Abstract
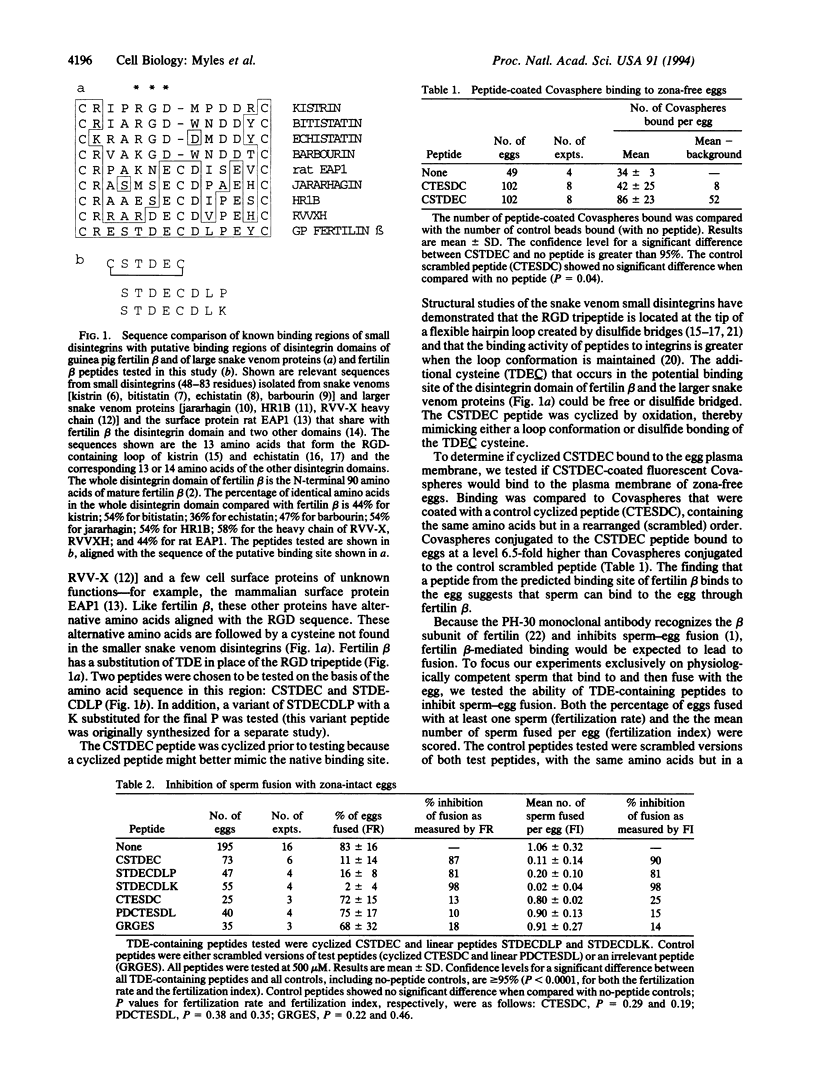
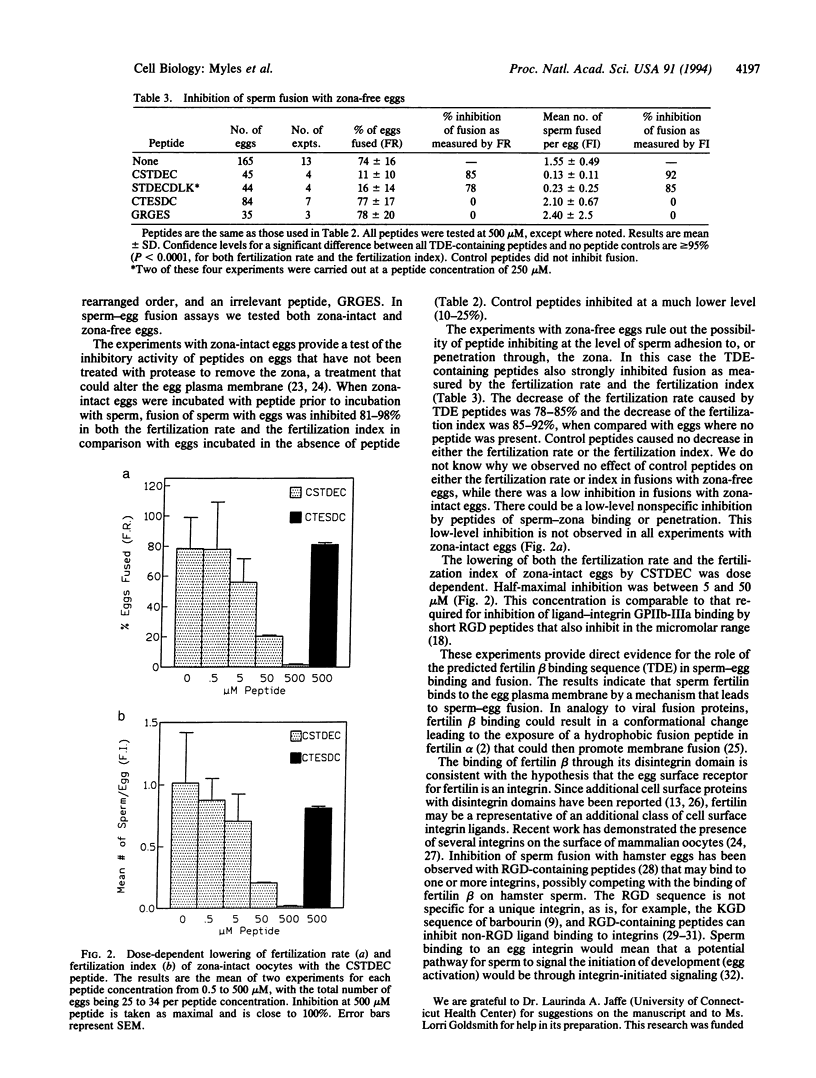
Fertilization and certain later stages in mammalian embryonic development require fusion between membranes of individual cells. The mechanism of eukaryotic cell-cell fusion is unknown, and no surface molecules required for this process have been unequivocally identified. The role of the sperm surface protein fertilin in sperm-egg fusion was tested by using peptide analogues of a potential integrin binding site in the fertilin beta subunit. Peptide analogues that include a TDE sequence from the disintegrin region of fertilin beta are able to bind to the egg plasma membrane and strongly inhibit sperm-egg fusion. These results show that the disintegrin domain of fertilin beta binds to the egg plasma membrane and that this binding is required for membrane fusion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Lazarus R. A., Dennis M. S., Wagner G. Solution structure of kistrin, a potent platelet aggregation inhibitor and GP IIb-IIIa antagonist. Science. 1991 Jul 26;253(5018):445–448. doi: 10.1126/science.1862345. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Myles D. G., Primakoff P., White J. M. Proteolytic processing of a protein involved in sperm-egg fusion correlates with acquisition of fertilization competence. J Cell Biol. 1990 Jul;111(1):69–78. doi: 10.1083/jcb.111.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel C. P., White J. M. Structure, function and evolutionary relationship of proteins containing a disintegrin domain. Curr Opin Cell Biol. 1992 Oct;4(5):760–765. doi: 10.1016/0955-0674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Boldt J., Howe A. M., Preble J. Enzymatic alteration of the ability of mouse egg plasma membrane to interact with sperm. Biol Reprod. 1988 Aug;39(1):19–27. doi: 10.1095/biolreprod39.1.19. [DOI] [PubMed] [Google Scholar]
- Bronson R. A., Fusi F. Evidence that an Arg-Gly-Asp adhesion sequence plays a role in mammalian fertilization. Biol Reprod. 1990 Dec;43(6):1019–1025. doi: 10.1095/biolreprod43.6.1019. [DOI] [PubMed] [Google Scholar]
- Calvete J. J., Wang Y., Mann K., Schäfer W., Niewiarowski S., Stewart G. J. The disulfide bridge pattern of snake venom disintegrins, flavoridin and echistatin. FEBS Lett. 1992 Sep 14;309(3):316–320. doi: 10.1016/0014-5793(92)80797-k. [DOI] [PubMed] [Google Scholar]
