Abstract
Cortical and thalamocortical activity is highly state dependent, varying between patterns of activity that are conducive to accurate sensory-motor processing, to states in which the brain is largely off-line and generating internal rhythms irrespective of the outside world. The generation of rhythmic activity occurs through the interaction of stereotyped patterns of connectivity together with intrinsic membrane and synaptic properties. One common theme in the generation of rhythms is the interaction of a positive feedback loop (e.g. recurrent excitation) with negative feedback control (e.g. inhibition, adaptation, or synaptic depression). The operation of these state-dependent activities has wide ranging effects from enhancing or blocking sensory-motor processing to the generation of pathological rhythms associated with psychiatric or neurological disorders.
Introduction
The forebrain is a network of coupled oscillators - even repetitive action potential generation is a type of oscillation. The high degree of interconnectivity between cortical neurons and between the cortex and thalamus, together with intrinsic membrane and synaptic properties, gives rise to a number of state-dependent network oscillations[1-3]. Currently we understand well the mechanisms of generation of three of these oscillations: slow, spindle, and gamma waves. Slow and spindle waves occur largely during slow-wave sleep, while gamma waves are present throughout brain states, but are most prominent in the alert and attentive animal. Reviewing the cellular and network mechanisms of these rhythms is instructive, pointing us towards the possible basis for network activity that is not yet well understood. Interestingly, all of these rhythms depend upon an excitatory or activating component (e.g. recurrent excitation, inward currents) interacting with an inhibitory or refractory component (e.g. return inhibition or adaptation). The unique properties of these network oscillations arise in part from the time it takes to complete one cycle, to the subtypes of neuron involved and their density of involvement, to the pattern of propagation and synchronization.
Slow Wave Sleep Activity
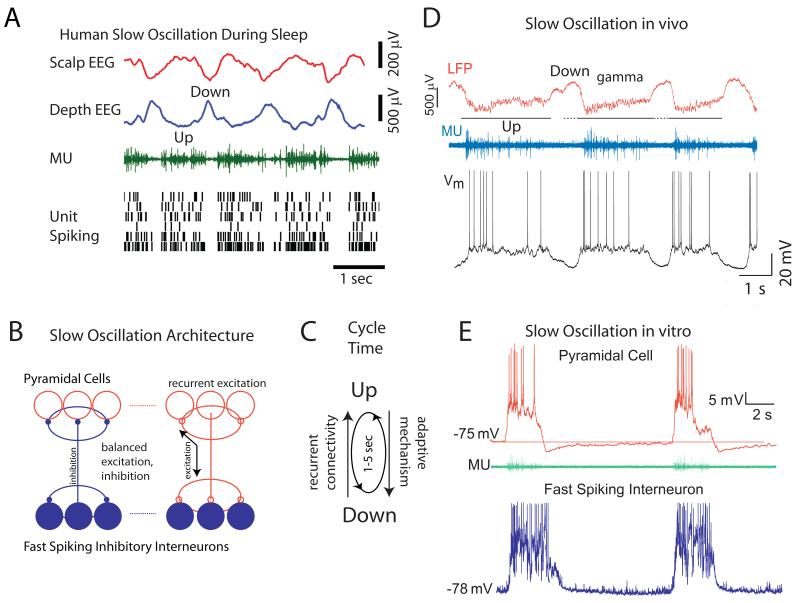
A fundamental characteristic of slow wave sleep is the presence of slow (0.5-4 Hz) rhythms in the EEG [1]. Intracellular recordings from cortical neurons revealed that a major generator of these slow rhythms is the so-called cortical slow oscillation[3-5]. The slow oscillation is characterized by alternating sequences of Up and Down states, generated within the cortex, but which are influenced by, and distributed to, subcortical structures such as the thalamus, basal ganglia, brainstem, and cerebellum[2-4, 6, 7]. The Up state of the slow oscillation results from intracortical recurrent excitation that is roughly balanced with recurrent local inhibition [8, 9]. The transition from the Down to Up state occurs when a strong enough (but not too strong) excitatory volley, either spontaneous or driven, enters into a local cortical network whose refractory mechanism has recovered sufficiently from the occurrence of the last Up state[8, 10, 11]. The subsequent activation of excitatory neurons results in an amplification that initiates even more excitatory neurons to discharge, in a positive feedback loop. This recurrent excitation not only activates excitatory cortical neurons, but also local inhibitory interneurons, particularly fast spiking cells[12], subsequently dampening and controlling the amplitude and spatial spread of the recurrent excitation. Since both the degree to which cortical excitatory and inhibitory neurons are excited depends upon the amplitude of the recurrent excitatory signal, the two increase and decrease together, resulting in a proportionality or “balance”[9, 11]. This balance, however, is only on average and moment to moment fluctuations in the dominance of excitation or inhibition cause rapid fluctuations in the membrane potential, typically in the gamma frequency range (Fig. 2C), and the initiation of action potentials (see Figs. 1A, 2). During the generation of the Up state, refractory mechanisms build up, such as the activation of Ca2+ and Na+ dependent K+ conductances in pyramidal cells[8, 10], synaptic depression[13], and perhaps even metabolic changes[14]. Owing to the buildup of refractory mechanisms, the recurrent networks become less able to maintain activity, and the network eventually and suddenly fails, resulting in a rapid transition to the Down state (Figs. 1A, 2).
