Abstract
Background Context
Use of Bone Morphogenetic Protein (BMP) as an adjunct to spinal fusion surgery proliferated following Food and Drug Administration (FDA) approval in 2002. Major safety concerns emerged in 2008.
Purpose
To examine whether published concerns about the safety of BMP altered clinical practice.
Study Design/Setting
Analysis of the National Inpatient Sample from 2002 through 2012.
Patient Sample
Adults (age >20) undergoing an elective fusion operation for common degenerative diagnoses, identified using codes from the International Classification of Diseases, 9th revisions, Clinical Modification (ICD-9-CM).
Outcome Measures
Proportion of cervical and lumbar fusion operations, over time, that involved BMP.
Methods
We aggregated the data into a monthly time series and reported the proportion of cervical and lumbar fusion operations, over time, that involved BMP. Auto Regressive Integrated Moving Average, a regression model for time series data, was used to test whether there was a statistically significant change in the overall rate of BMP use following a FDA Public Health Notification in 2008. The study was funded by federal research grants, and no investigator had any conflict of interests.
Results
Use of BMP in spinal fusion procedures increased rapidly until 2008, involving up to 45.2% of lumbar and 13.5% of cervical fusions. BMP use significantly decreased following the 2008 FDA Public Health Notification and revelations of financial payments to surgeons involved in the pivotal FDA approval trials. For lumbar fusion, the average annual increase was 7.9 percentage points per year from 2002 to 2008, followed by an average annual decrease of 11.7 percentage points thereafter (p = <0.001). Use of BMP in cervical fusion increased 2.0% per year until the FDA Notification, followed by a 2.8% per year decrease (p = 0.035).
Conclusions
Use of BMP in spinal fusion surgery declined subsequent to published safety concerns and revelations of financial conflicts-of-interest for investigators involved in the pivotal clinical trials.
BACKGROUND
Recombinant human Bone Morphogenetic Protein-2 (BMP) obtained Food and Drug Administration (FDA) approval in 2002 as an adjunct to a single level anterior lumbar spinal fusion operation.[1] Partly because BMP serves as an alternative to harvesting iliac crest bone graft, its use initially proliferated, including off-label procedures such as posterior lumbar fusion, posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF) and cervical fusion.[2, 3] Concerns regarding its safety in cervical fusion prompted the Food and Drug Administration (FDA) to issue a Public Health Notification in July 2008.[4] Additional reports questioning the safety and off-label use of BMP in cervical and lumbar fusions [5–12] subsequently led professional societies to make recommendations about the appropriate use of BMP in spinal fusion.[13]
We wanted to examine whether clinical practice has changed in recent years in response to these publications. We used a nationally representative discharge registry, available from the Agency for Healthcare Research and Quality (AHRQ), to assess trends in the proportion of all fusion operations that included BMP. We tested whether there was a statistically significant reduction in the rates of BMP use following the FDA Public Health Notification.
METHODS
Data source
We examined AHRQ’s Nationwide Inpatient Sample (NIS)[14], a component of the Health Care Utilization Project (HCUP), from 2002–2012. NIS is a nationally representative sample of discharge summaries from non-federal hospitals in the United States commonly used to describe trends in inpatient procedures. Participating hospitals submit uniform patient demographics, discharge disposition, hospital charges, and diagnosis and procedure codes from the International Classification of the Diseases, 9th revision, Clinical Modification (ICD-9-CM) to AHRQ’s central distributor. Survey weighting and sampling design variables are included to produce national estimates of utilization. We applied the revised 2012 longitudinal weights created for trend analyses.
Denominator data for reporting population-based rates were obtained from the U.S. Census, with stratification by sex and 5-year increments of age.[15]
Identifying admissions for fusion surgery with and without BMP
We identified adults (age > 20) undergoing elective fusion admissions for diagnoses of back pain, disc herniation, spinal stenosis, spondylolisthesis or scoliosis in NIS, grouping them by vertebral region involved. Because we were primarily interested in the discretionary use of BMP in elective fusion surgery for degenerative conditions, we excluded non-elective admissions, and fusions associated with diagnosis codes for fracture, dislocation, or spinal cord injury. We also excluded patients with diagnosis codes for congenital or other spinal anomaly (Table 1). A variable provided by NIS was used to include only those admissions that were “elective”, as reported by participating hospitals. Use of BMP was identified based on the coding of ICD-9-CM procedure code 84.52 (“insertion of recombinant BMP”).
Table 1.
