Abstract
Helicobacter pylori infection contributes to development of diverse gastric and extra-gastric diseases. The infection is necessary but not sufficient for development of gastric adenocarcinoma. Its eradication would eliminate a major worldwide cause of cancer death, so there is much interest in identifying how, if, and when this can be accomplished. There are several mechanisms by which H pylori contributes to development of gastric cancer. Gastric adenocarcinoma is one of many cancers associated with inflammation, which is induced by H pylori infection, yet the bacteria also cause genetic and epigenetic changes that lead to genetic instability in gastric epithelial cells. H pylori eradication reduces both. However, many factors must be considered in determining whether treating this bacterial infection will prevent cancer or only reduce its risk—these must be considered in designing reliable and effective eradication therapies. Furthermore, H pylori infection has been proposed to provide some benefits, such as reducing the risks of obesity or childhood asthma, although there are no convincing data to support the benefits of H pylori infections.
Keywords: bacteria, stomach cancer, prevention, Barrett's esophagus, esophageal adenocarcinoma
Helicobacter pylori infection is etiologically related to gastric cancer. Elimination of H pylori will dramatically reduce the incidence of gastric cancer. However, it is not clear how to reliably cure the infection or whether there might be populations in which H pylori might provide some benefit. There are several animal models of H pylori-induced carcinogenesis7-9, but unless the bacteria are combined with a chemical carcinogen, none of these models reliably produce a malignancy similar to that observed in humans. More importantly, curing the infection in animal models frequently results in resolution of the malignancy or dysplastic changes, calling into question their relevance to human gastric adenocarcinoma 9, 10. We review the effects of H pylori infection and challenges to and benefits of its eradication. For reviews of basic issues related to the ability of H pylori to survive on the surface of the stomach, the role of virulence factors in the pathogenesis of gastric cancer, H pylori induced inflammation and genetic instability in the gastric mucosa, and on the history of H pylori-related disease see 1, 2, 3, 4,5, 6.
H pylori as the Primary Cause of Gastric Cancer
H pylori infection is necessary but not sufficient for development of H pylori-associated gastric cancer 11, similar in concept to hepatitis B and C viruses and human papilloma virus. The infection is required for gastric cancer to develop, but H pylori infection alone is not sufficient for gastric carcinogenesis—other events are also involved. However, H pylori is not the only cause of gastric cancer—other less common causes account for 3%–5% of gastric adenocarcinomas and include infection with Epstein-Barr virus, genetic abnormalities in the host, autoimmune gastritis, and possibly proximal cancers related to esophageal adenocarcinoma. Therefore, even in the absence of H pylori, gastric adenocarcinoma would almost but not completely disappear.
Gastric cancer is a major cause of cancer death worldwide. Japan has a particularly high burden of gastric cancer, so in February 2013 the Japanese government approved insurance coverage for a gastric cancer prevention program 12, 13 that includes H pylori screening and treatment (primary prevention), as well as post-treatment surveillance (secondary prevention for people with atrophic gastritis). In November 2014 the World Health Organization published an IARC working group report entitled H pylori Eradication as a Strategy for Preventing Gastric Cancer; this report resulted from a conference held in December 2013 14. In addition, recommendations of the Kyoto Global Consensus Conference on Helicobacter pylori gastritis (held in January 2014) were published in early 2015 15. Those recommendations state: “H pylori gastritis should be defined as an infectious disease, even when patients have no symptoms and irrespective of complications such as peptic ulcers and gastric cancer”, “H pylori-infected individuals should be offered eradication therapy, unless there are competing considerations”, and “eradication of H pylori reduces the risk of gastric cancer. The degree of risk reduction depends on the presence, severity, and extent of atrophic damage at the time of eradication” 15.
Overall, it appears the tide has turned toward H pylori eradication and the question of whether it can eliminate gastric cancer has become moot—similar to asking whether eradication of polio virus infections would eradicate polio. The current issue is how to eradicate H pylori in the most efficient and cost-effective manner. For example, should the entire population of Japan be treated for infection? Should high-risk and high-prevalence groups in regions of low gastric cancer incidence, such as the United States (US), be treated? The magnitude of the problem is illustrated by the fact that Japan and Korea alone, which each have a high prevalence of gastric cancer, have approximately 80 million H pylori-infected individuals. Whereas H pylori eradication may be possible in Japan and Korea, eradication in other countries with many infected people, such as India, is probably unlikely due to costs, the presence of other important infectious diseases, and the immense numbers of people that would require treatment. In addition, in India and other developing countries, there is high risk for reinfection due to poor sanitation and low standards of living. Vaccination is a possibility, but progress toward a preventive or preventive and therapeutic vaccine has been disappointing and funding for vaccine research is scarce 16. So far in the 21st century we have greatly increased our understanding the pathogenesis of H pylori-related diseases and mucosal immunology, so many problems of H pylori vaccine development no longer seem insurmountable.
H pylori-associated Gastric Cancer
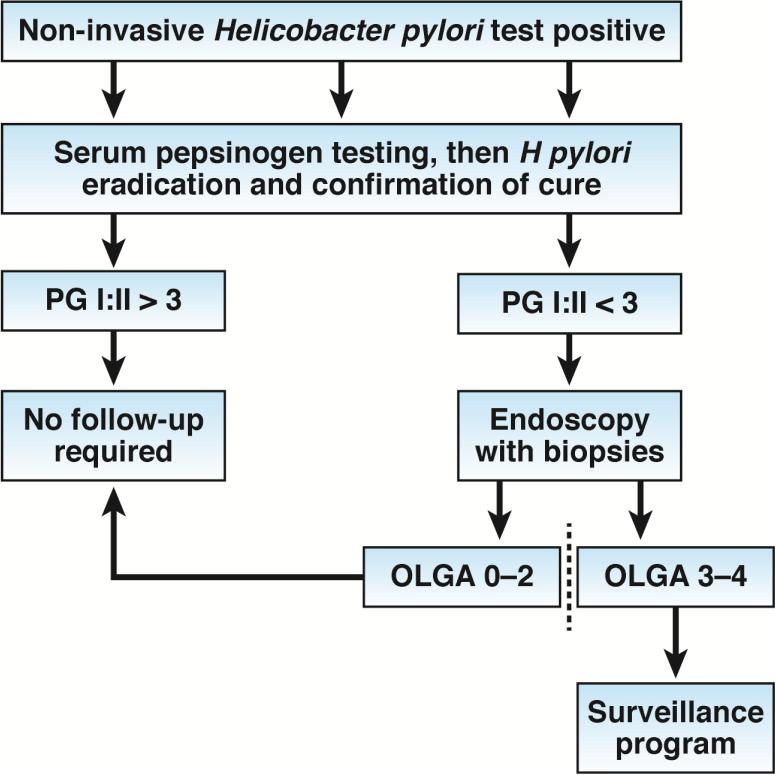
Atrophic gastritis, the precursor to gastric cancer, leads to little or no secretion of acid, which alters the gastric microbiome 6. The outcome of each individual infection is unpredictable, as is the rate of progression of the gastric mucosal damage. However, further progression is stopped by eradication. Eradicating H pylori before the development of atrophic changes can essentially eliminate cancer risk. Depending on the degree and extent of atrophic changes, eradication can stop and possibly partially reverse, the changes that occurred with atrophy and thus reduce associated cancer risk. As such, there is a point in the progression of the disease when a significant risk of cancer development remains despite H pylori eradication. It is at this point that secondary prevention (eg, endoscopic surveillance) programs may become cost-effective in reducing gastric cancer deaths 15, 17, 18. Candidates who might be suitable for non-invasive, secondary prevention surveillance programs can be identified based on serum levels of pepsinogen, which can be used to determine their risk for gastric cancer (Figure 1). Such an approach obviates the need for endoscopy for most subjects; those potentially at risk can then be specifically identified using validated histologic staging systems such as the Operative Link on Gastritis Assessment (OLGA) or OLGIM (Figure 1) 6, 15, 19, 20. Importantly, assays that measure serum levels of pepsinogen cannot accurately identify patients with gastritis if they are receiving proton pump inhibitors or following H pylori eradication 6, 21, 22.
Figure 1. Screening and Follow-up Approaches.
