Abstract
We report a rare case of a patient with prosthetic valve endocarditis requiring implantation of a total artificial heart (TAH) as a bridge to heart transplantation. Gemella haemolysans, an unusual cause of PVE, was identified as the organism responsible only by 16s rRNA PCR analysis of surgical tissue samples. We also describe one of the first uses of combined TAH and veno-venous extracorporeal membrane oxygenation therapy in the setting of severe respiratory and cardiac failure. Implantation of a TAH may be considered in situations where more traditional reconstructive methods are not feasible.
Keywords: Total artificial heart, Gemella haemolysans, prosthetic valve endocarditis, extracorporeal membrane oxygenation, heart transplantation
Introduction
Prosthetic valve endocarditis (PVE) is a serious disease with significant mortality.1 Treatment requires prolonged antimicrobial therapy, usually with a combination of multiple agents. Surgical therapy is also frequently necessary. Although implantation of a total artificial heart (TAH) is typically used as a bridge to heart transplantation in patients with severe biventricular heart failure,2 implantation of a TAH as a treatment for severe PVE has rarely been reported.3 We describe a patient with severe culture negative PVE due to Gemella haemolysans who underwent TAH implantation, initially in conjunction with veno-venous extracorporeal membrane oxygenation (VV-ECMO), as a bridge to successful heart transplant.