Number of patients meeting inclusion and exclusion criteria
| 2002 | 2007 | 2012 | All | |
|---|---|---|---|---|
| Included admissions | ||||
| Fusion surgery for degeneration | 282101 | 338645 | 427760 | 3993454 |
| Excluded Diagnosis | ||||
| Fracture or dislocation | 7728 | 11897 | 17220 | 136384 |
| Spinal Cord Injury | 1064 | 1737 | 3055 | 20440 |
| congenital or other anomaly | 9015 | 11309 | 18755 | 143500 |
| Inflammatory spondylopathy | 420 | 663 | 1795 | 10248 |
| Excluded Procedures | ||||
| Artificial disc replacement | 0 | 1962 | 1700 | 14837 |
| Open treatment of fracture | 1717 | 3133 | 5010 | 35875 |
| Spacer or dynamic stabilizing device | 0 | 523 | 1355 | 6564 |
| Other spine procedures | 32466 | 42258 | 67525 | 549205 |
| Excluded Comorbidity | ||||
| Cancer | 2006 | 4059 | 6005 | 45594 |
| Neurological impairment | 1294 | 2674 | 3470 | 26794 |
| Immune deficiency | 349 | 613 | 995 | 6743 |
| Intraspinal Abscess | 453 | 944 | 1635 | 10796 |
| Osteomyelitis | 1269 | 1778 | 2820 | 21839 |
| Pregnancy | 24 | 5 | 25 | 330 |
| Other exclusions | ||||
| Trauma | 11527 | 6780 | 8410 | 82774 |
| drug abuse | 342 | 998 | 1195 | 9966 |
| Age under 20 | 8823 | 8438 | 10865 | 102867 |
| Not elective admission | 26924 | 31600 | 43890 | 411512 |
| Summary Inclusion and Exclusion | ||||
| All inclusion criteria | 282101 | 338645 | 427760 | 3993454 |
| Any exclusion criteria | 80666 | 98619 | 144415 | 1237233 |
| Final cohort size | 201435 | 240026 | 283345 | 2756221 |
Note: Estimates based on weighted sample from the Nationwide Inpatient Sample
Covariates
Variables that describe each admission include patient demographics (age, sex, and race), insurance status, length-of-stay, admission charges, and median income for the zip code where the patient lives, a proxy for socioeconomic status. We recoded race and ethnicity variables into “white”"black”, or “other”. Race and ethnicity was not reported by all hospitals and was therefore not used to adjust rates. Insurance was grouped into “Medicare”"Medicaid”"Private Insurance”, and “Other”. The latter category included “self-pay” and “charity”, or was unavailable from the source hospital. Length of stay was grouped into categories of one, two, three, four, or five or more days.
We relied on diagnosis and procedure codes to calculate Quan’s version of the Charlson comorbidity index[16] and to classify patients by surgical indication, surgical approach used, and vertebral region.[17] Procedure codes that first became available beginning in 2004 were also used to describe combined anterior and posterior surgical approaches, stabilizing instrumentation, and 3 or more disc levels fused.
Analysis
We aggregated the data into a monthly time series and reported the proportion of fusion operations, over time, that involved BMP. We then used Auto Regressive Integrated Moving Average (ARIMA), a regression model for time series data, to test whether there was a statistically significant change in the rate of BMP use following the FDA Public Health Notification in 2008. We separately modeled the use of BMP in lumbar and cervical fusions, controlling for patient age, sex, and comorbidity.
An ARIMA models an outcome over time, incorporating two key components of the effects of time: a moving average process and autoregression. For the moving average process, the rate of BMP use is estimated by smoothing across successive months to reduce the idiosyncratic components of the monthly data. The autoregressive component adjusts future estimates based on serial autocorrelation in the time series data, that is, the rate of BMP use at time “t” is correlated to its rate at time “t-1”.
Shocks in a time series, such as the potential influence of the FDA Public Health Notification on BMP, can have a time-limited effect on the moving average component and a sustained effect on the autoregressive component, affecting all future estimates of the series. We included a 1-month autoregressive lag operator, as suggested by examining a serial correlogram, inspecting Aikaike’s Information Criteria, and by observing a reduction in the standard errors of the coefficients. Significance testing for discontinuity in the regression coefficient over time following the FDA Public Health notification was performed on the first difference between successive time points (called “stationary”, or first derivative in time-series analyses).
This study was exempt from IRB review because the data have been deemed a public data set by the Committee for the Protection of Human Subjects at Dartmouth.
