Abstract
After spinal cord injury (SCI), poor ability of damaged axons of the central nervous system (CNS) to regenerate causes very limited functional recovery. Schwann cells (SCs) have been widely explored as promising donors for transplantation to promote axonal regeneration in the CNS including the spinal cord. Compared with other CNS axonal pathways, injured propriospinal tracts display the strongest regenerative response to SC transplantation. Even without providing additional neurotrophic factors, propriospinal axons can grow into the SC environment which is rarely seen in supraspinal tracts. Propriospinal tract has been found to respond to several important neurotrophic factors secreted by SCs. Therefore, the SC is considered to be one of the most promising candidates for cell-based therapies for SCI. Since many reviews have already appeared on topics of SC transplantation in SCI repair, this review will focus particularly on the rationale of SC transplantation in mediating descending propriospinal axonal regeneration as well as optimizing such regeneration by using different combinatorial strategies.
Keywords: spinal cord injury, axonal regeneration, descending propriospinal tract, Schwann cell, peripheral nerve, transplantation
Introduction
After spinal cord injury (SCI), rostrocaudal axonal regeneration is crucial for significant functional recovery; however, neurons of the mature central nervous system (CNS) are believed to have low regenerative ability. In some central axons, an early growth response may be seen, but this response is abortive and generally does not create meaningful connections (Hall and Berry, 1989; Steward et al., 2008; Zeng et al., 1994). The abortive regeneration of CNS axons contributes heavily to the poor recovery observed after SCI. In the early 1980s the elegant experiments done by Aguayo and colleagues demonstrated that the peripheral nerve (PN) milieu, mainly composed of Schwann cells (SCs), was more favorable for regeneration of injured CNS axons than the CNS environment (Bray et al., 1987; David and Aguayo, 1981; Richardson et al., 1980; Richardson et al., 1982; Richardson et al., 1984). Since then, many therapeutic strategies have been established through transplantation of either a PN segment containing SCs or SCs isolated from the PN (Decherchi and Gauthier, 2000; Houle, 1991; Houle et al., 2006; Iannotti et al., 2003; Levi et al., 2002; Oudega et al., 2001; Xu et al., 1995b; Xu et al., 1997). These experiments strongly support the premise of using SCs for repair after SCI (Wiliams and Bunge, 2012). To date, most regeneration studies after SCI have focused on long supraspinal pathways that project from the brain or brainstem to the spinal cord such as the corticospinal (CST) and rubrospinal (RST) tracts. However, regeneration of these long supraspinal tracts is difficult to achieve (Deng et al., 2013; Guest et al., 1997a; Kanno et al., 2014; Lee et al., 2013; Papastefanaki et al., 2007; Tuszynski et al., 1998; Xu et al., 1995a). On the contrary, although greatly understudied, propriospinal tracts (PSTs) possess greater innate regenerative capacity, and have been shown to strongly respond to PN/SC grafts (Deng et al., 2013; Iannotti et al., 2003; Xu et al., 1995b; Xu et al., 1997). The regenerative response of the PSTs could even result in significant functional recovery after SCI (Deng et al., 2013). In this review, we will discuss results obtained from both PN and SC transplantation into injured spinal cords. We will discuss some unique characteristics of the PSTs, their responses to SC transplantation, and their limitations.
1 The Propriospinal tract (PST)
The propriospinal tract (PST) is important in mediating and maintaining a variety of normal spinal functions including reflexes, posture, and locomotion (Cowley et al., 2008; Jankowska, 1992; Kostyuk and Vasilenko, 1979). The neurons within the PST constitute an uninterrupted cell column and their axons project either unilaterally or bilaterally in the rostrocaudal plane and directly affect motoneurons and interneurons in multiple cord segments (Szentagothai, 1964). Anatomically, PSTs are classified as either “short” or “long” PST based on the distances of their axon projections (Cowley et al., 2010).
Although argument still exists as to what defines a short or long PN, we consider short PSTs (sPSTs) as those spanning less than six spinal segments, whereas long PSTs (lPSTs) project further than six spinal segments (Flynn et al., 2011). Short propriospinal pathways interconnecting several neighboring segments are located both in the lateral and ventral funiculus (Sterling and Kuypers, 1968), The sPSTs with cell bodies medially located in the grey matter often project contralaterally, while the sPSTs with cell bodies laterally located project ipsilaterally. The sPSTs can project bidirectionally (Burton and Loewy, 1976; Matsushita, 1970; Menetrey et al., 1985; Petko and Antal, 2000). In line with classical studies by Romanes and Sprague who demonstrated a medio-lateral division between motoneurons and their target muscle groups, within the limb enlargements (Romanes, 1951; Sprague, 1948), Kuypers and colleagues proposed a somatotopic organization of sPSTs. The sPSTs originating in neurons within the ventromedial grey matter (lamina VIII and the medial portion of lamina VII), innervate and influence motoneurons supplying axial muscles as their axons terminate within and around medial motoneurons pools. Correspondingly, the soma of sPSTs located in lateral regions (lateral parts of laminae VII) innervate motoneurons supplying more distal limb muscles, as their axons terminate in the vicinity of the lateral motoneuron pools (Molenaar and Kuypers, 1978; Sterling and Kuypers, 1968). lPSTs that are involved in locomotor activity reciprocally connect cervical and lumbar enlargements and are concentrated in the ventral quadrants (Giovanelli Barilari and Kuypers, 1969). The anatomical distinction that can be made with respect to lPST is whether their cell bodies are located rostrally (within the cervical enlargement) and project caudally, or vice-versa. These two populations are termed long descending PST (ldPST) and long ascending PST (laPST), respectively (Giovanelli Barilari and Kuypers, 1969; Matsushita and Ueyama, 1973; Molenaar and Kuypers, 1978). The function of ldPSTs is involved in feed-forward inhibition of supraspinal command and reciprocal connection of cervical and lumbar motor circuits (Alstermark et al., 1991a; Alstermark et al., 1999; Isa et al., 2006). The laPST system was found to play an important role in locomotion by coupling neural activity in cervical and lumbar enlargements (Cote et al., 2012; Miller et al., 1973). Propriospinal neurons receive strong and convergent supraspinal innervations including those from the corticospinal (CST), rubrospinal (RST), reticulospinal (ReST) and vestibulospinal (VST) tracts (Alstermark et al., 1987; Alstermark et al., 1991b; Illert et al., 1977; Kostyuk and Vasilenko, 1978; Nishimura et al., 2009; Robbins et al., 1992; Skinner et al., 1979). Such signal relay has significance in transporting supraspinal command down to the spinal cord not only in normal physiological but also in pathological conditions (Figure 1).
Figure 1. Schematic diagram of descending propriospinal system.