The approach is based on initially identifying those with H pylori infections and assessing the health of the gastric mucosa using non-invasive testing with a locally or regionally validated IgG H pylori serology and serum pepsinogen tests. Those without H pylori infection or atrophic gastritis would require no further evaluation or follow up. All those with H pylori infections would undergo eradication therapy; eradication can be confirmed using non-invasive tests such as the urea breath or stool antigen assays. After H pylori eradication, those with non-atrophic gastritis would require no further follow up. Those with suspected atrophic gastritis (based on pepsinogen level) would undergo endoscopy for further risk stratification by a validated histologic staging system. Patients cured of H pylori infection and healed, non-atrophic gastritis would require no further follow up. Those with confirmed atrophic gastritis (e.g., OLGA stage III or IV) would enter a long-term endoscopic surveillance program. Because their cancer risk if likely to decrease with time, these patients might also be included in studies of surveillance intervals or to determine whether anti-inflammatory or anti-oxidant adjuvant therapies could further reduce the risk. There are not enough data available to make recommendations for patients who have undergone H pylori eradication therapy but still have mild atrophy (e.g., OLGA I and II scores). Further studies are needed to determine the best management strategies for these individuals.
The ability of H pylori eradication to prevent gastric carcinogenesis depends on a patient's level of cancer risk at the time that H pylori is eradicated. Patients with non-atrophic gastritis can expect complete or nearly complete protection. Those with irreversible changes to the gastric mucosa have a significant risk, but can be assured that their risk will not increase and will likely decrease. Risk stratification also allows for identification of patients who might benefit from a post-H pylori eradication secondary cancer prevention program. The benefits of H pylori eradication extend also to those at highest risk for cancer death, such as patients who have early gastric cancer (gastric adenocarcinoma confined to the mucosa and submucosa of the stomach, with or without regional lymph node metastases). Among patients who have had successful endoscopic removal of an early gastric tumor but remain infected with H pylori, the risk of metachronous gastric cancer ranges from 1% to >4% per year. H pylori eradication reduces that risk approximately 3-fold 23, 24.
The evidence that H pylori eradication reduces gastric cancer risk has led to questions of what H pylori eradication accomplishes and how to best to use this knowledge. H pylori contributes to gastric carcinogenesis by producing persistent acute-on-chronic inflammation and genetic and epigenetic changes that contribute to genetic instability in the gastric epithelium. During tumor progression, gastric cancer cells acquire the ability to evade immune destruction, suppress the immune response, and begin to invade surrounding tissues3, 6, 25. Interactions between H pylori and other members of the gastric microbiome, endogenous factors, and exogenous factors can also produce carcinogens within the stomach 6. Environmental factors, especially diet, are important determinants of risk for populations (i.e., different diets or methods of food preservation can affect the severity of H pylori-induced gastric mucosal damage and cancer risk) 5, 26.
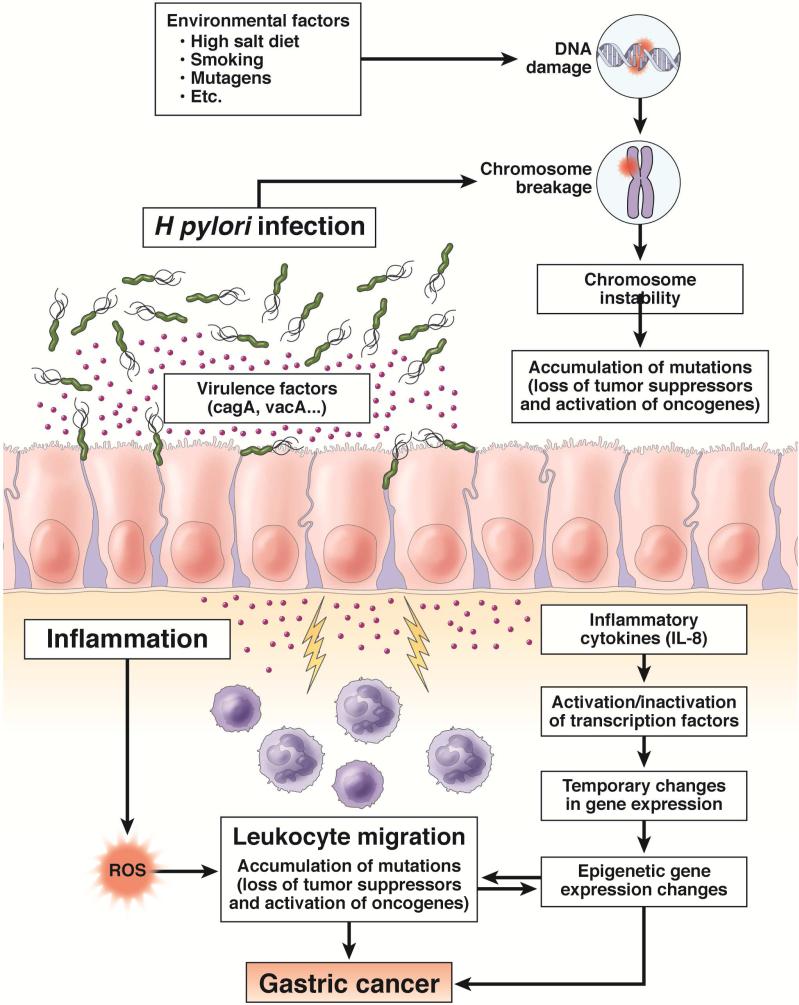
H pylori-induced inflammation leads to high gastric endothelial cell turnover and a microenvironment that is high in reactive oxygen and nitrogen species, increasing opportunities for DNA damage and somatic mutations 3, 27-29 (Figure 2). H pylori can induce methylation of multiple CpG islands, especially at sites encoding tumor suppressors such as E-cadherin 30. H pylori also stimulates activation-induced cytidine deaminase, which alters nucleotides 31-33. Furthermore, H pylori infection can lead to double-stranded breaks in DNA and alter expression of microRNAs to increase genetic instability 3, 6. Many, if not most, of these H pylori-related events (such as hypermethylation) are reversed following H pylori eradication 34-36 (Figure 3). Any increase following H pylori eradication would also reduce overgrowth of non-H pylori bacteria and potentially reduce or eliminate their deleterious effects 6.
Figure 2. Interactions Among Inflammation, Bacteria, and the Epithelium Leading to Gastric Cancer.
We show the interaction of H pylori, environmental factors, and inflammation in the pathogenesis of gastric cancer. Each plays important roles leading to progressive chromosome instability. H pylori-induced inflammation leads to high gastric endothelial cell turnover and a microenvironment that is high in reactive oxygen and nitrogen species, increasing opportunities for DNA damage and somatic mutations. ROS = Reactive oxygen species. Adapted from references 3, 28
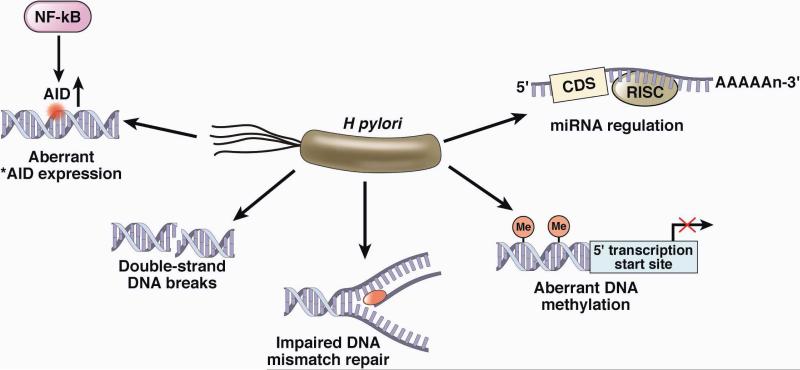
Figure 3. H pylori Infection Leads to Genetic Instability of Epithelial Cells.
H pylori can induce methylation of multiple CpG islands and also acting through stimulation of NF B stimulates activation-induced cytidine deaminase (AID), which alters nucleotides. Furthermore, the interaction of H pylori with the cell can result in double-stranded breaks in DNA as well as alter the expression of microRNAs and impair DNA mismatch repair all of which serve to increase genetic instability. Adapted from reference 6, with permission
Gastric cancer risk increases with infection with more virulent strains of H pylori, such a CagA-positive strains 2. Attempts to associate a specific H pylori-related disease with an individual putative virulence factor have produced inconsistent results, possibly because most virulence factors are frequently found together in the more virulent strains 2. None of the recognized virulence factors have been associated with a specific disease. However, they are typically associated with an increase in the inflammatory response and possibly can best be considered as markers for the severity of inflammation 2. Importantly, all H pylori strains cause gastric inflammation and disease; no avirulent strains have been identified. The difference in gastric cancer risk between the most- and the least-virulent strains is probably less than 3-fold, prompting the recommendation that all H pylori infections be eradicated, irrespective of virulence factors 2, 15.