RESULTS
Rates & volume of fusion operations
The age and sex-adjusted rate of lumbar and cervical spinal fusion operations for degenerative diagnoses in the U.S. was 116.8 per 100,000 in 2012 (95%CI 116.5, 117.2), an increase of 26.3% from the rate of 92.5 (95%CI 92.2, 92.9) in 2002. In 2012, the most recent year of our analysis, 57.8% were lumbar (n = 162,685) and 42.2% were cervical (n = 118,915; Table 2).
Table 2.
Annual age- and sex-adjusted population rate (per 100,000) and volume of fusion operation in the United State, along with proportion that involved Bone Morphogenetic Proteins (BMP)
| Year | Lumbar fusion Volume |
Rate (per 100,000) | Percent with BMP | Cervical fusion Volume |
Rate (per 100,000) | Percent with BMP |
|---|---|---|---|---|---|---|
| 2002 | 103371 | 51.6 | 1.05 | 97685 | 48.4 | 0.27 |
| (51.3 – 51.9) | (48.1 – 48.7) | |||||
| 2003 | 108735 | 53.7 | 11.92 | 103257 | 50.4 | 2.18 |
| (53.3 – 54.0) | (50.1 – 50.7) | |||||
| 2004 | 111314 | 54.3 | 23.51 | 94739 | 45.8 | 4.79 |
| (54.0 – 54.6) | (45.6 – 46.1) | |||||
| 2005 | 118457 | 57.1 | 31.43 | 98130 | 46.8 | 4.99 |
| (56.7 – 57.4) | (46.5 – 47.1) | |||||
| 2006 | 129449 | 60.3 | 37.79 | 104278 | 48.3 | 8.59 |
| (59.9 – 60.6) | (48.0 – 48.6) | |||||
| 2007 | 131599 | 60.4 | 41.18 | 107518 | 49.2 | 11.27 |
| (60.1 – 60.7) | (48.9 – 49.5) | |||||
| 2008 | 161352 | 73.0 | 43.17 | 117612 | 53.2 | 9.60 |
| (72.6 – 73.3) | (52.9 – 53.5) | |||||
| 2009 | 162342 | 72.4 | 41.37 | 115854 | 51.9 | 8.86 |
| (72.1 – 72.8) | (51.6 – 52.2) | |||||
| 2010 | 174445 | 76.1 | 43.27 | 118938 | 52.1 | 7.66 |
| (75.8 – 76.5) | (51.8 – 52.4) | |||||
| 2011 | 173897 | 74.5 | 31.56 | 130836 | 56.5 | 5.87 |
| (74.2 – 74.9) | (56.2 – 56.8) | |||||
| 2012 | 162685 | 68.4 | 25.44 | 118915 | 50.6 | 5.08 |
| (68.1 – 68.7) | (50.3 – 50.9) |
Note: Estimates based on weighted sample from the Nationwide Inpatient Sample
Note: Denominator data from the United State Census
Note: Values in parenthesis represent 95% confident intervals
Back pain, disc herniation and spinal stenosis combined to account for 53.9% of the lumbar fusion indications, while spondylolisthesis and scoliosis accounted for 36.6% and 8.6%, respectively. Among cervical fusions, neck pain, disc herniation, and stenosis accounted for 18.7%, 51.2%, and 25.8% of the fusion admissions, respectively.
Factors associated with BMP use
Although characteristics of patients undergoing fusion with BMP were similar to those without BMP, surgical indication and operative features differed (Table 3). Patients who received BMP were more likely to have a surgical indication of back pain (e.g. spondylosis) or scoliosis. They were also significantly more likely to undergo anterior or combined surgical approaches, receive stabilizing instrumentation, undergo fusions of three or more disc levels, or have had previous spine operations. Patients undergoing lumbar fusion with BMP were also more likely to have a fusion combined with decompression.
Table 3.
