Abstract
Stimulus-coupled incretin secretion from enteroendocrine cells plays a fundamental role in glucose homeostasis, and could be targeted for the treatment of type-2 diabetes. Here, we investigated the expression and function of transient receptor potential (TRP) ion channels in enteroendocrine L-cells producing glucagon-like peptide-1 (GLP-1). By microarray and qPCR analysis we identified trpa1 as an L-cell enriched transcript in the small intestine. Calcium imaging of primary L-cells and the model cell line GLUTag revealed responses triggered by the TRPA1 agonists allyl-isothiocyanate (AITC, mustard oil), carvacrol and polyunsaturated fatty acids, that were blocked by TRPA1 antagonists. Electrophysiology in GLUTag cells showed that carvacrol induced a current with characteristics typical of TRPA1 and triggered the firing of action potentials. TRPA1 activation caused an increase in GLP-1 secretion from primary murine intestinal cultures and GLUTag cells; an effect that was abolished in cultures from trpa1−/− mice or by pharmacological TRPA1 inhibition. These findings present TRPA1 as a novel sensory mechanism in enteroendocrine L-cells, coupled to the facilitation of GLP-1 release, which may be exploitable as a target for treating diabetes.
Introduction
The global rise in the prevalence of type 2 diabetes makes it one of the biggest health and socio-economic burdens of the 21st century. One of the newest and most successful strategies for the treatment of type 2 diabetes is based on mimicking, or enhancing, the endogenous action of incretin hormones. Glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) are incretin peptides released from K and L-cells, respectively, located in the epithelial layer of the gastrointestinal tract. GIP and GLP-1 potentiate glucose-dependent insulin secretion, while GLP-1 additionally suppresses appetite, glucagon secretion and gastric emptying (1). These effects have been exploited pharmacologically through the use of long-acting GLP-1 mimetics or inhibitors of incretin inactivation by dipeptidyl peptidase-4 (DPP4) (2). Incretin therapy has significant benefits over older insulinotropic medications like sulphonylureas, as it does not bypass the glucose sensing machinery of the pancreatic β-cell and thus promotes insulin secretion in relation to ambient plasma glucose levels, minimising the risk of hypoglycaemia. In addition, GLP-1 mimetics are associated with sustained weight loss (3; 4), whilst DPP4 inhibitors appear to be weight neutral (5); a difference that might reflect the higher effective concentrations of active GLP-1 reached with the former. Importantly, the substantially increased release of endogenous GLP-1 seen after Roux-en-Y gastric bypass surgery is likely to contribute towards the observed high rates of resolution of type 2 diabetes (6; 7). Understanding the mechanisms that underlie endogenous GLP-1 secretion could therefore facilitate the development of novel therapies based on potentiating the post-prandial incretin effect.
Over the past decade we and others have investigated the mechanisms underlying nutrient-coupled incretin secretion (8), aided by the development of model enteroendocrine cells lines (including GLUTag and STC-1) as well as primary intestinal epithelial cell cultures. As hormone release is dependent upon elevated cytosolic Ca2+, mechanisms of stimulus-coupled incretin secretion are commonly associated with membrane depolarisation and voltage-gated Ca2+ channel activation, or the release of Ca2+ from intracellular stores (9-12). Although numerous ion channels and G-protein coupled receptors (GPCRs) have been shown to facilitate these mechanisms, the potential contribution of the transient receptor potential (TRP) superfamily of ion channels has been relatively unexplored. TRP ion channels form a family of non-selective, cation conducting channels that are responsive to a variety of physical, chemical and thermal stimuli, and play fundamental roles in sensory physiology (13). They have well established roles in conferring appreciation for bitter, sweet and umami tastes (14), as well as the regulation of gastrointestinal motility and absorption (15). Emerging data support a role for TRP channels in modulating both incretin and insulin secretion. Oral administration of the TRPV1 agonist capsaicin, for example, increased plasma GLP-1 and insulin concentrations, and improved glucose tolerance in mice (16). TRPA1 agonists have been shown to regulate insulin secretion from rat pancreatic beta cells, and to increase peptide YY (PYY) release and suppress food intake and gastric emptying in mice (17; 18). TRPA1 is well characterised as a detector of non-nutrient food components such as cinnamaldehyde (cinnamon), gingerol (ginger), allicin (garlic) and allyl-isothiocyanate (AITC, mustard oil), but has also been identified as a putative polyunsaturated fatty acid (PUFA) sensor (19; 20). 1g and 3g doses of cinnamon were found to increase GLP-1 levels in humans (21). Despite these findings, however, the functional contribution of TRP channels to the regulation of enteroendocrine cell physiology is currently unclear.
In this study we investigated the expression and function of TRP channels in enteroendocrine cells. We show that trpa1 is selectively expressed in L-cells of the small intestine, is functionally active and is involved in nutrient and non-nutrient-coupled GLP-1 secretion.
Methods
Animals
Animal procedures were approved by local ethical review committees and conformed to UK Home Office regulations. Experiments were performed using mice on a C57B/6 background. Trpa1−/− mice were kindly gifted by Drs. Kelvin Kwan and David Corey (Harvard Medical School, Boston, MA) (22). GLU-Cre mice have been described previously (23). To enable Ca2+-monitoring in small intestinal L-cells these were crossed with commercially available ROSA26-GCamp3 reporter mice (24) (Jax stock 014538), resulting in expression of the genetically encoded Ca2+-sensor in L-cells, and with ROSA26-tdRFP mice to facilitate L-cell identification (25).
