Abstract
Background
Hemorrhagic shock is a leading cause of morbidity and mortality in surgery and trauma patients. Despite a large number of preclinical trials conducted to develop therapeutic strategies against hemorrhagic shock, there is still an unmet need exist for effective therapy for hemorrhage victims. Wnt/β-catenin signaling controls developmental processes and cellular regeneration owing to its central role in cell survival and proliferation. We therefore hypothesized that the activation of Wnt signaling reduces systemic injury caused by hemorrhagic shock.
Methods
Adult male Sprague-Dawley rats underwent hemorrhagic shock by controlled bleeding of the femoral artery to maintain a mean arterial pressure (MAP) of 30 mmHg for 90 min, followed by resuscitation with crystalloid equal to two times the shed blood volume. After resuscitation, animals were infused with Wnt agonist (5 mg/kg) or Vehicle (20% DMSO in saline). Blood and tissue samples were collected 6 h after resuscitation for analysis.
Results
Hemorrhagic shock increased serum levels of AST, lactate, and LDH. Treatment with Wnt agonist significantly reduced these levels by 40%, 36%, and 77%, respectively. Wnt agonist also decreased BUN and creatinine by 34% and 56%, respectively. Treatment reduced lung myeloperoxidase activity and IL-6 mRNA by 55% and 68% respectively and, significantly improved lung histology. Wnt agonist treatment increased Bcl-2 protein to Sham values and decreased cleaved caspase-3 by 46% indicating attenuation of hemorrhage-induced apoptosis in the lungs. Hemorrhage resulted in significant reductions of β-catenin protein levels in the lungs as well as down-regulation of a Wnt target gene, Cyclin-D1, while Wnt agonist treatment preserved these levels.
Conclusions
The administration of Wnt agonist attenuated hemorrhage-induced organ injury, inflammation and apoptosis. This was correlated with preservation of the Wnt signaling pathway. Thus, Wnt/β-catenin activation could be protective in hemorrhagic shock.
Keywords: Hemorrhagic shock, Wnt agonist, Wnt signaling, apoptosis, inflammation, lung injury
Introduction
Trauma is the fifth leading cause of death overall and the number one cause of death for patients below 40 years of age1–3. About 40% of these deaths are due to bleeding1. Despite advances in our understanding of the pathophysiology of trauma, there has been little improvement in trauma outcome over the past several decades. Although hemorrhage itself can be controlled, optimal resuscitation strategy to minimize reperfusion injury and tissue damage remains elusive. As such, there is still an unmet need in effective resuscitation strategies for hemorrhagic shock.
Hemorrhagic or hypovolemic shock is characterized as a functional alteration of the circulatory system. The primary treatment is to address the bleeding and frequently, this requires surgical intervention. Replacement of blood loss via fluid resuscitation is used to maintain blood pressure and is necessary to continue oxygen delivery to the ischemic tissues. The return of oxygen to ischemic tissues, however, initiates reperfusion injury. Early in reperfusion, endothelial cells exhibit morphological damage including cellular swelling, loss of adherence to basement membrane and adherence of activated leukocytes4. Endothelial damage is found throughout the vascular system and the postcapillary venule is the common site for inflammatory response due to reperfusion injury. The damaged endothelium also produces oxygen radicals such as superoxide and hydrogen peroxide5.
Wnt proteins belong to a family of secreted cysteine-rich glycoproteins. Signaling by Wnt proteins is involved in a wide array of developmental and physiological processes while dysregulation leads to diseases6–8. Wnt can activate β-catenin dependent (canonical) and independent (non-canonical) pathways9,10. The hallmark of the canonical pathway is the regulated degradation of β-catenin. In the absence of Wnt, β-catenin is phosphorylated by GSK3 and subjected to ubiquitination and proteasomal degradation resulting in a low level of cytoplasmic β-catenin. Upon Wnt stimulation, Wnt ligand forms a complex with the cell-surface receptor Frizzled (Fz) and initiates a cascade of events eventually inhibiting proteasomal degradation of β-catenin. The accumulated β-catenin enters the nucleus and interacts with transcription factors TCF (T-cell factor)/LEF (lymphoid enhancing factor) to activate Wnt target genes (reviewed in11).
Wnt signaling plays a role in vascular endothelial survival and proliferation12,13. The compound 2-amino-4-[3,4-(methlyenedioxy)benzylamino]-6-(3-methoxyphenyl)pyrimidine (Wnt agonist) was recently identified as the activator of the Wnt signaling pathway14,15. Since hypovolemic shock is characterized as a functional abnormality in the circulatory system and that endothelium is the primary target for reperfusion injury, we sought to determine whether Wnt agonist could ameliorate organ damage caused by hypovolemia associated reperfusion injury.