Clinical Research Findings
H pylori eradication eliminates the noxious stimulus and promotes resolution of inflammation. However, resolution of inflammation is a highly coordinated process regulated by anti-inflammatory molecules, including lipid mediators such as lipoxins and resolvins. This brings up the issue of whether and/or when H pylori-induced inflammation has resolved 37. Gastric cancer development is associated with inflammation. Increasing our ability to identify whether inflammation has resolved (or not), and why, should provide new insights factors that determine cancer risk after H pylori eradication and help identify strategies to further reduce cancer risk.
People who have undergone removal of an early gastric tumor have a high rate of metachronous cancer, and could provide a high-risk population for clinical studies, which could be performed in a reasonable period of time with a reasonable sample size. For example, this population may be ideal for studies of risk factors for metachronous cancers or for randomized controlled trials of gastric cancer prevention (such as with anti-oxidants, cyclooxygenase II inhibitors, etc). These individuals might also be studied to identify biomarkers of disease recurrence and progression.
There is evidence that it might be possible to reverse atrophic gastritis or gastric atrophy. For example, studies performed before H pylori was discovered found that corticosteroid therapy for patients with atrophic gastritis or atrophy (autoimmune and H pylori-associated diseases) produced a partial recovery of parietal and chief cells 38-41. These observations have not been confirmed since patients began receiving H pylori eradication therapy. It might be unethical to use high-dose steroid therapy to attempt to reverse atrophic changes, but it might be possible to study patients with atrophic gastritis who receive steroids for other conditions.
Studies in patients and animals receiving tamoxifen showed regression of intestinal metaplasia. Partial reversal of intestinal metaplasia was also observed in animals given ADP ribosylation inhibitors, such as Olaparib, or prostaglandin E2 42, 43. However, prostaglandin E2 is believed to be involved in development of colon cancer, so it probably shouldn't be given to patients 37. Nonetheless, these intriguing findings indicate that it may be possible to at least partially reverse atrophic gastritis 42, 44. Evidence for a direct role of intestinal metaplasia or spasmolytic polypeptide-expressing metaplasia in gastric carcinogenesis is circumstantial; it is possible that reversal of atrophy may result in detectable changes (eg, related to transdifferention) that do not, however, significantly modify cancer risk 45. Studies designed to reverse atrophic changes therefore must also assess changes in cancer risk such, as reduced genetic instability in the involved mucosa.
Finally, whole-genome sequencing analyses of gastric cancers have begun to identify factors that promote gastric carcinogenesis. This information has been used to develop tiered molecular classification systems that relate genetic changes to different etiologies of gastric cancer (eg, H pylori or Epstein Barr virus) 46, 47. These types of studies could lead to better design of cancer treatment and pathogenesis.
Eradication Therapy
Reducing gastric cancer from a major clinical problem and cause of cancer death to a rare disease requires that we reliably eradicate or prevent of H pylori infections. Theoretically, H pylori cause a relatively straight-forward gastric infection—they are an organism that is susceptible to many antibiotics. Traditionally, studies of antimicrobial therapy for other infections have been performed and interpreted under conditions of well-defined drug susceptibility. In other infectious diseases the development of antimicrobial resistance produces rapid changes in recommendations and practice. H pylori has been an exception in that treatment regimens that vary in dose, duration, and composition are often compared, and data have been interpreted without consideration for patterns or effects of drug resistance.
The rapid decrease in efficacy of clarithromycin-containing regimens has produced a clinical dilemma, because these are often the only therapies approved by local regulatory authorities. In response to this decrease in efficacy, new combinations of the drugs, such as sequential therapy, were introduced and claimed to be superior. However, in the absence of clarithromycin resistance, the new 4-drug clarithromycin and metronidazole-containing regimens (eg, sequential, concomitant, or hybrid regiments) all have similar high levels of efficacy. New formulations, such as sequential therapy, contain a third antimicrobial agent (metronidazole or tinidazole), so it can only appear to be superior to triple therapy in populations in which the level of clarithromycin resistance is modest and metronidazole resistance is low. After repeatedly proving that sequential therapy was superior to triple therapy in an Italian population, researchers tested sequential therapy in populations with higher levels of metronidazole resistance, and found it to be ineffective (78% eradication in a study in Korea, 85% in a study in France, and 84% in a study in Spain). A regimen is considered to be effective only if it reproducibly eradicates the infection in 90% or more of the treated patients. So, sequential therapy is not superior to triple therapy in only certain populations 48.
The focus on testing for superiority via comparative studies with a regimen known to be ineffective in a specific population (eg, because of clarithromycin resistance), instead of a focus on understanding the mechanisms of efficacy (such as differences in resistance), has delayed our understanding the factors responsible for the efficacy and failure of the 4-drug combinations by more than a decade. We now recognize that we must collect data on the effects of resistance to each antimicrobial agent, separately and in combination, before we collect data on patient outcomes, to determine if the results are generalizable rather than population specific. During this learning process many thousands of patients were randomly assigned to groups given regimens known to be ineffective in those populations and thereby experienced unnecessary treatment failures and their consequences.
Lessons learned
Study participants are randomly assigned to groups to produce study populations that are equivalent in all respects. In retrospect, in many comparative studies, the outcome was entirely dependent on the pattern of drug-resistance in the population studied. In a population with a low prevalence of resistance to 14-day treatment with clarithromycin, 10-day sequential therapy would be either equivalent or triple therapy slightly superior. Sequential therapy could therefore be considered to be effective and superior to other treatments in Italy, yet ineffective in Korea. These results are entirely explained by differences in patterns of drug resistance, which were typically not assessed before studies were initiated. Findings from many, or even most comparative studies, cannot be applied to other populations unless they are accompanied by resistance data. Meta-analyses have compounded these errors by combining studies from the same geographic location and then making general conclusions about efficacy.
Potentially inaccurate conclusions
In analyzing the results of any study of antimicrobial agents, it is important to determine the effect of drug resistance on efficacy. Efficacy should be presented in terms of proportions of subjects cured, with 95% confidence intervals. Some comparative trials and meta-analyses provide results in terms of odds ratio and discuss superiority, even though no regimen produced an acceptable cure rate49. To be clinically useful, a reagent must eradicate strains susceptible to the antibiotics tested in at least 90% of subjects. Any claim of superiority from a comparison of 2 otherwise good or excellent drugs must be accompanied by an explanation of an otherwise unexpected result. Differences in effectiveness often appeared because the bacteria were resistant to 1 of the drugs tested.
In the past, claims of superiority often resulted when a new combination was compared with a drug already shown to provide an unacceptably low rate of cure in the study population. The suspicion that investigators may have chosen a known inferior regimen in an attempt to make their study regimen look more effective can be tested by examining the justifications for sample sizes. Randomization would reliably produce study groups that differed in relation to the effects of resistance on only 1 regimen, so there would be no valid hypothesis.
The principles of informed consent also require that potential subjects be informed of all that is known by the investigators and all published information on issues that might influence their willingness to participate (including they might receive a regimen known to have an unacceptably low cure rate in that population). In 1952, Sir Heneage Ogilvie called the process of disguising (eg, calling an inferior regimen “standard therapy”) or withholding information that might influence participation “medical fraud” 50. In either instance (i.e., no valid hypothesis or lack of truly informed consent) the study would be unethical51.
Patient-specific Therapy
H pylori eradication therapy is relatively simple; health-care workers must consider only drug availability, acceptability (such as whether patients might have allergies or the drugs have side effects), cost, and known or suspected patterns of resistance, based on prior experience with the drug in the same population. Whenever possible, only regimens that reliably yield >90%, preferably >95%, treatment success should be used. Data on which drugs each patients’ infection is likely to be resistant or susceptible to should be considered. Pretreatment tests are not widely available, although rapid molecular tests for clarithromycin resistance are practical and commercially available 52. The best advice for areas where susceptibility tests are not yet available is to use what works reliably locally and the follow up with patients to insure eradication of infection and to detect trends of antimicrobial resistance. There are now many regimens available that will reliable provide 95% or greater treatment success per protocol when used to eradicate susceptible strains. These include 14-day therapy with clarithromycin or metronidazole triple therapy, sequential therapy, concomitant therapy, and levofloxacin triple therapy (see Table 1 and supplemental material) 53, 54.