Primary enteroendocrine and GLUTag cell culture
Processing and culture of primary enteroendocrine was as described previously (26). Briefly, mice aged 10-24 weeks were killed by cervical dislocation and colonic (entire colon/rectum), ileal (10 cm region adjacent to caecum) or duodenal (10 cm region adjacent to stomach) tissue was taken. Intestinal tissue was cleaned thoroughly with PBS and the outer muscle layer removed. Tissue was digested using collagenase type XI and cell suspensions were plated either onto 24-well plates (GLP-1 secretion analysis) or 35 mm glass-bottomed dishes (Ca2+ imaging), pre-treated with 1% v/v matrigel (BD Biosciences, Oxford, UK). For ileal and duodenal cultures, the ROCK inhibitor Y-27632 dihydrochloride (10 μM) was added to final cell suspensions before plating.
GLUTag cells were maintained in 75 cm2 flasks with DMEM (1000 mg/L glucose) supplemented with 10% fetal bovine serum, L-glutamine and penicillin/streptomycin at 37°C, 5% CO2. Cells for experimental use were plated as described for primary cultures, or for electrophysiology were plated onto 35 mm plastic dishes. All cultures were analysed within 48 hours of plating.
Compounds
Allylisothiocyanate (AITC), carvacrol (Car), arachidonic acid (AA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), HC-030031 (HC) and A-967079 (A-9) and other reagents except where indicated, were obtained from Sigma Aldrich (Poole, UK). For in vitro experiments, stock solutions (x1000 working concentration) of AITC, AA, EPA and DHA were made in DMSO, and carvacrol was made in ethanol. Compounds were diluted to working concentrations on the day of use. Respective vehicle controls were used to confirm the specificity of each compound tested.
Microarray analysis
Isolated RNA from cell populations of different intestinal regions was extracted as previously described, and converted to cDNA which was then used for microarray analysis (27) using Affymetrix Murine 430 2.0 and Affymetrix ST 1.0 GeneChips.
Quantitative RT-PCR
Isolation and extraction of RNA was performed as previously described (27). The appropriate amount of first-strand cDNA template was mixed with specific TaqMan primers (Applied Biosystems, Foster City, CA, USA), water and PCR Master Mix (Applied Biosystems), and quantitative RT-PCR was conducted using a 7900HT Fast Real-Time PCR system (Applied Biosystems). Results were normalised to β-actin from the same sample. The following primer pairs were used (Applied Biosystems): trpa1: Mm01227437_m1; trpc1: Mm00441975_m1; trpc3: Mm00444690_m1; trpm7: Mm00457998_m1. Experiments were performed on at least three independently isolated cDNA samples.
Ca2+ imaging
GLUTag cells were incubated with 5 μM fura-2-acetoxymethyl ester (fura-2-AM; Invitrogen, UK) in a 1 mM glucose supplemented standard extracellular buffer (concentrations in mM: 143.4 NaCl, 4.5 KCl, CaCl2 2.6, MgCl2 1.2, HEPES 10, pH corrected to 7.4 using NaOH) for 15 minutes at 37°C followed by 15 minutes at room temperature. Cells were then washed three times with glucose-free extracellular solution (as above) and changes in Ca2+ levels were assessed by measuring the change in ratiometric fluorescence (excitation 340 ± 10 nm/380 ± 4 nm) at ≥510 nm. Measurements of intracellular Ca2+ dynamics from primary cultures were performed using intestinal cultures from GLU-Cre/ROSA26-GCaMP3/ROSA26-tdRFP mice. L-cells were identified by the presence of RFP fluorescence and changes in intracellular Ca2+ levels were represented by a change in the intensity of GFP fluorescence (excitation 480 ± 10 nm). Imaging experiments were performed using an Olympus IX71 microscope with a 40x oil immersion objective, fitted with a monochromator (Cairn Research, UK) and OrcaER camera (Hamamatsu, Japan). Images were acquired at 1 Hz and analysed, after background subtraction, using MetaFluor software (Molecular Devices, USA).
Electrophysiological analysis
Electrophysiological experiments were performed using an Axopatch 200B and Digidata 1440A (Axon Instruments, USA). Fire-polished filamented borosilicate patch pipettes coated with beeswax, with a resistance of 2.5-4 MΩ were used (Harvard apparatus, USA). For current clamp experiments the following solutions were used. Standard extracellular buffer as above. Intracellular solution (mM): 107 KCl, 1 CaCl2, 7 MgCl2, 11 EGTA, 10 HEPES, 5 K2ATP (pH 7.2 with KOH). For voltage clamp experiments, following whole-cell configuration, series resistance was compensated by 70% and the following solutions were used. Extracellular solution (mM): 115 NaCl, 5 CsCl, 5 CoCl2, 20 TEA-Cl2, 10 4-Aminopyridine, 5 EDTA, 10 HEPES, 0.3 μM tetrodotoxin (pH 7.4 with NaOH). Intracellular solution (mM): 107 CsCl, 5 MgCl2, 11 EGTA, 10 HEPES, 5 Na2ATP (pH 7.2 with CsOH). Recordings were acquired at 25 kHz and filtered (low-pass Bessel filter) at 10 kHz.