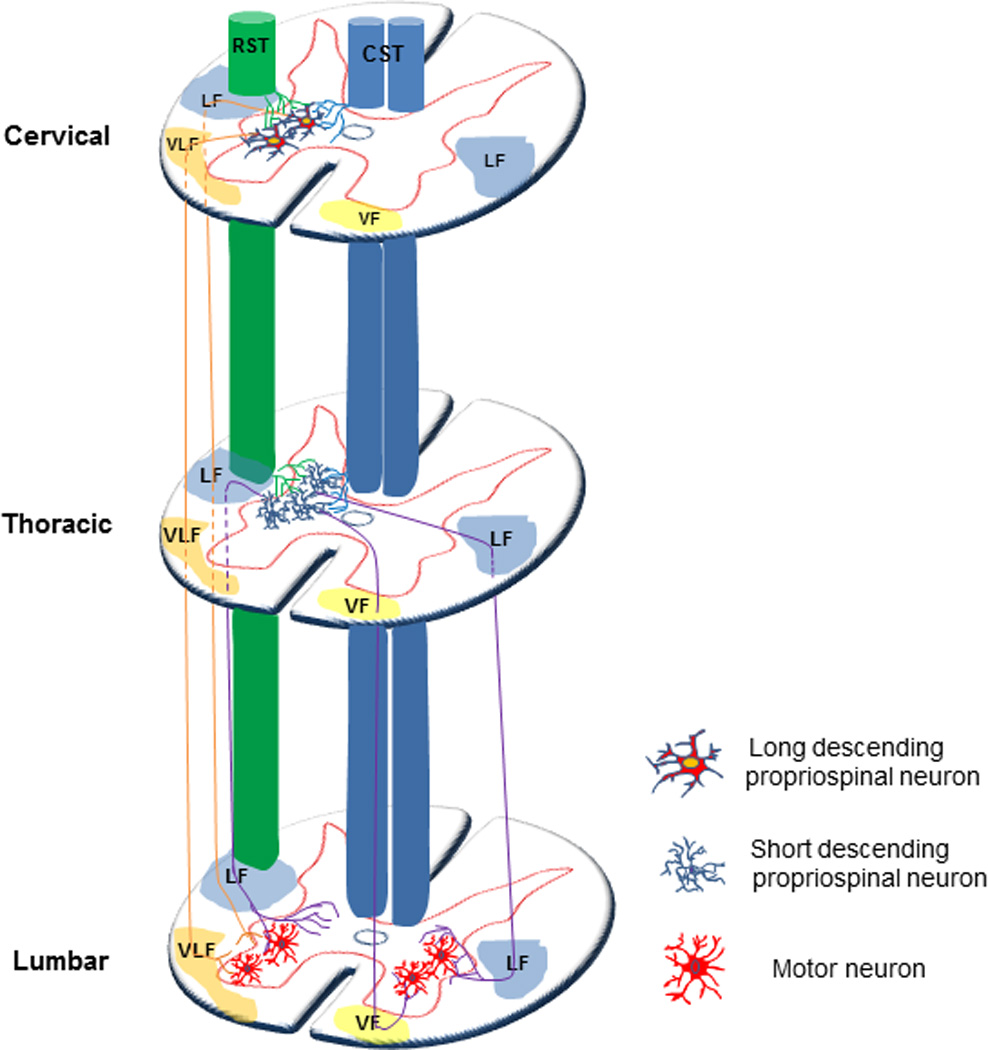
Short propriospinal pathways interconnecting several neighboring segments are located both in the lateral and ventral funicula. The sPSTs with cell bodies medially located in the grey matter often project contralaterally, while the sPSTs with cell bodies laterally located project ipsilaterally. The sPSTs can project bidirectionally. The sPSTs originating in neurons within the ventromedial grey matter (lamina VIII and the medial portion of lamina VII), innervate medial motoneurons pools. Correspondingly, the soma of sPSTs located in lateral regions (lateral parts of laminae VII) innervate the lateral motoneuron pools. lPSTs that are involved in locomotor activity reciprocally connect cervical and lumbar enlargements and are concentrated in the ventral quadrants. Propriospinal neurons receive convergent supraspinal innervations including those from the corticospinal (CST), rubrospinal (RST). lPSTs: long propriospinal tracts; sPSTs: short propriospinal cord tracts; CST: corticospinal tract; RST: rubrospinal tract; LF: lateral funicula; VLF: ventral lateral funicula; VF: ventral funicula; LF: lateral funicula.
Several critical literatures concluded that supraspinal axons, which usually fail to regenerate through and beyond the lesion site, form ‘new’ contacts with spared intraspinal or propriospinal circuits projecting past a SCI lesion to lumbar segments. Such supraspinal-propriospinal reorganization formed an anatomical ‘bridge’ allowing transmission of descending signals below the lesion to activate the lumbar locomotor central pattern generator (CPG) (Bareyre et al., 2004; Courtine et al., 2008; Cowley et al., 2008; Vavrek et al., 2006). Such plasticity occurred based on the intact propriospinal system spared following an incomplete SCI. However, for a severe injury such as a complete SCI, axonal regeneration through and beyond the injury is required to achieve meaningful functional recovery. Descending propriospinal axons (dPSTs) are uniquely suited for reestablishing connections across the lesion since they show greater growth responses after SCI than long-tract axons (Deng et al., 2013; Iannotti et al., 2003; Xu et al., 1995b; Zhang et al., 2009). Therefore, the plasticity of CST axons that innervate dPST neurons and subsequent regeneration of dPST axons beyond the lesion site may provide an alternative pathway or “functional relay” for transmission of supraspinal motor command down to the spinal cord to promote motor recovery.
2 Schwann cells mediate endogenous repair of PNS and CNS injuries
The peripheral environment has long been shown to be permissive for CNS axonal regeneration (David and Aguayo, 1985; Horvat et al., 1989; Salame and Dum, 1985). SCs are the major component of the grafted nerve that promotes such regeneration. Developmentally, SCs derive from the neural crest (Bhatheja and Field, 2006). Neural crest cells give rise to SC precursors from which immature SCs are generated. The immature SCs then differentiate into either myelin-forming or non-myelin-forming SCs (Corfas et al., 2004; Jessen and Mirsky, 2005). SCs are very important for guiding axonal growth and producing myelin sheath for peripheral axons. SCs are also essential for endogenous repair of peripheral and central axons after injury (Arthur-Farraj et al., 2012; Oudega et al., 2005). SCs are involved very early in segmenting and incorporating degraded damaged myelin and recruiting macrophages for removal of myelin debris. Early after axonal injury, SCs in the distal nerve dedifferentiate into non-myelinating SCs and proliferate extensively. These newly formed non-myelinating SCs migrate within reorganized connective tissue to form column-like structures known as bands of Bungner (Arthur-Farraj et al., 2012; Jessen and Mirsky, 2005). This proliferative response is accompanied by downregulation of myelin-associated molecules that inhibit axonal regeneration, and upregulated cell adhesion molecules including neural cell adhesion molecule (NCAM), N-cadherin, L1, and several other trophic factors, creating a permissive environment for the repair of injured axons (Arthur-Farraj et al., 2012; Jessen and Mirsky, 2008; Svaren and Meijer, 2008; Taveggia et al., 2010). For different types of SCI such as contusion, laceration, and photochemical lesions, SCs can migrate into the injured spinal cord and myelinate or ensheathe a large number of regenerating axons, and more importantly, establish nodes of Ranvier with normal ion channel patterns on central axons and can maintain action potential conduction for over a year (Black et al., 2006). In experimental SCI, transplantation of either peripheral nerve or isolated and purified SCs establishes conduits for axon regeneration and remyelination, replaces lost glial cells, and improves neurological function (Honmou et al., 1996). In this review, we define both PN and purified SC transplantation as SC transplantation for repair after SCI.