Characteristics associated with BMP use in lumbar and cervical fusion
| Lumbar fusion |
Cervical fusion |
|||||
|---|---|---|---|---|---|---|
| No BMP | BMP | p-value | No BMP | BMP | p-value | |
| Age | ||||||
| Age (mean) | 56.2 | 55.1 | <0.001 | 52.8 | 53.9 | <0.001 |
| Age 20 to 65 (%) | 67.6 | 71.3 | <0.001 | 82.2 | 80.1 | <0.001 |
| Age 65 or older (%) | 32.4 | 28.7 | 17.8 | 19.9 | ||
| Sex | ||||||
| Male (%) | 43.5 | 43.4 | 0.415 | 47.6 | 48.0 | 0.349 |
| Female (%) | 56.5 | 56.6 | 52.4 | 52.0 | ||
| Race | ||||||
| White (%) | 83.3 | 84.9 | <0.001 | 82.6 | 83.4 | 0.015 |
| Black (%) | 6.8 | 6.1 | 8.6 | 8.4 | ||
| Asian (%) | 6.1 | 5.3 | 4.9 | 4.3 | ||
| Other or Multiple (%) | 3.9 | 3.7 | 3.9 | 3.9 | ||
| Comorbidity | ||||||
| None(%) | 67.8 | 67.8 | 0.534 | 71.3 | 68.5 | <0.001 |
| One(%) | 24.7 | 24.6 | 22.6 | 24.2 | ||
| Two or more (%) | 7.6 | 7.7 | 6.1 | 7.3 | ||
| Income | ||||||
| One (low income) (%) | 20.8 | 20.2 | <0.001 | 21.1 | 21.4 | 0.002 |
| Two (%) | 26.2 | 26.2 | 25.3 | 24.6 | ||
| Three (%) | 26.8 | 28.1 | 26.5 | 27.8 | ||
| Four (high income) (%) | 26.2 | 25.6 | 27.1 | 26.2 | ||
| Insurance | ||||||
| Medicare (%) | 34.9 | 31.9 | <0.001 | 23.4 | 26.6 | <0.001 |
| Medicaid (%) | 4.7 | 4.2 | 5.3 | 4.4 | ||
| Commercial (%) | 47.2 | 50.6 | 58.9 | 57.5 | ||
| Other or uninsured (%) | 13.2 | 13.2 | 12.4 | 11.5 | ||
| Length of stay | ||||||
| One day (%) | 7.5 | 6.9 | <0.001 | 63.2 | 54.2 | <0.001 |
| Two days (%) | 17.3 | 17.8 | 20.7 | 22.6 | ||
| Three days (%) | 28.2 | 28.2 | 7.7 | 9.7 | ||
| Four days (%) | 21.3 | 20.6 | 3.3 | 5.0 | ||
| Five or more days (%) | 25.7 | 26.5 | 5.0 | 8.6 | ||
| Diagnosis | ||||||
| Axial (%) | 20.4 | 23.7 | <0.001 | 18.1 | 20.3 | <0.001 |
| Herniation (%) | 21.2 | 20.4 | 51.6 | 44.2 | ||
| Stenosis (%) | 12.6 | 8.9 | 25.7 | 27.4 | ||
| Listhesis (%) | 36.9 | 36.1 | 2.2 | 3.2 | ||
| Scoliosis (%) | 8.0 | 9.9 | 2.0 | 3.4 | ||
| Operative characteristics | ||||||
| Decompression with fusion (%) | 59.2 | 64.0 | <0.001 | 85.3 | 77.3 | <0.001 |
| Stabilizing instrumentation (%) | 49.3 | 71.2 | <0.001 | 40.2 | 66.4 | <0.001 |
| Three or more levels fused (%) | 9.5 | 12.8 | <0.001 | 13.3 | 22.5 | <0.001 |
| Previous spine surgery (%) | 6.4 | 7.5 | <0.001 | 1.7 | 4.9 | <0.001 |
| Operative approach | ||||||
| Posterior (%) | 84.7 | 74.4 | <0.001 | 7.2 | 13.3 | <0.001 |
| Anterior (%) | 7.2 | 12.4 | 89.0 | 80.8 | ||
| Combined (%) | 8.2 | 13.2 | 3.8 | 5.9 | ||
Note: Estimates based on weighted sample from the Nationwide Inpatient Sample
Rate of BMP use
We observed a 44.7% decrease in the rate of BMP use with lumbar fusion, from a peak use of 45.2% to 25.0% by the end of 2012. Similarly, we found a 56% reduction in BMP use for cervical fusion, from a peak of 13.5% to 6.0% in 2012.
Following its FDA approval, use of BMP with fusion increased rapidly until 2008, involving up to 45.2% of lumbar and 13.5% of cervical fusions. After the 2008 FDA Public Health Notification, the rate of BMP use decreased 0.978 percentage points per month for lumbar fusion and 0.231 percentage points for cervical fusion. This compared to pre-notification increases of 0.658 for lumbar fusion and 0.167 for cervical fusion (Table 4). For lumbar fusion, this translates into an average annual increase of 7.9 percentage points per year from 2002 to 2008, followed by an average annual decrease of 11.7 percentage points thereafter (p-value <0.001; pre-post trend). Similarly, BMP use in cervical fusion increased 2.0 percentage points per year until the FDA Notification, followed by an average decrease of 2.8 percentage point per year (p = 0.035; pre-post trend). Changes in using BMP were not explained by changes in patient age, sex or comorbidity.