GLP-1 analysis from primary intestinal and GLUTag cultures
After 24 hours in culture, wells was washed three times with standard extracellular buffer supplemented with 0.1% BSA (fatty-acid free) and 10 mM glucose. Test compounds were diluted to their working concentration in the same extracellular buffer and added to each well (250 μL). Cells were incubated at 37°C for 2 hours, and solutions then removed and centrifuged at 2000 RCF for 5 minutes. Supernatants were snap frozen on dry ice. For primary intestinal cultures, supernatant and lysate samples were collected. GLP-1 contents were measured using a total GLP-1 assay (Meso Scale Discovery, Gaithersburg, MD, USA). For primary cultures, results were calculated as a percentage of GLP-1 content per well and were normalised to the control well (extracellular buffer alone) measured in parallel on the same day. For experiments where voltage-gated ion channels were blocked, the extracellular solution was replaced with that used for voltage clamp analysis (see above).
Assessment of GLP-1 release, in vivo
Male mice were fasted overnight before receiving AITC or vehicle (PBS) by oral gavage (10 mL/kg). Five minutes after the gavage, each animal was anaesthetised (isoflurane) and a terminal blood sample was taken. For intestinal perfusion studies, fasted male mice were anaesthetised and underwent a laparotomy prior to receiving an intra-duodenal bolus injection (0.6 mL) of carvacrol,vehicle (PBS) or positive control (20% w/vol glucose in PBS). A portal vein blood sample was taken at 5 minutes post injection. The method was adapted from a protocol previously described for colonic stimulation (28). Blood samples were collected in EDTA, centrifuged at 13,000 g for 90 seconds and the plasma was collected and used for GLP-1 analysis.
Data analysis
Results are expressed as mean ± SEM, unless otherwise stated. Statistical analysis was performed using GraphPad Prism (version 5.01, San Diego, CA, USA). For mRNA expression and GLP-1 secretion data, one-way ANOVA with Bonferroni post-hoc analysis was performed on log-transformed values, as these data were heteroscedastic. Electrophysiological and Ca2+ imaging data were assessed using repeated measures ANOVA with Bonferroni post-hoc analysis, or Student’s paired t-test, as appropriate. Values were regarded significant if p<0.05.
Results
TRP expression profiles within discrete enteroendocrine cell populations
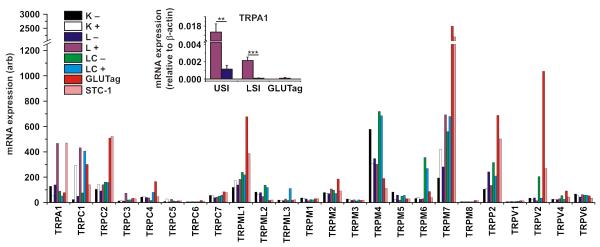
Microarray analysis was used to compare expression of mRNAs encoding TRP channels in primary murine K and L cell populations as well as in GLUTag and STC-1 cell lines (27). The profiling of TRP channel expression using the Affymetrix 430 2.0 array is shown in figure 1. Of the 24 channels analysed, only 6 (trpa1, trpc1, trpc3, trpc4, trpc5 and trpm7) exhibited at least 2-fold higher expression in one or more primary enteroendocrine cell population compared with their respective negative controls. An independent microarray analysis of L-cells and controls from the upper small intestine was performed using Affymetrix ST 1.0 arrays (figure S1a), and confirmed the selective and/or high expression of trpa1, trpc1, trpc3 and trpm7, but not trpc4 and trpc5. Of the 4 candidates identified from both screens, TRPA1 is the only channel currently known to be potently activated by compounds commonly present in food, and was therefore chosen for further investigation. In contrast to a previous report (16), which detected TRPV1 immunohistochemically in STC-1 cells, we only found very low trpv1 mRNA expression in all the analysed cell types, with no evidence of enteroendocrine cell-specific expression.
Figure 1. Expression profiles of TRP channel mRNAs within discrete enteroendocrine cell populations.
Main figure shows microarray analysis for the TRP superfamily. Expression of each TRP mRNA was assessed by RMA analysis from murine small intestinal K (K+) and L (L+) cells, colonic L-cells (LC+), as well as GLUTag and STC-1 cell lines. Each primary enteroendocrine cell population was compared to their respective negative population (K−, L− and LC−). Inset shows real-time quantitative PCR analysis of trpa1 mRNA in the upper small intestine (USI) and lower small intestine (LSI) within murine L+ and L− cell populations, as well as GLUTag cells. Values are normalised to the expression of β-actin from the same cell populations (n≥3, **p<0.01, ***p<0.001, two-way unpaired Student’s t-test).
To confirm the fidelity of the microarray screens, targeted quantitative PCR was performed to assess trpa1 expression levels in L-cells of the small and large intestine as well as in GLUTag cells (figure 1 inset). As observed in the microarray screen, trpa1 expression was enriched in L-cell populations compared with controls, was higher in upper than lower small intestinal L-cells and was detectable at low levels in GLUTag cells. It was not detectable in colonic L-cells (data not shown). Expression of trpc1, trpc3 and trpm7 was also confirmed using targeted quantitative analysis, further validating the expression profiles identified by the microarrays (figure S1b-d).