- Chen Y., Pitzenberger S. M., Garsky V. M., Lumma P. K., Sanyal G., Baum J. Proton NMR assignments and secondary structure of the snake venom protein echistatin. Biochemistry. 1991 Dec 17;30(50):11625–11636. doi: 10.1021/bi00114a004. [DOI] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Plow E. F. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990 Feb 25;265(6):3440–3446. [PubMed] [Google Scholar]
- Dennis M. S., Henzel W. J., Pitti R. M., Lipari M. T., Napier M. A., Deisher T. A., Bunting S., Lazarus R. A. Platelet glycoprotein IIb-IIIa protein antagonists from snake venoms: evidence for a family of platelet-aggregation inhibitors. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2471–2475. doi: 10.1073/pnas.87.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z. R., Gould R. J., Jacobs J. W., Friedman P. A., Polokoff M. A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J Biol Chem. 1988 Dec 25;263(36):19827–19832. [PubMed] [Google Scholar]
- Gould R. J., Polokoff M. A., Friedman P. A., Huang T. F., Holt J. C., Cook J. J., Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990 Nov;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kline D., Kline J. T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992 Jan;149(1):80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., Smith M. A., Andrieux A., Ryckwaert J. J., Marguerie G., Ginsberg M. H. Evidence that arginyl-glycyl-aspartate peptides and fibrinogen gamma chain peptides share a common binding site on platelets. J Biol Chem. 1987 Jan 25;262(3):947–950. [PubMed] [Google Scholar]
- Myles D. G. Molecular mechanisms of sperm-egg membrane binding and fusion in mammals. Dev Biol. 1993 Jul;158(1):35–45. doi: 10.1006/dbio.1993.1166. [DOI] [PubMed] [Google Scholar]
- Paine M. J., Desmond H. P., Theakston R. D., Crampton J. M. Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family. J Biol Chem. 1992 Nov 15;267(32):22869–22876. [PubMed] [Google Scholar]
- Perry A. C., Jones R., Barker P. J., Hall L. A mammalian epididymal protein with remarkable sequence similarity to snake venom haemorrhagic peptides. Biochem J. 1992 Sep 15;286(Pt 3):671–675. doi: 10.1042/bj2860671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Scarborough R. M. GPIIb-IIIa: the responsive integrin. Cell. 1991 May 3;65(3):359–362. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Hyatt H. An antisperm monoclonal antibody inhibits sperm fusion with zona-free hamster eggs but not homologous eggs. Fertil Steril. 1986 Sep;46(3):489–493. [PubMed] [Google Scholar]