Figure 2.
Network mechanisms mediating the generation of the slow oscillation. A. Slow oscillation is prevalent in the human neocortex during sleep. Local field and multiple unit recordings from implanted electrodes in the human cortex reveals Down states to be associated with cessation of network activity, while Up states are mediated by the persistent discharge of cortical networks. B. Schematic of the basic network architecture underlying the generation of slow oscillation. Fast spiking inhibitory interneurons contact one another and local pyramidal cells, while pyramidal cells contact both each other and local inhibitory interneurons. This architecture insures that inhibition is roughly proportional to excitation in the local network, promoting the generation of persistent activity. C. The presence of an adaptive mechanism (e.g. spike frequency adaptation; synaptic depression) results in “flips” between periods of activity (Up) and inactivity (Down), approximately once every second. D. Simultaneous recording of the local field potential, multiple unit activity, and intracellular synaptic/action potentials in a pyramidal cell during the generation of the slow oscillation in vivo. E. Whole cell recordings from a pyramidal cell and a fast spiking interneuron during the generation of the slow oscillation in vitro. A from [16]; C from [9]; D from [12].
Figure 1.
State dependent activity in cortical and thalamocortical networks. A. Slow wave sleep is associated with the generation of Up and Down states of the slow oscillation and spindle waves. The transition to waking is associated with an abolition of these network oscillations, the loss of the Down state of the slow oscillation, and the increased prevalence of rhythmic activity in the gamma frequency range. B. Recent recordings in head-fixed mice differ from the recordings in cats (A), and demonstrate the presence of slow oscillatory activity during quiet resting without movement. Walking on a cylinder results in a suppression of the slow rhythmic activity. Cessation of walking results in the return of the slow rhythmic activity. Recording was obtained from a putative fast spiking (parvalbumin positive) interneuron in the primary visual cortex. C. Schematic diagram of basic thalamocortical circuit for the generation of rhythmic activities. The slow oscillation is generated within the cortex as a relatively balanced recurrent interaction of excitatory and inhibitory neurons. Gamma frequency oscillations are also generated within the cortex, as an interaction of excitatory and inhibitory neurons. Spindle waves are generated during sleep in the thalamus as an interaction of thalamic reticular GABAergic neurons and thalamocortical relay cells. These rhythms interact with one and another, owing to the interconnected nature of the forebrain. Networks of inhibitory interneurons and intracortical connections are important for dynamic control of cortical state and oscillations[69-71]. A is from [5]; B is from [38].