Table 4.
Time series regression model for the monthly change in the proportion of Bone Morphogenetic Protein use with lumbar and cervical fusion in the United States in relationship to the FDA Public Health Notification
| Lumbar fusion | Cervical fusion | |
|---|---|---|
| Change in % monthly BMP use | ||
| Post FDA trend | −0.978*** | −0.231 |
| (−3.96) | (−1.66) | |
| Change in percent female | 7.089 | −1.469 |
| (0.57) | (−0.31) | |
| Change in mean age | −0.597* | −0.0844 |
| (−2.01) | (−0.35) | |
| Change in mean charlson score | 1.113 | −7.402 |
| (0.13) | (−1.75) | |
| Constant | 0.658*** | 0.167* |
| (3.83) | (2.28) | |
| ARMA | ||
| time lag (1 month) | −0.344*** | −0.319*** |
| (−3.80) | (−3.68) | |
| Sigma | ||
| Constant | 1.646*** | 0.870*** |
| (19.28) | (16.71) | |
| Pre vs. post trend p-value | 0.000*** | 0.035* |
| Number of months | 123 | 123 |
t statistics in parentheses
Estimates based on weighted sample from the Nationwide Inpatient Sample
p < 0.05,
p < 0.01,
p < 0.001
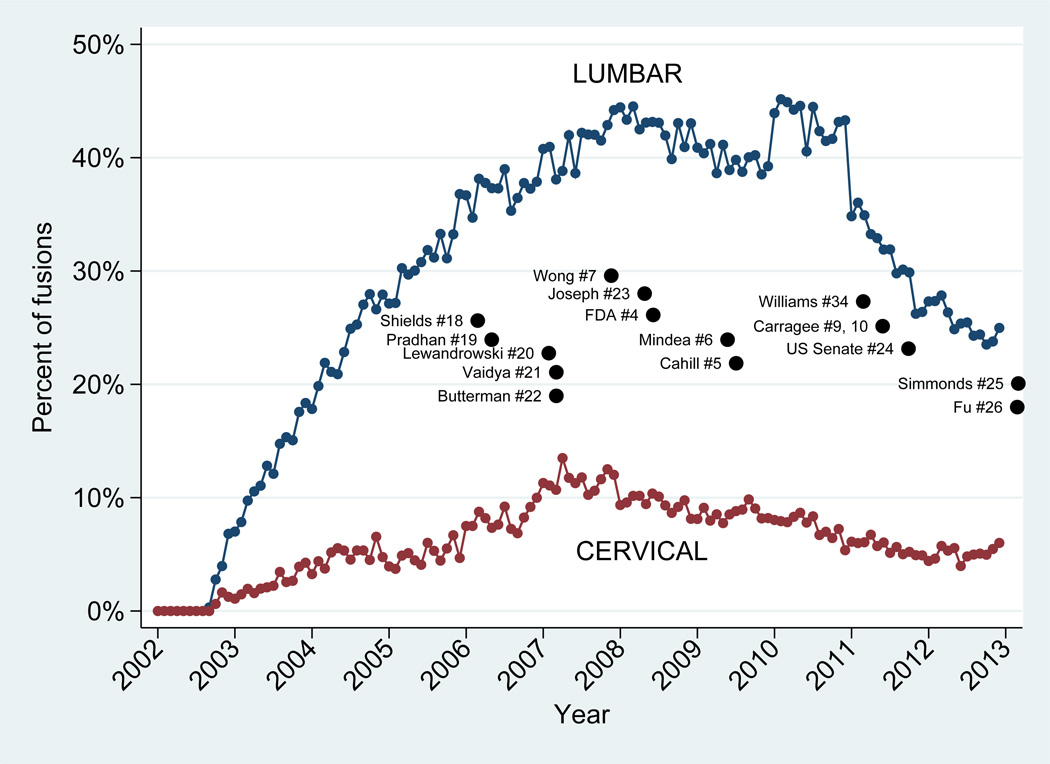
Decreased BMP use continued through 2012. Figure 1 illustrates the percent of lumbar and cervical fusions over time that involved BMP, and in relationship to published safety reports. The figure is annotated by the publication dates of studies by Shields[18], Pradham[19], Lewandrowski [20], Vaidyn[21], and Butterman[22] which were among the first case series to report wound complication, osteolysis, and dysphagia with BMP in anterior cervical fusion. Those by Mindea,[6] Wong, [7] Joseph,[23] and Carragee [10] were the first to raise concerns about postoperative radiculitis and retrograde ejaculation with lumbar fusions involving BMP. Reports that likely had the greatest visibility included the FDA Public Health notification[4], Cahill and colleagues study in the Journal of the American Medical Association,[5] an entire issue of The Spine Journal dedicated to the use of BMP,[9] a US Senate Finance Committee report,[24] and the reanalysis of pivotal trial data through the Yale University Open Data Access Project (YODA).[25, 26]
Figure 1.