3 Schwann cell mediates propriospinal axonal regeneration
3.1 Advantage of using Schwann cells/PN
The PN auto-graft is one of the earliest experimental treatments to promote CNS axonal regeneration after SCI. A PN graft not only provides supportive SCs but also promotes the survival of axotomized spinal cord neurons by upregulating the expression of nitrous oxide (NO) and further activation of the NO-dependent cyclic-GMP pathway, a survival effector, in these neurons (Yick et al., 1999). Moreover, nerve grafts induced expression of growth factors such as NGF and BDNF in the host spinal cord and attenuated delayed glial scar formation at the interface of the caudal spinal cord, which is crucial for successful regeneration (Kuo et al., 2011). Dissociated SCs were later utilized as a transplantation strategy to promote axon regeneration. After transplantation, the initially dissociated SCs align predominantly parallel to the length of the implant. Thus, dissociated SCs alone could both elicit axonal ingrowth and serve as an effective substrate or bridge for growing axons (Murray and Fischer, 2001). Compared to peripheral nerve grafts, one unique advantage of using purified SCs is the potential to engineer them to overexpress growth-promoting factors and/or adhesion molecules to enhance axon growth (Wiliams and Bunge, 2012).
3.2 Schwann cells/PN transplantation promotes propriospinal regeneration
The majority of regenerated axons in the grafted PN/SCs have been shown to be of spinal cord origin. Propriospinal neurons whose axons regenerated into the SC/PN grafts originated within Rexed lamina III-VII, the medial portion of lamina VIII, and lamina X and were distributed throughout the spinal grey matter as far rostral and caudal as C3 and S3 (Kao et al., 1977; Paino et al., 1994; Richardson et al., 1980; Richardson et al., 1982; Richardson et al., 1984; Xu et al., 1995b; Xu et al., 1997). The density of labeled neurons was the greatest in sections closest to the graft and diminished progressively at increasing rostral and caudal distances. Approximately half of the labeled cells were located in a 4 mm region surrounding the graft (Paino et al., 1994; Xu et al., 1995b). A bilateral distribution of retrograde-labeled propriospinal neurons within the spinal cord was observed, with a significantly higher occurrence of labeled cells on the ipsilateral side were reported in both PN nerve graft and isolated SCs graft models (Decherchi and Gauthier, 2000; Iannotti et al., 2003). However, due to the fact that intrinsic spinal cord neurons, especially long projecting propriospinal neurons, have various projection patterns including projection distance, laterality, or branching within the graft (Saywell et al., 2011; Siebert et al., 2010; Tuszynski and Steward, 2012; Verburgh and Kuypers, 1987), analysis of the size, distribution, and cytological features failed to yield specific identification of usual destinations of these labeled neurons. In addition to the anatomical differences,Siebert et al. (2010) observed phenotypic variation in the post-injury response to axotomy between ldPST neurons and sdPST neurons. The ldPST neurons lacked both cell death and regenerative responses, and down-regulated many genes important for regrowth. Instead of mounting a robust early response exhibited by short thoracic propriospinal neurons, ldPST neurons become relatively dormant or quiescent (Siebert et al., 2010). This study demonstrated that ldPST neurons respond more like supraspinal neurons than sdPST neurons following low thoracic axotomy. This study did not combine propriospinal neuronal response with any other treatment such as the use of SC transplantation, and no information is available concerning how SC transplantation might affect the posttraumatic response of propriospinal neurons.
3.3 Optimal transplantation time
Some inhibitory influences associated with the mature CNS are downregulated over time after injury. The injury site environment may become more favorable for axonal growth at later time points following injury. For example, in the acute phase, regeneration of propriospinal axons into grafted PN is often hindered by a lesion cavity between the cord stumps and the grafts, which is caused by coalescence of several small cysts. Delayed spinal cord grafting after the trauma appeared to prevent such cyst formation (Kao et al., 1977). In addition, Wardrope and Wilson found that the lytic enzymes released by the damaged axons were no longer found in the extracellular space one week following injury (Wardrope and Wilson, 1986). Moreover, it is likely that the differences between acute and delayed transplant injury conditions are not restricted to the environment alone. Rather, there may be differences in the ability of neurons themselves to mount a regenerative response. The process of re-exposing the lesion site 2 weeks after injury and clearing away the glial scar at the injury site prior to transplantation may actually elicit a “conditioning lesion” (Neumann and Woolf, 1999; Richardson et al., 1984). That is, neurons that have been injured previously may be primed to upregulate cellular and molecular programs associated with axonal growth. After axotomy at a distance from the cell body, a strong initial inflammatory as well as early regeneration and cell death responses occur. An early up-regulation of several growth factor receptors, as well as a down-regulation of receptors to several factors that inhibit axonal growth may indicate that potential therapies to protect PST neurons from early cell death post-axotomy and to maximize and sustain the early regenerative response should be applied during an acute phase. On the other hand, when acutely injured propriospinal axons displayed a strong growth capacity, chronically injured axons are more impaired in their propensity to regenerate. Decherchei and Gauthier found that a time window of three weeks post-lesion is a critical period for axonal regeneration after which injured neurons may attenuate regeneration potential (Decherchi and Gauthier, 2000). Sandrow et al. also observed a 50% reduction in the mean number of contributing propriospinal neurons after extended delay (Sandrow et al., 2008). Siebert and colleagues further confirmed that the thoracic propriospinal neurons mounted a very dynamic response following low thoracic injury. In the chronic phase, the transitory enhanced expression of regeneration-associated genes diminished. Contrarily, gene expressions of several inhibitory receptors for axonal growth were initially down regulated but recovered to control level in later periods post-injury (Siebert et al., 2010).Therefore, acute or sub-acute phase (one or two weeks after injury) is the optimal time window for the treatment of the propriospinal axonal regeneration.
3.3 Combination with neurotrophic factors
Another therapeutic approach for axonal regeneration involves the use of exogenous neurotrophic factors. The combination of neurotrophic factors with transplants heightens the regenerative effort of injured neurons. Exogenous application of neurotrophic factors increases the intrinsic capacity of mature neurons for regrowth, and prevents atrophy of axotomized neurons (Coumans et al., 2001). Several observations indicated that GDNF, GFRα1, and GFRα2 mRNAs were highly expressed in the ventral horn, and moderately expressed in the intermediate zone and dorsal horn of the spinal gray matter, and injury induced up-regulation of receptor genes for GDNF shortly after SCI (Satake et al., 2000; Widenfalk et al., 2001). These observations suggest that propriospinal neurons may respond to GDNF and regenerate axons into the GDNF-enriched graft. GDNF may exert a direct effect via receptors expressed on injured axons or via retrograde transport from the site of implantation to the cell body of injured axons. Intervention to rescue injury-induced death of propriospinal neurons should start quickly following injury, prior to onset of cell death to sustain their survival and regenerative responses (Paratcha et al., 2001; Trupp et al., 1999 Seibert’s 2010). Neurotrophins combined with SC transplantation act synergistically to maximize neuroprotective or regenerative responses. After transplantation of a SC-seeded guidance channel into an acutely transected spinal cord, approximately two-thirds of a total 2,500 myelinated and unmyelinated axons of propriospinal origin were present within the transplant cable (Xu et al., 1997). If combined with GDNF, a significant and synergistic increase in axonal regeneration and myelination occurred. Retrograde tracing revealed that GDNF-induced enhancement of axonal regeneration mainly originated from propriospinal neurons (Iannotti et al., 2003; Zhang et al., 2009). Alternatively, other factors like BDNF failed to increase the number of axons that regenerated back into the spinal cord which may be attributed to an insensitivity of propriospinal axons to BDNF (Tom et al., 2013). Application of neurotrophic factors is more important in chronic injuries when growth factor related gene expressions are greatly reduced (Dolbeare and Houle, 2003; Tom et al., 2009).