Even very small (0.5 × 0.5 mm) regions of the neocortex can generate the slow oscillation, and layer 5 appears to have the lowest threshold in most cortical regions[8], although layers 2/3 may also initiate this rhythm in some cortical areas or circumstances[12, 15]. While the slow oscillation was once thought to be restricted to periods of slow wave sleep, animal studies now suggest that it may occur in the waking state, particularly during periods of inattentiveness or drowsiness (Fig. 1B). Down states may occur in local cortical regions[16, 17], and presumably represent brief periods of disrupted processing in that cortical area. Indeed, the density of Down states, or slow waves, in cortical activity increases with time awake, such that there is a peak of such activity at the beginning of slow wave sleep, and a subsequent slow dissipation of slow waves and Down states with sleep[18]. Since the slow oscillation can initiate anywhere in the cortex, it may occur either very locally, or rapidly propagate throughout the cortical network [8, 10, 16, 19-21], depending in large part on state. During deep slow wave sleep, local cortical networks may exhibit broad synchrony through recurrent connections which allow the transitions between Up and Down states to occur rapidly and nearly synchronously in distributed cortical networks that are interconnected by long range axons[22]. In other circumstances, such as during drowsiness or less deep sleep, Down states may be more local, and lack broad synchrony across the cortex[16, 17]. Recent investigations have revealed that the thalamus also contributes to the initiation and frequency of occurrence of slow waves in the cortex[23-26]. Removal or inactivation of the thalamus results in a dramatic, but temporary, decrease in Up state occurrence in the cortex, and the thalamus may have its own mechanisms for generating low frequency oscillations.[27]
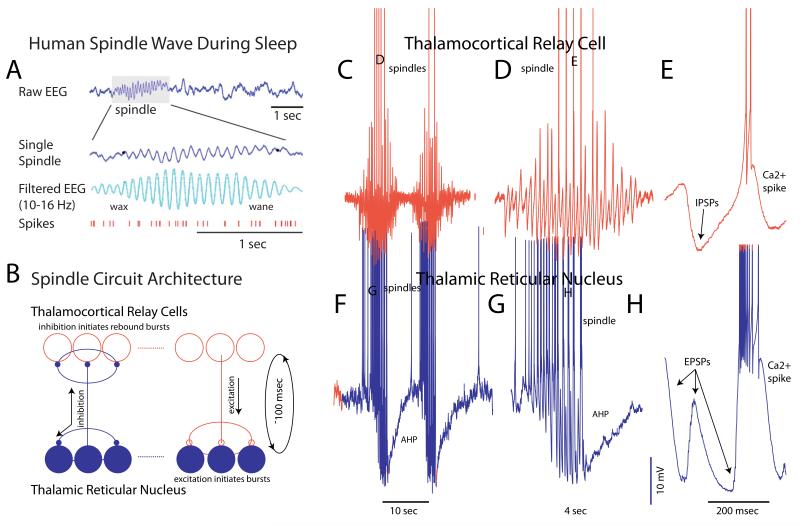
In addition to the slow oscillation, the cellular mechanisms of generation of sleep spindles are also well understood. Andersen and Andersson, through a series of seminal studies, revealed that this sleep-related oscillation was generated through a circuitous interaction between inhibitory and excitatory neurons in the thalamus[28]. Subsequent in vivo and in vitro studies expanded upon these findings, revealing great detail about this slow-wave sleep rhythm[3, 29]. Spindle waves appear as a waxing and waning 7-16 Hz oscillation in the human (and other mammals) EEG during slow wave sleep. They are generated as a circuitous interaction between the GABAergic neurons of the thalamic reticular nucleus (nRt) and thalamocortical relay cells (Fig. 3) [29-31]. Essentially, activation of thalamic reticular cells, either by excitation from the cortex, thalamus, or prethalamic structures, results in the activation of GABAA-receptor mediated hyperpolarization and inhibition of thalamocortical neurons (Fig. 3). In the slow-wave sleep state, these thalamocortical neurons are hyperpolarized, in part owing to the withdrawal of neuromodulatory transmitters[29]. The depolarizing ending phase of IPSP can initiate a low threshold Ca2+ spike when thalamocortical neurons are in this state, and thus initiate a burst of action potentials (although it is not clear if these Ca2+ spike-mediated bursts are absolutely essential for the generation of spindles; [32]). Since thalamocortical neurons excite nRt neurons, these GABAergic cells are once again activated, resulting in the initiation of the next cycle of the spindle wave. The spindle wave waxes as the oscillation gains strength from the increased participation of neurons in the oscillation, as synaptic barrages in both nRt and thalamocortical cells become larger and larger (Fig. 3), and the oscillation propagates throughout the thalamocortical network by recruiting connected cells into the oscillation[33]. The waning of the oscillation likely involves multiple mechanisms, including hyperpolarization of nRt neurons (Fig. 3F, G) which, in later stages of the spindle wave, reduces their responsiveness to incoming barrages of EPSPs[34], depolarization of thalamocortical cells owing to the activation of a Ca2+ sensitive adenylate cyclase and the subsequent activation of the h-current[35], synaptic depression owing to repetitive activation of synaptic connections between nRt and thalamocortical relay cells, and desynchronization[36]. Recent in vivo investigations of the waning of spindle waves supports hyperpolarization of nRt neurons as a primary mechanism[37], as opposed to desynchronization of thalamocortical networks.