DISCUSSION
Concerns about morbidity, blood loss, longer operating time, and limited autograft availability have prompted spine surgeons to look for alternatives in fusion procedures, such as bone morphogenetic protein.[27–29] Expanded use of BMP has led to re-analysis of original studies to re-evaluate donor site morbidity,[30] rate of revision surgery,[11] adverse events[5, 12], and patient-reported outcomes.[25] Reports of adverse events have been limited to small case series, but are consistent with analyses of population-based administrative data.[5, 11, 31–33] Pivotal trials that led to FDA approval of BMP have been accused of design flaws and industry influence.[9, 24, 26, 34] An investigation by the United States Senate Finance Committee concluded that “Medtronic was involved in drafting, editing, and shaping the content of medical journal articles authored by its physician consultants who received significant amounts of money through royalties and consulting fees from Medtronic.”[24] A re-analysis of patient-level data and a meta-analysis of the industry-sponsored FDA trials independently concluded that BMP had no clinical advantage over iliac crest bone graft, and its risks were understated in journal publications.[25] In their combined summary, the Annals of Internal Medicine Editorial Board reported that "Early journal publications misrepresented the effectiveness and harms through selective reporting, duplicate publication, and underreporting".[35]
In light of this emerging evidence, The North American Spine Society advocates to keep BMP as an option only for patients undergoing anterior lumbar fusions who have a high risk of non-union, poor or inadequate graft bed, and revision fusions (except in males with a reproductive priority.)[13] These indications for appropriate use seem clinically reasonable, but they lack clinical evidence.
Our estimates of the use of BMP using more recent data are similar to earlier estimates. We found that BMP use with lumbar fusion peaked at 43% by 2008. Cahill reported a rate of BMP use of 40% for lumbar fusions and 9% for cervical fusions in 2006 NIS data.[5] Among fusion cases performed between 2004 through 2007 reported in the Scoliosis Research Society registry, the rate of BMP use was 13% for anterior cervical fusions, 38% for thoracolumbar fusion with a combined approach, and 51% for transforaminal lumbar interbody fusions.[33] The decrease in BMP use that we report parallels Medronic’s worldwide revenue for biologic products, decreasing from $840 million in fiscal year 2009 to $471 million in fiscal year 2014.[36] Our finding of greater BMP use among patients undergoing more complex fusion procedures is consistent with our prior report.[11]
Our study included a representative sample designed to produce national estimates of inpatient procedure rates over the full spectrum of adults receiving inpatient care from non-federal hospitals. The limitations of our study include the reliance on claims data, which lack some clinical detail such as disease severity, specific vertebral level fused, or the amount of BMP used. While some have raised concerns that using claims data may not be accurate, their use in classifying spine surgery patients by indications for fusion has been validated, and fusion procedures appear to be coded accurately.[17] Though they lack clinical detail, claims data provide a population-level perspective lacking in clinical trial, patient regisitries, and clinical series research designs. While we sought to focus primarily on degenerative spinal problems, we could not distinguish between varying forms of scoliosis on the basis of ICD-9-CM codes, or determine whether the rate of BMP use was different depending on the type of scoliosis a patient had. Using NIS data, we cannot link successive admissions for the same patient in order to examine reoperation rates. Therefore, we cannot know whether the changing pattern of BMP use was associated with changing patterns of adverse events, readmission, repeat spinal surgery, or ectopic bone formation. Patient-reported outcomes are not available in NIS.
FDA approval studies often use ideal settings, highly skilled surgeons, carefully selected patients, high-volume hospitals, and strict protocol adherence. Once approved, products may diffuse into widespread clinical practice, among surgeons with varying experience, in less controlled settings, with expanded indications, and perhaps in less rigorously selected patients. The safety profile may therefore be worse than in the FDA Safety and Effectiveness reports, and this may expose patients to unnecessary harms and preventable complications. By the time Cahill’s study was published, BMP had been on the market for seven years and was used in up to 44% of lumbar fusions and 13% of cervical fusions.