3.4 Graft-host interface
To achieve meaningful functional regeneration, it is necessary to promote axons to regenerate beyond the lesion site, reenter the host spinal cord and reconnect with target neurons. Following SCI, CNS axons do not regenerate spontaneously due to the presence of inhibitory molecules associated with glial scar and CNS myelin at the lesion site, as well as the shortage of neurotrophic support in the host spinal cord. PN or isolated SC transplantation could improve glial environment to certain extent, however, such transplantation did not elicit regeneration of propriospinal axons beyond the graft environment, despite robust ingrowth of axons into the graft (Deng et al., 2011; Wiliams and Bunge, 2012). Without additional treatment at the graft-host interface, regenerating axons within the SC graft are hindered by properties of the interface similar to the SC-CNS parenchymal interface seen at the dorsal root entry zone (DREZ), which is normally impenetrable by regenerating sensory axons in the adulthood (Fernandez et al., 1985; Guest et al., 1997b; Moissonnier et al., 1996; Xu et al., 1999). More recently, dorsal root axons have been found to regenerate into CNS territory in a dorsal root crush model; however, they rapidly stalled and then remained completely immobile or stable, even after conditioning lesions that enhanced growth along the root (Di Maio et al., 2011). The molecular environment encountered by regenerating axons including connective tissue and host astrocytes at the distal graft/host interface, appears to be more important for progress of regrowth than quantity of fibers within the graft. To promote regenerated axons within the PN graft into the host spinal cord where they can establish functional connections, different therapeutic combinations aimed at modifying glial scar at the graft-host interface have been tested. In 2004, Chau et al. used chondroitinase ABC (ChABC) to directly digest the glial scar caudal to a SC-seeded transplant after acute SCI. By 1 month post ChABC treatment, significant propriospinal axons regenerated through the graft-host interface and extended into the host cord as far as 5 mm (Chau et al., 2004). However, no functional evaluation was performed in this study. Tom et al. (2008, 2009) also applied ChABC to the distal graft-host interface in an acute PN graft model allowing regenerating axons to extend beyond the graft spanning the cavity and promoted some functional recovery. However, the sources of axon outgrowth in these studies were not discerned (Tom and Houle, 2008; Tom et al., 2009). It is quite possible that these regenerated axons primarily originated from propriospinal neurons.
In addition to modifying the glial scar at the graft-host interface, activation of injured neurons by different growth factors will add another important dimension to axonal regeneration (Dolbeare and Houle, 2003; Storer et al., 2003). For example, Lee et al. combined the transplantation of multiple PN autografts (PNGs), embedded within acidic fibroblast growth factor (aFGF)-laden fibrin matrix, and ChABC delivered to both the graft and at the graft/host interfaces after a complete spinal transection (Lee et al., 2013). In this experiment, descending propriospinal axons were found to regenerate beyond the lesion site. Interestingly, another trophic factor GDNF was found to modify the SC grafthost interface, promote the migration of host astrocytes into the SC bridge, reduce reactive astrogliosis, macrophage infiltration, and cavitation (Deng et al., 2011; Iannotti et al., 2003). Within the SC graft, elongated processes of astrocytes extended parallel to the graft axis and in close alignment with regenerated axons (Figure 2). This morphological change of reactive astrocytes at the graft-host interface proved permissive for regenerative axon growth. However, the origins of regenerated axons were not traced in these studies. Recently, William and Bunge reported that, compared to pre-gelled bridges, fluid bridges of SCs and Matrigel affected morphology and distribution of host astrocytes, induced immature astrocyte characteristics and elongated processes into the bridge forming a tight-meshwork that walled off the graft-host interface (Williams et al., 2013). This modification was closely associated with enhanced regeneration of brainstem axons across the rostral interface and improvement in hindlimb locomotion. Deng et al. expanded upon the previous work and constructed a continuous growth-promoting pathway in adult rats, formed by transplantation of SCs overexpressing GDNF both in the lesion gap and caudal host cord (Deng et al., 2013) (Figure 3). This pathway, extending from the cut axonal ends to the site of innervation in the distal spinal cord, promoted regeneration of dPST axons through and beyond the lesion gap of a spinal cord hemisection. Within the distal host spinal cord, regenerated dPST axons were myelinated and formed synapses with host neurons providing anatomical basis for its functional recovery.
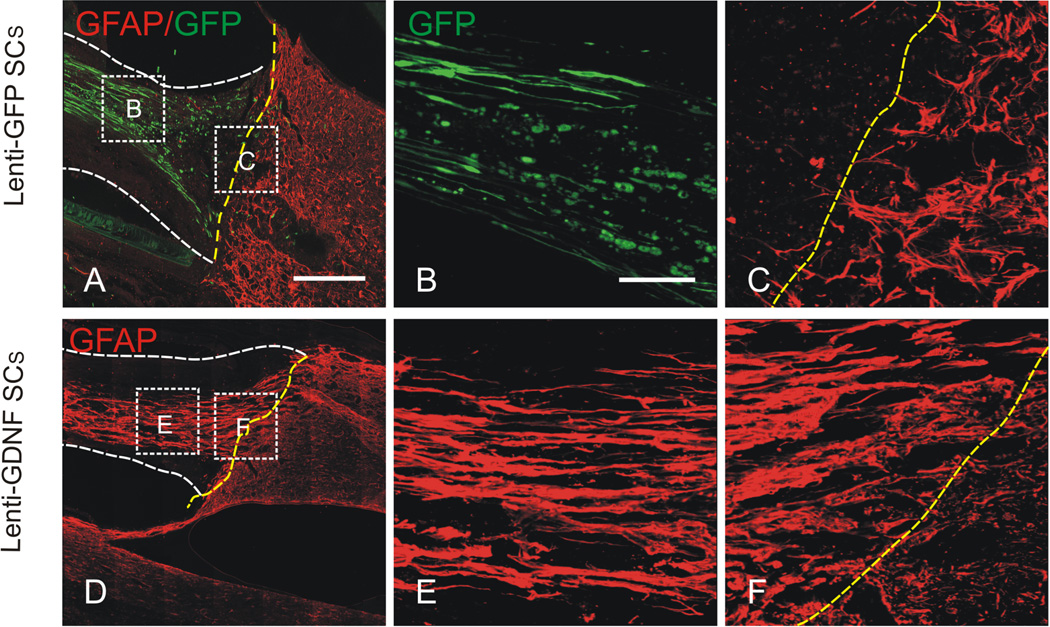
Figure 2. GDNF induced migration of host astrocytes into the Schwann cell (SC) grafts.
Images shown in (A-F) are representative photomicrographs of the caudal graft-host interface from grafts that contained 1) lenti-GFP SCs, 2) lenti-GDNF SCs. (C-E) In the Lenti-GFP SC graft, a dense meshwork of hypertrophic astrocytes, labeled with GFAP, was seen at the host side of the caudal graft-host interface (yellow dashed line). Note that host astrocytes did not migrate into the SC graft in the absence of GDNF. The survival of grafted SCs, evidenced by GFP-staining, with elongated processes extending along the axis of the graft was clearly seen. (D-F) In the lenti-GDNF SC graft, remarkably more host astrocytes migrated into the graft environment for considerable distances. (B,C) and (E,F) are high magnifications of boxed areas of the graft proper and caudal graft-host interface in A and D, respectively. Yellow dashed lines indicate the graft-host interfaces. White dash lines in (A, C, D, F) depict the graft proper. Scale bars A, D: = 400µm; B, C, E, F, = 100µm. Modified from Deng et al. (2011.