Materials and Methods
Male Sprague-Dawley (SD) rats weighing 275–325 g were purchased from Charles River Laboratories (Wilmington, MA) and used in this study. The rats were housed under 12 h light/dark cycle and fed a standard Purina rat chow diet. The rats were fasted for 10 h prior to the procedure. Animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and the project was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Animal Model of Hemorrhagic Shock
The model of hemorrhagic shock was previously described in detail by us16–19. Briefly, the rats were anesthetized with isoflurane inhalation. The right femoral vein and artery, and the left femoral artery were cannulated with PE-50 tubings. Right arterial catheter was used to monitor pressure and heart rate via a blood pressure analyzer (BPA; Digi-Med, Louisville, KY), the left one was utilized for blood withdrawal. The venous catheter was used for fluid resuscitation. The rats were rapidly bled to 30 mm Hg and maintained for 90 min by either further withdrawal of blood or infusion of small volumes of Ringer’s lactate. At the end of 90 min, the rats were resuscitated with two times the shed blood volume in the form of Ringer’s lactate (i.e., low-volume resuscitation) over a 60-min period. The shed blood was not used for resuscitation and the animals were not heparinized. The Sham animals underwent the same surgical procedure but were not bled or resuscitated.
Administration of Wnt Agonist
At 15 min after the initiation of resuscitation, 1 ml Vehicle (20% Dimethyl sulfoxide [DMSO] in normal saline) or Wnt agonist (5 mg/kg BW in Vehicle) was infused through the femoral vein catheter over a period of 45 min. Blood and tissue samples were harvested 4 h post-resuscitation (i.e., 6.5 h from the beginning of hemorrhage). Wnt agonist (2-Amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine) was purchased from Calbiochem, San Diego, CA).
Measurement of serum levels of organ injury indicators
Serum concentrations of aspartate aminotransferase (AST), lactate, lactate dehydrogenase (LDH), Blood Urea Nitrogen (BUN) and creatinine were determined by using assay kits according to manufacturer’s instructions (Pointe Scientific, Lincoln Park, MI).
Lung histology
Lung tissues were fixed in 10% buffered formalin and embedded in paraffin. The tissue blocks were cut into 5 µm sections, transferred to glass slides, stained with hematoxylin and eosin, dehydrated and mounted. Morphologic examinations were performed by using light microscopy. Lung injury was assessed as absent, mild, moderate or severe injury (score 0–3) based on previously published criteria20,21.
Lung MPO activity
Lung tissues were homogenized in KPO4 buffer containing 0.5% hexa-decyl-trimethyl-ammonium bromide. Upon centrifugation, supernatant was measured of MPO activity as previously described22,23.
Lung IL-6 mRNA expression
Total RNA was extracted from the lungs using Tri-Reagent (Molecular Research Center, Cincinnati, OH). RNA (4 µg) was reverse-transcribed and analyzed by real time PCR using primers specific for rat IL-6 (NM_012589). Rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. The primer sequences are the following: IL-6 forward: 5’-AGG GAG ATC TTG GAA ATG AGA AAA-3’ and reverse: CAT CAT CGC TGT TCA TAC AAT CAG-3’; GAPDH forward: 5’-ATG ACT CTA CCC ACG GCA AG-3’ and reverse: 5’-CTG GAA GAT GGT GAT GGG TT-3’. Each cycle consisted of 30 sec at 94°C, 30 sec at 60°C, and 45 sec at 72°C.
Western blotting
Lung tissues were homogenized in lysis buffer containing protease inhibitors. The extracted protein (50 µg) were fractionated on a Bis-Tris gel and transferred to 0.2 µm nitrocellulose membrane. The membranes were blocked and incubated overnight with rabbit polyclonal cleaved caspase-3 (Cell Signaling, Danvers, MA), rabbit polyclonal Bcl-2, mouse monoclonal β-catenin or mouse monoclonal cyclin D1 antibodies (Santa Cruz Biotech, Dallas, TX). The membranes were then incubated with horseradish peroxidase-labeled secondary antibodies, reacted with chemiluminiscence, exposed to radiograph film and band densities were determined using NIH ImageJ software. The bands were normalized against β-actin protein levels.
Statistical analysis
All data are expressed as mean ± SE and analyzed by one way analysis of variance (ANOVA) and compared using Student Newman Keul’s (SNK) test for multiple comparisons. The differences in values were considered significant if P < 0.05.
Results
Wnt agonist attenuated tissue damage following hemorrhagic shock
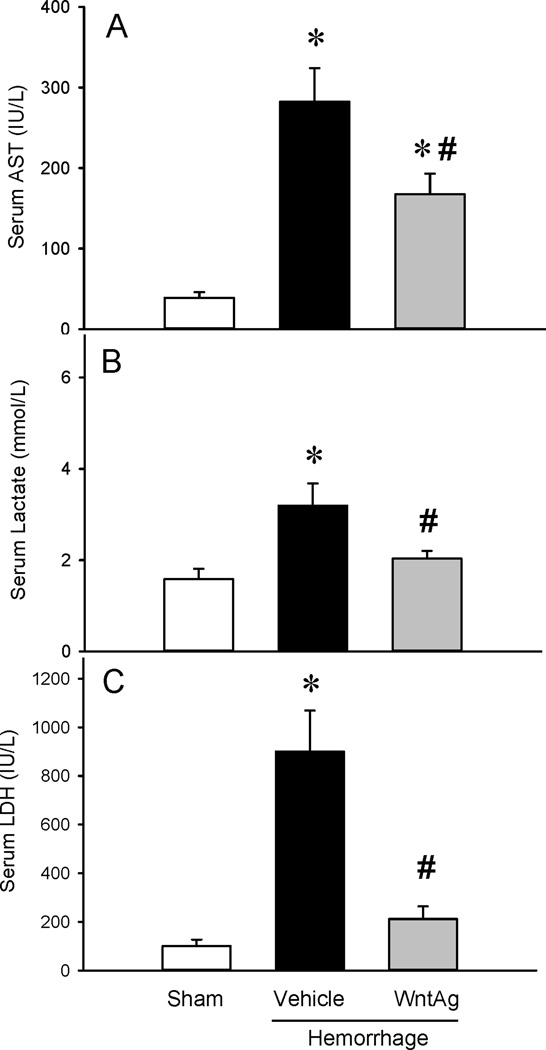
As shown in Figure 1, hemorrhagic shock significantly increased serum levels of AST, lactate and LDH by 658%, 121% and 840% respectively, as compared to sham-operated animals. Wnt agonist decreased these levels by 40%, 36% and 77%, respectively (Figures 1A–C). Hemorrhagic shock also increased serum levels of BUN and creatinine by 260% and 200%, respectively. Treatment with Wnt agonist decreased them by 34% and 56%, respectively (Figures 1C–D). Treatment with Wnt agonist during resuscitation in hemorrhagic shock reduced tissue damage and preserved renal function.
