Abstract
Objective: Cerebrovascular abnormalities have been reported in adult patients with Pompe disease. The objective was to study these abnormalities by (1) determining the diameter and mean flow velocity (MFV) of large cerebral arteries and (2) estimating cerebral blood flow (CBF), resistance index (RI) and cerebrovascular reactivity (CVR) as functions of resistance vessels.
Methods: In ten adults with Pompe disease and twenty controls, the diameter, peak systolic (PSV) and end-diastolic velocities (EDV) of arteries supplying the brain were quantified by MR angiography and sonography. MFV, RI and CBF were calculated. CVR in the middle cerebral artery (MCA) was determined by hyperventilation and acetazolamide injection.
Results: MR angiography revealed dilation of cerebral arteries predominantly in the posterior circulation. Dilative arteriopathy was found in three patients; two of them showed vertebrobasilar dolichoectasia. Despite of the dilative arteriopathy, the MFV was normal, indicating increased CBF and dilated resistance vessels. RI of all examined arteries and CVR of MCA were normal.
Conclusion: The data suggest that dilation of small and large cerebral arteries is a common feature in adults with Pompe disease. Increased CBF might be the consequence of dilated resistance vessels. However, dysfunction of resistance vessels was rarely found.
Synopsis: In adults with Pompe disease, dilation of small and large cerebral arteries is a common feature and might be associated with increased cerebral blood flow.
Keywords: Adults with Pompe disease, Dilative arteriopathy and dolichoectasia, Cerebral blood flow, Cerebrovascular reactivity
Introduction
Pompe disease (glycogenosis type II, MIM #232300) is a rare autosomal–recessive lysosomal storage disease caused by mutations in the GAA gene encoding the enzyme acid α-glucosidase. This results in decreased enzyme activity and intracellular glycogen accumulation (Pompe 1932; Joshi et al. 2008; van der Ploeg et al. 2010). Glycogen accumulation was found within endothelial cells and smooth muscle cells of cerebral arteries. Additionally, aneurysmal dilations of small cerebral arteries predominantly in the cerebellar cortex were reported in an adult with Pompe disease (Kretzschmar et al. 1990). Otherwise, in three children excessive accumulation of glycogen within endothelial cells was shown to reduce the lumen in cerebral arteries (Garancis 1968). Both abnormalities could disturb the cerebral blood flow regulation by small arteries, arterioles and capillaries (resistance vessels). Dilation, elongation and tortuosity (dolichoectasia) of large cerebral arteries were also reported. Cerebrovascular symptoms in adults with Pompe disease are headache, cerebral compression symptoms, ischemia or haemorrhage (Matsuoka et al. 1988; Kretzschmar et al. 1990; Laforêt et al. 2008; Sacconi et al. 2010).
The objective of this study was to evaluate these cerebrovascular abnormalities by (1) determining the diameter and mean flow velocity (MFV) of large cerebral arteries and (2) estimating cerebral blood flow (CBF), resistance index (RI) and cerebrovascular reactivity (CVR) as functions of resistance vessels.
Patients and Methods
Patients
We prospectively investigated ten adult Pompe patients of our outpatient department (age 46 ± 12 years, five females). Diagnosis of Pompe disease was confirmed by deficiency of α-glucosidase and corresponding gene mutations in the GAA. With the exception of the siblings P5 and P8, all patients received enzyme replacement therapy (ERT). All measurements were compared to age- and sex-matched control groups. MR and duplex sonography control values were taken from historic age- and sex-matched patients (n = 20, age 50 ± 14 years, ten females) with normal MR and sonography findings. In nine additional subjects, CVR was determined by Doppler sonography (age 46 ± 12 years, four females). Informed consent was received from all patients and CVR controls. The local ethics committee approved the study.
Gd-MRA
Imaging was performed using a 1.5-Tesla MR machine (Magnetom Sonata Vision, Siemens, Erlangen, Germany). MRA images were obtained after the injection of gadolinium contrast agent with an axial FLASH 3D sequence (TR 3.7 ms, TE 1.4 ms, flip angle 30° and a spatial resolution of 0.8 mm with isometric voxels). In addition a time-of-flight angiography (TR 37 ms, TE 7 ms, flip angle 25°, a spatial resolution of 0.5 mm with isometric voxels) was performed. Due to claustrophobia, patient P5 was examined by CT angiography (SOMATOM Sensation 64, Siemens). The diameter of the distal internal carotid artery (distal ICA, 1 cm proximal of the terminal end), the middle cerebral artery (MCA at M1 segment, 1 cm distal of its origin), the anterior cerebral artery (ACA at A1 segment, 1 cm distal of its origin), the vertebral artery (at V4 segment, 1 cm proximal of the basilar artery origin), and basilar artery (BA, 1 cm distal of the BA origin) were measured, blinded by a neuroradiologist (Fig. 1b). In both MRA sequences, the diameters were determined in the raw images. A diameter over three times the standard deviation (SD) of control values was defined as dilative arteriopathy. The elongation and tortuosity of cerebral arteries were visually assessed. The terms dolichoectasia and fusiform aneurysm were used to describe dilated arteries with elongation and tortuosity. White matter lesions were analysed by fluid-attenuated inversion recovery (FLAIR) images (TR 8,000 ms, TE 129 ms, TI 2,500 ms, slice thickness 5 mm) and classified by Fazekas score (grade 0 = absence; grade 1 = punctate; grade 2 = early confluent; or grade 3 = confluent lesions).