Table 1.
Common Eradication Regimens and Resistance
| Therapy | Clari Susceptible | Clari Resistant | Met Resistant | Met-Clari Resistant | Levo^ Resistant | Population result^^ <90% cures** |
|---|---|---|---|---|---|---|
| Clari Triple 7* 93% | 0-10%** | 93% | 0-10%** | 93% | Clari >4% | |
| Clari Triple 14 98% | 0-49%** | 98% | 0-49%** | 98% | Clari >10% | |
| Sequential 10 94% | 80% | 75% | 0-10%** | 94% | Met and with dual resistance | |
| Sequential 14* 98% | 88% | 75% | 0-49%** | 98% | Met and with dual resistance | |
| Concomitant 4* | 98% | 97% | 98% | 0-49%** | 98% | only with dual resistance |
| Levo Triple 14* | 97% | 97% | 97% | 97% | 0-49%** | Levo >10% |
| Bismuth quad 10 | 93% | 93% | 85% | 85% | 93% | Met >37% |
| Bismuth quad 14 | 98% | 98% | 95% | 95% | 98% | Adherence issues primarily |
The cure rate for a population will fall below 90% when resistance exceeds the percent shown using the therapy shown.
7 and 10 day therapies not recommended as they either ineffective (levo) or are less effective (clari)
7 day 10%, 14 day 20% success with PPI + amoxicillin used for calculations when resistance was present as the represent western populations. Results depend in part of prevalence of CYP2Y19 polymorphisms as poor PPI metabolism tends to increase treatment success with this dual therapy.
Levofloxacin
Note: In the US, treatment naïve patients are expected to be resistant to clarithromycin (10%-20%) or metronidazole 20%-40%, levofloxacin (more than 30%), but not amoxicillin, tetracycline, or rifabutin <1%. Patients previously treated with macrolide, metronidazole, or quinolone are expected to have high rates of resistance, so susceptibility testing is recommended.
In most of the US, resistance to the combination of clarithromycin and metronidazole is rare, making it our first choice. If the patient's infection does not respond to this therapy, bismuth quadruple therapy is given. Infections that are not eradicated by either regimen should be cultured and tested for susceptibility or the patient should be sent to a center with expertise in resistant infections. It is possible to predict the outcome of most therapeutic regimens, provided there is local or regional data available on drug susceptibility (see supplemental data).
Identifying Treatment Failure
13C urea breath tests are frequently used to detect H pylori infection. However, false-positive results have been reported from Spain and Korea 55, 56. These occurred for results were near the cut-off value, such as between a delta over baseline between the cut-off (such as 3) and 10 55-57. The problem is most common for patients with atrophic gastritis, likely due to the presence of non-H pylori urease containing organisms 55-57. It was proposed that the addition of citric acid, which suppresses the delta over baseline value in uninfected individuals and increases the value in subjects with H pylori infection, should reduce the problem but this strategy requires direct testing 55, 56, 58. False-positive results lead to the erroneous conclusion that antibiotic treatment failed, frequently in subjects who have already experienced multiple treatment failures 56. As such, in areas where atrophic gastritis is common, patients with results from a 13C- urea breath tests between the cut-off and a delta over baseline value of approximately 10 should not be considered as treatment failures until the finding is confirmed with a different test, such as a stool antigen assay or histologic analysis. In regions where atrophic gastritis is common and the urea breath tests does not contain citric acid, urea breath tests results may fall into this gray zone for more than 10% of cases 56. Studies are needed to determine whether citric or malic acid adjuvant therapy can reduce the frequency of false-positive results.
Future studies should provide more data to guide selection of patient-specific therapy. New molecular methods that detect specific resistance using stool or biopsy specimens will likely be developed to allow therapy to be tailored based on susceptibility. We predict a renewal of interest in amoxicillin and high-dose proton pump inhibitor dual therapy. This regimen to be appears highly successful in Asia, but they have fared less well in western populations. Areas for potential advances include more reliable control of intragastric pH and a better understanding of outcome in relation to amoxicillin pharmokinetics. Tetracycline continues to be unavailable or difficult to obtain in many countries; studies are needed to learn whether doxycycline can be reliable substituted. Until such studies are available it is best still avoided. New drugs such as solithromycin could eventually prove useful.
Is the Only Good H pylori a Dead H pylori?
Any claim that a major human pathogen might also provide a meaningful health benefit, and that plans to eradicate it should be reconsidered, is guaranteed to elicit interest from the press. H pylori was no exception and the press has continued to carry stories regarding a possible relationship between reductions in H pylori infections and the increased prevalence of esophageal adenocarcinoma, childhood asthma, and obesity. H pylori infection has been proposed protect against these disorders. In this instance, the term protection means that there is an inverse correlation in an epidemiology study. However, just because 2 events are associated does not mean that one causes the other. For example, a study reported a correlation between the number of storks in Brandenburg, Germany, and the birth rate in Berlin 59.
It could be possible that factors associated with decreases in H pylori infection also led to the recognition of unrecognized positive associations. For example, there has long been an interest in a possible role of chronic infection with atherosclerosis 60. The journal Global Heart devoted a large portion of the June 2014 issue to atherosclerosis in ancient humans. Computed tomography studies found that ancient humans had extensive atherosclerosis, even though the people analyzed were from different locations, had a wide range of diets and lifestyles, and died at relatively young ages 61, 62. The researchers proposed that chronic inflammation induced by microbial and parasitic diseases might have been responsible 61, 62. The incidence of myocardial infarctions decreased significantly in the second half of the 20th century in the US, indicating a concomitant change in an important environmental factor(s) involved in arthrosclerosis 63. Could on of those factors have been H pylori infection?
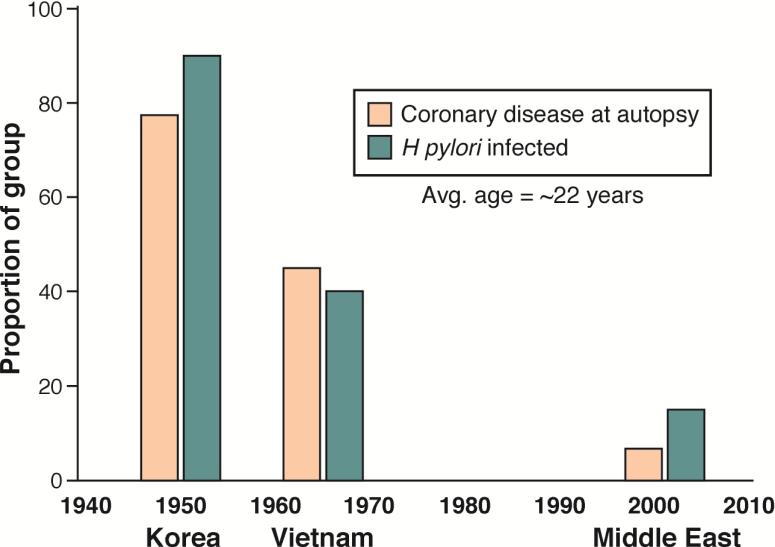
H pylori has been with humans since we traveled out of Africa more than 50,000 years ago 5, 64. The bacteria have been proposed to cause a variety of extra-gastric diseases and metabolic derangements, either directly, through molecular mimicry, or indirectly, by producing chronic inflammation 65-67. The H pylori-related disease peptic ulcer has long been associated with an increased risk for cardiovascular disease 68. The decrease in cardiovascular mortality was recently linked with the prevalence of H pylori in the US 69. Examination of the hearts of young soldiers who died during the second half of the 20th century (i.e., during the Korean, Vietnam, and Gulf wars) revealed a progressive decrease in significant coronary atherosclerosis. Over the same time period, there has also been a decrease in the prevalence of H pylori infection in the US population (Figure 4) 70-73. Although these 2 events might be unrelated, H pylori infection has been linked with systemic inflammation, atherosclerosis, lipid disorders, heart disease, alternations in vitamin B12 metabolism, and changes in the microbiome. We provide a testable hypothesis that coronary arthrosclerosis might be associated with a preventable factor.