Ca2+ response following TRPA1 agonism in primary enteroendocrine cells
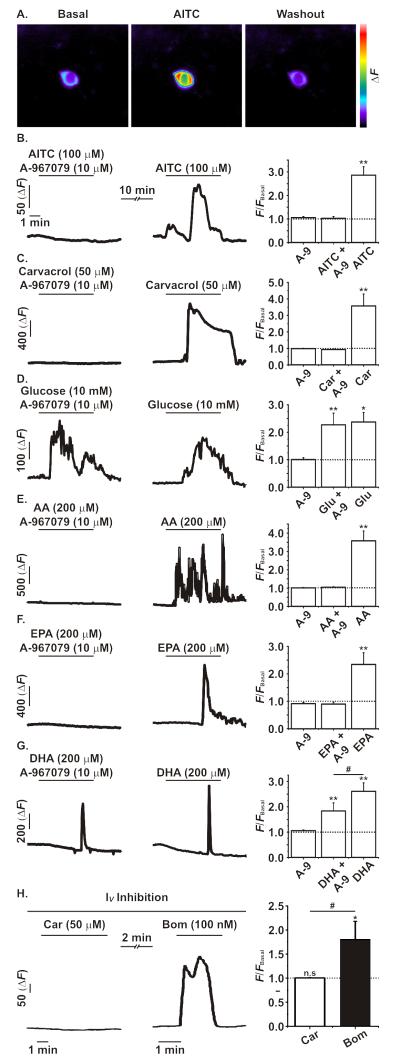
To assess whether the high expression of trpa1 mRNA in upper small intestinal L-cells represents a functional TRPA1 ion channel population, calcium imaging was performed on primary L-cells cultured from the duodenum of GLU-Cre/ROSA26-GCaMP3 mice. These mice exhibit L-cell specific expression of the GCaMP3 protein, a genetically-encoded Ca2+ sensor that enables intracellular Ca2+ levels to be monitored as a function of GFP fluorescence (24) (figure 2a). Consistent with the high expression of trpa1 mRNA, the TRPA1 agonist allyl-isothiocyanate (AITC; 100 μM) caused a significant increase in GCaMP3 fluorescence in GLU-Cre/ROSA26-GCaMP3 primary L-cells (figure 2b; figure S2). This response was completely inhibited when AITC was co-applied with the specific TRPA1 inhibitor, A-967079 (10 μM). To further validate the functional expression of TRPA1 ion channels, the action of an alternative agonist, carvacrol (50 μM), was investigated. As observed following the application of AITC, carvacrol induced a large Ca2+ transient in GLU-Cre/ROSA26-GCaMP3 primary L-cells, which was also completely inhibited following TRPA1 inhibition (figure 2c). To confirm the specificity of TRPA1 inhibition by A-967079, we tested its effects on responses to glucose. Consistent with previous findings (26), 10 mM glucose caused an increase in intracellular Ca2+ in L-cells, an effect that was reproducible and unaffected by the co-application of A-967079 (figure 2d).
Figure 2. Ca2+ imaging analysis of TRPA1 in primary small intestinal L-cells using GLU-Cre/ROSA26-GCaMP3 mice.
A. Representative images showing the change in GCaMP3 fluorescence before, during and after the application of AITC (100 μM) to a primary duodenal L-cell cultured from GLU-Cre/ROSA26-GCaMPe mice. B and C. The application of AITC (100 μM) or carvacrol (Car, 50 μM) caused a robust and significant increase in intracellular Ca2+ as measured by GCaMP3 fluorescence; an effect that was inhibited by the co-application of the TRPA1 inihibitor A-967079 (A-9, 10 μM). D. Glucose (10 mM) application significantly increased intracellular Ca2+ levels independent of TRPA1 inhibition. E-G. The addition of polyunsaturated acids AA, EPA and DHA (all at 200 μM) significantly increased intracellular Ca2+ levels. The co-application of A-967079 fully inhibited the effects of AA and EPA, but only partially inhibited the effect of DHA on intracellular Ca2+ levels. H. Carvacrol (Car; 50 μM) caused no change in intracellular Ca2+ levels during a complete voltage-gated ion channel block (see methods). In contrast, the subsequent application of the Gq activator bombesin (Bom; 100 nM) did cause a significant increase in intracellular Ca2+ levels. All experiments were n≥5, */#p<0.05, **p<0.01, repeated measures ANOVA with Bonferroni post-hoc test; * indicates significance level from baseline and # indicates significance level between groups. The dotted line on each graph represents the respective baseline value.
As TRPA1 has been reported to respond to a number of polyunsaturated fatty acids (PUFA) (19), we examined the effects of arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on duodenal GLU-Cre/ROSA26-GCaMP3 primary L-cells. AA, EPA and DHA (200 μM each) caused robust increases in intracellular Ca2+ (figure 2e-g). Whilst the increase of intracellular Ca2+ caused by AA and EPA was prevented by A-967079, the effect of DHA was only partially inhibited, suggesting the additional activation of a TRPA1-independent mechanism.
We hypothesised that the Ca2+ responses could arise from direct Ca2+-entry through TRPA1, recruitment of intracellular Ca2+-stores or depolarisation-dependent activation of voltage-gated Ca2+ channels. When we used a cocktail of inhibitors (see methods) to prevent activation of voltage gated ion channels, AITC (100 μM) failed to elicit a response (figure 2h) whereas bombesin (100 nM), a Gq-activator releasing Ca2+ from intracellular stores, still increased intracellular Ca2+ levels. These findings suggest that TRPA1 activation increases depolarisation-dependent voltage-gated Ca2+ entry.
We investigated whether GLUTag cells could be used as a model system for monitoring TRPA1 activity. Despite the relatively low expression of trpa1 mRNA, AITC induced robust calcium transients in a subpopulation (20/107; 19%) of GLUTag cells, supporting their use for the electrophysiological characterisation of TRPA1.
