects without LBP in the left TA muscle (F = 34.8, P < 0.001). During the MOVE phase, subjects with LBP demonstrated higher activation of bilateral EO, ES, IO, and RA muscles, and left RF and right TA muscles (range F = 12.3-60.3, P < 0.001) than subjects without LBP.
Figure 4.
Summary of mean normalized integrated surface EMG during the PRE phase for: A) the unsupported (ULR) and B) the supported (SLR leg raising tasks. In the left panel, white bars represent the control group, while black represent the age-, sex-matched subgroup of persons with LBP prior to receiving treatment. In the right panel, grey and striped bars represent change in EMG amplitude the STB or MSI groups, respectively.
For objective 2, treatment had little effect on ΔEMG in either the PRE or MOVE phases. There were no significant effects of treatment group, task, or muscle in either phase (range F = 0.03 -1.24, range P = 0.248-1.000 and range F = 0.06-4.0, range P = 0.055-0.999).
Discussion
Objective 1 demonstrated impaired APA function in persons with LBP during the leg raising protocol. APA impairments primarily manifested as contralateral force application in persons with LBP during both the ULR and SLR tasks (which do and do not regularly elicit an APA in persons without LBP, respectively). The amplitude of loading was higher for subjects with LBP compared to subjects without LBP during the SLR task; thus, subjects with LBP use the same strategy despite the unique APA requirements of each task. Although subjects with LBP demonstrated similar muscle activation amplitudes to subjects without LBP in the PRE phase, they had higher activation amplitude across most trunk and leg muscles in the MOVE phase. In objective 2, both treatment programs demonstrated similar improvements in ODI and NPR after 6 weeks, yet neither treatment altered the impaired ability to contextually modulate the APA. Post-treatment, receiving neither STB nor MSI treatment altered the amplitude or timing of contralateral leg loading. Fz onset percentage did increase for both tasks (ULR, SLR), indicating that neither treatment was effective in modifying the APA strategy so that the postural strategy used was specific to task requirements in subjects with LBP. However, there were significant changes in the timing of Fz loading between groups across both tasks. The STB group initiated loading later, while the MSI group loaded earlier post-treatment; however, changes in loading were similar for both the ULR and SLR tasks, indicating an inability to modulate to task requirements. In addition, mean amplitude in ΔEMG was similar between treatment groups, indicating that neither was preferential in modifying muscle activation patterns used during the PRE or MOVE phases.
An important strength of this study was the effective masking of lab personnel who tested subjects on the APA paradigm, reducing potential testing bias. Also, a novel APA paradigm featuring leg raising tasks that do and do not require an APA in healthy adults (ULR and SLR, respectively) was used [28]. This protocol offers a unique advantage as its tasks were not specifically trained during either of the treatment programs; in contrast, other studies have tested the Abdominal Drawing-in Maneuver as an outcome measure and as part of treatment [43,44]. Our protocol assessed transfer tasks to determine if treatment modulates overall s function, not just effects on trained exercises. Also, the leg raise tasks used in our study were slow (in contrast to rapid arm raises [45]), which is more relevant to common activities of daily living. The primary weakness of this study was that treatment effects were only assessed immediately following treatment. Some studies suggest that treatment specific differences may be more apparent at later follow-up intervals [29,45]. Clinical data from this trial are presented in Henry et al (2014) [29], which demonstrated that both STB and MSI treatment protocols could significantly reduce pain and increase function immediately post-treatment, and that these improvements were maintained 12 months post-treatment [29]. The persistence of the APAs impairment despite these clinical improvements may predispose these patients to recurrence of their LBP, an issue that has been commonly reported in chronic LBP [1]. It remains to be determined whether these treatment paradigms are insufficient to re-establish the APA response or if the duration of the treatment program was simply too short to elicit these changes.
Although the APA-associated outcomes have been compared between persons with and without LBP (objective 1), results have been largely inconsistent. Most of these studies examine trunk muscle activation onset during a rapid arm-raising task and have reported delayed APA onset times [46], delays in certain sub-groups, side-specific delays, or no delays at all [15-19]. None of these studies used a comparison task that does not require an APA (e.g. SLR) to determine the extent of the APA impairment or examined APA function during a task that reliably elicits an APA at slower speeds, which may better reflect activities of daily living. In this study, we used two leg-raising tasks, either requiring or not requiring an APA, in order to better understand the ability of persons with LBP to modulate the APA according to task requirements. Lomond et al. [28] have established that, in persons without LBP, the SLR task is less likely to demonstrate contralateral limb loading (8.9% of trials) compared to ULR trials (100%). When loading did occur in the SLR task, its amplitude was lower than during the ULR task. In this study, subjects with LBP demonstrated a clear inability to discriminate between these task requirements, consistently loading the contralateral limb in both tasks. Although this study failed to demonstrate a delay in the APAs, it did demonstrate a clear inability for persons with LBP to modulate its movement strategy between tasks with differing requirements to elicit an APA, even following treatment.
