Abstract
Objectives
Methamphetamine (MA) addiction has no known effective pharmacotherapy. Small trials showed beneficial effects for oral naltrexone in amphetamine users. Trials in alcohol dependent subjects showed better response in persons with the A118G single nucleotide polymorphism (SNP) of the µ-opioid receptor. We conducted a pharmacogenetic trial of sustained release intramuscular naltrexone to examine the role of the A118G SNP in MA dependence.
Method
All eligible A118G subjects screened were enrolled; an equal number of wild type (A118A) subjects were selected using modified urn randomization, balanced on gender and frequency of recent MA use. Enrolled subjects received a single 380 mg naltrexone injection and weekly psychotherapy for four weeks. Self-report of MA use and urine toxicology for MA was assessed twice weekly. Urine samples with <1,000 ng/mL of MA were considered negative.
Results
Eleven A118G and 11 A118A subjects were enrolled. There were no significant differences between the groups in days of abstinence from MA use (11.5 v. 14.8, respectively, p = 0.51), number of MA-negative urine samples (1.7 v. 1.8, respectively, p = 0.97), consecutive MA-negative urine samples (1.0 v. 1.5, respectively, p = 0.91), or number of MA-negative urine samples before first relapse (0.9 v. 1.5, respectively, p = 0.86).
Conclusions
Although A118G polymorphism has been shown to be associated with improved treatment response to naltrexone among alcoholics, whether this polymorphism impacts naltrexone treatment response among MA users is unclear at this time.
Keywords: Methamphetamine, Naltrexone, A118G SNP, Clinical trial, Polymorphism
BACKGROUND
Methamphetamine (MA) addiction remains a significant public health problem with no known effective pharmacotherapy. A small placebo controlled randomized clinical trial demonstrated the efficacy of naltrexone in reducing amphetamine use in amphetamine-dependent individuals (Jayaram-Lindstrom, Hammarberg et al. 2008). The United States Food and Drug Administration (FDA) approved both oral and sustained release intramuscular naltrexone for treatment of alcohol dependence and opioid dependence. The use of either the oral or extended-release intramuscular (IM) formulation of naltrexone is not associated with the development of tolerance or dependence, and there is no known discontinuation syndrome (Alkermes 2005; U.S Food and Drug and Administration 2007). Thus, naltrexone appears to have a very low abuse liability, which makes it attractive as a treatment for substance dependence.
Naltrexone is an opioid receptor antagonist. The endogenous opioid system is intimately tied to the effects of many drugs of abuse. Differences in activity of genes coding for receptors in this system may not only produce different effects of drug use, but might potentially produce different treatment responses. In a retrospective meta-analysis, Arias, et al. (Arias, Feinn et al. 2006) concluded that the A118G single nucleotide polymorphism (SNP) of the μ-opioid receptor (OPRM1) was not a risk factor for the development of addiction, but pointed to studies in which it seemed to modulate pharmacological and therapeutic response to opioid receptor antagonists (Lotsch, Skarke et al. 2002; Wand, McCaul et al. 2002; Hernandez-Avila, Wand et al. 2003). Oslin, et al. pooled data from three clinical trials and found that alcohol dependent subjects who were A118G hetero- or homozygous had an enhanced response to naltrexone (significantly lower rates of relapse and a longer time to return to heavy drinking) (Oslin, Berrettini et al. 2003). However, these findings had not been verified in a prospective study.