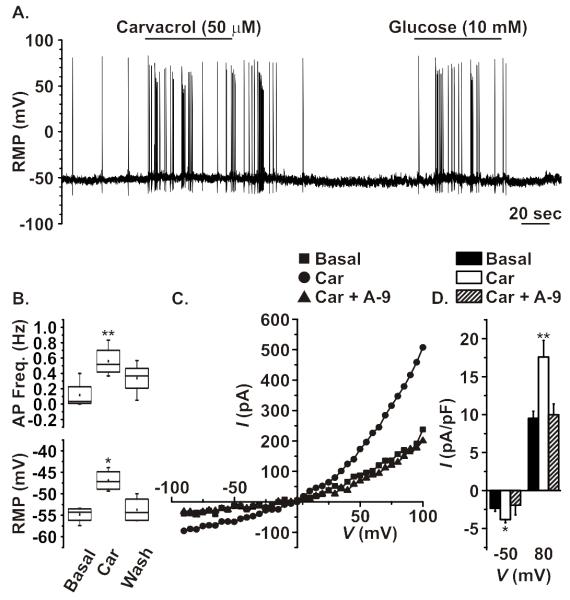
TRPA1 agonists potentiate electrical activity in GLUTag cells
GLUTag cells were used to investigate whether TRPA1 activation modifies cellular electrical activity. Due to its ability to activate TRPA1 reversibly through non-covalent interactions (20), the effect of carvacrol (50 μM) on action potential frequency was assessed using whole-cell current clamp. Carvacrol application caused a rapid increase in the rate of action potential firing from GLUTag cells, which was quickly reversed following washout (figure 3a/b). Subsequent responses to a glucose stimulus were unaffected (figure 3a). The carvacrol-triggered increase in action potential frequency was associated with depolarisation of the resting membrane potential (from −54.9 ±0.9 mV to −46.9 ±1.3 mV, n=4), consistent with the activation of a TRPA1-like depolarising current (figure 3b) (29).
Figure 3. Electrophysiological analysis of TRPA1 in GLUTag cells.
A. Representative current-clamp recording showing the increase in action potential frequency following the application of TRPA1 agonist carvacrol (50 μM). The effect of carvacrol was reversible and did not affect responses to the subsequent application of glucose (10 mM). B. The addition of carvacrol (Car) significantly and reversibly increased action potential frequency and depolarised the plasma membrane (n=4). C. Representative current-voltage trace showing the effects of carvacrol application on increasing the outward and inward-rectifying properties of an isolated TRP-like current. The change in rectification properties was fully reversed following the co-application of A-967079 (A-9, 10 μM). D. Carvacrol caused a significant increase in the inward current at −50 mV (typical resting membrane potential) and outward current at +80 mV, which was reversed following the co-application of A-967079 (n=7, *p<0.05, **p<0.01, two-way paired Student’s t-test)
To further investigate the origin of the membrane depolarisation, current-voltage analysis was performed on GLUTag cells using whole-cell voltage clamp. To isolate TRPA1-like currents, blockers of voltage-gated Na+, K+ and Ca2+ channels were used (see methods). Addition of carvacrol activated an outwardly rectifying current, with characteristics typical of TRPA1 (figure 3d) (20), and was reversed by co-application of A-967079 (10 μM). Consistent with the observed shift in resting membrane potential and increase in action potential frequency, carvacrol significantly increased the magnitude of the depolarising current measured at −50 mV, the typical resting membrane potential of GLUTag cells (30).
TRPA1 agonist-coupled GLP-1 secretion in enteroendocrine cells
In GLUTag cells, AITC (100 μM) caused an ~2-fold increase in GLP-1 secretion, independent of the basal glucose concentration (figure 4a; figure S3a). This response was completely inhibited when AITC was co-applied with the TRPA1 inhibitor, HC-030031 (50 μM). Importantly, HC-030031 did not impair secretion triggered by glucose, consistent with the lack of effect of TRPA1-antagonism on glucose-induced Ca2+ responses (figure S3b; figure 2d). AA, EPA and DHA (200 μM each) all caused ~4-fold elevations in GLP-1 secretion, which were significantly inhibited by co-application of HC-030031 (figure 4b-d).
Figure 4. The effect of TRPA1 agonism on GLP-1 secretion from GLUTag cells.
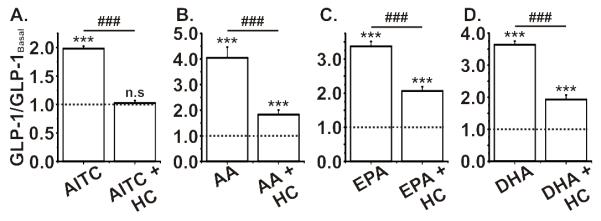
A. AITC (100 μM) incubation with GLUTag cells caused a significant increase in GLP-1 secretion over two hours, which was fully inhibited when AITC was co-incubated with HC-030031 (50 μM). B-D. The incubation of polyunsaturated fatty acids (PUFA), AA, EPA and DHA (all at 200 μM), also caused a significant and robust increase in GLP-1 secretion from GLUTag cells. The co-incubation with A-967079 significantly reduced, but did not abolish, the effect of PUFA-induced GLP-1 secretion. All experiments were n= ≥6, ***/###p<0.001, one-way ANOVA with Bonferroni post hoc test; * indicates significance level from baseline and # indicates significance level between groups. The dotted line on each graph represents the respective baseline value.