Figure 1. Changes in serum levels of systemic injury and renal function markers.
(A) AST, (B) Lactate, (C) LDH, (D) BUN, (E) Creatinine in Sham, Vehicle and Wnt agonist (WntAg)-treated rats at 6 h after hemorrhagic shock. Data are expressed as mean ± SE and compared by one-way ANOVA and Student Neuman Keul’s (SNK) test. *P<0.05 vs. Sham and #P<0.05 vs. Vehicle.
Wnt agonist reduced lung injury and inflammation following hemorrhagic shock
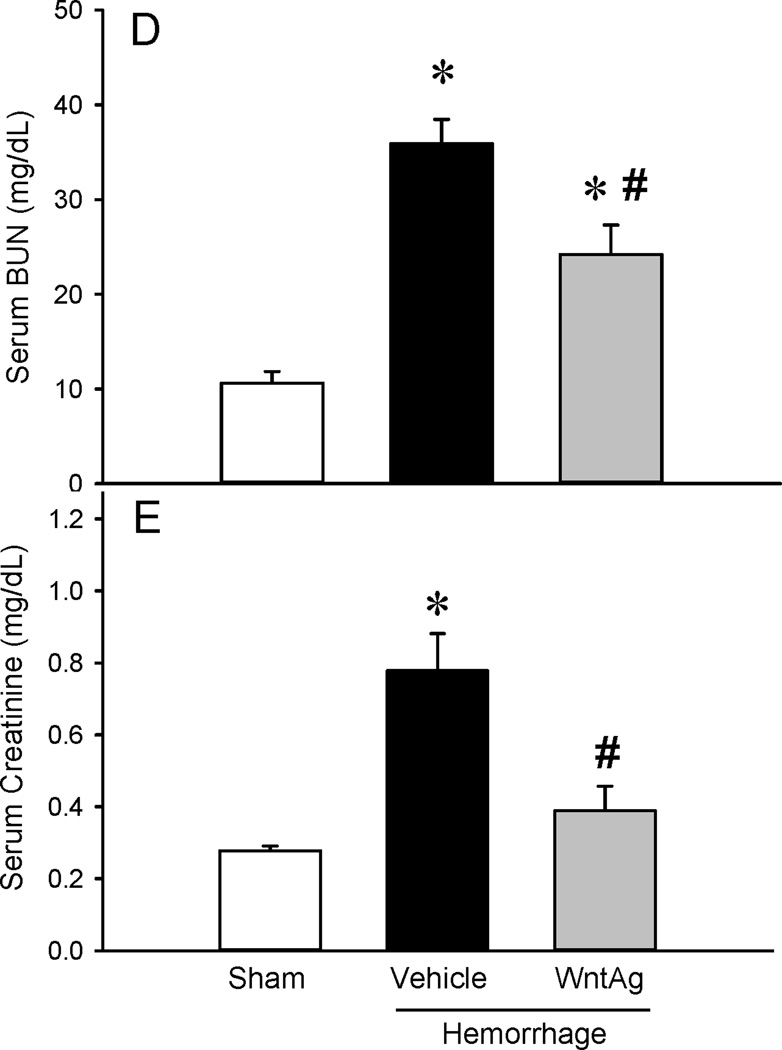
As shown in Figure 2A, vehicle treated animals following hemorrhagic shock presented with edema, tissue infiltration, intralveolar hemorrhage and debris, neutrophil margination, hyperemia and congestion, and cellular hyperplasia in the lungs. Treatment with Wnt agonist significantly improved lung architecture. Lung injury score was markedly increased after hemorrhagic shock and treatment with Wnt agonist reduced these levels by 46% (Figure 2B). As a measure of inflammation, lung IL-6 mRNA expression (tissue cytokine expression) and MPO activity (tissue neutrophil infiltration) were analyzed following treatment with Wnt agonist during hemorrhagic shock. The lung IL-6 mRNA was increased by 93-fold after hemorrhagic shock and treatment with Wnt agonist decreased it by 68% (Figure 3A). The lung MPO activity was also increased by 14-fold after hemorrhagic shock and treatment decreased it by 55% (Figure 3B).