There is a growing interest in pharmacogenomics as a tool to develop safe and effective medications tailored to a person’s genetic makeup. According to prior studies the frequency of the A118G polymorphism can be as high as 49% in certain populations and therefore a pharmacogenomic intervention could be of particular interest for these groups (Kreek, Nielsen et al. 2004). Therefore, based on the findings of previous studies, we conducted a pilot pharmacogenetic clinical trial to investigate whether the A118G allele is associated with differential response to naltrexone treatment compared to the wild type (WT; A118A). The most innovative features of this pilot trial were a) the use of sustained release IM naltrexone to improve adherence and decrease variability in drug response and b) the recruitment of participants in a prospective study to compare their response to naltrexone by their pharmacogenetic status. However, recruiting samples with equal representation of both the A118G SNP and the WT was challenging because the A118G SNP is relatively uncommon in Caucasian dominated areas (Kreek, Nielsen et al. 2004). Thus, a proportionally larger number of subjects had to be screened. The conventional approach to a pharmacogenomic efficacy trial would be to recruit equal numbers of A118G and WT subjects, and to randomly assign them to naltrexone or placebo. However, we wished to use a more efficient design. We therefore selected a design that allowed us to maximize the knowledge gained from the number of subjects that we could screen and enroll. The key finding that we would have expected from a conventional 2 × 2 design was that subjects receiving naltrexone who had the A118G polymorphism would have had superior outcomes to subjects in any of the three other groups – naltrexone/WT, placebo/A118G, and placebo/WT. This suggested that a more efficient design in this exploratory phase of medication development would be comparing the naltrexone/A118G group to one other group. We would anticipate a significant difference between the naltrexone/A118G group and the placebo/WT sample. However, interpretability would be limited, as both drug and genotype would differ. A placebo/A118G sample would provide an interesting comparison, but would require screening an excessive number of subjects. Comparing the effects of naltrexone on subjects with and without the A118G SNP would provide important data such as effect size that could be used to guide the design of subsequent, more definitive studies. Therefore we chose an economical approach to this pharmacogenetic efficacy trial and compared the effects of naltrexone on subjects with and without the A118G SNP.
METHODS
Subjects
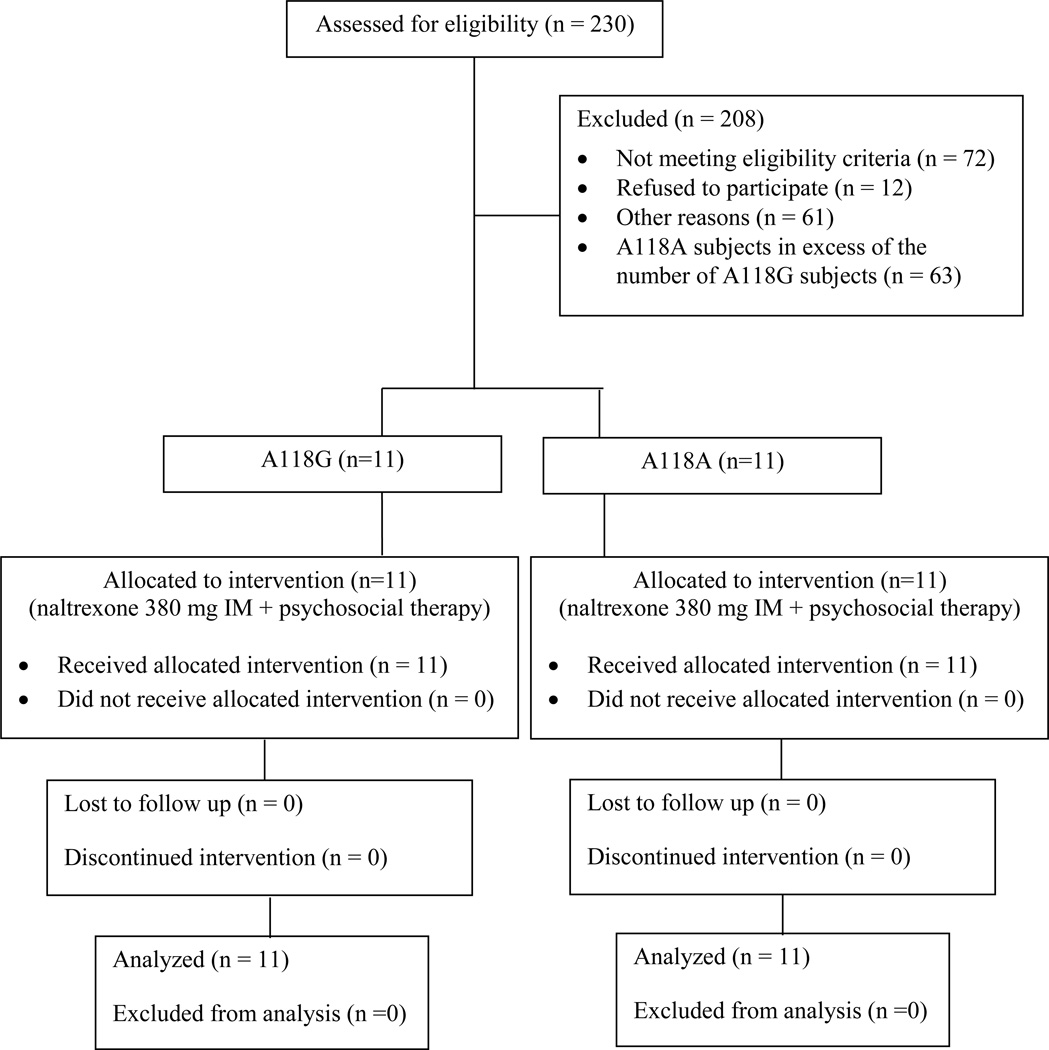
A total of 230 subjects were screened for eligibility. Inclusion criteria were: age between 18 and 50 years, MA dependent according to DSM-IV criteria, seeking treatment for MA dependence, in good physical health, and without other psychiatric diagnoses (except nicotine, marijuana, or alcohol dependence) as determined by DSM-IV. Exclusion criteria were: pregnancy or lactation, dependence on alcohol requiring medical detoxification, court-mandated drug abuse treatment, use of oral or depot naltrexone within the previous 90 days, and regular use of opioid containing medications. The study was designed to include a 1:1 ratio of subjects with A118A and A118G genotypes and some A118A subjects were therefore excluded at random (see Study Design, below). To exclude subjects using opioids, we evaluated them for any history of opioid use, conducted multiple urine analyses to test for opioids, and also performed the naloxone challenge test (Sadock, Sadock et al. 2005). Subjects received a subcutaneous injection of 0.8 mg of naloxone and were observed for 20 minutes for signs and symptoms of opioid withdrawal. Subjects did not receive naltrexone if there were signs and symptoms of opioid withdrawal.