CONCLUSIONS
We found that the use of BMP in spinal fusion operations declined subsequent to published safety concerns and amid revelations of financial conflicted investigators involved in the pivotal trials. While there was no evidence of preferential use of BMP based on patient characteristics, it was more commonly used in patients undergoing more complex types of spinal fusion operations.
The changing practice of BMP use with spinal fusion in relationship to emerging concerns about it safety and efficacy underscores the importance for post-approval surveillance of emerging technologies. Developing ongoing, systematic, population-based methods to monitor the safety and effectiveness of emerging technologies may help curtail surgical complications and maximize the safe use of spinal products.
ACKNOWLEDGEMENTS
Funding for the study was provided by grant #HS021695 from the Agency for Healthcare Research and Quality; and grant #P60AR062799 from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases. The findings and conclusions expressed are solely those of the author(s) and do not necessarily represent the views of any agency of the Federal Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brook I. Martin, The Dartmouth Institute for Health Policy and Clinical Practice; and of The Department of Orthopaedic Surgery at Dartmouth-Hitchcock Medical Center, One Medical Center Dr., Lebanon, NH 03756, (603) 653-9167, Brook.I.Martin@Dartmouth.edu.
Jon D. Lurie, Departments of Medicine, Orthopaedics, and of The Dartmouth Institute, One Medical Center Dr. Lebanon, NH 03756, (603) 653-3575, Jon.D.Lurie@Dartmouth.edu.
Richard A. Deyo, Department of Family Medicine, Department of Medicine, Department of Public Health & Preventive Medicine, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., Portland, OR. 97239, (503) 494-1694, deyor@ohsu.edu.
Anna N.A. Tosteson, Departments of Medicine, and Community and Family Medicine, and The Dartmouth Institute, One Medical Center Dr. Lebanon, NH 03756, (603) 653-3519, Anna.N.A.Tosteson@Dartmouth.edu.
Farrokh Reza Farrokhi, Virginia Mason Medical Center, 1100 9th Ave; Mail stop X7-NS; Seattle, WA 98111, 206-223-7525, farrokh.farrokhi@vmmc.org.
Sohail K. Mirza, Professor of Orthopaedic Surgery, and The Dartmouth Institute, Chair, Department of Orthopaedics, One Medical Center Dr. Lebanon, NH 03756, (603) 653-6090, Sohail.K.Mirza@Dartmouth.edu.
REFERENCES
- 1.Food and Drug Administration. Summary of Safety and Effectiveness Data (InFUSE Bone Graft/LT-Cage Lumbar Tapered Fusion Device) 2002 [Google Scholar]
- 2.Lad SP, Nathan JK, Boakye M. Trends in the use of bone morphogenetic protein as a substitute to autologous iliac crest bone grafting for spinal fusion procedures in the United States. Spine. 2011;36(4):E274–E281. doi: 10.1097/BRS.0b013e3182055a6b. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 3.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine. 2010;35(19):1794–1800. doi: 10.1097/BRS.0b013e3181ecf6e4. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. FDA Public Health Notification: Life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. Report issued July 1, 2008.
- 5.Cahill KS, Chi JH, Day A. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 6.Mindea SA, Shih p, Song JK. Recombinant human bone morphogenetic protein-2-induced radiculitis in elective minimally invasive transforaminal lumbar interbody fusions: a series review. Spine. 2009;34(14):1480–1484. doi: 10.1097/BRS.0b013e3181a396a1. (Phila Pa 1976); discussion 1485. [DOI] [PubMed] [Google Scholar]
- 7.Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2) Spine J. 2008;8(6):1011–1018. doi: 10.1016/j.spinee.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Owens K, Howard JM, Witten JL. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J. 2010 doi: 10.1007/s00586-010-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ. Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J. 2011;11(6):511–516. doi: 10.1016/j.spinee.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Ching A, Matsen L, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Use of Bone Morphogenetic Proteins in Spinal Fusion Surgery for Older Adults with Lumbar Stenosis: Trends, Complications, Repeat Surgery, and Charges. Spine. 2011 doi: 10.1097/BRS.0b013e31821bfa3a. (Phila Pa 1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smoljanovic T, Bojanic I. Commentary: An evolving perception of the risk of rhBMP-2 use for anterior spinal interbody fusions. Spine J. 2011;11(6):520–521. doi: 10.1016/j.spinee.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 13.North American Spine Society. NASS Coverage Policy Recommendations. [Accessed 11/21/2014];Recombinant Human Bone Morphogenetic Protein (rhBMP-2) 2014 Available from: https://www.spine.org/Documents/PolicyPractice/CoverageRecommendations/rhBMP.pdf.