Figure 4. Relationship Between Coronary Artery Disease and H pylori Infection in the US.
Data on coronary artery disease were collected from histologic analyses of autopsies performed on soldiers who died in the Korean, Vietnam, and Middle East wars 70-72. They are compared with the prevalence of H pylori infection in the US population during the same time periods, based on the premise that infection at age 22 did not change during the lifetimes of this cohort 73.
In a review article, Julie Parsonnet considered the evidence that H pylori reduces the risk for of immune regulator disorders such as asthma, esophageal cancer, Barrett's esophagus, gastroesophageal disease, inflammatory bowel disease, infectious diseases such as tuberculosis and gastroenteritis, and obesity 74. Overall, she was skeptical of the associations and concluded “the mere fact that H pylori is disappearing from the human host without substantive intervention might indicate that, in many countries, it is providing little benefit”. 74.
Esophageal disease
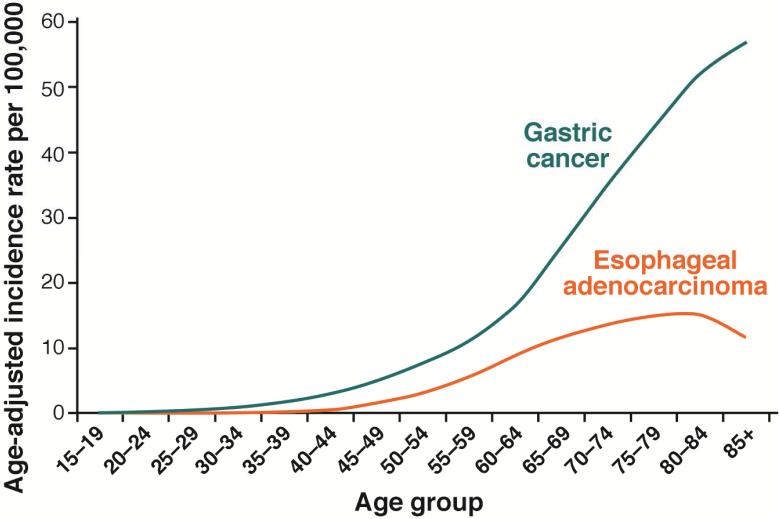
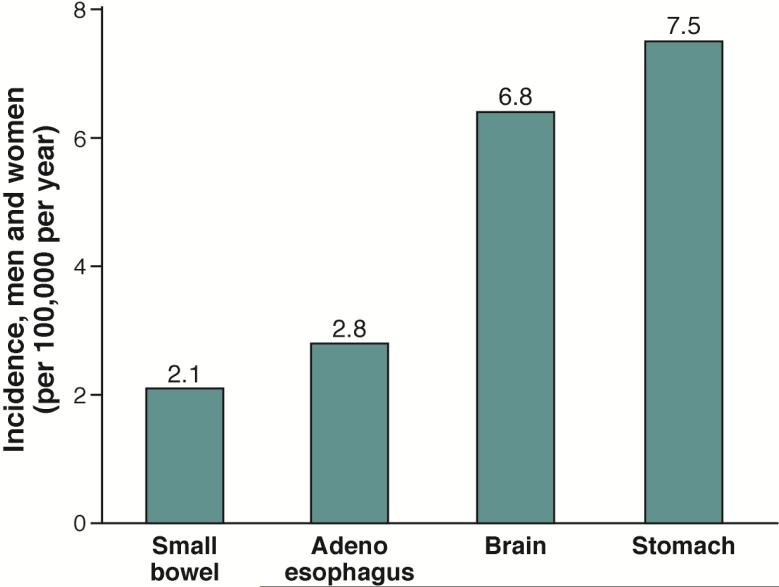
The role of H pylori in development of esophageal adenocarcinoma, Barrett's esophagus, and gastroesophageal reflux disease was discussed in the most recent Maastricht consensus report (see 75). These esophageal problems are all are acid reflux-related diseases. H pylori infection can promote and inhibit acid reflux; antral predominant gastritis is associated with increased acid secretion and an increased risk for reflux esophagitis, whereas H pylori-induced corpus gastritis reduces acid secretion and thereby prevents or reduces the severity of reflux. According to the model, the bacterium behaves as a biologic secretory or anti-secretory agent 76, 77 that promotes, prevents, or has no effect on gastroesophageal reflux, depending on the pattern and extent of gastritis. Importantly, the more H pylori reduces acid secretion, the more protection it provides against acid reflux and the greater the risk of gastric cancer. Gastric cancer is a common disease that affects all races and both sexes and, until the mid-20th century was the leading cause of cancer deaths worldwide. Esophageal adenocarcinoma is primarily a rare disease of white men (i.e., the incidence of esophageal adenocarcinoma is more similar to that of small bowel adenocarcinoma) (Figure 5) 78. Despite the continuing decrease in the incidence of gastric carcinoma in the US, the risk of developing adenocarcinoma of the stomach remains higher than the risk of adenocarcinoma of the esophagus in the highest risk group (i.e., men in the US) (Figure 6) 79.
Figure 5. Age-adjusted US Incidence Rates of Gastric and Esophageal Adenocarcinoma (2006–2010).
Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. 79
Figure 6. Incidence of Cancers in the US.
Incidence values are based on data collected in 2011 by the Surveillance, Epidemiology, and End Results Program .
Non-esophageal Diseases
Ptolemy proposed that the earth was the center of the universe. He had observed that when he looked at the northern sky he (and everyone after him) saw that the stars circled around the earth. Thomas Henry Huxley said in 1870 “The great tragedy of science is the slaying of a beautiful hypothesis by an ugly fact”. Although thousands of experiments can support a hypothesis, it only takes 1 finding to disprove it. For example, despite countless observations over more than a thousand years that supported Ptolemy's model, experiments performed with the first telescopes rapidly disproved it. We will therefore not review each association for validity but provide evidence that H pylori infection does not cause non-esophageal diseases such as asthma or obesity.
Asthma
The initial studies relating H pylori prevalence with asthma were based on the concept that the incidence of asthma was increasing and it would be useful to search for the cause 80. The most-recent studies found that asthma incidence has peaked or is decreasing 81, 82. A study of asthma trends in England from 2001 to 2005 involved 333,294 individuals from all socioeconomic groups and all ages. Their results can be used to test whether there was a link between H pylori infection and changes in asthma incidence 82. The UK, as a developed country, has experienced a steady reduction in the prevalence of H pylori infection, which is currently likely concentrated in immigrants and the lower socioeconomic strata. We would therefore predict that any changes related to the decrease in H pylori infection would tend to be concentrated in the higher socioeconomic strata. In that study, the incidence rate of asthma decreased significantly in all groups over the interval studied (from 6.9/1000 patient-years; 95% confidence intervals [CI], 6.8–7.0 to 5.2/1000 patient-years; 95% CI, 5.1–5.3 ) (P<.001).
The decrease occurred in all socioeconomic groups, as assessed by deprivation quintiles. For example, the incidence in quintile 5 (the least affluent and most likely to have H pylori infection) decreased 24.2%, from an age-standardized rate/1000 patient-years in 2001 of 8 (95% CI, 7.7–8.2) to 6.0 in 2005 (95% CI, 5.8–6.2), whereas in quintile 1 (the most affluent) the incidence rate decreased 26.5%, from 6.3 (95% CI, 6.1–6.5) to 4.6 (95% CI, 4.4–4.8). The greatest effect was in children under 5 years of age (a decrease of 38.4%). A decrease in childhood asthma, compared with other developed countries, has also been observed 83, 84. Overall, these data are not consistent with the hypothesis that H pylori infection protects against childhood asthma.
The asthma association studies led to studies in mice showing that H pylori infection protected against asthma, possibly by promoting immune tolerance 85. Further studies showed that 2 H pylori antigens (ϒ-glutamyl transpeptidase and VacA) induced T-regulatory cells in the gastric mucosa of mice, resulting in development of tolerance and reduced allergic responses 86. Of interest, induction of T-regulatory cells in the gastric mucosa was also an important step in the establishment and maintenance of H pylori-induced gastric adenocarcinoma 87, 88.