Study design
Subjects were screened for eligibility criteria, including genotype. Subjects could be excluded prior to or after genotyping based on criteria other than genotype. Subjects and all study staff who had contact with subjects were kept blind to genotype. All A118G subjects who met eligibility criteria were offered entry into the study. Subjects without the A118G SNP (WT, A118A) were selected 1:1 using modified urn randomization in which the urn function was used to increase the probability that groups would be balanced on gender and frequency of recent MA use (17 or more days in the 30 days prior to screening; modified urn randomization code available upon request. Subjects were matched based on gender and frequency of MA use because studies have shown that these factors influence treatment outcomes (Elkashef, Rawson et al. 2008; Pettinati, Kampman et al. 2008; Greenfield, Back et al. 2010).
This was a four-week, open-label study using injectable naltrexone. All enrolled subjects received a single 380 mg IM injection of sustained release naltrexone at the beginning of week one and returned for twice-weekly visits. Self-report of MA use in past 30 days, primary route of administration, Amphetamine Withdrawal Questionnaire (AWQ) score, and Attention Deficit and Hyperactivity Disorder (ADHD) diagnosis were assessed at baseline. Self-report of MA use, urine toxicology for MA, and adverse events were assessed during the weekly visits. Subjects also received manual-driven, individual psychosocial therapy once per week during the treatment phase. A checklist similar to the ones used in other trials of FDA–approved medications (Grabowski, Rhoades et al. 2004; Mooney, Herin et al. 2009; Galloway, Buscemi et al. 2011) was used to assess the prevalence of 34 potential adverse events, selected a priori on the basis of likelihood of occurrence and severity. The treatment phase was followed by a one-week follow-up period in which safety of IM naltrexone was assessed. The study was approved by the Institutional Review Board of the California Pacific Medical Center Research Institute (ClinicalTrials.gov Identifier: NCT00984360).
Genetic assay
DNA was extracted from blood using QIAamp DNA Blood Midi Kit (Qiagen Sciences, Inc., Germantown, MD) and analyzed for the A118G polymorphism using the DpnII restriction fragment length polymorphism. Following published methods (Gelernter, Kranzler et al. 1999; Bruehl, Chung et al. 2008), PCR primers were designed (5’-ccg tca gta cca tgg aca gca gcg gtg-3’ and 5’-gtt cgg acc gca tgg gtc gga cag at-3’) that incorporate mismatched bases to produce an artificial restriction site in a 154-bp PCR product. Amplification consisted of 30 cycles of 95°C for 30 s, 69.7°C for 30 s, and 72°C for 30 s, with a 5 minute final extension at 72°C. Amplification products were subjected to restriction digest with DpnII at 37°C for 3 h. Digestion products were examined by electrophoresis on 3% agarose gel, with persistence of the 154-bp band indicating homozygous A118A genotype and cleavage to a 129-bp band indicating the presence of the A118G allele. Genotyping was conducted by individuals blinded to clinical study data and hypotheses.