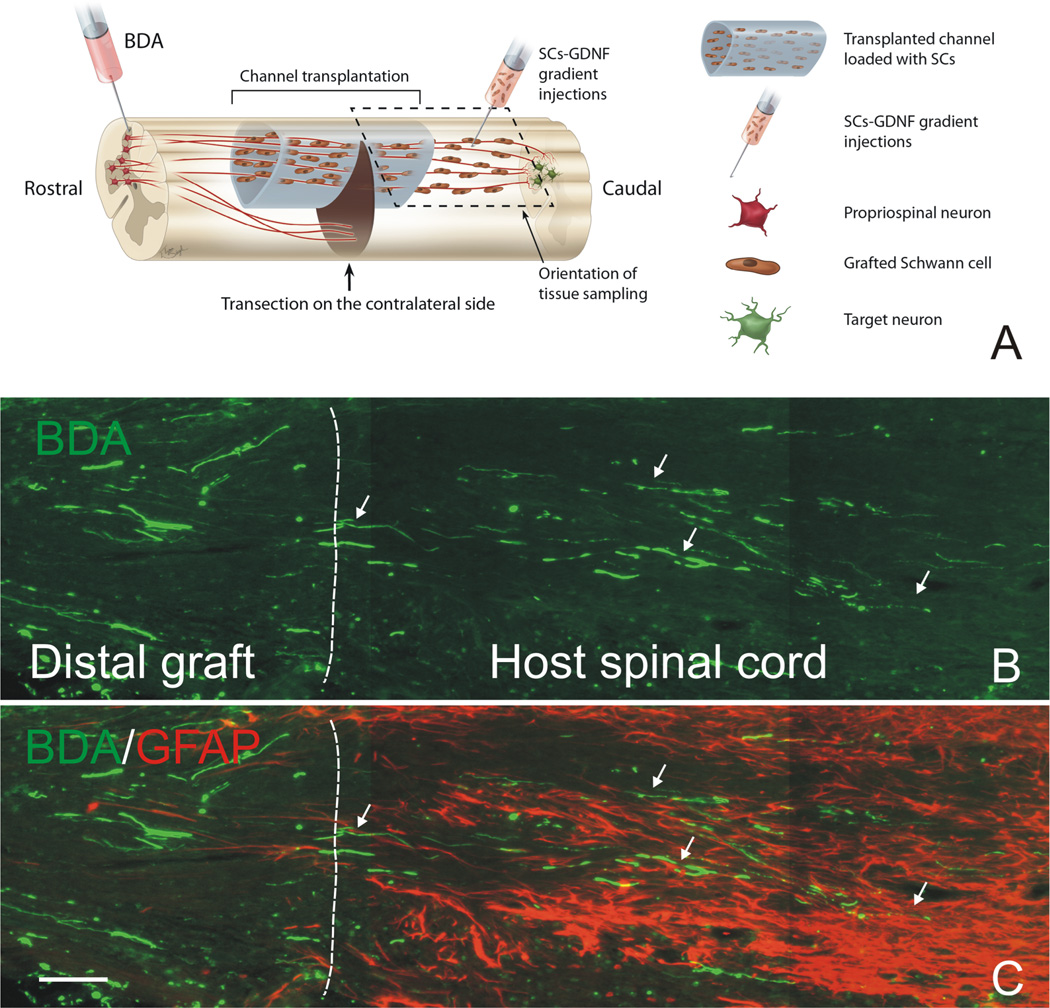
Figure 3. Descending propriospinal axons regenerate across the caudal graft-host interface and grew back into the distal host spinal cord.
(A) Schematic drawing shows the experimental strategy and how tissue was sampled. Note that a continuous growth permissive pathway was established by graft of Schwann cell over-expressing GDNF (SCs-GDNF) both in the lesion and caudal spinal cord to promote the axonal regrowth beyond the graft-host interface. (B) BDA-anterogradely labeled propriospinal axons (green, arrows) were found to penetrate through the distal graft-host interface (white dashed line) and to elongate within the distal host spinal cord only in the group injected with SCs-GDNF into the caudal host tissue. (C) The distal graft-host interface was demarcated by GFAP-labeled astrocytes (red). B&C Scale Bar=100µ m. Modified from Deng et al. (2013).
3.5 Functional recovery
Anatomical and physiological investigations have confirmed that the intraspinal network of propriospinal neurons plays a critical role in motor reflexes, voluntary movement, and sensory processing. A population of propriospinal neurons located in the upper cervical segments is critical for certain CST-dependent forelimb motor tasks. Their role is to transmit CST input, as well as convergence input from the rubro-, tecto-, and reticulo-spinal tracts, to motoneurons in segments C6 to T1 that innervate the forelimb (Flynn et al., 2011; Isa et al., 2013). These connections may be important for preparatory movements of the hindlimbs prior to and during targeted reaching by the forelimbs (Kostyuk and Vasilenko, 1978). Two special populations of propriospinal neurons were long projecting neurons, including laPST neurons projecting from the lumbosacral enlargement rostrally to the cervical enlargement, and ldPST neurons projecting caudally from the cervical enlargement to the lumbosacral enlargement. These two types of long distance PST neurons send reciprocal projections between the upper and lower limb segments and function in the regulation and fine-tuning of locomotion, limb coordination, and postural support (Flynn et al., 2011). In addition, the ability of propriospinal neurons to activate and coordinate spinal CPGs makes them ideally suited to facilitate significant locomotor recovery. Even non-specific electrical stimulation of propriospinal networks (leading to lumbar CPG activation) following SCI results in significant locomotor performance improvements (Ballion et al., 2001; Cowley et al., 2008; Juvin et al., 2005). Short thoracic propriospinal neurons arise from the thoracic levels of the spinal cord and their axons project only a few segments in both the rostral and caudal directions. These neurons are primarily involved in regulating axial musculature and postural mechanisms, working in concert with ldPST and laPST (Flynn et al., 2011).
It has been suggested that the propriospinal system plays a key role in functional recovery after SCI (Jane et al., 1964; Selzer, 1978). Several recent studies have provided compelling evidence supporting this notion (Bareyre et al., 2004; Courtine et al., 2008; Vavrek et al., 2006). These publications reported motor improvement in animals following SCI via generation of de novo intraspinal circuits involving propriospinal neurons. These data suggest that descending supraspinal signaling may be reestablished through new connections with intact propriospinal neurons projecting past the lesion site and contact neurons and circuits that can shape motor function. A recent study by Fenrich and Rose (2009) further emphasizes the importance of commissural propriospinal neurons in recovery from SCI. They demonstrated that severed axons of commissural propriospinal neurons can regenerate and make functional synaptic connections with spinal motoneurons (Fenrich and Rose, 2009).
A basic function of an axon is to conduct action potentials. There are some studies in which electrophysiological testing was applied after different grafting paradigms following SCI. Pinzon et al. showed that propriospinal neurons, primarily within laminae IV, V, and VII, of the adjacent cord segments regenerated axons through a PN graft and gave rise to evoked potentials characterized by low amplitude, long latency responses and undetectable late responses with low voltage stimulation, compared to evoked responses in normal animals (Pinzon et al., 2001). The approximate conduction velocities of the identified responses are in the range of myelinated axons indicating that the regenerated propriospinal axons were myelinated. Deng et al. demonstrated that provision of a SC-GDNF growth promoting pathway promoted dPST axons to regenerate through a hemisection lesion to enter the caudal host spinal cord. These regenerated axons conducted action potentials across the lesion gap (Deng et al., 2013). The issue remains that limited number of regenerating axons grew back into the host spinal cord despite the use of different kinds of combinatorial treatment strategies.