To date, there has been only one other study comparing the effects of physical therapy treatment on the APAs (objective 2). Tsao and Hodges [45] examined the long-term effects of motor control training on the APA during a rapid arm raise. Immediately following treatment (segmental trunk stabilization approach focusing on transversus abdominis and multifidus muscle control in a variety of patient positions), muscle activation onset was earlier and less variable, and these changes were maintained through 6 months of follow-up. In the current study STB treatment (which included retraining of transversus abdominis and multifidus but also included strengthening all trunk muscles and modification of functional activities) did modify contralateral force application and increased late-phase muscle activation. These changes, however, were similar across tasks and, for the SLR task, were in the opposite direction of modulation exhibited by people without LBP. Together, these results suggest that motor control changes following treatment reflect a global change in movement strategy, rather than a specific modification of the APA itself.
The primary implication of this study is that STB and MSI exercise treatments, whose goal is to teach patients how to modulate their postural control (e.g. APAs), may be ineffective at doing so, particularly for tasks that were not specifically trained during treatment. Despite some success in demonstrating altered APAs, we have also demonstrated that these changes in movement strategies are potentially non-specific and likely inappropriate for some movement conditions. Therefore, treatment programs might benefit from additional practice and variation in task context to improve modulation of postural control and transfer of learning beyond treated exercises.
Further research should focus on the long-term consequences of physical therapy treatments on the motor control of movements with a variety of task requirements. Newer treatment programs should be modified to include more practice and variation of task performance in order to determine the correct dose, duration, and treatment length so as to elicit effective modulation of postural control. In addition, research should examine central nervous system changes prior to and following treatment in order to ascertain the neural mechanism(s) by which treatments are or are not successful so that treatments can be further modified to improve treatment outcomes.
Conclusion
This study suggests that persons with LBP demonstrate impaired APAs compared to healthy subjects without LBP. However, the impaired movement strategies are not limited to tasks that require an APA. These impairments persisted or were amplified after 6 weeks of two different physical therapy treatments designed to encourage an APA response: STB and MSI treatments. The inability for persons with LBP to modulate their movement strategy to task requirements may represent a primary risk factor for reoccurrence of LBP [20, 46]. Therefore, future physical therapy interventions should aim to more thoroughly characterize the extent of movement impairments and ensure that the intervention includes adequate repetition and variation of impaired movements in order to generate lasting central neural changes that result in persistent and adaptable improvements in movement coordination across different tasks and contexts.
Supplementary Material
Acknowledgements
The authors would also like to acknowledge the Vermont physical therapists that participated in this study: Justine Dee, MS PT, Matthew Odachowski, MPT and Jeffrey Albertson, MPT of Dee Physical Therapy, South Burlington; Janet Carscadden, PT and Andrea Trombley, MS, PT of Evolution Physical Therapy and Yoga, Burlington; Rose Bernier, PT, Traci Glanz, PT, Lucia Ryan, PT, Diane Stevens, PT and Sonya Worth, PT, PGDipHSc of Fletcher Allen Health Care, Burlington; Candice Brueck, MPT of Timberlane Physical Therapy, South Burlington; Karen Westervelt, PT, PGDipHSc and Jane Eliason, PT of Copley Hosptial, Morrisville, Vermont. We further thank Rebecca Ouellette-Morton for assistance with data collection.