Figure 3.
Network mechanisms underlying the generation of spindle waves. A. Spindle waves occur during slow wave sleep in the human EEG and appear as a waxing and waning 10-16 Hz rhythm. The activity of cortical pyramidal cells are mildly modulated by the occurrence of spindle waves. B. Circuit architecture underlying the generation of spindle waves. Thalamic reticular neurons, which are GABAergic, innervate each other and thalamocortical relay cells. Thalamocortical relay cells do not innervate each other, but do innervate thalamic reticular neurons. The ability to generate low threshold Ca2+ spikes in both of these cell types promotes the generation of spindle waves (see C-H). The cycle time between a burst of activity in the thalamic reticular nucleus to a rebound burst of spikes in the connected thalamocortical relay cells, and the subsequent re-excitation of the thalamic reticular neurons, is about 100 msec, and thus this rhythm favors frequencies around 10 Hz. C-E. Intracellular recordings of spindle waves in a thalamocortical relay neuron revealing the waxing and waning of IPSPs arriving from activity in the thalamic reticular nucleus. Some of these IPSPs result in the generation of a rebound low threshold Ca2+ spike and the initiation of a burst of action potentials. F-H. Intracellular recordings of spindle waves in a thalamic reticular neuron revealing how the arrival of barrages of EPSPs from bursts of spikes in thalamocortical relay cells initiates low threshold Ca2+ spikes and bursts of action potentials, thus renewing the next cycle of the oscillation. Note the prominent hyperpolarization in thalamic reticular neurons during and following each spindle wave. This hyperpolarization is responsible, in part, for the waxing and waning of spindles. A from [72]; C-H from [30, 31, 73].
Waking Activity
Traditionally, the waking state has been associated with an “activated” EEG, meaning a suppression of slow (< 4 Hz) rhythmic activity, and an increased prevalence, either in absolute or relative terms, of higher frequencies, particularly in the gamma-frequency range (30-80 Hz)[5]. Recent intracellular recordings in waking mice have complicated this view, suggesting that rhythmic activity at 3-5 Hz can occur in the neocortex of head-fixed and stationary mice. This oscillatory activity is strongly suppressed by movement, such as walking or whisking[38-40] (Fig. 1B).
One important question that arises from these studies is: Why is there slow rhythmic activity in the waking, stationary mouse? One key variable that has not been accurately addressed in these studies is the precise state of the animal. In our recordings from stationary awake mice we find that cortical state varies constantly, ranging from slow oscillatory to activated [41], and correlates with task engagement (McGinley, Zagha, McCormick unpublished observations), suggesting that mice may have a strong propensity to exhibit slow oscillatory activity in cortical networks during periods of non-task engagement.
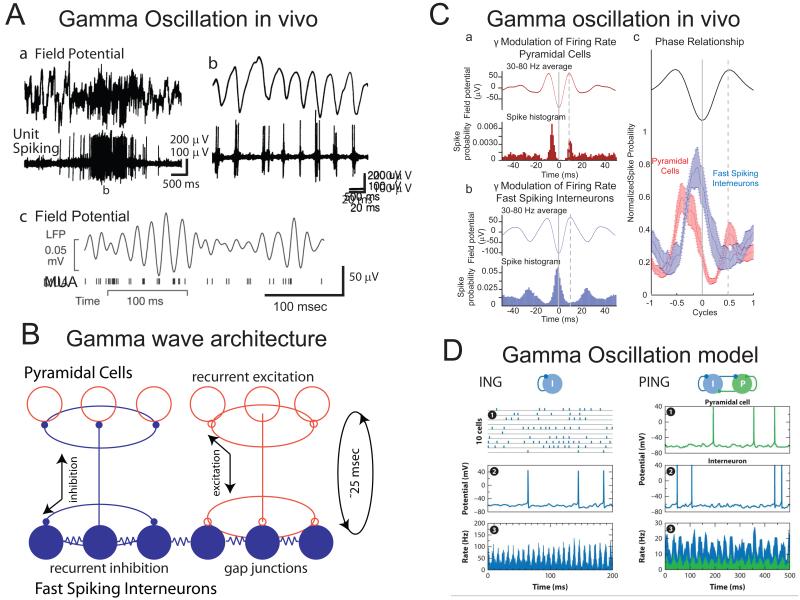
Besides the suppression of slow rhythmic activity, the attentive or active waking state is also associated with an increased prevalence of activity in the gamma (30-80 Hz) range[1, 42]. Over the past decade the two distinct cellular mechanisms for generation of synchronized activity in this frequency range have been put forward[42]: 1) an interaction between interneurons (ING, INterneuron Gamma); or 2) an interaction between interneurons and excitatory principle cells (PING, Principle-INterneuron Gamma). When both of these mechanisms are involved, it is known as PINGING (Fig. 4D). It is instructive to consider a few experiments in rat hippocampal slices, which provide evidence for these mechanisms. The ING mechanism occurs when interneurons are activated either with strong afferent stimulation or through the addition of