Data analysis
In clinical trials of treatments for MA dependence, there is no universally accepted definition of a primary outcome variable that represents clinically significant improvement. Therefore we used both self-report and biological specimens (i.e., urine samples) to evaluate naltrexone effectiveness in subjects with A118G polymorphism compared to the WT. We compared self-reports of number of days of abstinence, number of consecutive days of abstinence, and number of days abstinent before first relapse. We also compared the total number of MA-negative urine samples, number of consecutive MA-negative urine samples, and number of MA-negative urine samples before first relapse between the two groups. ‘First relapse’ was the first time a subject used MA after the first period of abstinence while in the study (abstinence could have started prior to or after start of naltrexone therapy). For this study, MA-negative urine was defined as a negative qualitative screen with a cutoff of 1,000 ng/ml. The study population was compared on the basis of genetic polymorphism. Independent sample t tests were used for continuous variables and Pearsons chi-square tests for categorical variables. Statistical significance was set at p<0.05 and all tests were 2-sided. Data was analyzed using R statistical software (version 2.10).
RESULTS
A total of 230 MA treatment-seeking subjects were screened, of which, 175 subjects were genotyped. Of these, 51 subjects had A118G genotype, of which 11 subjects met eligibility criteria and were enrolled in the study. Of the 124 A118A subjects, 11 subjects who matched A118G subjects in gender and frequency of MA use were enrolled. Baseline characteristics of these two groups are presented in Table 1. The groups did not vary significantly in measured characteristics except A118A subjects were more likely to be Caucasians. Of the 51 A118G subjects, 33 were Caucasians, 9 were Asians, 2 were black or African American, 1 was American Indian or Alaskan Native, 4 were Native Hawaiian or Other Pacific Islander, and 1 was of mixed racial origin. Of the 124 A118A subjects, 105 were Caucasians, 1 was Asian, 8 were black or African American, 1 was American Indian or Alaskan Native, 1 were Native Hawaiian or Other Pacific Islander, and 7 were of mixed racial origin.
Table 1.
Baseline clinical characteristics by genotype
| A118G (n=11) |
A118A (n=11) |
95% CI of difference |
p | |
|---|---|---|---|---|
| Age, years – mean (SEM) | 39.5 (2.0) | 40.7 (1.7) | −5.71 – 3.35 | 0.69 |
| Male - N (%) | 6 (55%) | 9 (82%) | 0.02 – 2.47 | 0.36 |
| Caucasian - N (%) | 5 (45%) | 11 (100%) | 0.00 – 0.60 | 0.01 |
| Days of MA use - mean (SD) | 17.4 (8.3) | 21.2 (9.7) | −4.23 – 11.83 | 0.39 |
| MA negative urine samples - N (%) | 1 (9.1%) | 4 (36%) | −0.08 – 0.56 | 0.31 |
| Smoking as primary route of administration - N (%) | 9 (82%) | 6 (55%) | 0.40 – 48.8 | 0.36 |
| AWQ withdrawal score (0–40) - mean (SEM) | 15.7 (2.2) | 12.4 (1.8) | −9.16 – 2.56 | 0.53 |
| ADHD diagnosis - N (%) | 1 (9%) | 0 (0%) | 0.02 - ∞ | 1 |
Table 2 compares the self-reported MA use between the two groups. There were no significant differences between A118G and A118A groups in mean number of days of abstinence from MA use (11.5 v. 14.8, respectively, p = 0.51), mean number of consecutive days of abstinence from MA use (5.6 v. 10.6, respectively, p = 0.29), and mean number of days abstinent from MA use before first relapse (3.5 v. 9.0, respectively, p = 0.28).
Table 2.