Case
A 40 year old woman had congenital aortic coarctation, repaired at age 4, and a biscupid aortic valve with subsequent symptomatic aortic stenosis requiring bioprosthetic aortic valve replacement and root enlargement at age 32. Five months prior to admission, she developed progressive shortness of breath, lower extremity edema, fevers with temperatures to 39°C, myalgias and night sweats. She was treated empirically with oseltamivir, doxycycline, and eventually with clarithromycin and a short course of prednisone for possible bronchitis, without improvement. At a referring facility, a transthoracic echocardiogram was suggestive of endocarditis with a paravalvular aortic root abscess. She was treated with vancomycin and ceftriaxone but two days later she developed pulmonary edema, an elevated Troponin-I to 74 ng/ml (normal <0.4), and an elevated white blood cell count to 21,000 per cubic millimeter. She was transferred to our institution where a repeat transthoracic echocardiogram (Fig. 1) showed new systolic dysfunction, regional akinesis and an ejection fraction of 36% (previously normal) with possible left ventricular thrombus. There was a large abscess around the bioprosthetic aortic valve abutting the coronary ostia with dehiscence of the aortic root from the aortic annulus. Gentamicin and rifampin were added to her prior antibiotics.
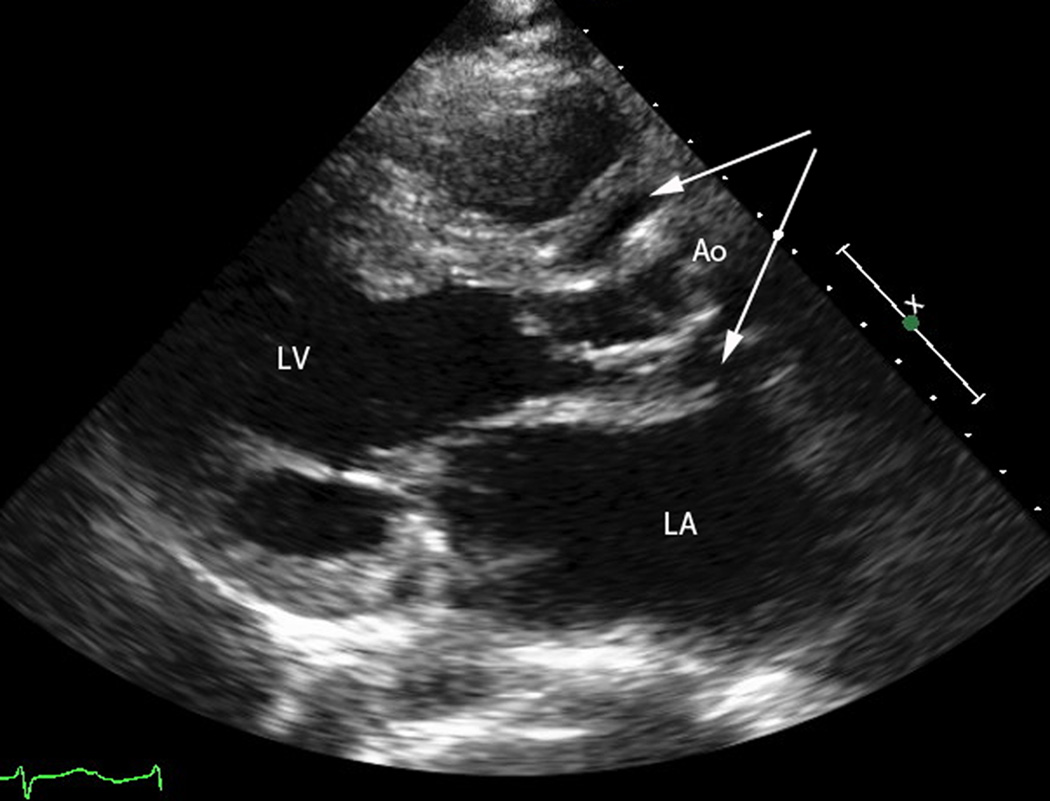
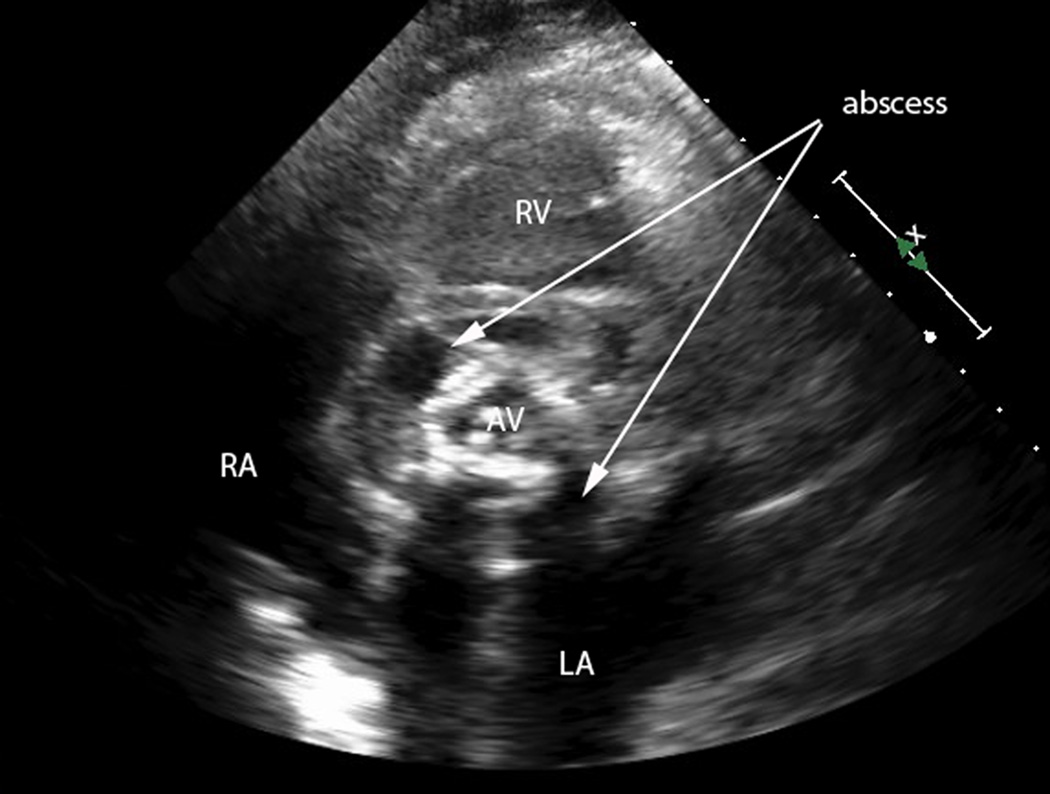
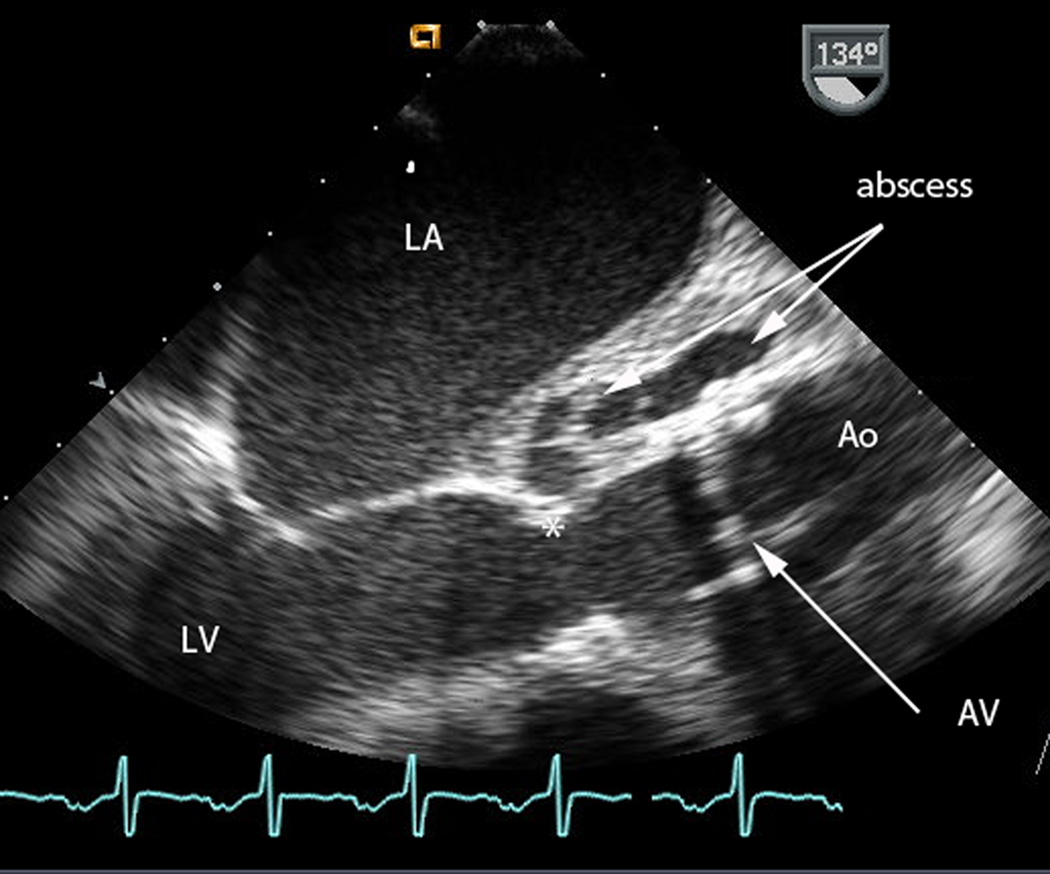
Figure 1.
a) Transthoracic echocardiogram, parasternal long axis view. The left ventricle (LV) ejects to the ascending aorta (Ao). Surrounding the aortic root is a large abscess (arrows).
b) Transthoracic echocardiogram, parasternal short axis view. Measurement markers along the right of the image represent 1 cm distances. The aortic valve (AV) is seen in the center of the image. Surrounding the valve is a large, complex abscess (arrows) which extends 1–2 cm circumferentially around the AV.
c) Intraoperative transesophageal echocardiogram, taken just before placement of the total artificial heart. Measurement markers along the right of the image represent 1 cm distances. The mechanical aortic valve (AV) is dehisced from the aortic valve attachment (*), and a large abscess (arrows) is seen in the aortomitral intervalvular fibrosa.
Following hemodynamic stabilization, she was taken to the operating room for exploration and definitive therapy. She was found to have an infection involving both coronary ostia, a root abscess extending into the right atrium, and insufficient tissue to perform a root reconstruction. She therefore underwent extensive debridement mandating reconstruction with a TAH.