One important question is whether a small number of regenerated propriospinal neurons can influence functional recovery. The ability of propriospinal neurons to contribute to functional recovery following complete spinal cord transection has been demonstrated in the lamprey (Selzer, 1978). The role for propriospinal neurons to enhance recovery following SCI and cell transplantation in the mammal has been understudied. Kinematic analysis during treadmill walking in spinal cord-transected rodents with open-ended SC grafts has provided no clear evidence of fore- to hind-limb coordination although animals grafted with SC-filled channels exhibited more frequent and longer episodes of alternating dorsal stepping (Guest et al., 1997b). Animals recovered some behavioral function when a continuous growth-promoting pathway of SCs-GDNF was provided (Deng et al., 2013). Interestingly, significant differences existed only in stride length but not other parameters between the SCs-GDNF-treated group and the other treatment groups in footprint analysis indicating that functional restoration occurs in more proximal than distal muscles, which could be innervated by different spinal pathways (Deng et al., 2013). Several reasons could account for these inexplicit results. First, after SCI, the original anatomical structure is interrupted. Axonal regeneration is disorderly along the rostral-caudal orientation under transplantation interference. Second, in addition to the heterogeneous types of propriospinal neurons, the target neurons of regenerated axons are basically randomly chosen which induced complicated behavioral results and sometimes worsened the motor function recovery. Third, although axonal regeneration, remyelination, and synaptic formation in our studies appear to be functional, it is unclear how efficient these connections were in transducing information. It is possible that the regenerated axons remained in a pathological state with decreased conduction velocities even after regeneration, possibly caused by chronic demyelination. Fourth, although we show evidence that regenerated axons are myelinated, we do not know the extent of myelination along the axons or whether the thickness of myelin surrounding the axons approaches normal. Last but not least, despite the observation that regenerated axons formed new synapses on host neurons, the efficiency of these connections might not be optimal.
3.6 New sources Schwann cells for transplantation
Due to the great potential to amplify and genetically manipulate SCs in vitro, transplantation of purified SCs has become an important strategy for experimental and clinical treatment for SCI. In addition to the expansion of isolated SCs from PN, Schwann cell precursors (SCPs) with a SC phenotype derived from bone marrow stromal cells, subcutaneous fat tissue, skin, or even human umbilical cord have recently been created in vitro (Agudo et al., 2008; Ban et al., 2009; Biernaskie et al., 2007; Chi et al., 2010; Kamada et al., 2011; Park et al., 2010; Someya et al., 2008; Yan-Wu et al., 2011). These SC-like cells display impressive regenerative potential in vivo following SCI. These cells resemble PN-derived SCs in terms of expression of SC markers, as well as the ability to fill the lesion and myelinate central and peripheral axons (Biernaskie et al., 2007). However, the SCPs may exhibit striking differences from mature SCs upon transplantation into the CNS. For example, integration ability into host tissue exceeds PN-derived SCs. They may be able to modify adjacent host tissues, specifically reducing reactive gliosis (Biernaskie et al., 2007). In addition, after PN injury, SCs located in the distal segment of the injury become activated. They increased proliferative ability and growth factor expression which enhances their regeneration-permissive capacity and make them an attractive cell type for promoting axonal regeneration (Dinh et al., 2007; Rasouli et al., 2006). Although several studies have taken advantage of these various sources of SCs for axonal regeneration, no further information is available concerning their effects on propriospinal axonal regeneration.
4 Summary
Since being described over one hundred years ago by Sir Charles Sherrington (Flynn et al., 2011), the propriospinal system has been shown to be important for normal spinal cord physiology as well as functional recovery after SCI in all mammals. However, the relative contribution of the propriospinal system to functional recovery in man can only be speculated at this stage. Studies in animal models of SCI provide compelling evidence that propriospinal neurons are the most promising targets for therapeutic interventions after SCI compared to neurons originated from other CNS regions such as in the sensorimotor cortex and the red nucleus. Nevertheless, several important questions remain. For example, in severe SCI particularly in complete SCI, can regenerated propriospinal axons reestablish synaptic connections with distal spinal neurons? Can regenerated propriospinal neurons receive supraspinal innervation? Can propriospinal regeneration serve as a functional relay for motor and sensory recovery? What are intrinsic mechanisms underlying regenerative response of propriospinal neurons to axotomy? Can propriospinal regeneration be further enhanced via different combinatorial strategies such as activity-dependent synaptic reorganization? Do different phenotypes of propriospinal neurons have different regenerative capacity? Do propriospinal neurons in different species have different capacity for regeneration? Since SC transplantation has been approved by FDA for clinical trials (Guest et al., 2013), addressing these questions would further facilitate the translation of SC transplantation to clinical treatment of SCI. To promote a complete functional regeneration, several critical steps have been proposed which may include (i) enhancing the intrinsic regenerative capacity of injured neurons, (ii) manipulating the interaction between the grafted SCs and host astrocytes making the graft-host interface more permissive for axon growth, and (iii) reducing or removing inhibitory molecules associated with the glial scar. These strategies may enhance the permissivity of the off-ramp as well as the on-ramp for axon growth. It will be very exciting to explore additional combinatory strategies to recruit the propriospinal system on the backbone of SC transplantation to target functional regeneration following SCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agudo M, et al. Schwann cell precursors transplanted into the injured spinal cord multiply, integrate and are permissive for axon growth. Glia. 2008;56:1263–1270. doi: 10.1002/glia.20695. [DOI] [PubMed] [Google Scholar]
- Alstermark B, et al. Subpopulations and functions of long C3-C5 propriospinal neurones. Brain Res. 1987;404:395–400. doi: 10.1016/0006-8993(87)91402-8. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Tantisira B. Pyramidal excitation in long propriospinal neurones in the cervical segments of the cat. Exp Brain Res. 1991a;84:569–582. doi: 10.1007/BF00230969. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Isa T, Tantisira B. Integration in descending motor pathways controlling the forelimb in the cat. 18. Morphology, axonal projection and termination of collaterals from C3-C4 propriospinal neurones in the segment of origin. Exp Brain Res. 1991b;84:561–568. [PubMed] [Google Scholar]
- Alstermark B, et al. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C(3)-C(4) propriospinal neurons in the Macaca fuscata. J Neurophysiol. 1999;82:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. doi: 10.1046/j.0953-816x.2001.01794.x. [DOI] [PubMed] [Google Scholar]
- Ban DX, et al. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat's spinal cord injury. Brain Res. 2009;1256:149–161. doi: 10.1016/j.brainres.2008.11.098. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Waxman SG, Smith KJ. Remyelination of dorsal column axons by endogenous Schwann cells restores the normal pattern of Nav1.6 and Kv1.2 at nodes of Ranvier. Brain. 2006;129:1319–1329. doi: 10.1093/brain/awl057. [DOI] [PubMed] [Google Scholar]
- Bray GM, et al. The use of peripheral nerve grafts to enhance neuronal survival, promote growth and permit terminal reconnections in the central nervous system of adult rats. J Exp Biol. 1987;132:5–19. doi: 10.1242/jeb.132.1.5. [DOI] [PubMed] [Google Scholar]