Source(s) of Support: This study (NIH2R01HD040909) was funded by the National Institutes of Health, USA, and awarded to SM Henry as Principal Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson GB, Frymoyer JW. The Epidemiology of Spinal Disorders. Lippincott-Raven Publishers; Philadelphia, PA: 1997. [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88:21–24. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and Health Status Among Adults With Back and Neck Problems. The Journal of the American Medical Association. 2008;299(6):656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 4.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Archives of Internal Medicine. 2009;169(3):251. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol. 2004;92(6):3255–3265. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- 6.Hodges PW, Richardson CA. Inefficient muscular stabilisation of the lumbar spine associated with low back pain: A motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 7.Hodges PW, Richardson C. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999 Sep;80:1005–1012. doi: 10.1016/s0003-9993(99)90052-7. 1999. [DOI] [PubMed] [Google Scholar]
- 8.Ritter P, Villrunger A. Simultaneous EEG-fMRI. Neuroscience & Biobehavioural Reviews. 2006;30(6):823–838. doi: 10.1016/j.neubiorev.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Van Dillen LR, Maluf KS, Sahrmann SA. Further examination of modifying patient-preferred movement and alignment strategies in patients with low back pain during symptomatic tests. Manual Ther. 2009;14:52–60. doi: 10.1016/j.math.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs JV, Henry SM, Nagle KJ. Low back pain associates with altered activity of the cerebral cortex prior to arm movements that require postural adjustment. Clin Neurophysiol. 2010;121(3):431–440. doi: 10.1016/j.clinph.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massion J. Movement, posture and equilibrium: Interaction and coordination. Progress in Neurobiology. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 12.Moseley GL, Hodges PW. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci. 2006;120(2):474–476. doi: 10.1037/0735-7044.120.2.474. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs JV, Henry SM, Nagle KJ. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behav Neurosci. 2009;123:455–458. doi: 10.1037/a0014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mok NW, Brauer SG, Hodges PW. Failure to use movement in postural strategies leads to increased spinal displacement in low back pain. Spine. 2007;32(19):E537–546. doi: 10.1097/BRS.0b013e31814541a2. [DOI] [PubMed] [Google Scholar]
- 15.Silfies SP, Mehta R, Smith SS, Karduna AR. Differences in Feedforward Trunk Muscle Activity in Subgroups of Patients With Mechanical Low Back Pain. Archives of Physical Medicine and Rehabilitation. 2009;90(7):1159–1169. doi: 10.1016/j.apmr.2008.10.033. 7// [DOI] [PubMed] [Google Scholar]
- 16.Marshall P, Murphy B. Delayed abdominal muscle onsets and self-report measures of pain and disability in chronic low back pain. Journal of Electromyography and Kinesiology. 2010;20(5):833–839. doi: 10.1016/j.jelekin.2009.09.005. 10// [DOI] [PubMed] [Google Scholar]
- 17.Allison GT, Morris SL, Lay B. Feedforward responses of transversus abdominis are directionally specific and act asymmetrically: implications for core stability theories. J Orthop Sports Phys Ther. 2008 May;38(5):228–237. doi: 10.2519/jospt.2008.2703. [DOI] [PubMed] [Google Scholar]
- 18.Tsao H, Druitt TR, Schollum TM, Hodges PW. Motor Training of the Lumbar Paraspinal Muscles Induces Immediate Changes in Motor Coordination in Patients With Recurrent Low Back Pain. The Journal of Pain. 2010;11(11):1120–1128. doi: 10.1016/j.jpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Gubler D, Mannion AF, Schenk P, et al. Ultrasound Tissue Doppler Imaging Reveals No Delay in Abdominal Muscle Feed-Forward Activity During Rapid Arm Movements in Patients With Chronic Low Back Pain. Spine. 2010;35(16):1506–1513. doi: 10.1097/BRS.0b013e3181c3ed41. 1510.1097/BRS.1500b1013e3181c1503ed1541. [DOI] [PubMed] [Google Scholar]
- 20.Sahrmann SA. Diagnosis and Treatment of Movement Impairment Syndromes Vol 1st. Mosby, Inc; St. Louis, MO: 2002. Movement impairment syndromes of the lumbar spine. p. 5. [Google Scholar]
- 21.Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. Journal of Orthopaedic & Sports Physical Therapy. 2003;33(3):126–142. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]
- 22.Hicks GE, Fritz JM, Delitto A, McGill SM. Preliminary development of a clinical prediction rule for determining which patients with low back pain will respond to a stabilization exercise program. Arch Phys Med Rehabil. 2005;86(9):1753–1762. doi: 10.1016/j.apmr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Richardson C, Jull G, Hodges P, Hides J. Therapeutic exercise for spinal segmental stabilization in low back pain: Scientific basis and clinical approach. Churchill Livingstone; Edinburgh: 1999. [Google Scholar]