Figure 2. Changes in histological appearance of the lung.
(A) Representative photographs of lung tissue sections stained with hematoxylin-eosin (original magnification ×200) of Sham, Vehicle and Wnt agonist treatment in rats at 6 h after hemorrhagic shock. (B) The injury score of the lung tissue sections are shown. Data are expressed as mean ± SE and compared by one-way ANOVA and SNK test. *P<0.05 vs. Sham and #P<0.05 vs. Vehicle.
Figure 3. Changes in the lung IL-6 mRNA and MPO activity.
(A) Total RNA from lung tissues was extracted, reverse-transcribed and examined for IL-6 mRNA expressions by real time PCR. The fold change was calculated based on GAPDH levels. (B) MPO activity in the lungs. Data are expressed as mean ± SE and compared by one-way ANOVA and SNK test. *P<0.05 vs. Sham and #P<0.05 vs. Vehicle.
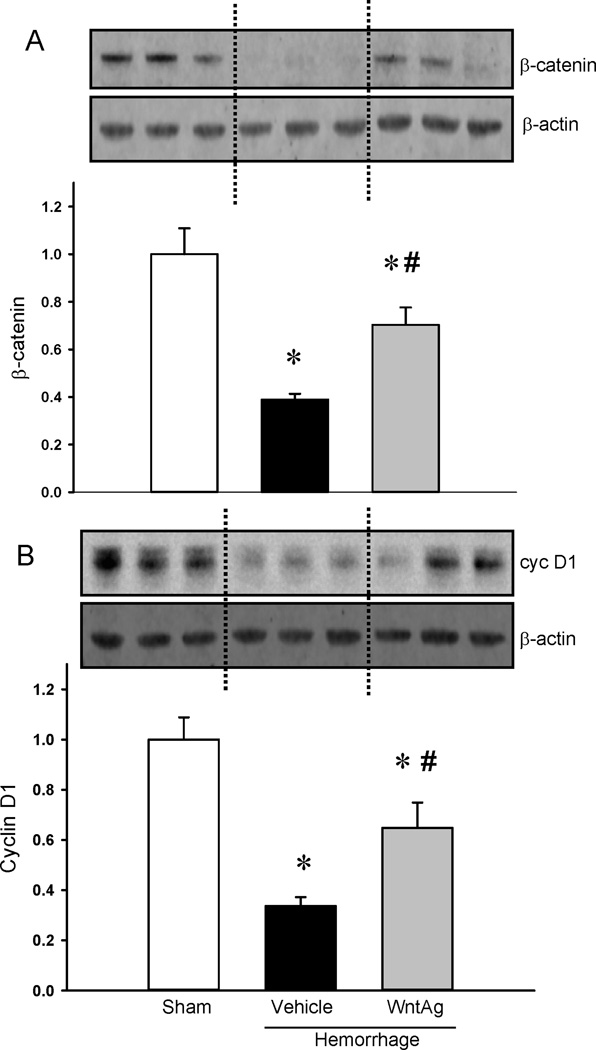
Wnt agonist attenuated apoptosis in the lungs and preserved Wnt/β-catenin signaling following hemorrhagic shock
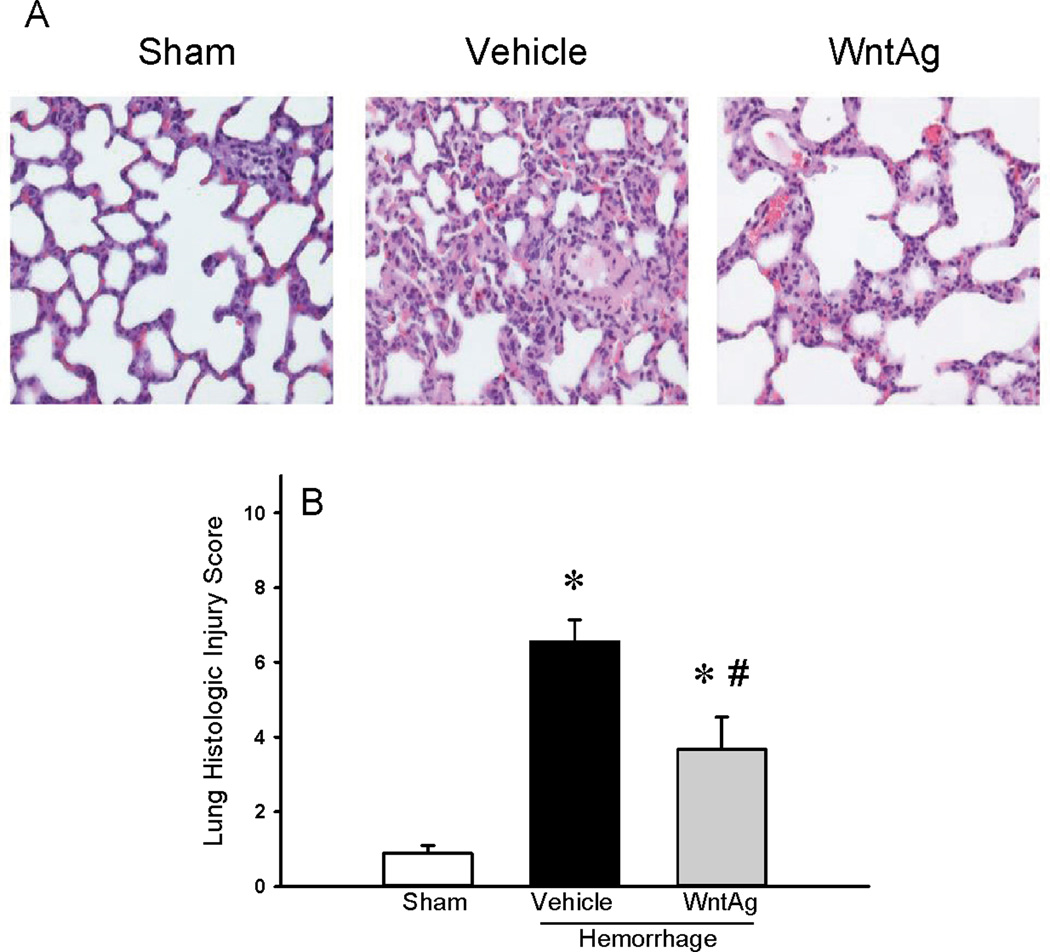
In the vehicle treated animals after hemorrhagic shock, cleaved caspase-3 was increased by 5.5-fold whereas treatment with Wnt agonist significantly attenuated these levels by 46% (Figure 4A). On the other hand, Bcl-2 was markedly decreased by 35% in vehicle treated animals while significantly increased with Wnt agonist treatment and restored to sham values (Figure 4B). Both β-catenin and cyclin D1 in the lungs were significantly decreased while Wnt agonist markedly preserved these levels (Figure 5A–B).