- Burton H, Loewy AD. Descending projections from the marginal cell layer and other regions of the monkey spinal cord. Brain Res. 1976;116:485–491. doi: 10.1016/0006-8993(76)90495-9. [DOI] [PubMed] [Google Scholar]
- Chau CH, et al. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Chi GF, et al. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010;222:304–317. doi: 10.1016/j.expneurol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Corfas G, et al. Mechanisms and roles of axon-Schwann cell interactions. J Neurosci. 2004;24:9250–9260. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote MP, et al. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol. 2012;3:330. doi: 10.3389/fphys.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumans JV, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal neurons are sufficient for bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol. 2008;586:1623–1635. doi: 10.1113/jphysiol.2007.148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal transmission of the locomotor command signal in the neonatal rat. Ann N Y Acad Sci. 2010;1198:42–53. doi: 10.1111/j.1749-6632.2009.05421.x. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal regeneration after crush injury of rat central nervous system fibres innervating peripheral nerve grafts. J Neurocytol. 1985;14:1–12. doi: 10.1007/BF01150259. [DOI] [PubMed] [Google Scholar]
- Decherchi P, Gauthier P. Regrowth of acute and chronic injured spinal pathways within supra-lesional post-traumatic nerve grafts. Neuroscience. 2000;101:197–210. doi: 10.1016/s0306-4522(00)00343-2. [DOI] [PubMed] [Google Scholar]
- Deng LX, et al. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011;229:238–250. doi: 10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LX, et al. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33:5655–5667. doi: 10.1523/JNEUROSCI.2973-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio A, et al. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci. 2011;31:4569–4582. doi: 10.1523/JNEUROSCI.4638-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh P, et al. Transplantation of preconditioned Schwann cells following hemisection spinal cord injury. Spine (Phila Pa 1976) 2007;32:943–949. doi: 10.1097/01.brs.0000261408.61303.77. [DOI] [PubMed] [Google Scholar]
- Dolbeare D, Houle JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J Neurotrauma. 2003;20:1251–1261. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, Rose PK. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J Neurosci. 2009;29:12145–12158. doi: 10.1523/JNEUROSCI.0897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E, et al. Peripheral nerve autografts to the injured spinal cord of the rat: an experimental model for the study of spinal cord regeneration. Acta Neurochir (Wien) 1985;78:57–64. doi: 10.1007/BF01809242. [DOI] [PubMed] [Google Scholar]
- Flynn JR, et al. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Giovanelli Barilari M, Kuypers HG. Propriospinal fibers interconnecting the spinal enlargements in the cat. Brain Res. 1969;14:321–330. doi: 10.1016/0006-8993(69)90113-9. [DOI] [PubMed] [Google Scholar]
- Guest J, Santamaria AJ, Benavides FD. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant. 2013;18:682–689. doi: 10.1097/MOT.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest JD, et al. Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J Neurosci Res. 1997a;50:888–905. doi: 10.1002/(SICI)1097-4547(19971201)50:5<888::AID-JNR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Guest JD, et al. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol. 1997b;148:502–522. doi: 10.1006/exnr.1997.6693. [DOI] [PubMed] [Google Scholar]
- Hall S, Berry M. Electron microscopic study of the interaction of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J Neurocytol. 1989;18:171–184. doi: 10.1007/BF01206660. [DOI] [PubMed] [Google Scholar]
- Honmou O, et al. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat JC, et al. Formation of functional endplates by spinal axons regenerating through a peripheral nerve graft. A study in the adult rat. Brain Res Bull. 1989;22:103–114. doi: 10.1016/0361-9230(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Houle JD. Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve grafts. Exp Neurol. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- Houle JD, et al. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti C, et al. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379–393. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundberg A, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 3. Convergence on propriospinal neurones transmitting disynaptic excitation from the corticospinal tract and other descending tracts. Exp Brain Res. 1977;29:323–346. doi: 10.1007/BF00236174. [DOI] [PubMed] [Google Scholar]
- Isa T, et al. Properties of propriospinal neurons in the C3-C4 segments mediating disynaptic pyramidal excitation to forelimb motoneurons in the macaque monkey. J Neurophysiol. 2006;95:3674–3685. doi: 10.1152/jn.00103.2005. [DOI] [PubMed] [Google Scholar]
- Isa T, Kinoshita M, Nishimura Y. Role of Direct vs. Indirect Pathways from the Motor Cortex to Spinal Motoneurons in the Control of Hand Dexterity. Front Neurol. 2013;4:191. doi: 10.3389/fneur.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane JA, Evans JP, Fisher LE. An Investigation Concerning the Restitution of Motor Function Following Injury to the Spinal Cord. J Neurosurg. 1964;21:167–171. doi: 10.3171/jns.1964.21.3.0167. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J, Morin D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci. 2005;25:6025–6035. doi: 10.1523/JNEUROSCI.0696-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada T, et al. Transplantation of human bone marrow stromal cell-derived Schwann cells reduces cystic cavity and promotes functional recovery after contusion injury of adult rat spinal cord. Neuropathology. 2011;31:48–58. doi: 10.1111/j.1440-1789.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Kanno H, et al. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci. 2014;34:1838–1855. doi: 10.1523/JNEUROSCI.2661-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CC, Chang LW, Bloodworth JM., Jr Axonal regeneration across transected mammalian spinal cords: an electron microscopic study of delayed microsurgical nerve grafting. Exp Neurol. 1977;54:591–615. doi: 10.1016/0014-4886(77)90259-x. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Vasilenko DA. Propriospinal neurones as a relay system for transmission of cortico-spinal influences. J Physiol (Paris) 1978;74:247–250. [PubMed] [Google Scholar]
- Kostyuk PG, Vasilenko DA. Spinal interneurons. Annu Rev Physiol. 1979;41:115–126. doi: 10.1146/annurev.ph.41.030179.000555. [DOI] [PubMed] [Google Scholar]
- Kuo HS, et al. Acid fibroblast growth factor and peripheral nerve grafts regulate Th2 cytokine expression, macrophage activation, polyamine synthesis, and neurotrophin expression in transected rat spinal cords. J Neurosci. 2011;31:4137–4147. doi: 10.1523/JNEUROSCI.2592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, et al. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J Neurosci. 2013;33:10591–10606. doi: 10.1523/JNEUROSCI.1116-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AD, et al. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg. 2002;96:197–205. doi: 10.3171/spi.2002.96.2.0197. [DOI] [PubMed] [Google Scholar]
- Matsushita M. The axonal pathways of spinal neurons in the cat. J Comp Neurol. 1970;138:391–417. doi: 10.1002/cne.901380402. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ueyama T. Ventral motor nucleus of the cervical enlargement in some mammals; its specific afferents from the lower cord levels and cytoarchitecture. J Comp Neurol. 1973;150:33–52. doi: 10.1002/cne.901500103. [DOI] [PubMed] [Google Scholar]
- Menetrey D, de Pommery J, Roudier F. Propriospinal fibers reaching the lumbar enlargement in the rat. Neurosci Lett. 1985;58:257–261. doi: 10.1016/0304-3940(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Miller S, Reitsma DJ, van der Meche FG. Functional organization of long ascending propriospinal pathways linking lumbo-sacral and cervical segments in the cat. Brain Res. 1973;62:169–188. doi: 10.1016/0006-8993(73)90626-4. [DOI] [PubMed] [Google Scholar]