pharmacological agents[43, 44]. Whole-cell recordings from pyramidal cells up to 1 mm apart reveal synchronized and rhythmic ~40 Hz IPSPs. This rhythmic inhibition is blocked by GABAA-R antagonists but not AMPA-R or NMDA-R antagonists, suggesting that the inhibitory population can generate a 40 Hz oscillation with tonic activation. The basis of the oscillation is spiking followed by GABAA-mediated inhibition and a spike afterhyperpolarization, both of which have durations of approximately 25 msec, leading to a propensity to oscillate at approximately 40 Hz. Coupling between neurons, mainly through synaptic connections, but also perhaps through gap junctions, forms a synchronizing mechanism[42, 43].
Figure 4.
Recurrent interactions of excitatory and inhibitory cortical neurons generates higher frequency (gamma) oscillations. A. Gamma oscillations in the cortical field potential are associated with modulating firing rates in cortical neurons. Aa-Ab were recorded from the anesthetized cat primary visual cortex during the presentation of an optimal stimulus. Note the rhythmic discharge of cortical neurons in relation to the local gamma oscillation. Ac. Example of gamma oscillation in the field potential and multiple unit activity in the visual cortex of an awake, behaving primate during an attention task. B. Neuronal architecture for generation of gamma oscillations. Fast spiking neurons, which are interconnected by gap junctions, innervate one another and local pyramidal cells. Local pyramidal cells supply recurrent excitation through their local connections to each other and interneurons. The cycle time from discharge of fast spiking interneurons, to recovery from inhibition and discharge of pyramidal cells (which drives the next cycle) is approximately 25 msec, thus resulting in a 40 Hz rhythm. C. The timing of discharge of pyramidal and fast spiking interneurons supports a PING or PINGING model of gamma generation. Pyramidal cells discharge, on average, just prior to the discharge of fast spiking interneurons. The average discharge rate of pyramidal cells can be an order of magnitude weaker than fast spiking interneurons, since there are far more of the former than the later. D. Models of gamma-oscillation generation that rely upon either the ING (interneuron-interneuron interaction) mechanism or PING (pyramidal-interneuron interaction) mechanism. The feature that distinguishes these two models is that in PING, interneuron firing is dependent upon pyramidal cell activity in a phasic manner, such that pyramidal cells fire just prior to interneurons, on average. Aa, Ab from [74]; Ac from [75]; C from [47]; D from [42].
The PING mechanism has been studied in hippocampal slices that are activated with muscarinic agonists[45]. As opposed to the ING mechanism, this oscillation is blocked by both GABAA-R and AMPA-R antagonists, indicating that a gamma oscillation can arise from an interaction between excitatory and inhibitory neurons. Here, the cycle time between activation of an inhibitory neuron by EPSPs, to inhibitory phasing of activity in the excitatory neuron by IPSPs, forms the basis of the gamma oscillation (Fig. 4).
The main difference between PING and ING is how inhibitory interneurons are driven to spike and consequently the phase relationship between pyramidal and interneuronal firing[46]. In PING, interneurons are phasically excited on a cycle-by-cycle basis by the local principle cell population, and then provide rapid feedback inhibition. The cycle renews when positive feedback in the recurrently-connected principle cells excites the interneurons again. In contrast, ING posits that interneurons are excited by a general depolarization owing to the release of neuromodulators or glutamatergic inputs in a non-phasic manner. Both PING and ING rely on the inhibitory population to provide synchronized inhibition to the principle cells.