Figure 4. Changes in apoptosis markers in the lungs.
Protein lysates were subjected to Western blotting and examined for cleaved caspase-3 (A) and Bcl-2 (B) proteins. β-actin was used as the internal control. The ratio between the protein and β-actin for Sham was considered as 1.0. Data are expressed as mean ± SE and compared by one-way ANOVA and SNK test. *P<0.05 vs. Sham and #P<0.05 vs. Vehicle.
Figure 5. Changes in Wnt/β-catenin signaling mediators.
Protein lysates from the lungs were examined for β-catenin (A) and Cyclin-D1 (B) expressions. β-actin was used as the internal control. The ratio between the protein and β-actin for Sham is considered as 1.0. Data are expressed as mean ± SE and compared by one-way ANOVA and SNK test. *P<0.05 vs. Sham and #P<0.05 vs. Vehicle.
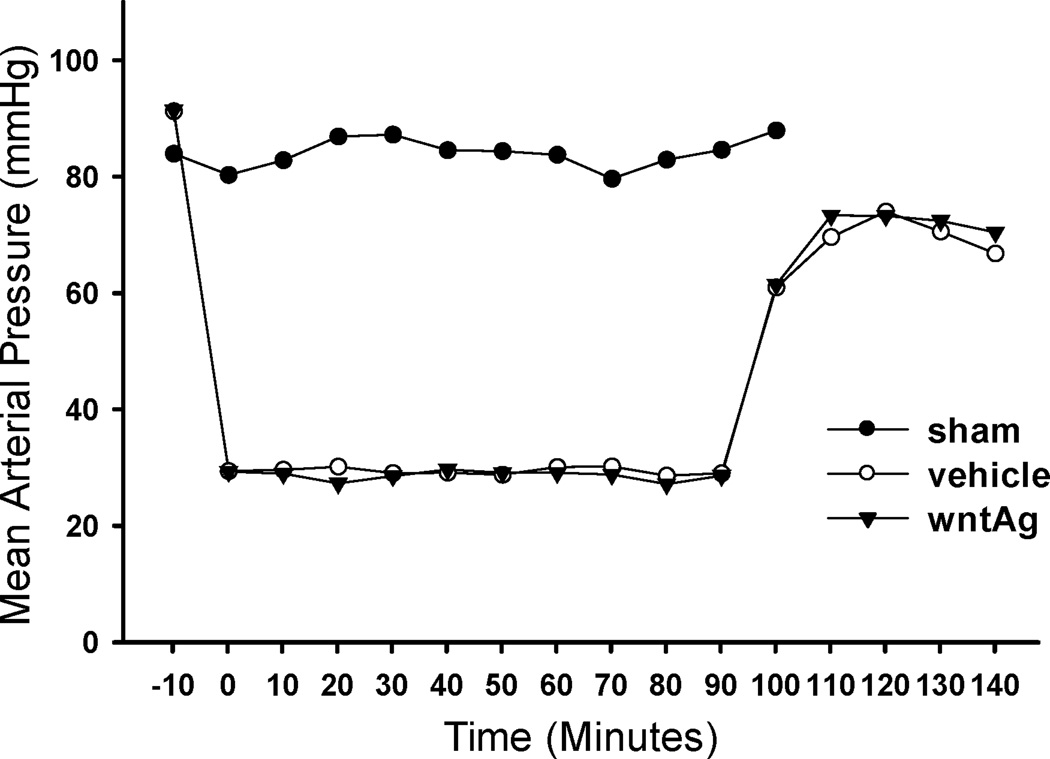
Wnt agonist did not alter mean arterial pressure from vehicle following hemorrhagic shock
In hemorrhaged animals, resuscitation with two times the shed blood volume of Ringer’s lactate (i.e., low volume resuscitation) significantly increased the mean arterial pressure (MAP). Interestingly, Wnt agonist treatment did not significantly alter the MAP from the vehicle group during the observation period of 140 min from the initiation of hemorrhage. These data indicate that the mechanism of its action in hemorrhagic shock is not at the hemodynamic parameter level (Figure 6).