Self-report outcomes by genotype
| A118G | A118A | 95% CI of difference |
p | |
|---|---|---|---|---|
| Days abstinent - mean (SD) | 11.5 (10.1) | 14.8 (10.5) | −5.86 – 12.46 | 0.51 |
| Consecutive days abstinent - mean (SD) | 5.6 (6.2) | 10.6 (10.9) | −2.89 – 12.89 | 0.29 |
| Days abstinent before first relapse - mean (SD) | 3.5 (5.8) | 9.0 (12.3) | −3.05 – 14.05 | 0.28 |
Table 3 compares the urinalysis results between the two groups. There were no significant differences between groups in mean number of MA-negative urine samples (1.7 v. 1.8, respectively, p = 0.97), mean number of consecutive MA-negative urine samples (1.0 v.1.5, respectively, p =0.91, or mean number of MA-negative urine samples before first relapse (0.9 v. 1.5, respectively, p = 0.86).
Table 3.
Urinalysis (UA) results
| Outcome – mean (SD) | A118G | A118A | 95% CI | p |
|---|---|---|---|---|
| Negative UAs | 1.7 (2.6) | 1.8 (3.2) | −2.49 – 2.69 | 0.97 |
| Consecutive negative UAs | 1.0 (1.6) | 1.5 (2.8) | −1.53 – 2.53 | 0.91 |
| Negative UAs before first relapse | 0.9 (1.8) | 1.5 (3.2) | −1.71 – 2.91 | 0.86 |
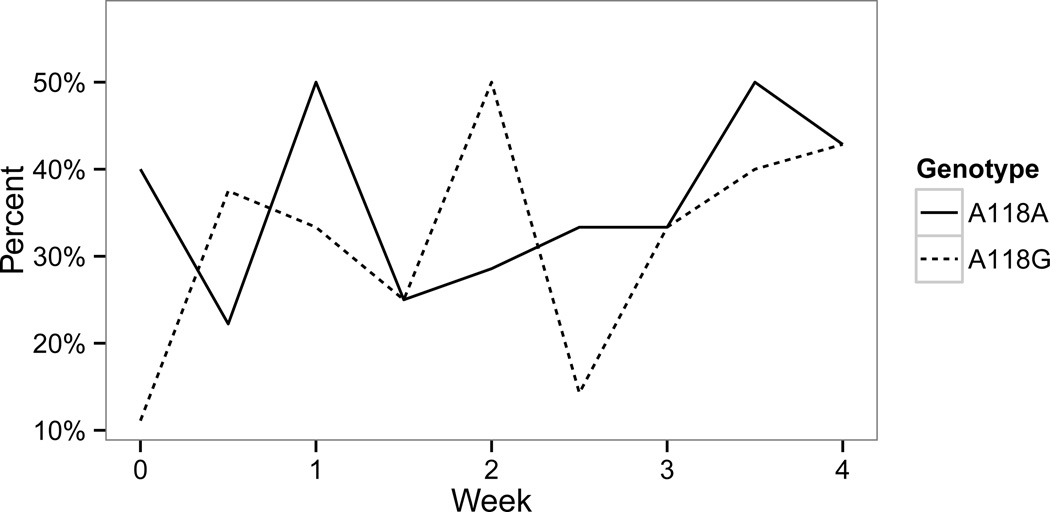
Figure 2 shows the percentage of MA-negative urine samples in both groups over the 4-week study. There was no statistically significant difference in the percentage of MA-negative urine samples between the two groups.
Figure 2. Percentage of MA-negative urine samples.
There were no serious adverse events. Sixteen subjects reported nausea at one or more of their twice-weekly visits and six subjects reported soreness at the injection site which improved over time and did not require intervention.
DISCUSSION
To date, small clinical trials have shown that naltrexone significantly attenuates subjective effects of amphetamine in healthy controls and amphetamine dependent subjects, and reduces craving and relapse to amphetamine use (Jayaram-Lindstrom, Wennberg et al. 2004; Jayaram-Lindstrom, Hammarberg et al. 2008; Jayaram-Lindstrom, Konstenius et al. 2008). Although pharmacogenetic trials demonstrated that alcohol dependent subjects with the A118G SNP of the μ-opioid receptor responded better to treatment with naltrexone (had lower rates of relapse and longer times before return to heavy drinking) (Oslin, Berrettini et al. 2003), no such pharmacogenetic trial had been conducted to investigate efficacy of naltrexone in the treatment of amphetamine dependence in subjects with the A118G SNP compared to the WT.