In the PING mechanism, principle cells are the source of excitation for interneurons, so they should fire slightly before interneurons in the gamma cycle. This has been reported in several studies in vivo (Fig. 4) in either anesthetized or awake, behaving animals[47-49]. Although there is abundant evidence for the involvement of fast-spiking interneurons in the generation of higher frequency oscillations[1, 47, 50], the involvement of other types of interneurons is as of yet largely unknown. Intracellular recordings from other subtypes of interneurons (e.g. SOM, NPY, VIP, 5HT3a) in vitro during the generation of Up states, a period when gamma oscillations are prevalent[21, 51, 52], have revealed a relative lack of participation of these interneuron subtypes in this oscillation[12], suggesting that the fast spiking subtype of interneuron is the workhorse of higher frequency oscillation generation. The activity of different subtypes of interneurons is, however, state dependent[53] and it remains to be determined if fast spiking interneurons dominant in gamma generation in all conditions. In cats, ferrets, and monkeys, a special subpopulation of pyramidal neurons named “chattering” cells intrinsically oscillates in the gamma frequency range, generating high frequency bursts that are synchronized with higher frequency network oscillations[54, 55]. While this special subpopulation of excitatory neurons may contribute to the generation of gamma oscillations, they have not yet been observed in rodents, suggesting that their contribution is not essential.
Rhythms Interact in the Healthy and Disordered Brain
The forebrain represents a system of coupled oscillators, resulting in the generation of interacting rhythms. For example, the transition from a Down to Up state can trigger a spindle wave, resulting in the appearance of a “K-complex” in the EEG during sleep[56, 57]. Multiple types of rhythms can become nested, where the prevalence and phase of each rhythm influences that of the other, even in distant structures, such as between the neocortex, paleocortex, and hippocampus [1, 2, 58-61]. Disease states that result in the loss of appropriate interactions between cell types and structures can also result in a disorganization of these rhythms, either in their prevalence, synchrony, frequency, or distribution[62-65]. Epilepsy, for example, appears as a state in which normal forebrain oscillations are exaggerated to such an extent that they become parasitic, preventing normal brain function, and thus of clinical importance[66-68]. Understanding the nature of network oscillations and their pathological counterparts will lead to a more complete understanding of forebrain function and dysfunction.
Highlights.
Cortical and thalamocortical activity are characterized by multiple states that strongly influence sensory processing and behavior
During slow wave sleep, cortical networks generate the slow oscillation while the thalamus may generate spindle waves and the interaction of the two can generate K-complexes
During active waking, slow rhythmic activity is largely abolished and higher frequency rhythms, such as gamma waves, are prevalent
During quiescent waking and immobility, rodents can exhibit prominent oscillatory activity in cortical and thalamocortical networks. These oscillations are associated with decreased performance on detection tasks
Different patterns of rhythmic activity interact to generate complex nested rhythms
Acknowledgements
Supported by NIH 5R01N2026143 (DAM) and 1F132DC012449 (MM) and the Kavli Institute of Neuroscience at Yale.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Buzsaki G. Rhythms of the Brain. Oxford University Press; New York, NY: 2006. [Google Scholar]
- 2.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137(4):1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 4.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12(12):4701–11. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85(5):1969–85. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 6.Ros H, et al. Neocortical networks entrain neuronal circuits in cerebellar cortex. J Neurosci. 2009;29(33):10309–20. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77(4):1697–715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3(10):1027–34. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 9.Haider B, et al. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26(17):4535–45. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Compte A, et al. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89(5):2707–25. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- 11.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423(6937):288–93. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 12**.Tahvildari B, et al. Selective functional interactions between excitatory and inhibitory cortical neurons and differential contribution to persistent activity of the slow oscillation. J Neurosci. 2012;32(35):12165–79. doi: 10.1523/JNEUROSCI.1181-12.2012. This study demonstrated that different subtypes of interneurons exhibit dramatically different patterns of excitatory and inhibitory synaptic input during the generation of the Up state. Fast spiking interneurons were the only interneurons consistently and strongly involved in the persistent activity of the Up state in vitro.