- Primakoff P., Hyatt H., Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol. 1987 Jan;104(1):141–149. doi: 10.1083/jcb.104.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudek V., Atkinson R. A., Pelton J. T. Three-dimensional structure of echistatin, the smallest active RGD protein. Biochemistry. 1991 Jul 30;30(30):7369–7372. doi: 10.1021/bi00244a003. [DOI] [PubMed] [Google Scholar]
- Scarborough R. M., Rose J. W., Hsu M. A., Phillips D. R., Fried V. A., Campbell A. M., Nannizzi L., Charo I. F. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991 May 25;266(15):9359–9362. [PubMed] [Google Scholar]
- Scarborough R. M., Rose J. W., Naughton M. A., Phillips D. R., Nannizzi L., Arfsten A., Campbell A. M., Charo I. F. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J Biol Chem. 1993 Jan 15;268(2):1058–1065. [PubMed] [Google Scholar]
- Shebuski R. J., Ramjit D. R., Bencen G. H., Polokoff M. A. Characterization and platelet inhibitory activity of bitistatin, a potent arginine-glycine-aspartic acid-containing peptide from the venom of the viper Bitis arietans. J Biol Chem. 1989 Dec 25;264(36):21550–21556. [PubMed] [Google Scholar]
- Takeya H., Nishida S., Miyata T., Kawada S., Saisaka Y., Morita T., Iwanaga S. Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992 Jul 15;267(20):14109–14117. [PubMed] [Google Scholar]
- Takeya H., Oda K., Miyata T., Omori-Satoh T., Iwanaga S. The complete amino acid sequence of the high molecular mass hemorrhagic protein HR1B isolated from the venom of Trimeresurus flavoviridis. J Biol Chem. 1990 Sep 25;265(27):16068–16073. [PubMed] [Google Scholar]
- Tarone G., Russo M. A., Hirsch E., Odorisio T., Altruda F., Silengo L., Siracusa G. Expression of beta 1 integrin complexes on the surface of unfertilized mouse oocyte. Development. 1993 Apr;117(4):1369–1375. doi: 10.1242/dev.117.4.1369. [DOI] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Wolfsberg T. G., Bazan J. F., Blobel C. P., Myles D. G., Primakoff P., White J. M. The precursor region of a protein active in sperm-egg fusion contains a metalloprotease and a disintegrin domain: structural, functional, and evolutionary implications. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10783–10787. doi: 10.1073/pnas.90.22.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Fertilization of guinea pig eggs in vitro. Anat Rec. 1972 Sep;174(1):9–19. doi: 10.1002/ar.1091740103. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Setoguchi M., Higuchi Y., Akizuki S., Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2(6):585–591. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]