- 14.AHRQ. HCUPnet, Healthcare Cost and Utilization Project. [8/11/2010]; Available from: http://hcupnet.ahrq.gov/
- 15.Ruggles S, Alexander JT, Genadek K, Goeken R, Schroeder MB, Sobek M. Integrated Public Use Microdata Series: Version 5.0 [Machine-readable database] Minneapolis: University of Minnesota; 2010. [Google Scholar]
- 16.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CM administrative data. Med Care. 2002;40(8):675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Martin BI, Lurie JD, Tosteson ANA, Deyo RA, Tosteson TD, Weinstein J, Mirza SK. Indications for spine surgery: validation of an administrative coding algorithm to classify degenerative diagnoses. Spine. 2014 doi: 10.1097/BRS.0000000000000275. (Phila Pa 1976), In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 19.Pradhan BB, Turner AW, Zatushevsky MA, Cornwall GB, Rajee SS, Bae HW. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine. 2006;31(10):E277–E284. doi: 10.1097/01.brs.0000216442.12092.01. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 20.Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J. 2007;7(5):609–614. doi: 10.1016/j.spinee.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buttermann GR. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426–435. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine. 2007;32(25):2885–2890. doi: 10.1097/BRS.0b013e31815b7596. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 24.United States Senate Finance Committee. Staff report on Medtronic's influence on clinical studies. [Accessed 11/26/2014];International journal of occupational and environmental health. 2013 12(2):67–76. doi: 10.1179/2049396713Y.0000000020. In addition to: http://www.finance.senate.gov/newsroom/chairman/release/?id=b1d112cb-230f-4c2e-ae55-13550074fe86. [DOI] [PubMed] [Google Scholar]
- 25.Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158(12):877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 26.Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158(12):890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 27.Sasso RC, LeHuec JC, Shaffrey C. Spine Interbody Research Group, Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;(18 Suppl):S77–S81. doi: 10.1097/01.bsd.0000112045.36255.83. [DOI] [PubMed] [Google Scholar]
- 28.Burkus JK, Sandhu HS, Gornet MF. Influence of rhBMP-2 on the healing patterns associated with allograft interbody constructs in comparison with autograft. Spine. 2006;31(7):775–781. doi: 10.1097/01.brs.0000206357.88287.5a. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 29.Gupta MC, Maitra S. Bone grafts and bone morphogenetic proteins in spine fusion. Cell Tissue Bank. 2002;3(4):255–267. doi: 10.1023/A:1024605128411. [DOI] [PubMed] [Google Scholar]
- 30.Carragee EJ, Bono CM, Scuderi GJ. Pseudomorbidity in iliac crest bone graft harvesting: the rise of rhBMP-2 in short-segment posterior lumbar fusion. Spine J. 2009;9(11):873–879. doi: 10.1016/j.spinee.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Martin BI, Mirza SK, Franklin GM, Lurie JD, Mackenzie TA, Deyo RA. Hospital and surgeon variation in complications and repeat surgery following incident lumbar fusion for common degenerative diagnoses. Health Serv Res. 2013;48(1):1–25. doi: 10.1111/j.1475-6773.2012.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin BI, Franklin GM, Deyo RA, Wickizer TM, Lurie JD, Mirza SK. How do coverage policies influence practice patterns, safety, and cost of initial lumbar fusion surgery? A populationbased comparison of workers' compensation systems. Spine J. 2013 doi: 10.1016/j.spinee.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams BJ, Smith JS, Shaffrey CI, et al. Does BMP increase the incidence of perioperative complications in spinal fusion? A comparison of 55,862 cases of spinal fusion with and without BMP. Spine. 2011 doi: 10.1097/BRS.0b013e318216d825. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 34.Mirza SK. Folly of FDA-approval studies for bone morphogenetic protein. Spine J. 2011;11(6):495–499. doi: 10.1016/j.spinee.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Laine C, Guallar E, Goodman SN, et al. Closing in on the truth about recombinant human bone morphogenetic protein-2: evidence synthesis, data sharing, peer review, and reproducible research. Ann Intern Med. 2013;158(12):916–918. doi: 10.7326/0003-4819-158-12-201306180-00012. [DOI] [PubMed] [Google Scholar]
- 36.Medtronic. [Accessed 11/21/2014];Quarterly revenue reports. Available from http://investorrelations.medtronic.com/phoenix.zhtml?c=76126&p=quarterlyEarnings.