- 13.Bazhenov M, et al. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J Neurosci. 2002;22(19):8691–704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham MO, et al. Neuronal metabolism governs cortical network response state. Proc Natl Acad Sci U S A. 2006;103(14):5597–601. doi: 10.1073/pnas.0600604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csercsa R, et al. Laminar analysis of slow wave activity in humans. Brain. 2010;133(9):2814–29. doi: 10.1093/brain/awq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–69. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyazovskiy VV, et al. Local sleep in awake rats. Nature. 2011;472(7344):443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curie T, et al. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36(3):311–23. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheroziya M, Timofeev I. Global intracellular slow-wave dynamics of thez thalamocortical system. J Neurosci. 2014;34(26):8875–93. doi: 10.1523/JNEUROSCI.4460-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–70. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Mejias M, et al. Slow and fast rhythms generated in the cerebral cortex of the anesthetized mouse. J Neurophysiol. 2011;106(6):2910–21. doi: 10.1152/jn.00440.2011. [DOI] [PubMed] [Google Scholar]
- 22.Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (< 1 Hz) cortical oscillation. J Neurophysiol. 1995;73(1):20–38. doi: 10.1152/jn.1995.73.1.20. [DOI] [PubMed] [Google Scholar]
- 23*.David F, et al. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33(50):19599–610. doi: 10.1523/JNEUROSCI.3169-13.2013. This study demonstrates that the thalamus contributes an important component to the rate or recurrent of the Up state of the slow oscillation in the cortex.
- 24**.Poulet JF, et al. Thalamic control of cortical states. Nat Neurosci. 2012;15(3):370–2. doi: 10.1038/nn.3035. Activation of the thalamus can result in activation of the cortex, supporting a role of the thalamus in cortical activation.
- 25*.Lemieux M, et al. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 2014;34(16):5689–703. doi: 10.1523/JNEUROSCI.1156-13.2014. Lesions of the thalamus result in marked, but temporary, alterations in the generation of slow oscillatory activity in the cortex, and more persistent changes in higher frequency activity and synchronization. These results support an important role of the thalamus in cortical activities.
- 26.Hughes S, Crunelli V. UP states rise from the depths. Nat Neurosci. 2013;16(2):115–7. doi: 10.1038/nn.3313. [DOI] [PubMed] [Google Scholar]
- 27.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13(1):9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen P.a.A., S. A. Physiological Basis of the Alpha Rhythm. Appleton, Century, Crofts; 1968. p. 235. [Google Scholar]
- 29.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 30.Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol. 1995;483(Pt 3):665–85. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483(Pt 3):641–63. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, et al. Sleep spindles are generated in the absence of T-type calcium channel-mediated low-threshold burst firing of thalamocortical neurons. Proc Natl Acad Sci U S A. 2013;110(50):20266–71. doi: 10.1073/pnas.1320572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim U, Bal T, McCormick DA. Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J Neurophysiol. 1995;74(3):1301–23. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- 34.Kim U, McCormick DA. Functional and ionic properties of a slow afterhyperpolarization in ferret perigeniculate neurons in vitro. J Neurophysiol. 1998;80(3):1222–35. doi: 10.1152/jn.1998.80.3.1222. [DOI] [PubMed] [Google Scholar]
- 35.Luthi A, McCormick DA. Ca(2+)-mediated up-regulation of Ih in the thalamus. How cell-intrinsic ionic currents may shape network activity. Ann N Y Acad Sci. 1999;868:765–9. doi: 10.1111/j.1749-6632.1999.tb11354.x. [DOI] [PubMed] [Google Scholar]
- 36.Bonjean M, et al. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31(25):9124–34. doi: 10.1523/JNEUROSCI.0077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Bartho P, et al. Ongoing network state controls the length of sleep spindles via inhibitory activity. Neuron. 2014;82(6):1367–79. doi: 10.1016/j.neuron.2014.04.046. Investigation of the mechanisms of termination of spindle waves during natural sleep concludes that hyperpolarization of thalamic reticular cells is likely a key component.
- 38.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80(2):350–7. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9(5):608–10. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 40**.Polack PO, Friedman J, Golshani P. Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci. 2013;16(9):1331–9. doi: 10.1038/nn.3464. Walking results in activation of visual cortex in mice. This activation is associated with the suppression of slow oscillatory activity and a small depolarization of pyramidal cells, perhaps through the release of norepinephrine.