Overall, animal studies showed that H pylori possess specific antigens that might mediate alterations in the immune system to promote tolerance and reduce asthma. An alternate possibility is that since H pylori infection correlates with poor household hygiene, any protection observed was likely related to differences in household hygiene (the hygiene hypothesis), with the presence of H pylori serving as a marker for poor household hygiene. This hypothesis has been tested in vivo and in vitro. The first test was designed to separate H pylori from other elements in the hygiene hypothesis. In Malaysia, very few Malay people have H pylori infections but in many areas, hygiene is substandard. According to the H pylori hypothesis, childhood asthma should be a common problem among the Malay because of the absence of H pylori. It is not 89.
A second test included mouse models and was designed to examine the observation that the presence of dogs in a home was associated with a reduced risk of asthma 90. Investigators collected house dust from a residence with a pet dog and from 1 with no pets and used the 2 house dust samples to try to protect mice from asthma. The dust from the family with the pet dog protected mice from asthma, and was associated with remodeling of the gut microbiome related, at least in part, to the presence of Lactobacillus johnsonii. The authors concluded that protection against asthma “is likely a combinatorial effect based on multiple pathways and resulting microbial products encoded by co-colonizers resulting in presentation of a specific suite of microbial ligands and a distinct profile of microbial metabolites to the innate and acquired components of the host immune system”.
For a more detailed discussion of models of asthma, differences in the immune responses of mice vs humans, and roles of environmental factors (diet and commensals) in allergy and asthma development, see 91. Overall, the studies do not support the hypotheses that increases in childhood asthma were related to the absence of H pylori or reduced immune system stimulation by specific H pylori antigens. One must therefore conclude that the hypothesis is wrong.
Obesity
Obesity is an extremely complicated problem. Fundamentally, obesity develops via an imbalance between energy intake and expenditure. In the 20th century, there were enormous changes in the production and availability of food, related to the green revolution, to increases in standards of living, and to improvements to the transportation infrastructure. Giant companies began producing calorie-dense processed foods, sugary soft drinks, and fast foods while the proportion of the population that performed intensive physical work decreased. In 1939 E. Parmalee Prentice wrote Hunger and History in which he reviewed the world's literature on hunger from antiquity to the 1930's, discussing famines, hunger, and food production 92. He stated that a history of hunger had become possible because for the first time the world had entered a phase in which there were large regions without risk of famine.
This book was followed in 1952 by The Geography of Hunger, in which Josue de Castro examined the problem of world hunger (two-thirds of the world's population was thought to suffer from hunger at that time) 93. He related a study by Rigoberto Aguillar, who in 1944 examined 10,000 poor children in Mexico City. Aguillar found 5000 children to have clear signs of dietary deficiency; children 10 to 12 years old appeared to be no older than age 4 or 5. In 1945 de Castro visited Mexico City where he found “innumerable cases of vitamin deficiency in children” 93. A half century later the problem had changed to an increasing concern about obesity among Mexican children 94-96.
It has been proposed that the increasing widespread obesity might be related to alterations in gastric physiology, due to the absence of H pylori 74, 97. However, there is little data to support that hypothesis other than conflicting data from comparisons of obesity rates among those with and without H pylori infection 98, 99. Obesity is a manifestation of an imbalance between energy intake and expenditure, so the absence of H pylori might increase appetite and intake. However, it not clear whether absence of H pylori might also encourage behaviors that reduce energy consumption.
The natural decrease in the prevalence of H pylori occurred first in the proportion of the population with high socioeconomic status, and that decrease continues among people of low socioeconomic status, especially during childhood. The infected and uninfected populations differ in many ways, some of which might affect obesity. These differences include the consumption of processed foods and high-calorie fast foods, as well as in the amount of physical activity. Available studies have rarely considered variables other than H pylori prevalence and body mass index, and none have taken into account the marked changes in the food industry and its effects on eating habits.
The food industry has had remarkable effects on human behavior (eg, on a mother's choice of breakfast food for her school children). The industry has steadily improved its ability to produce delicious high-calorie foods. Innovations that encourage consumption include the discovery of bliss points, and how to combine ingredients to largely negate satiety signals, which normally limit consumption 100, 101. These innovations by the food industry have resulted in increased consumption of processed foods dense in sugar and fat, and of extra calories contained in soft drinks and fast foods 100, 101. The modern life style also encourages sedentary activities due to television, computers, video games, and smart phones, all of which also increase opportunities to snack. In addition, overall physical activity has decreased (i.e., most ride rather than walk).
H pylori infection has long been rare among Malays (~60% of the population of Malaysia) but was common and is now decreasing among Chinese (~30% of the population) and Indians (~10%) subpopulations. Malaysia might be considered a poster child of a developing country as it has undergone rapid industrialization and urbanization with rapid increases in standards of living. However, these changes been associated with westernization of the diet, increased caloric intake, and reduced energy expenditure 102, 103. The change to a sedentary lifestyle with increased intake of calorie-dense foods (eg, McDonald's entered in 1982, Pizza Hut in 1984, and Domino's Pizza in 1997) has resulted in an increasing prevalence of obesity (eg, 0.7% in 1990 to more than 10% in 2004) and this has occurred in among the Malays, Chinese, and Indians, irrespective of differences in H pylori prevalence 89, 102-104. The recent increase in obesity is a complex issue and there is only weak circumstantial evidence for a significant role of decreasing H pylori infection in the obesity epidemic. A meaningful causative association between H pylori and obesity is unlikely.
Supplementary Material
Acknowledgements
The author thanks Mohammad H. Shakatreh for his help and expertise in preparing figure 5 and Aaron P. Thrift, for his assistance in providing data for figure 6.
Support: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center, and DK067366. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: The author is a unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H pylori infection. He is a also a paid consultant for RedHill Biopharma regarding novel H pylori therapies and for Otsuka Pharmaceuticals regarding diagnostic testing. The author has received royalties from Baylor College of Medicine patents covering materials related to 13C-urea breath test.