Once complete, she suffered from profound respiratory failure with poor oxygenation and high peak airway pressures consistent with a systemic inflammatory response. With a PaO2 of 46 mmHg, a PaCO2 of 78 mmHg and a pH of 7.14 on aggressive ventilator settings she was placed on VV-ECMO for support. Cannulation was accomplished via the common femoral vein into the intrahepatic vena cava and return directly into the right atrium with a cannula placed parallel to the right-sided inflow (tricuspid) valve. VV-ECMO flows were brought up to around 3 L/min which quickly stabilized gas exchange.
Over the ensuing four days, her respiratory function improved such that VV-ECMO could be weaned. All blood and tissue cultures from her aortic and right atrial abscess remained negative. However, 16s rRNA PCR identified G. haemolysans in all tissue specimens. Pathology of her explanted heart showed both ischemic and embolic infarcts of varying ages (ie several weeks).
Antibiotics were changed to ampicillin and gentamicin which continued for another four weeks and she was maintained on suppressive oral amoxicillin. She underwent successful heart transplantation six months later and continues to do well seven months afterwards.
Discussion
Prosthetic valve endocarditis involves between 1–6% of prosthetic valves and may account for up to 1/3 of all cases of infective endocarditis (IE).1,4 PVE continues to be a serious disease with high mortality. Therapy requires prolonged use of intravenous antimicrobials, usually with a combination of agents.5 Whether surgery provides additional benefit in routine cases of PVE is not clear.6,7 However, in certain individuals, urgent valve surgery is critical. Indications for surgery include severe heart failure, myocardial abscess, valve dehiscence, new conduction disturbance, or large vegetations.8 Our patient met several of these criteria.
Due to displacement of the aortic root from the base of the heart leading to distortion of the coronary arteries, large myocardial infarction and right ventricular dysfunction, aortic root reconstruction was not possible.9 An intraoperative judgment that a complex reconstruction would not be tolerated resulted in an unconventional use of the TAH. TAH has been used to provide mechanical circulatory support in patients with severe biventricular heart failure. 61–68% of patients with a SynCardia TAH survived to transplant in recent reports, with a mean time until transplant of 87–97 days.3,10 The use of a TAH in the management of severe IE has only rarely been described.3
The combined use of TAH and VV-ECMO has been previously reported.11 Due to the presence of the mechanical valves, technical considerations for cannulation are imperative. In this case, the ECMO inflow was placed in the IVC, and the outflow was placed parallel to the right sided inflow valve directly in the right atrium. This arrangement resulted in excellent support with no ECMO flow limitation and without concern for valvular interference by the cannulae.
The most common organisms associated with PVE include coagulase negative staphylocci, Staphylococcus aureus, enterococci and Streptococcus viridans group organisms.4 G. haemolysans is a gram positive coccus, often found as a commensal organism in the upper respiratory, gastrointestinal and genitourinary tract, and occasionally causes both localized and disseminated infections. It is an uncommon cause of endocarditis, with only about 20 cases reported in the literature and rarely associated with PVE.12 Most documented cases of G. haemolysans endocarditis have been associated with previous valvular damage in the setting of poor dentition or dental manipulation which was not the situation with this patient. Antimicrobial therapy for G. haemolysans IE would typically include penicillin or ampicillin in combination with gentamicin.5,13 Our patient received four weeks of ampicillin and gentamicin following TAH implantation. Oral amoxicillin was continued as suppressive therapy until after heart transplantation given the possibility that the new artificial device had become infected at the time of implantation.
Culture-negative endocarditis occurs in approximately 5–15% of IE.14,15 Molecular methods using 16s rRNA PCR may be extremely helpful in identifying a causative agent using valve tissue at the time of surgery.16 This technique may find organisms that are difficult to grow or whose growth has been suppressed by prior antibiotic treatment, such as the G. haemolysans seen here.17 While susceptibility testing by PCR is not presently available, identification of the etiologic agent may be enough to allow revision of the antimicrobial regimen, as was seen in this case. We recommend that all patients with culture-negative IE who undergo valve replacement have tissue samples examined using this method.