- 41**.Zagha E, et al. Motor cortex feedback influences sensory processing by modulating network state. Neuron. 2013;79(3):567–78. doi: 10.1016/j.neuron.2013.06.008. Feedback from the motor cortex can strongly and rapidly control the state of somatosensory cortex, suggesting that corticocortical feedback pathways are important for cortical activation and arousal.
- 42.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jefferys JG, Traub RD, Whittington MA. Neuronal networks for induced ‘40 Hz’ rhythms. Trends Neurosci. 1996;19(5):202–8. doi: 10.1016/s0166-2236(96)10023-0. [DOI] [PubMed] [Google Scholar]
- 44.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373(6515):612–5. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 45.Fisahn A, et al. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394(6689):186–9. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 46.Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63(6):727–32. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasenstaub A, et al. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47(3):423–35. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Vinck M, et al. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron. 2013;80(4):1077–89. doi: 10.1016/j.neuron.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csicsvari J, et al. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37(2):311–22. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 50.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Compte A, et al. Spontaneous high-frequency (10-80 Hz) oscillations during up states in the cerebral cortex in vitro. J Neurosci. 2008;28(51):13828–44. doi: 10.1523/JNEUROSCI.2684-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Destexhe A, et al. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30(7):334–42. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentet LJ, et al. Membrane potential dynamics of GABAergic neurons in the barrel cortex of behaving mice. Neuron. 2010;65(3):422–35. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274(5284):109–13. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 55.Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci. 2000;20(13):4829–43. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cash SS, et al. The human K-complex represents an isolated cortical down-state. Science. 2009;324(5930):1084–7. doi: 10.1126/science.1169626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82(3):671–86. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 58.Isomura Y, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52(5):871–82. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 59.Scheffzuk C, et al. Selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice. PLoS One. 2011;6(12):e28489. doi: 10.1371/journal.pone.0028489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30(7):350–6. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14(11):506–15. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrarelli F, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 63.Ferrarelli F, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–48. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandal MJ, et al. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62(3):1504–18. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75(6):963–80. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62(5):612–32. doi: 10.1016/j.neuron.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kostopoulos GK. Spike-and-wave discharges of absence seizures as a transformation of sleep spindles: the continuing development of a hypothesis. Clin Neurophysiol. 2000;111(Suppl 2):S27–38. doi: 10.1016/s1388-2457(00)00399-0. [DOI] [PubMed] [Google Scholar]
- 68.Leresche N, et al. From sleep spindles of natural sleep to spike and wave discharges of typical absence seizures: is the hypothesis still valid? Pflugers Arch. 2012;463(1):201–12. doi: 10.1007/s00424-011-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69**.Fu Y, et al. A cortical circuit for gain control by behavioral state. Cell. 2014;156(6):1139–52. doi: 10.1016/j.cell.2014.01.050. The activation of VIP interneurons in layer 1 by movement and arousal may inhibit other interneurons that inhibit the apical dendrite of pyramidal cells, thus leading to disinhibition of pyramidal cells and an increase in the effectiveness of their excitatory dendritic inputs.
- 70.Lee S, et al. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16(11):1662–70. doi: 10.1038/nn.3544. This study identified a circuit by which motor cortex inputs disinhibit the apical dendrites of pyramidal neurons in primary somatosensory cortex. Specifically, motor cortex neurons preferentially synapse onto VIP-containing interneurons, which inhibit apical dendrite-targeting SOM-containing interneurons. The authors demonstrate that this circuit is engaged during active movement (whisking).
- 71**.Pi HJ, et al. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503(7477):521–4. doi: 10.1038/nature12676. This study characterized a disinhibitory local circuit in auditory sensory and prefrontal cortices of mice. VIP-containing interneurons inhibit apical dendrite-targeting SOM-containing interneurons, which in turn disinhibit pyramidal neurons. This circuit was engaged during learning of an auditory discrimination task, in particular following reinforcement signals.
- 72.Andrillon T, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31(49):17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim U, Sanchez-Vives MV, McCormick DA. Functional dynamics of GABAergic inhibition in the thalamus. Science. 1997;278(5335):130–4. doi: 10.1126/science.278.5335.130. [DOI] [PubMed] [Google Scholar]
- 74.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86(5):1698–702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Womelsdorf T, et al. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439(7077):733–6. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]