We next tested the response of primary ileal epithelial cultures to TRPA1 stimulation. Consistent with the effects observed in GLUTag cells, both AITC and carvacrol caused a significant increase in GLP-1 release, which was inhibited by HC-030031 (figure 5a,b). AITC failed to trigger GLP-1 secretion when Ca2+-entry through voltage-gated Ca2+-channels was prevented, whilst bombesin still enhanced GLP-1-secretion (figure 5c). Application of AA, EPA and DHA also caused a significant increase in GLP-1 release, greater than that observed following AITC or carvacrol stimulation (figure 5d-f). However, unlike in GLUTag cells, PUFA-stimulated GLP-1 secretion from ileal cultures was unaffected by HC-030031. To further confirm that the stimulatory effects of AITC and carvacrol were TRPA1-specific, ileal cultures were tested from trpa1−/− mice. Both AITC and carvacrol induced significantly smaller increases in GLP-1 release from trpa1−/− ileal cultures compared to age-matched trpa1+/+ littermates, mirroring the reduced level of GLP-1 secretion observed following TRPA1 inhibition with HC-030031 (figure 5g,h). Application of 15 mg/kg AITC by gavage increased plasma GLP-1 concentrations in vivo, but this response was not reduced in trpa1−/− mice, while lower doses did not significantly increase GLP-1 levels (figure 6 and b). Injection of carvacrol directly into the duodenum of anaesthetised mice, bypassing potential problems with gastric emptying, also only produced a trend towards increased GLP-1 secretion (figure S4).
Figure 5. The effect of TRPA1 agonism on GLP-1 secretion from small intestinal trpa1+/+ and trpa1−/− enteroendocrine cells.
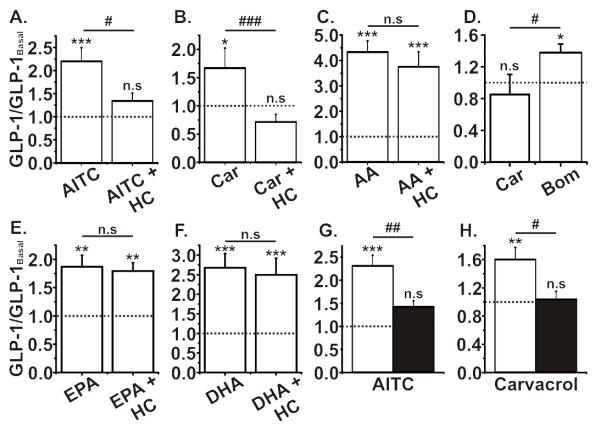
A and B. Both AITC (100 μM) and carvacrol (50 μM) caused a significant increase in GLP-1 secretion from murine ileal cultures and were fully inhibited by the TRPA1 inhibitor HC-030031 (50 μM). C. Incubation of carvacrol (Car; 50 μM) in the presence of a complete voltage-gated ion channel block had no effect on GLP-1 secretion from murine ileal cultures, however, a significant increase in GLP-1 secretion was observed following a similar incubation with bombesin (Bom; 100 nM) (n=5-6, */#p<0.05, unpaired Student’s t-test). D-F. The polyunsaturated fatty acids AA, EPA and DHA (all at 200 μM), all caused robust and significant increases in GLP-1 secretion and were not affected by co-incubation with HC-030031 (50 μM). G and H. The effect of AITC and carvacrol to increase GLP-1 secretion from murine ileal cultures was abolished in trpa1−/− mice (black bars) compared to WT (white bars). Experiments were n=≥6 wells from ≥3 mice, unless otherwise indicated, */#p<0.05, **/##p<0.01, ***/###p<0.001, one-way ANOVA with Bonferroni post hoc test; * indicates significance level from baseline and # indicates significance level between groups. The dotted line on each graph represents the respective baseline value.
Figure 6. In vivo effects of AITC.
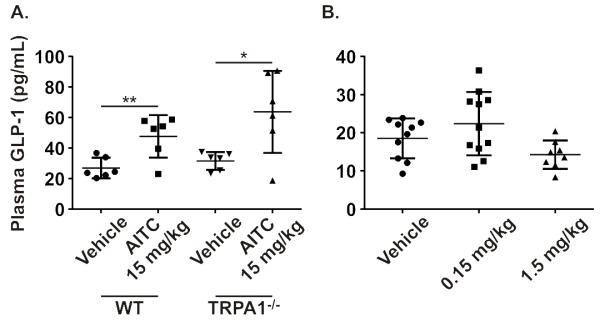
A. Administration of AITC (15 mg/kg) or vehicle (PBS) by oral gavage caused a significant increase in plasma GLP-1 levels at five minutes in WT mice that was also present in the trpa1−/− (n=6). B. The administration of AITC at 1.5 and 0.15 mg/kg failed to cause any significant increase in plasma GLP-1 levels (n=8-11; two-way unpaired Student’s t-test).
TRPV1 agonism has no effect on intracellular Ca2+ levels or GLP-1 secretion in enteroendocrine L-cells
High concentrations of AITC have been shown to activate TRPV1 (31), and TRPV1 activation by capsaicin has been reported to increase plasma GLP-1 concentrations in mice (16). We therefore examined whether TRPV1 channels are functionally expressed in L-cells, and might account for the observed non-specific increase in plasma GLP-1 levels by AITC in vivo. Consistent with the low trpv1 mRNA levels, however, application of the specific TRPV1 agonist, capsaicin (100 nM), caused no change in GCaMP3 fluorescence in primary colonic L-cells (figure 7a). Furthermore, incubation of primary ileal, colonic or GLUTag cultures with capsaicin (100 nM) did not increase in GLP-1 secretion (figure 7b).
Figure 7. Analysis of functional TRPV1 in primary enteroendocrine cells and GLUTag cells.