References
- 1.Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaoka Y, Graham DY. Helicobacter pylori virulence and cancer pathogenesis. Future Oncol. 2014;10:1487–1500. doi: 10.2217/fon.14.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanada K, Graham DY. Helicobacter pylori and the molecular pathogenesis of intestinal-type gastric carcinoma. Expert Rev Anticancer Ther. 2014;14:947–954. doi: 10.1586/14737140.2014.911092. [DOI] [PubMed] [Google Scholar]
- 4.Hardbower DM, Peek RM, Jr., Wilson KT. At the Bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:201–212. doi: 10.1189/jlb.4BT0214-099R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol. 2013;23:492–501. doi: 10.1016/j.semcancer.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Fox JG, Gonda T, et al. Mouse models of gastric cancer. Cancers (Basel) 2013;5:92–130. doi: 10.3390/cancers5010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu S, Yang M, Nam KT. Mouse models of gastric carcinogenesis. J Gastric Cancer. 2014;14:67–86. doi: 10.5230/jgc.2014.14.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto T, Toyoda T, Mizoshita T, et al. Helicobacter pylori infection and gastric carcinogenesis in rodent models. Semin Immunopathol. 2013;35:177–190. doi: 10.1007/s00281-012-0357-1. [DOI] [PubMed] [Google Scholar]
- 10.Freedberg DE, Abrams JA, Wang TC. Prevention of gastric cancer with antibiotics: can it be done without eradicating Helicobacter pylori? J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju148. [DOI] [PubMed] [Google Scholar]
- 11.Bracken MB. Risk, chance, and causation. Yale University Press; New Haven: 2013. [Google Scholar]
- 12.Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol. 2014;49:1–8. doi: 10.1007/s00535-013-0897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132:1272–1276. doi: 10.1002/ijc.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IARC Helicobacter pylori working group . Helicobacter pylori eradication as a strategy for preventing gastric cancer. Vol. 8. International Agency for Research on Cancer; Leon: 2014. [Google Scholar]
- 15.Sugano K, Tack J, Kuipers EJ, et al. Kyoto Global Consensus Report on Helicobacter pylori gastritis. Gut. 2015 doi: 10.1136/gutjnl-2015-309252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czinn SJ, Blanchard T. Vaccinating against Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. 2011;8:133–140. doi: 10.1038/nrgastro.2011.1. [DOI] [PubMed] [Google Scholar]
- 17.Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol. 2013;27:933–947. doi: 10.1016/j.bpg.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Areia M, Dinis-Ribeiro M, Rocha GF. Cost-utility analysis of endoscopic surveillance of patients with gastric premalignant conditions. Helicobacter. 2014 doi: 10.1111/hel.12150. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Correa P, Di MF, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650–658. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 20.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Kaise M, Miwa J, Fujimoto A, et al. Influence of Helicobacter pylori status and eradication on the serum levels of trefoil factors and pepsinogen test: serum trefoil factor 3 is a stable biomarker. Gastric Cancer. 2012 doi: 10.1007/s10120-012-0185-y. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Miki K, Ichinose M, et al. Changes in evaluation of the pepsinogen test result following Helicobacter pylori eradication therapy in Japan. Inflammopharmacology. 2007;15:31–35. doi: 10.1007/s10787-006-0009-y. [DOI] [PubMed] [Google Scholar]
- 23.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SB, Park JM, Lim CH, et al. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: A Meta-Analysis. Helicobacter. 2014;19:243–248. doi: 10.1111/hel.12146. [DOI] [PubMed] [Google Scholar]
- 25.Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657–1666. doi: 10.3748/wjg.v20.i7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Weber A, Graham DY. Age-, period- and cohort effects on gastric cancer mortality. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3359-0. in press. [DOI] [PubMed] [Google Scholar]
- 27.Hanada K, Uchida T, Tsukamoto Y, et al. Helicobacter pylori-infection introduces DNA double-strand breaks in host cells. Infect Immun. 2014;82:4182–4189. doi: 10.1128/IAI.02368-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang WJ, Du Y, Zhao X, et al. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20:4586–4596. doi: 10.3748/wjg.v20.i16.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham DY, Matsueda S, Shiotani A. Changing the natural history of metachronous gastric cancer after H pylori eradication. Japanese J Helicobacter Res. 2015 in press. [PMC free article] [PubMed] [Google Scholar]
- 30.Chan AO, Lam SK, Wong BC, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003;52:502–506. doi: 10.1136/gut.52.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conticello SG. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008;9:229. doi: 10.1186/gb-2008-9-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu T, Marusawa H, Endo Y, et al. Inflammation-mediated genomic instability: roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–1206. doi: 10.1111/j.1349-7006.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perri F, Cotugno R, Piepoli A, et al. Aberrant DNA methylation in non-neoplastic gastric mucosa of H pylori infected patients and effect of eradication. Am J Gastroenterol. 2007;102:1361–1371. doi: 10.1111/j.1572-0241.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- 35.Chan AO, Peng JZ, Lam SK, et al. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55:463–468. doi: 10.1136/gut.2005.077776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung WK, Man EP, Yu J, et al. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin Cancer Res. 2006;12:3216–3221. doi: 10.1158/1078-0432.CCR-05-2442. [DOI] [PubMed] [Google Scholar]
- 37.Lee HN, Na HK, Surh YJ. Resolution of inflammation as a novel chemopreventive strategy. Semin Immunopathol. 2013;35:151–161. doi: 10.1007/s00281-013-0363-y. [DOI] [PubMed] [Google Scholar]
- 38.Ardeman S, Chanarin I. Steroids and addisonian pernicious anemia. N Engl J Med. 1965;273:1352–1355. doi: 10.1056/NEJM196512162732502. [DOI] [PubMed] [Google Scholar]
- 39.Jeffries GH, Todd JE, Sleisenger MH. The effect of prednisolone on gastric mucosal histology, gastric secretion, and vitamin B 12 absorption in patients with pernicious anemia. J Clin Invest. 1966;45:803–812. doi: 10.1172/JCI105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siurala M, Peltola P, Varis K. The effect of long-term treatment with prednisone upon the state of the human gastric mucosa. Scand J Gastroenterol. 1968;3:407–412. doi: 10.3109/00365526809180137. [DOI] [PubMed] [Google Scholar]
- 41.Baggett RT, Welsh JD. Observations on the effects of glucocorticoid administration in pernicious anemia. Am J Dig Dis. 1970;15:871–881. doi: 10.1007/BF02236052. [DOI] [PubMed] [Google Scholar]
- 42.Toller IM, Altmeyer M, Kohler E, et al. Inhibition of ADP ribosylation prevents and cures Helicobacter-induced gastric preneoplasia. Cancer Res. 2010;70:5912–5922. doi: 10.1158/0008-5472.CAN-10-0528. [DOI] [PubMed] [Google Scholar]
- 43.Toller IM, Hitzler I, Sayi A, et al. Prostaglandin E2 prevents Helicobacter-induced gastric preneoplasia and facilitates persistent infection in a mouse model. Gastroenterology. 2010;138:1455–67. doi: 10.1053/j.gastro.2009.12.006. 1467. [DOI] [PubMed] [Google Scholar]
- 44.Goldenring JR. Gastric intestinal metaplasia and tamoxifen: can we reverse the inevitable? Dig Dis Sci. 2014;59:1078–1079. doi: 10.1007/s10620-014-3088-4. [DOI] [PubMed] [Google Scholar]
- 45.Mesquita P, Raquel A, Nuno L, et al. Metaplasia--a transdifferentiation process that facilitates cancer development: the model of gastric intestinal metaplasia. Crit Rev Oncog. 2006;12:3–26. doi: 10.1615/critrevoncog.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 47.Razzak M. Genetics: New molecular classification of gastric adenocarcinoma proposed by The Cancer Genome Atlas. Nat Rev Clin Oncol. 2014;11:499. doi: 10.1038/nrclinonc.2014.138. [DOI] [PubMed] [Google Scholar]
- 48.Gisbert JP, Calvet X, O'connor A, et al. Sequential therapy for Helicobacter pylori eradication: A critical review. J Clin Gastroenterol. 2010;44:313–325. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Huang X, Yao L, et al. Advantages of moxifloxacin and levofloxacin-based triple therapy for second-line treatments of persistent Helicobacter pylori infection: a meta analysis. Wien Klin Wochenschr. 2010;122:413–422. doi: 10.1007/s00508-010-1404-3. [DOI] [PubMed] [Google Scholar]
- 50.Ogilvie H. Whither medicine? Lancet. 1952;2:820–824. doi: 10.1016/s0140-6736(52)92350-7. [DOI] [PubMed] [Google Scholar]
- 51.Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol. 2010;8:1032–1036. doi: 10.1016/j.cgh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–88. doi: 10.1038/nrgastro.2010.210. [DOI] [PubMed] [Google Scholar]
- 53.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol. 2014;8:21–28. doi: 10.1586/17474124.2014.859522. [DOI] [PubMed] [Google Scholar]
- 55.Calvet X, Sanchez-Delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis. 2009;48:1385–1391. doi: 10.1086/598198. [DOI] [PubMed] [Google Scholar]