In summary, we describe a rare case in which TAH was used as bridge to transplantation for severe culture-negative PVE. In addition, we describe one of the first uses of combined TAH and VV-ECMO for both cardiac and respiratory support. We emphasize the utility of 16s rRNA PCR to help identify the causative agent when cultures are negative. Implantation of a TAH may be considered in situations where more traditional reconstructive methods are not feasible anatomically or physiologically.
Acknowledgments
Conflicts of Interest and Source of Funding: Levy WC has received research funding from HeartWare and Thoratec. Mokadam NA is a consultant and surgical preceptor for SynCardia.
Footnotes
For the remaining authors, none were declared.
References
- 1.Nataloni M, Pergolini M, Rescigno G, Mocchegiani R. Prosthetic valve endocarditis. J Cardiovasc Med. 2010;11:869–883. doi: 10.2459/JCM.0b013e328336ec9a. [DOI] [PubMed] [Google Scholar]
- 2.Copeland JG, Smith RG, Arabia FA, et al. Total artificial heart bridge to transplantation: A 9-year experience with 62 patients. J Heart and Lung Transplant. 2004;23:823–831. doi: 10.1016/j.healun.2003.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Copeland JG, Copeland H, Gustafson M, et al. Experience with more than 100 total artificial heart implants. J Thorac Cardiovas Surg. 2012;143:727–734. doi: 10.1016/j.jtcvs.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 5.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis - Diagnosis, antimicrobial therapy, and management of complications. Circulation. 2005;111:E394–E434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 6.Tleyjeh IM, Kashour T, Zimmerman V, Steckelberg JM, Wilson WR, Baddour LM. The role of valve surgery in infective endocarditis management: A systematic review of observational studies that included propensity score analysis. Am Heart J. 2008;156:901–909. doi: 10.1016/j.ahj.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Hill EE, Herregods M-C, Vanderschueren S, Claus P, Peetermans WE, Herijgers P. Management of prosthetic valve infective endocarditis. Am J Cardiol. 2008;101:1174–1178. doi: 10.1016/j.amjcard.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2008;52:E1–E142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Lopes S, Calvinho P, de Oliveira F, Antunes M. Allograft aortic root replacement in complex prosthetic endocarditis. Eur J Cardiothorac Surg. 2007;32:126–130. doi: 10.1016/j.ejcts.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch ME, Nguyen A, Mastroianni C, et al. SynCardia temporary total artificial heart as bridge to transplantation: current results at La Pitié hospital. Ann Thorac Surg. 2013;95:1640–1646. doi: 10.1016/j.athoracsur.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Spiliopoulos S, Dogan G, Guersoy D, Serrano MR, Koerfer R, Tenderich G. Veno-venous extracorporeal membrane oxygenation with a bicaval dual-lumen catheter in a SynCardia total artificial heart patient. J Cardiothorac Surg. 2013;8:179. doi: 10.1186/1749-8090-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan R, Urban C, Rubin D, Segal-Maurer S. Subacute endocarditis caused by Gemella haemolysins and a review of the literature. Scand J Infect Dis. 2004;36:885–888. doi: 10.1080/00365540410024916. [DOI] [PubMed] [Google Scholar]
- 13.Buu-Hoï A, Sapoetra A, Branger C, Acar JF. Antimicrobial susceptibility of Gemella haemolysins, isolated from patients with subacute endocarditis. Eur J Clin Microbiol. 1982;1:102–106. doi: 10.1007/BF02014200. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st Century. Arch Intern Med. 2009;169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsouli A, Massad MG. Current issues in the diagnosis and management of blood culture-negative infective and non-infective endocarditis. Ann Thorac Surg. 2013;95:1467–1474. doi: 10.1016/j.athoracsur.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Marin M, Munoz P, Sanchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine. 2007;86:195–202. doi: 10.1097/MD.0b013e31811f44ec. [DOI] [PubMed] [Google Scholar]
- 17.Fournier PE, Thuny F, Richet H, et al. Comprehensive diagnostic strategy for blood culture-negative endocarditis: A prospective study of 819 new cases. Clin Infect Dis. 2010;51:131–140. doi: 10.1086/653675. [DOI] [PubMed] [Google Scholar]