Here we present the first pharmacogenetic efficacy trial to assess the effect of naltrexone in MA dependent individuals with and without the A118G SNP of OPRM1 gene. The existence of a pharmacogenetic marker of efficacy such as the A118G SNP presents both opportunities and challenges. If unrecognized, its signal can be lost among subjects who have the more common wild type; if unrecognized and unevenly distributed between treatment groups, it can cause confounding. Like other variables that might predict outcome, using a valid marker to balance between groups can greatly increase statistical power. Additionally, in our study about 29% of the population who were genotyped had A118G SNP; other studies have reported even higher frequency of the polymorphism in Asians (Kreek, Nielsen et al. 2004). Thus findings of this study may be of potential relevance to populations with high frequency of A118G SNP.
Another feature of this study was the use of injectable, sustained release naltrexone to ensure patient adherence. In most clinical trials, patient adherence is far from perfect, increasing variability in response, and decreasing power. In a previous clinical trial assessing the effects of oral naltrexone on amphetamine dependence, only 62.5% of patients in the naltrexone group were adherent. Further, in that trial, adherence to medication was correlated with reduction in drug use, indicating the importance of controlling this factor. In this trial, naltrexone was administered as an IM slow-release formulation to eliminate the need for daily dosing and ensure adherence for the duration of the study. The once a month dose of IM naltrexone (380 mg) was approximately 1/3rd compared to that of oral naltrexone for the same duration (50 mg once a day for 28 days). However, due to higher bioavailability, the exposure to naltrexone over 28 days following IM administration is approximately four-fold higher than that with oral administration (U.S Food and Drug and Administration 2010).
In our trial, there was no evidence for differences between A118G and WT MA dependent subjects. Despite trials demonstrating better response to naltrexone treatment among A118G alcohol dependent patients, in our study A118G and WT MA dependent subjects did not significantly differ in days of abstinence or number of MA-negative urine samples. There may be several reasons for this difference. The reward pathway in alcoholism is mediated by endogenous opioids (Gianoulakis 2004). In both humans and mice, it has been demonstrated that the A118G allele of the OPRM1 gene confers a more robust dopamine response to alcohol in the ventral striatum, compared to that seen in A118A homozygotes (Ramchandani, Umhau et al. 2011). This increased reward-related dopamine response seen in A118G individuals can account for their better response to naltrexone compared to A118A individuals who are alcohol dependent. Studies in rodents have shown that psychostimulant drugs such as cocaine and amphetamine also cause endorphin release in the brain reward system (Olive, Koenig et al. 2001). There is also evidence for the involvement of the opioid system in human psychostimulant dependence (Guterstam, Jayaram-Lindstrom et al. 2013). However, we did not find any evidence to support the hypotheses that the release of endogenous opioids and OPRM1 genetics influence the reward, reinforcement, or other aspects of stimulant use, in contrast to effects observed in alcohol use. Our results are supported by findings from a recent placebo-controlled positron emission tomography study which demonstrated that an intravenous dose of amphetamine does not cause any acute opioid release in the healthy human brain reward system (Guterstam, Jayaram-Lindstrom et al. 2013). These findings therefore suggest that the hypothesized role of the opioid system in mediating the effects of amphetamine needs to be further investigated in animal models as well as in human subjects.
Treatment with naltrexone was well tolerated – it did not produce any serious adverse effects, and no dropouts were attributed to adverse events. This is consistent with earlier studies of naltrexone for alcohol and amphetamine-dependent patients (Croop, Faulkner et al. 1997; Jayaram-Lindstrom, Wennberg et al. 2005; Jayaram-Lindstrom, Hammarberg et al. 2008).
The findings of this study should be interpreted based on two notable limitations: small sample size and lack of placebo control groups. The sample size was small and therefore was not adequately powered to detect differences between A118A and A118G SNP groups and could not be stratified for additional analyses, e.g., based on ethnicity. Alternative recruitment methods, e.g., multicenter trials with access to ethnicities having higher frequency of polymorphism, may be necessary when conducting pharmacogenetic trials. Our study lacked control groups of placebo/A118G and placebo/WT, limiting the comparisons that could be tested. Based on data in alcohol dependence, we hypothesized that naltrexone would be of benefit predominantly for subjects with the A118G SNP, but alternate findings may have resulted if all interactions of genotype and medication were tested. In addition, treatment duration was short, although based on Jayaram-Lindstrom’s work (e.g., the change in craving was significant at 4 weeks) we considered it possible to observe a difference between groups within one month (Jayaram-Lindstrom, Hammarberg et al. 2008). Therefore, long term effects of naltrexone in this population are unknown. Trials in alcohol dependence show that the effects of naltrexone in subjects with the A118G allele increase over time. In the positive randomized, placebo-controlled trial of naltrexone for treatment of amphetamine dependence, patients received 12 weeks of treatment - extended naltrexone treatment might therefore yet reveal benefits for A118G vs WT MA users.