- 56.Kim N, Kwon YH, Lee JY, et al. The diagnostic validity of citric acid-free, high dose 13C-urea breath test after Helicobacter pylori eradication in Korea. Helicobacter. 2015 doi: 10.1111/hel.12189. in press. [DOI] [PubMed] [Google Scholar]
- 57.Kato C, Sugiyama T, Sato K, et al. Appropriate cut-off value of 13C-urea breath test after eradication of Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2003;18:1379–1383. doi: 10.1046/j.1440-1746.2003.03193.x. [DOI] [PubMed] [Google Scholar]
- 58.Graham DY, Malaty HM, Cole RA, et al. Simplified 13C-urea breath test for detection of Helicobacter pylori infection. Am J Gastroenterol. 2001;96:1741–1745. doi: 10.1111/j.1572-0241.2001.03867.x. [DOI] [PubMed] [Google Scholar]
- 59.Bracken MB. Risk, chance, and causation. Yale University Press; New Haven: 2013. [Google Scholar]
- 60.Sessa R, Pietro MD, Filardo S, et al. Infectious burden and atherosclerosis: A clinical issue. World J Clin Cases. 2014;2:240–249. doi: 10.12998/wjcc.v2.i7.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas GS, Wann LS, Allam AH,T, et al. Why did ancient people have atherosclerosis? Global Heart. 2014;9:229–237. doi: 10.1016/j.gheart.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Thompson RC, Allam AH, Zink A, et al. Computed tomographic evidence of atherosclerosis in the mummified remains of humans from around the world. Global Heart. 2014;9:187–196. doi: 10.1016/j.gheart.2014.03.2455. [DOI] [PubMed] [Google Scholar]
- 63.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 64.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–1585. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 65.Franceschi F, Tortora A, Gasbarrini G, et al. Helicobacter pylori and extragastric diseases. Helicobacter. 2014;19(Suppl 1):52–58. doi: 10.1111/hel.12159. [DOI] [PubMed] [Google Scholar]
- 66.Lamb DJ, El Sankary W, Ferns GA. Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. 2003;167:177–185. doi: 10.1016/s0021-9150(02)00301-5. [DOI] [PubMed] [Google Scholar]
- 67.Buzas GM. Metabolic consequences of infection and eradication. World J Gastroenterol. 2014;20:5226–5234. doi: 10.3748/wjg.v20.i18.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Editorial: Cardiovascular disease and peptic ulcer. Br Med J. 1974;3:760–761. doi: 10.1136/bmj.3.5934.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes WS. An Hypothesis: The dramatic decline in heart attacks in the United States is temporally related to the decline in duodenal ulcer and Helicobacter pylori infection. Helicobacter. 2014;19:239–214. doi: 10.1111/hel.12123. [DOI] [PubMed] [Google Scholar]
- 70.Enos WF, Holmes RH, Beyer J. Coronary disease among United States soldiers killed in action in Korea. JAMA. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- 71.McNamara JJ, Molot MA, Stremple JF, et al. Coronary artery disease in combat casualties in Vietnam. JAMA. 1971;216:1185–1187. [PubMed] [Google Scholar]
- 72.Webber BJ, Seguin PG, Burnett DG, et al. Prevalence of and risk factors for autopsy-determined atherosclerosis among US service members, 2001-2011. JAMA. 2012;308:2577–2583. doi: 10.1001/jama.2012.70830. [DOI] [PubMed] [Google Scholar]
- 73.Graham DY, Malaty HM, Evans DG, et al. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100:1495–1501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 74.Parsonnet J. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Vol. 8. Leon: 2014. Are there benefits of Helicobacter pylori infection? pp. 72–78. [Google Scholar]
- 75.Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 76.Graham DY. The changing epidemiology of GERD: geography and Helicobacter pylori. Am J Gastroenterol. 2003;98:1462–1470. doi: 10.1111/j.1572-0241.2003.07533.x. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Akiyama J, Graham DY. Current understandings of Helicobacter pylori, peptic ulcer and gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2006;52:235–248. [PubMed] [Google Scholar]
- 78.Dore MP, Graham DY. Helicobacter pylori and gastroesophageal reflux disease. In: Vela MF, Richter JE, Pandolfino JD, editors. Practical Manual of Gastroesophageal Reflux Disease. 1 ed. John Wiley & Sons, Ltd.; Hoboken: 2013. pp. 267–285. [Google Scholar]
- 79.SEER Cancer Statistics Review (CSR) 1975-2011. 2014.
- 80.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sears MR. Trends in the prevalence of asthma. Chest. 2014;145:219–225. doi: 10.1378/chest.13-2059. [DOI] [PubMed] [Google Scholar]
- 82.Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J R Soc Med. 2010;103:98–106. doi: 10.1258/jrsm.2009.090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Schayck CP, Smit HA. The prevalence of asthma in children: a reversing trend. Eur Respir J. 2005;26:647–650. doi: 10.1183/09031936.05.00019805. [DOI] [PubMed] [Google Scholar]
- 84.Ponsonby AL, Glasgow N, Pezic A, et al. A temporal decline in asthma but not eczema prevalence from 2000 to 2005 at school entry in the Australian Capital Territory with further consideration of country of birth. Int J Epidemiol. 2008;37:559–569. doi: 10.1093/ije/dyn029. [DOI] [PubMed] [Google Scholar]
- 85.Oertli M, Sundquist M, Hitzler I, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122:1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oertli M, Noben M, Engler DB, et al. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kandulski A, Wex T, Kuester D, et al. Naturally occurring regulatory T cells (CD4+, CD25 high, FOXP3+) in the antrum and cardia are associated with higher H pylori colonization and increased gene expression of TGF-beta1. Helicobacter. 2008;13:295–303. doi: 10.1111/j.1523-5378.2008.00612.x. [DOI] [PubMed] [Google Scholar]
- 88.Kandulski A, Malfertheiner P, Wex T. Role of regulatory T-cells in H. pylori-induced gastritis and gastric cancer. Anticancer Res. 2010;30:1093–1103. [PubMed] [Google Scholar]
- 89.Lee YY, Mahendra RS, Graham DY. Helicobacter pylori infection - A boon or a bane: Lessons from studies in a low-prevalence population. Helicobacter. 2013;18:338–346. doi: 10.1111/hel.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fujimura KE, Demoor T, Rauch M, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111:805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 92.Prentice EP. Hunger and History The Influence of hunger on human history. Harper & Brothers; New York: 1939. [Google Scholar]
- 93.de Castro J. The geography of hunger. Little, Brown and Company; Scranton: 1952. [Google Scholar]
- 94.Hurtado-Lopez EF, Macias-Rosales R. Focus of childhood obesity from pediatrics. Rev Med Inst Mex Seguro Soc. 2014;52(Suppl 1):S116–S119. [PubMed] [Google Scholar]
- 95.Safdie M, Cargo M, Richard L, et al. An ecological and theoretical deconstruction of a school-based obesity prevention program in Mexico. Int J Behav Nutr Phys Act. 2014;11:103. doi: 10.1186/s12966-014-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alvarez-Villasenor AS, George-Flores V. Overweight and obesity among children in nurseries. Rev Med Inst Mex Seguro Soc. 2014;52:606–609. [PubMed] [Google Scholar]
- 97.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lender N, Talley NJ, Enck P, et al. Review article: Associations between Helicobacter pylori and obesity--an ecological study. Aliment Pharmacol Ther. 2014;40:24–31. doi: 10.1111/apt.12790. [DOI] [PubMed] [Google Scholar]
- 99.Xu C, Yan M, Sun Y, Joo J, et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter. 2014 doi: 10.1111/hel.12153. in press. [DOI] [PubMed] [Google Scholar]
- 100.Morris M. Salt sugar fat: How the food giants hooked us. Random House; 2013. [Google Scholar]
- 101.Nestle M. How the food industry influences nutrition and health. University of California Press; Berkeley: 2002. Food politics. [Google Scholar]
- 102.Ismail MN, Chee SS, Nawawi H, Yusoff K, Lim TO, James WP. Obesity in Malaysia. Obes Rev. 2002;3:203–208. doi: 10.1046/j.1467-789x.2002.00074.x. [DOI] [PubMed] [Google Scholar]
- 103.Ismaii MN. The nutrition and health transition in Malaysia. Vol. 5. Public Health Nutrition; 2002. pp. 191–195. [DOI] [PubMed] [Google Scholar]
- 104.Rampal L, Rampal S, Khor GL, et al. A national study on the prevalence of obesity among 16,127 Malaysians. Asia Pac J Clin Nutr. 2007;16:561–566. [PubMed] [Google Scholar]
- 105.Cancer incidence in five continents. IARC Scientific Publications No. 155; Lyon: 2004. [PubMed] [Google Scholar]
- 106.Grossman MI. Closing remarks. Gastroenterology. 1978;74:487–488. [Google Scholar]
- 107.Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut. 2013;62:1262–1269. doi: 10.1136/gutjnl-2012-303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson WF, Camargo MC, Fraumeni JF, Jr., et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: Evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177–186. doi: 10.1016/j.cgh.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol. 2014;8:21–28. doi: 10.1586/17474124.2014.859522. [DOI] [PubMed] [Google Scholar]
- 111.Park CS, Lee SM, Park CH, et al. Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am J Gastroenterol. 2014;109:1595–1602. doi: 10.1038/ajg.2014.222. [DOI] [PubMed] [Google Scholar]
- 112.Salazar CO, Cardenas VM, Reddy RK, et al. Greater than 95% success with 14-day bismuth quadruple anti- Helicobacter pylori therapy: A pilot study in US Hispanics. Helicobacter. 2012;17:382–389. doi: 10.1111/j.1523-5378.2012.00962.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.