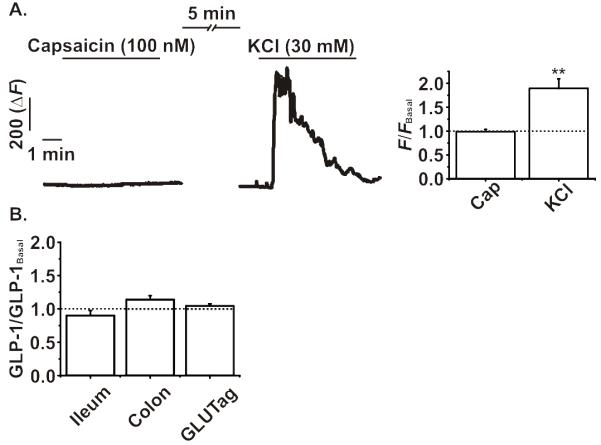
A. Addition of capsaicin (Cap, 100 nM) failed to elicit any significant change in intracellular Ca2+ levels as measured by GCaMP3 fluorescence in colonic L-cells from GLU-Cre/ROSA26-GCaMP3 mice. KCl (30 mM) was used to confirm the responsiveness of each L-cell analysed. B. Incubation of murine ileal or colonic intestinal cultures, or GLUTag cells, with capsaicin (100 nM) for two hours did not significantly alter GLP-1 secretion compared to basal (10 mM glucose). All experiments were n= ≥5, *p<0.05, repeated measures ANOVA or one-way ANOVA, both with Bonferroni post-hoc test. The dotted line on each graph represents the respective baseline value.
Discussion
Trpa1 was identified by expression analysis as an enteroendocrine cell transcript localised particularly to L-cells of the upper small intestine. In functional experiments, TRPA1 activation was shown to cause membrane depolarisation, action potential firing, Ca2+ entry and GLP-1 secretion. Responses were triggered by AITC and carvacrol as well as by PUFAs, and were largely abolished by TRPA1 antagonists or in mice lacking trpa1, clearly demonstrating a role for TRPA1 in GLP-1 secretion. Others have reported an elevation of murine plasma PYY after application of the TrpA1-agonists cinnamaldehyde or methyl syringate, which was blocked when ruthenium red or HC-030031 were coapplied (18). By contrast, although we observed an elevation of GLP-1 after AITC application in vivo, the effect was preserved in trpa1−/− mice. We believe that factors such as the relatively small effect size and the non-specific targets of higher concentrations of AITC, hindered the detection of significant TrpA1 mediated changes in plasma hormone concentrations. Trpa1 was also detected in a recent RNASeq analysis of transcripts enriched in gastric D-cells (unpublished data), suggesting that stimulation of D-cell somatostatin release might also be triggered by TRPA1 agonists in vivo, which would tend to counteract the secretion of GLP-1 (32).
TRPA1 channels are known to be activated by numerous dietary-related compounds such as those present in cinnamon, ginger and oregano as well as the Brassica genus of plants. Besides stimulation by AITC and carvacrol, we found that TRPA1 channels in duodenal L-cells were also activated by PUFAs, consistent with their putative role as a fatty acid sensor (19). Intracellular Ca2+ fluxes caused by AA, EPA or DHA were fully or partially inhibited by the co-application of a specific TRPA1 antagonist. By contrast, TRPA1 antagonism had either partial or no effect on PUFA-triggered GLP-1 secretion from GLUTag or primary ileal cultures, respectively. The reason for this discrepancy is unclear, but it could reflect differences in the duration of PUFA exposure between the experimental approaches. PUFAs, such as arachidonic acid, have well established roles in intracellular cell signalling as well as being an essential precursor of eicosanoid synthesis (33; 34). These alternative pathways could contribute to an increase in GLP-1 secretion, independent of TRPA1 activation, potentially explaining the mismatch between PUFA-associated changes in cellular Ca2+ and GLP-1 release. The additional discrepancy between GLUTag and primary L-cells, in terms of PUFA-induced GLP-1 secretion, could be due to the differences in the relative contributions of TRPA1-dependent and independent mechanisms that are downstream of PUFA stimulation.
The finding of trpa1 expression in upper small intestinal L-cells is consistent with a recent report that trpa1 was identified in cholecystokinin (CCK) containing enteroendocrine cells of the mouse intestinal tract (35). Although the authors did not specifically examine whether trpa1 was also co-expressed with GLP-1, we showed previously that approximately half of CCK-positive cells in the small intestine co-express GLP-1 (27). In the present manuscript we restricted our functional analysis to TRPA1, but further investigation is warranted into the roles of TRPC1, TRPC3 and TRPM7, which have putative functions in regulating intracellular Ca2+ levels by capacitative Ca2+ entry, or in Mg2+ homeostasis (36; 37). Another TRP channel, TRPV1, was recently reported to play a role in enhancing GLP-1 and insulin secretion in mice (16), but whether this effect was via direct enteroendocrine cell activation was unclear. Our findings that capsaicin had no effect on intracellular Ca2+ levels in murine colonic L-cells or on GLP-1 secretion and that the microarray signal for TRPV1 was very low in L-cells, suggest that TRPV1-associated GLP-1 secretion is not via direct activation of L-cells. As TRPV1 activation has been shown to augment gastric emptying in both mice and humans (38; 39), it is possible that the previously observed augmentation by capsaicin of glucose-triggered GLP-1 release was related to the rapid emptying of gastric glucose, delivering a larger glucose load to L-cell rich regions of the intestine.