- 24.Costa LOP, Maher CG, Latimer J, et al. Motor control exercise for chronic low back pain: a randomized placebo-controlled trial. Physical Therapy. 2009;89(12):1275–1286. doi: 10.2522/ptj.20090218. [DOI] [PubMed] [Google Scholar]
- 25.Brooks C, Kennedy S, Marshall PW. Specific Trunk and General Exercise Elicit Similar Changes in Anticipatory Postural Adjustments in Patients With Chronic Low Back Pain: A Randomized Controlled Trial. Spine. 2012 doi: 10.1097/BRS.0b013e31826feac0. [DOI] [PubMed] [Google Scholar]
- 26.Vasseljen O, Unsgaard-Tondel M, Westad C, Mork PJ. Effect of core stability exercises on feed-forward activation of deep abdominal muscles in chronic low back pain: a randomized controlled trial. Spine. 2012 Jun 1;37(13):1101–1108. doi: 10.1097/BRS.0b013e318241377c. [DOI] [PubMed] [Google Scholar]
- 27.Marshall PW, Murphy BA. Muscle Activation Changes After Exercise Rehabilitation for Chronic Low Back Pain. Archives of Physical Medicine and Rehabilitation. 2008;89(7):1305–1313. doi: 10.1016/j.apmr.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Lomond K,V, Henry S,M, Jacobs J,V, et al. Protocol to assess the neurophysiology associated with multi-segmental postural coordination. Physiological Measurement. 2013;34(10):N97. doi: 10.1088/0967-3334/34/10/N97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry SM, Van Dillen L, Ouellette-Morton RH, et al. Outcomes are not different for patient-matched vs. non-matched treatment in subjects with chronic, recurrent low back pain: a randomized, clinical trial. Spine. doi: 10.1016/j.spinee.2014.03.024. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Physical Therapy. 2001 Feb;81(2):776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 31.Childs JD, Fritz JM, Flynn TW. Treatments for back pain. Annals of Internal Medicine. 2005;142(10):874. doi: 10.7326/0003-4819-142-10-200505170-00023. [DOI] [PubMed] [Google Scholar]
- 32.Stratford P, Gill C, Westaway M, Binkley J. Assessing disability and change on individual patients: A report of a patient specific measure. Physiotherapy Canada. 1995;47(4):258–263. [Google Scholar]
- 33.Waddell G, McCulloch JA, Kummel E, Venner RM. Nonorganic physical signs in low-back pain. Spine. 1980;5(2):117–125. doi: 10.1097/00007632-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 34.McGorry RW, Webster BS, Snook SH, Hsiang SM. The relation between pain intensity, disability and the episodic nature of chronic and recurrent low back pain. Spine. 2000;25(7):834–841. doi: 10.1097/00007632-200004010-00012. [DOI] [PubMed] [Google Scholar]
- 35.Stratford PW, Spadoni G. The reliability, consistency, and clinical application of a numeric pain rating scale. Physiother Can. 2001;53(114):88–91. [Google Scholar]
- 36.Jacobs JV, Henry SM, Jones SL, Hitt JR, Bunn JY. A history of low back pain associates with altered electromyographic activation patterns in response to perturbations of standing balance. Journal of Neurophysiology. 2011;106(5):2506–2514. doi: 10.1152/jn.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermens H, Freriks B. Sensor loactions. 2006 http://www.seniam.org, 2011.
- 38.McGill SM. Low back exercises: evidence for improving exercise regimens. Physical Therapy. 1998;78(7):754–765. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- 39.Richardson C, Jull G. An historical perspective on the development of clinical techniques to evaluate and treat the active stabilising system of the lumbar spine. Australian Physiotherapy. 1995;1:5–13. 1995. [Google Scholar]
- 40.Melnick M, Saunders HD, Saunders R. Managing Back Pain: Daily Activities Guide for Back Pain Patients. The Saunders Group Inc.; Chaska, MN: 1998. [Google Scholar]
- 41.Drake JD, Fischer SL, Brown SH, Callaghan JP. Do exercise balls provide a training advantage for trunk extensor exercises? A biomechanical evaluation. J Manipulative Physiol Ther. 2006;29(5):354–362. doi: 10.1016/j.jmpt.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Larivière C, Arsenault AB, Gravel D, Gagnon D, Loisel P. Surface electromyography assessment of back muscle intrinsic properties. Journal of Electromyography and Kinesiology. 2003;13(4):305–318. doi: 10.1016/s1050-6411(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan P, Twomey L, Allison G, Sinclair J, Miller K. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother. 1997;43(2):91–98. doi: 10.1016/s0004-9514(14)60403-7. [DOI] [PubMed] [Google Scholar]
- 44.O'Sullivan PB, Twomey L, Allison GT. Altered abdominal muscle recuitment in patients with chronic back pain following a specific exercise intervention. J. Orthopaedic & Sports Physical Therapy. 1998;27(2):114–123. doi: 10.2519/jospt.1998.27.2.114. [DOI] [PubMed] [Google Scholar]
- 45.Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18(4):559–567. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Hodges PW. Pain and motor control: from the laboratory to rehabilitation. Journal of Electromyography and Kinesiology. 2011;21(2):220–228. doi: 10.1016/j.jelekin.2011.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.