Figure 6. Changes in mean arterial pressure.
Recordings of arterial blood pressure during the 90 min hemorrhage and the first 50 min of resuscitation in Sham, Vehicle and Wnt agonist treatment.
Discussion
The first sign of hypovolemic shock is a decrease in blood pressure. Compensatory mechanisms such as catecholamine release are able to maintain blood pressure and redistribute cardiac output to sustain the vital organs such as the heart and brain24. However, these compensatory mechanisms are limited and without interventions, severe cellular hypoxia and organ damage occur leading to multi organ failure and death25. While fluid resuscitation is effective in maintaining blood pressure and reoxygenation of cells and tissues, it leads to reperfusion injury and tissue damage. In the current study we showed that in hemorrhagic shock, administration of Wnt agonist during resuscitation attenuated systemic injury as evidenced by the significant decrease in the serum markers of liver enzymes, lactate, LDH, BUN and creatinine. The treatment also improved lung architecture, and reduced neutrophil infiltration to the lungs, IL-6 expression, and apoptosis markers. We also demonstrated that these protective effects of Wnt agonist were mediated by the Wnt/β-catenin pathways possibly via cyclin D1 gene expression.
The canonical Wnt pathway is featured by the regulated degradation of β-catenin. The β-catenin destruction complex is consisted of glycogen synthase kinase (GSK) 3α and 3 β, Casein Kinase 1, Adenomatous Polyposis, the scaffold protein Axin and transcription co-factor β-catenin. Upon Wnt stimulation, Wnt ligand forms a complex with the cell-surface receptor Frizzled (Fz) and initiates a cascade of events eventually inhibiting proteasomal degradation of β-catenin. The accumulated β-catenin enters the nucleus and interacts with transcription factors TCF (T-cell factor)/LEF (lymphoid enhancing factor) to activate Wnt target genes (reviewed in11). One such Wnt target gene is cyclin D1 which is a major regulator of the progression of cells into the proliferative stage of the cell cycle26,27. Our study showed that hemorrhagic shock significantly decreased beta-catenin protein in the lungs while treatment with Wnt agonist increased these levels during resuscitation. We also observed downregulation of Wnt target gene cyclin D1 after hemorrhagic shock and treatment with Wnt agonist increased cyclin D1 during resuscitation. These data suggest that Wnt signaling is disrupted in hemorrhagic shock and Wnt agonist was able to preserve the Wnt/β-catenin signaling.
Several reports point to the role of Wnt activation in modulating inflammatory responses. Wnt activation by Wnt2, one of the Wnt ligands, contributed to host protection in response to enteric bacteria by affecting signaling pathways related to cell proliferation and apoptosis by modulating inflammatory responses28. Overexpression of Wnt2 lowered IL-8 levels induced by bacterial infection in intestinal epithelial cells. Wnt2 signaling led to upregulation of β-catenin and promoted viability in intestinal epithelial cells during bacterial infection28. Wnt pathway ligand Wnt7b produced by macrophages, stimulated epithelial response in injured kidney to reestablish a developmental processes that initiated repair and regeneration29. Wnt4, a gene expressed in the developing kidney is re-expressed in the early phase of ischemia/reperfusion kidney and plays a key role in transcriptional regulation of cyclin D1 and cell cycle progression in renal tubular cells in acute renal failure. Thus, Wnt activation regulates inflammation and promotes repair and regeneration of injured tissue.
The mechanism by which Wnt signaling is protective in hemorrhagic shock has not been completely determined. Endothelium is the primary site for reperfusion injury caused by hemorrhagic shock. Endothelial cells are sensitive to hypoxia and they exhibit morphological damages including increase in cellular volumes and loss of cytoskeletal organization and decreased membrane fluidity4. The injury is initiated from leukocyte-endothelial adhesion, transendothelial migration, platelet-leukocyte aggregation and enhanced oxidant production5. Reperfusion injury diminishes the ability of mitochondria and contributes to reactive oxygen species production30. Mitochondria are also central to apoptotic pathways and play a major role in apoptotic cell death31,32. Several studies have been reported on the benefit of antioxidants during reperfusion33–35. The effect of nitric oxide on the endothelium has been heavily investigated and the current thinking is that the constitutively expressed nitric oxide synthase (NOS) is involved in neutrophil adhesion to the endothelium whereas the inducible NOS is associated with NF-κB activation and subsequent cytokine production36. There is evidence that NF-κB is a key factor of apoptosis via cross talk with the known apoptotic genes of the mitogen activated protein kinase family37. Studies have shown that Wnt signaling plays a major role in endothelial cell survival and proliferation12,13. It has been shown that MDR1 gene which encodes a p-glycoprotein expressed in the brain vasculature is a direct target of TCF4/β-catenin transcription complex38. In brain endothelial cells, Wnt agonist in vitro enhanced β-catenin and increased transcription of protein levels of p-glycoprotein39. It is plausible that Wnt agonist protects the endothelium from reperfusion injury via activation of the Wnt target genes (i.e., cyclin D1) during hemorrhagic shock.