- Moissonnier P, et al. Motoneurons of the injured spinal cord of the adult dog can grow lengthy axons into an autologous peripheral nerve graft. A retrograde axonal tracing study. Spinal Cord. 1996;34:320–325. doi: 10.1038/sc.1996.59. [DOI] [PubMed] [Google Scholar]
- Molenaar I, Kuypers HG. Cells of origin of propriospinal fibers and of fibers ascending to supraspinal levels. A HRP study in cat and rhesus monkey. Brain Res. 1978;152:429–450. doi: 10.1016/0006-8993(78)91102-2. [DOI] [PubMed] [Google Scholar]
- Murray M, Fischer I. Transplantation and gene therapy: combined approaches for repair of spinal cord injury. Neuroscientist. 2001;7:28–41. doi: 10.1177/107385840100700107. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Morichika Y, Isa T. A subcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Brain. 2009;132:709–721. doi: 10.1093/brain/awn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, et al. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- Oudega M, Moon LD, de Almeida Leme RJ. Schwann cells for spinal cord repair. Braz J Med Biol Res. 2005;38:825–835. doi: 10.1590/s0100-879x2005000600003. [DOI] [PubMed] [Google Scholar]
- Paino CL, et al. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J Neurocytol. 1994;23:433–452. doi: 10.1007/BF01207115. [DOI] [PubMed] [Google Scholar]
- Papastefanaki F, et al. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain. 2007;130:2159–2174. doi: 10.1093/brain/awm155. [DOI] [PubMed] [Google Scholar]
- Park HW, et al. Human mesenchymal stem cell-derived Schwann cell-like cells exhibit neurotrophic effects, via distinct growth factor production, in a model of spinal cord injury. Glia. 2010;58:1118–1132. doi: 10.1002/glia.20992. [DOI] [PubMed] [Google Scholar]
- Petko M, Antal M. Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord. J Comp Neurol. 2000;422:312–325. doi: 10.1002/(sici)1096-9861(20000626)422:2<312::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Pinzon A, et al. Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat thoracic spinal cord. J Neurosci Res. 2001;64:533–541. doi: 10.1002/jnr.1105. [DOI] [PubMed] [Google Scholar]
- Rasouli A, et al. Transplantation of preconditioned schwann cells in peripheral nerve grafts after contusion in the adult spinal cord. Improvement of recovery in a rat model. J Bone Joint Surg Am. 2006;88:2400–2410. doi: 10.2106/JBJS.E.01424. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982;237:147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Aguayo AJ. Regeneration of long spinal axons in the rat. J Neurocytol. 1984;13:165–182. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- Robbins A, Pfaff DW, Schwartz-Giblin S. Reticulospinal and reticuloreticular pathways for activating the lumbar back muscles in the rat. Exp Brain Res. 1992;92:46–58. doi: 10.1007/BF00230382. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951;94:313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Salame CG, Dum RP. Central nervous system axonal regeneration into sciatic nerve grafts: physiological properties of the grafts and histologic findings in the neuraxis. Exp Neurol. 1985;90:322–340. doi: 10.1016/0014-4886(85)90022-6. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, et al. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake K, et al. Up-regulation of glial cell line-derived neurotrophic factor (GDNF) following traumatic spinal cord injury. Neuroreport. 2000;11:3877–3881. doi: 10.1097/00001756-200011270-00054. [DOI] [PubMed] [Google Scholar]
- Saywell SA, et al. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol. 2011;105:806–826. doi: 10.1152/jn.00738.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ME. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Middleton FA, Stelzner DJ. Long descending cervical propriospinal neurons differ from thoracic propriospinal neurons in response to low thoracic spinal injury. BMC Neurosci. 2010;11:148. doi: 10.1186/1471-2202-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner RD, et al. Cells of origin of long descending propriospinal fibers connecting the spinal enlargements in cat and monkey determined by horseradish peroxidase and electrophysiological techniques. J Comp Neurol. 1979;188:443–454. doi: 10.1002/cne.901880307. [DOI] [PubMed] [Google Scholar]
- Someya Y, et al. Reduction of cystic cavity, promotion of axonal regeneration and sparing, and functional recovery with transplanted bone marrow stromal cell-derived Schwann cells after contusion injury to the adult rat spinal cord. J Neurosurg Spine. 2008;9:600–610. doi: 10.3171/SPI.2008.9.08135. [DOI] [PubMed] [Google Scholar]
- Sprague JM. A study of motor cell localization in the spinal cord of the rhesus monkey. Am J Anat. 1948;82:1–26. doi: 10.1002/aja.1000820102. [DOI] [PubMed] [Google Scholar]
- Sterling P, Kuypers HG. Anatomical organization of the brachial spinal cord of the cat. 3. The propriospinal connections. Brain Res. 1968;7:419–443. doi: 10.1016/0006-8993(68)90008-5. [DOI] [PubMed] [Google Scholar]
- Steward O, et al. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J Neurosci. 2008;28:6836–6847. doi: 10.1523/JNEUROSCI.5372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer PD, Dolbeare D, Houle JD. Treatment of chronically injured spinal cord with neurotrophic factors stimulates betaII-tubulin and GAP-43 expression in rubrospinal tract neurons. J Neurosci Res. 2003;74:502–511. doi: 10.1002/jnr.10787. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentagothai J. Propriospinal Pathways and Their Synapses. Prog Brain Res. 1964;11:155–177. [PubMed] [Google Scholar]
- Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6:276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Houle JD. Intraspinal microinjection of chondroitinase ABC following injury promotes axonal regeneration out of a peripheral nerve graft bridge. Exp Neurol. 2008;211:315–319. doi: 10.1016/j.expneurol.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, et al. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, et al. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol. 2013;239:91–100. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, et al. Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 1998;7:187–196. doi: 10.1177/096368979800700213. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrek R, et al. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Verburgh CA, Kuypers HG. Branching neurons in the cervical spinal cord: a retrograde fluorescent double-labeling study in the rat. Exp Brain Res. 1987;68:565–578. doi: 10.1007/BF00249799. [DOI] [PubMed] [Google Scholar]
- Wardrope J, Wilson DH. Peripheral nerve grafting in the spinal cord: a histological and electrophysiological study. Paraplegia. 1986;24:370–378. doi: 10.1038/sc.1986.55. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, et al. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiliams RR, Bunge MB. Schwann cell transplantation: a repair strategy for spinal cord injury? Prog Brain Res. 2012;201:295–312. doi: 10.1016/B978-0-444-59544-7.00014-7. [DOI] [PubMed] [Google Scholar]
- Williams RR, et al. Permissive Schwann Cell Graft/Spinal Cord Interfaces for Axon Regeneration. Cell Transplant. 2013 doi: 10.3727/096368913X674657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, et al. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995a;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Xu XM, et al. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995b;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Xu XM, et al. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu XM, et al. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Yan-Wu G, et al. Human umbilical cord-derived Schwann-like cell transplantation combined with neurotrophin-3 administration in dyskinesia of rats with spinal cord injury. Neurochem Res. 2011;36:783–792. doi: 10.1007/s11064-011-0402-9. [DOI] [PubMed] [Google Scholar]
- Yick LW, et al. Peripheral nerve graft and neurotrophic factors enhance neuronal survival and expression of nitric oxide synthase in Clarke's nucleus after hemisection of the spinal cord in adult rat. Exp Neurol. 1999;159:131–138. doi: 10.1006/exnr.1999.7134. [DOI] [PubMed] [Google Scholar]
- Zeng BY, et al. Regenerative and other responses to injury in the retinal stump of the optic nerve in adult albino rats: transection of the intraorbital optic nerve. J Anat. 1994;185(Pt 3):643–661. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]