Physiological relevance
Based on the success of incretin-based strategies for the treatment for type-2 diabetes, new therapeutic approaches are under evaluation, aiming to stimulate the endogenous release of GLP-1 and other gut peptides. Evidence supporting this approach comes from the field of bariatric surgery, which is not only successful for the treatment of morbid obesity, but additionally results in the remission of type-2 diabetes in many individuals. Rearrangement of the gastrointestinal tract during surgery limits or restricts food entering the stomach and/or the upper small intestine, exposing the unbypassed intestine to an enriched nutrient environment. Associated with this change in nutrient exposure are significantly elevated post-prandial GLP-1 and PYY levels (40), which likely contribute to improved insulin release and reduced appetite. Developing strategies that medically mimic bariatric surgery is a high priority.
The role of TRPA1 in GLP-1 secretion in humans is yet to be studied, but there is emerging evidence that TRPA1 dietary agonists can participate in the restoration of glycaemic control in patients with type-2 diabetes (41-44). A recent meta-analysis investigating the effects of dietary cinnamon, which contains the TRPA1 agonist cinnamaldehyde, showed that there was a significant improvement in levels of fasting plasma glucose, total cholesterol and triglyceride levels after 4 to 18 weeks of increased cinnamon intake (45). Our findings offer support for the investigation of whether small intestinal delivery of TRPA1 dietary agonists can regulate glycaemic control, and present TRPA1 as a potential therapeutic target for the treatment of type-2 diabetes.
Supplementary Material
Acknowledgements
GLP-1 immuno assays were performed by Keith Burling at the Medical Research Council Metabolic Diseases Unit, Cambridge.
Funding. This work was supported by Wellcome Trust grants (WT088357/Z/09/Z and WT084210/Z/07/Z) and a MRC_grant (MC_UU_12012/3) to F.M.G. and F.R.
Footnotes
Duality of interest. F.M.G. has received speaker’s fee from Novo Nordisk, Merck Sharp & Dohme and Eli Lily, and ad hoc consulting fees from Roche and Pfizer. F.R. has received speaker’s fees from Merck Sharp and Dohme and Novo Nordisk, and ad hoc consulting fees from AstraZeneca. No other conflicts of interest are declared.
References
- 1.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316–3323. doi: 10.2337/db13-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 6.Rhee NA, Vilsboll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab. 2012;14:291–298. doi: 10.1111/j.1463-1326.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezcurra M, Reimann F, Gribble FM, Emery E. Molecular mechanisms of incretin hormone secretion. Curr Opin Pharmacol. 2013;13:922–927. doi: 10.1016/j.coph.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers GJ, Tolhurst G, Ramzan A, Habib AM, Parker HE, Gribble FM, Reimann F. Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol. 2011;589:1081–1093. doi: 10.1113/jphysiol.2010.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56:2688–2696. doi: 10.1007/s00125-013-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 15.Boesmans W, Owsianik G, Tack J, Voets T, Vanden Berghe P. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011;162:18–37. doi: 10.1111/j.1476-5381.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao DS, Zhong L, Hsieh TH, Abooj M, Bishnoi M, Hughes L, Premkumar LS. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One. 2012;7:e38005. doi: 10.1371/journal.pone.0038005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MJ, Son HJ, Song SH, Jung M, Kim Y, Rhyu MR. The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One. 2013;8:e71603. doi: 10.1371/journal.pone.0071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motter AL, Ahern GP. TRPA1 is a polyunsaturated fatty acid sensor in mammals. PLoS One. 2012;7:e38439. doi: 10.1371/journal.pone.0038439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 21.Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, Darwiche G, Almér LO. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89:815–821. doi: 10.3945/ajcn.2008.26807. [DOI] [PubMed] [Google Scholar]
- 22.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2455. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 26.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick G, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 30.Reimann F, Gribble FM. Glucose-sensing in glucagon-like peptide-1-secreting cells. Diabetes. 2002;51:2757–2763. doi: 10.2337/diabetes.51.9.2757. [DOI] [PubMed] [Google Scholar]
- 31.Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Moss CE, Marsh WJ, Parker HE, Ogunnowo-Bada E, Riches CH, Habib AM, Evans ML, Gribble FM, Reimann F. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia. 2012;55:3094–3103. doi: 10.1007/s00125-012-2663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan WA, Blobe GC, Hannun YA. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell Signal. 1995;7:171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SC, Jhon DY, Bae YS, Kim JH, Rhee SG. Activation of phospholipase C-gamma by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996;271:18342–18349. doi: 10.1074/jbc.271.31.18342. [DOI] [PubMed] [Google Scholar]
- 35.Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014 doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 36.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debreceni A, Abdel-Salam OM, Figler M, Juricskay I, Szolcsanyi J, Mozsik G. Capsaicin increases gastric emptying rate in healthy human subjects measured by 13C-labeled octanoic acid breath test. J Physiol Paris. 1999;93:455–460. doi: 10.1016/s0928-4257(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros JV, Bezerra VH, Lucetti LT, Lima-Junior RC, Barbosa AL, Tavares BM, Magalhaes PJ, Santos AA, Cunha FQ, Soares PM, Souza MH. Role of KATP channels and TRPV1 receptors in hydrogen sulfide-enhanced gastric emptying of liquid in awake mice. Eur J Pharmacol. 2012;693:57–63. doi: 10.1016/j.ejphar.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 41.Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32:408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010;27:1159–1167. doi: 10.1111/j.1464-5491.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 43.Solomon TP, Blannin AK. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes Metab. 2007;9:895–901. doi: 10.1111/j.1463-1326.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- 44.Solomon TP, Blannin AK. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur J Appl Physiol. 2009;105:969–976. doi: 10.1007/s00421-009-0986-9. [DOI] [PubMed] [Google Scholar]
- 45.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.