In embryonic development, the canonical pathway has been associated with cell fate while non-canonical pathway has been involved in cellular polarity and cell movements40. In the current study, we showed that both β-catenin and cyclin-D1 in the lungs were significantly decreased after hemorrhagic shock in the lungs and that Wnt agonist markedly preserved these levels and therefore speculated that the canonical pathway is involved in the protective effects of Wnt agonist in shock. Future studies are needed to determine the role if any, of the non-canonical pathway in this protection.
Our studies indicated that administration of the Wnt agonist during resuscitation does not alter MAP from that of the vehicle group indicating that the Wnt agonist action is not at the level of improving blood pressure. However, the data clearly indicated that the Wnt agonist treatment protected the lungs from hemorrhage induced damage. Although it is not known whether Wnt agonist improves cardiovascular responses or remote organ functions in addition to the lungs, decrease in serum levels of organ injury markers indicate systemic potential benefit of the Wnt agonist after hemorrhagic shock and resuscitation.
Although we have not examined other tissues by histological approach, we measured organ injury markers, liver enzymes (AST, ALT), and kidney markers (BUN and creatinine). Wnt agonist treatment attenuated all injury markers examined and from this result, we can speculate that hemorrhage induced liver injury and kidney dysfunctions were ameliorated with Wnt agonist treatment. Likewise, we have shown protective effect of the Wnt agonist in the lungs as evidenced by a reduction in lung injury and inflammation as well as a decrease in apoptosis in the lungs. All of these effects were correlated with Wnt/β-catenin signaling in the lung tissue. Further analyses of the liver, kidneys or gut are needed to determine whether the protective effects vary among tissues.
While fixed pressure hemorrhage model used in the current study may limit clinical relevance, fixed volume resuscitation is rather beneficial. Fluid overload may lead to pulmonary edema, systemic inflammatory response, anemia, thrombocytopenia, electrolyte/acid-base abnormalities. Administration of excessive fluids increases the odds ratio of acute respiratory distress syndrome, pneumonia, multiple organ failure, blood stream infections and death. It can be argued that the model doesn’t reflect trauma caused by soft tissue injury. However, the model requires the cut down of the blood vessels which mimics tissue trauma. The time point chosen, i.e., 6.5 h after hemorrhage to examine the effect of Wnt agonist in shock was based on our previous studies where we observed significant alterations in tissue injury following hemorrhagic shock41. Future studies will address the long term effects such as survival advantage after Wnt agonist treatment in hemorrhagic shock.
In our hemorrhagic shock model, we did not use shed blood as resuscitation fluid instead we used low volume resuscitation with Ringer’s lactate (i.e., crystalloid). This resuscitation method was chosen because such fluids are readily available in clinical settings. However neither crystalloids nor colloids alone had any major benefit in trauma outcome42–44. Our studies suggest that Wnt agonist delivered in low volume crystalloid preserved the Wnt/β-catenin signaling pathway during resuscitation following hemorrhagic shock and was protective from systemic inflammation as well as the injury to the lungs.
In a pilot safety study, sham animals were treated with 5 mg/kg BW Wnt agonist and monitored the animals for 24 h. There was neither toxicity nor biological activity observed in sham animals. In future studies, in an attempt to develop this compound as a therapy for hemorrhagic shock, we will perform dose response studies and conduct the drug normal control with the highest dose tested.
In summary, Wnt agonist attenuated hemorrhage-induced systemic inflammation, organ injury and lung apoptosis. Wnt/β-catenin signaling is disrupted in hemorrhagic shock and Wnt activation preserved the Wnt/β-catenin signaling. Wnt signaling could be a potential target for therapy for resuscitation after hemorrhagic shock.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant R01 HL076179 to P.W.
Footnotes
This work was presented at the American College of Surgeons-Brooklyn and Long Island Chapter, 9 May 2013.
The authors declare that no competing interests exist.