CONCLUSIONS
We conducted an exploratory study of the influence of the A118G mu opioid receptor polymorphism on response to naltrexone in MA dependence treatment. In contrast to a previous randomized controlled trial we did not find evidence of efficacy, although this may have been due to the limitations of this study and differences in design from the previous study.
Figure 1. Study schema.
Selection of subjects in an open labeled trial to determine effects of A118G polymorphism on treatment response to injectable naltrexone among methamphetamine-dependent patients
ACKNOWLEDGEMENTS
We would like to thank Tri Luu for assisting us with genotyping our subjects, Raymond Buscemi for delivering the manual driven cognitive-behavioral therapy to subjects, Jayme Mulkey and Emma Olson for coordinating the study, Youfei Yu for assisting us in preparing the manuscript, and the subjects for participating in the study.
Source of Funding: This study was supported by Award Number NIH DA018179 from the National Institute on Drug Abuse. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest.
REFERENCES
- Alkermes I. VIVITROL™ (naltrexone for extended-release injectable suspension) Full Prescribing Information. 2005. [Google Scholar]
- Arias A, Feinn R, et al. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, et al. The mu opioid receptor A118G gene polymorphism moderates effects of trait anger-out on acute pain sensitivity. Pain. 2008;139(2):406–415. doi: 10.1016/j.pain.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop R, Faulkner E, et al. The safety profile of naltrexone in the treatment of alcoholism: results from a multicenter usage study. Archives of General Psychiatry. 1997;54(12):1130–1135. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, et al. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Galloway G, Buscemi R, et al. A Randomized, Placebo-Controlled Trial of Sustained-Release Dextroamphetamine for Treatment of Methamphetamine Addiction. Clinical Pharmacology and Therapeutics. 2011;89(2):276–282. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, et al. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4(5):476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous Opioids and Addiction to Alcohol and other Drugs of Abuse. Current Topics in Medicinal Chemistry. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29(5):969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, et al. Substance Abuse in Women. Psychiatric Clinics of North America. 2010;33(2):339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterstam J, Jayaram-Lindstrom N, et al. Effects of amphetamine on the human brain opioid system – a positron emission tomography study. International Journal of Neuropsychopharmacology. 2013;16:763–769. doi: 10.1017/S1461145712000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, et al. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118B(1):60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Hammarberg A, et al. Naltrexone for the Treatment of Amphetamine Dependence: A Randomized, Placebo-Controlled Trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Konstenius M, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008;33(8):1856–1863. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Wennberg P, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167–171. doi: 10.1080/08039480510023052. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindstrom N, Wennberg P, et al. Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol. 2004;24(6):665–669. doi: 10.1097/01.jcp.0000144893.29987.e5. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, et al. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med. 2004;5(1):85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Skarke C, et al. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12(1):3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, et al. Effects of oral methamphetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2009;101(1–2):34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, et al. Stimulation of Endorphin Neurotransmission in the Nucleus Accumbens by Ethanol, Cocaine, and Amphetamine. The Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, et al. A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav. 2008;33(5):651–667. doi: 10.1016/j.addbeh.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Molecular Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA, et al. Kaplan and Sadock's Pocket Handbook of Psychiatric Drug Treatment. Lippincott: Williams & Wilkins; 2005. Opioid Receptor Antagonists: Naltrexone, Nalmefene, and Naloxone; p. 181. [Google Scholar]
- U.S Food and Drug and Administration. Vivitrol (naltrexone for extended-release injectable suspension) 2007 from http://www.fda.gov/cder/foi/label/2007/021897s003lbl.pdf.
- U.S Food and Drug and Administration. Efficacy and Safety Background for the Advisory Committee: Vivitrol. Silver Spring, MD: Food and Drug Administration; 2010. [Google Scholar]
- Wand GS, McCaul M, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26(1):106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]