Author Contribution
MK-study design, data collection, data analysis, data interpretation, writing
WLY-literature search, study design, data analysis, data interpretation, critical revision
AJ-literature search, writing, critical revision
AK-data collection, data analysis, data interpretation
MG-data collection, data analysis, data interpretation
JN-provide resources, data interpretation, critical revision
GFC-provide resources, data interpretation, critical revision
PW-provide resources, study design, data interpretation, critical revision
References
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507–1511. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. [PubMed] [Google Scholar]
- 3.Nunez TC, Cotton BA. Transfusion therapy in hemorrhagic shock. Curr Opin Crit Care. 2009;15(6):536–541. doi: 10.1097/MCC.0b013e328331575b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Rushing GD, Britt LD. Reperfusion injury after hemorrhage: a collective review. Ann Surg. 2008;247(6):929–937. doi: 10.1097/SLA.0b013e31816757f7. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 11.Gao C, Xiao G, Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4(1):13. doi: 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263(2):384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- 13.Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8(1):43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuncewitch M, Yang WL, Molmenti E, Nicastro J, Coppa GF, Wang P. Wnt agonist attenuates liver injury and improves survival after hepatic ischemia/reperfusion. Shock. 2013;39(1):3–10. doi: 10.1097/SHK.0b013e3182764fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew Chem Int Ed Engl. 2005;44(13):1987–1990. doi: 10.1002/anie.200462552. [DOI] [PubMed] [Google Scholar]
- 16.Cui X, Wu R, Zhou M, Dong W, Ulloa L, Yang H, Wang H, Tracey KJ, Simms HH, Wang P. Adrenomedullin and its binding protein attenuate the proinflammatory response after hemorrhage. Crit Care Med. 2005;33(2):391–398. doi: 10.1097/01.ccm.0000153416.41398.a9. [DOI] [PubMed] [Google Scholar]
- 17.Wu R, Cui X, Dong W, Zhou M, Simms HH, Wang P. Mechanisms responsible for vascular hyporesponsiveness to adrenomedullin after hemorrhage: the central role of adrenomedullin binding protein-1. Ann Surg. 2005;242(1):115–123. doi: 10.1097/01.sla.0000167849.10599.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu R, Dong W, Zhou M, Cui X, Simms HH, Wang P. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137(2):200–208. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Chaung WW, Wu R, Ji Y, Dong W, Wang P. Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med. 2012;30(1):199–203. doi: 10.3892/ijmm.2012.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3(1):35–56. [PubMed] [Google Scholar]
- 21.Matsuda A, Jacob A, Wu R, Zhou M, Nicastro JM, Coppa GF, Wang P. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011;17(1–2):126–133. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike K, Moore EE, Moore FA, Read RA, Carl VS, Banerjee A. Gut ischemia/reperfusion produces lung injury independent of endotoxin. Crit Care Med. 1994;22(9):1438–1444. doi: 10.1097/00003246-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Cui T, Miksa M, Wu R, Komura H, Zhou M, Dong W, Wang Z, Higuchi S, Chaung W, Blau SA, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181(3):238–246. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulop A, Turoczi Z, Garbaisz D, Harsanyi L, Szijarto A. Experimental models of hemorrhagic shock: a review. Eur Surg Res. 2013;50(2):57–70. doi: 10.1159/000348808. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 27.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway . Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Lu R, Wu S, Zhang YG, Xia Y, Sartor RB, Sun J. Wnt2 inhibits enteric bacterial-induced inflammation in intestinal epithelial cells. Inflamm Bowel Dis. 2012;18(3):418–429. doi: 10.1002/ibd.21788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107(9):4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cairns CB, Moore FA, Haenel JB, Gallea BL, Ortner JP, Rose SJ, Moore EE. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997;42(3):532–536. doi: 10.1097/00005373-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Zhao Y, He X, Kim TH, Kuharsky DK, Rabinowich H, Chen J, Du C, Yin XM. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277(30):26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- 33.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, Korthuis RJ. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34(7):810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 34.Nelson SK, Gao B, Bose S, Rizeq M, McCord JM. A novel heparin-binding, human chimeric, superoxide dismutase improves myocardial preservation and protects from ischemia-reperfusion injury. J Heart Lung Transplant. 2002;21(12):1296–1303. doi: 10.1016/s1053-2498(02)00461-8. [DOI] [PubMed] [Google Scholar]
- 35.Flynn WJ, Jr, Pilati D, Hoover EL. Effect of allopurinol on venous endothelial dysfunction after resuscitated hemorrhagic shock. Int J Surg Investig. 1999;1(1):11–18. [PubMed] [Google Scholar]
- 36.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187(6):917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding WX, Yin XM. Dissection of the multiple mechanisms of TNF-alpha-induced apoptosis in liver injury. J Cell Mol Med. 2004;8(4):445–454. doi: 10.1111/j.1582-4934.2004.tb00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60(17):4761–4766. [PubMed] [Google Scholar]
- 39.Lim JC, Kania KD, Wijesuriya H, Chawla S, Sethi JK, Pulaski L, Romero IA, Couraud PO, Weksler BB, Hladky SB, et al. Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells. J Neurochem. 2008;106(4):1855–1865. doi: 10.1111/j.1471-4159.2008.05537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129(7):1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu R, Dong W, Qiang X, Ji Y, Cui T, Yang J, Zhou M, Blau S, Marini CP, Ravikumar TS, et al. Human vasoactive hormone adrenomedullin and its binding protein rescue experimental animals from shock. Peptides. 2008;29(7):1223–1230. doi: 10.1016/j.peptides.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alderson P, Schierhout G, Roberts I, Bunn F. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2000;(2):CD000567. doi: 10.1002/14651858.CD000567. [DOI] [PubMed] [Google Scholar]
- 43.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 44.Roberts I, Alderson P, Bunn F, Chinnock P, Ker K, Schierhout G. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004;(4):CD000567. doi: 10.1002/14651858.CD000567.pub2. [DOI] [PubMed] [Google Scholar]