Fig. 1.
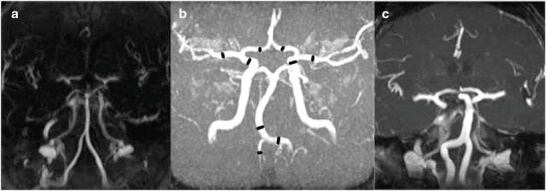
Gadolinium-enhanced MR angiography of three adults with Pompe disease, (a) normal vertebrobasilar arteries in P7, (b) tortuous but not significantly enlarged cerebral arteries in P2 (black markers present position of diameter and velocity measurements), (c) vertebrobasilar dolichoectasia in P10
Sonography
In the MCA, ACA, V4 and BA, at the same position as the MR diameter measurements, the peak systolic velocity (PSV) and the end-diastolic velocity (EDV) were angle-corrected and quantified by transcranial Doppler sonography (3-MHz probe, Acuson Sequoia™ 512, Siemens). Additionally, the diameter, the angle-corrected PSV and EDV in the vertebral artery at the proximal V2 segment (V2), the proximal ICA (proximal ICA, 1–2 cm after origin) and the common carotid artery (CCA, 2 cm before bifurcation) were measured with a 6-MHz probe. The MFV (in cm/s), the RI and the CBF (in mL/min) were calculated [MFV = (PSV + 2 * EDV)/3; RI = (PSV − EDV)/PSV; CBF = π * diameter2 * MFV/4]. The intima–media complex was determined at the distal CCA.
By head-mounted 2-MHz Doppler probes (Multi-Dop X4, DWL, Sipplingen, Germany), the function of resistance vessels in the MCA vascular bed was quantified in normocapnia and in a standardised setting by hyperventilation-induced vasoconstriction (CVRhyperventilation) and acetazolamide-induced vasodilation (CVRacetazolamide) (Eicke et al. 1999). CVR was calculated following 1 min of hyperventilation and 10 min after intravenous 1 g acetazolamide administration (CVR = 100 × [MFV stimulation − MFVbaseline]/MFVbaseline). A CVR outside the threefold standard deviation (SD) of control values was considered to be pathological.
Statistics
Values were given in mean ± SD; group differences were assessed with Mann–Whitney U tests. For Pearson correlations, the largest diameters were used.
Informed Consent and Ethics
Informed consent was obtained from all patients prior to inclusion. The study was approved by the local ethics committee of the Martin-Luther-University Halle-Wittenberg and followed the ethical standards of the Helsinki Declaration of 1975, as revised in 2000.
Results
Our adult patients with Pompe disease had mild to severe myopathy, and two of them (P3, P7) were on noninvasive ventilation support (Table 1). All patients were ambulatory, normocapnic (arterial pCO2 5.0 ± 0.5, range 4.1–5.7) and had normal kidney function. The incidence of cardiovascular risk factors was comparable to that of our control group (hypertension 40 vs. 60%, smoking 30 vs. 10%, hyperlipidaemia 40 vs. 50%, diabetes 20 vs. 40%, coronary heart disease 15 vs. 10%). None of the patients reported headache or symptoms related to cerebrovascular disease. FLAIR–MRI revealed punctate white matter lesions in 3/9 patients (P4, P8 and P9, each with Fazekas score 1). The duration between MR imaging and Duplex sonography was 49.7 ± 46.3 days in patients and 1.4 ± 1.4 days in controls.
Table 1.
Clinical characteristic from ten adult patients with Pompe disease
| Patient | P1 | P2 | P3 | P4a, b | P5 | P6 | P7 | P8b | P9a*,b | P10a* |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 21 | 37 | 44 | 44 | 46 | 47 | 54 | 56 | 64 | 71 |
| Sex | F | F | M | M | M | F | M | F | M | F |
| Duration of disease (years) | 10 | 10 | 4 | 12 | 10 | 12 | 9 | 16 | 11 | 30 |
| Duration of ERT (months) | 3 | 33 | 41 | 54 | 0 | 33 | 48 | 0 | 14 | 47 |
| GAA genotype | IVS1-13T>G | p.Leu552Pro | IVS1-13T>G | IVS1-13T>G | p.Pro493Leu | IVS1-13T>G | IVS1-13T>G | p.Pro493Leu | IVS1-13T>G | IVS1-13T>G |
| IVS9-1G>C | p.Pro493Leu | c.2136-7delGT | p.Trp499Arg | p.Cys103Gly | p.Leu552Pro | p.Cys103Gly | p.Cys103Gly | p.Gly309Arg | c.2481+102_2646+31del | |
| 6-minute walk test [m] | 510 | 295 | 380 | 130 | 420 | 375 | 380 | 420 | 120 | 60 |
| WGMS | 3 | 3 | 5 | 6 | 4 | 6 | 5 | 3 | 5 | 6 |
| Slow vital capacity (%) | 60 | 110 | 38, NVS | 53 | 94 | 65 | 50, NVS | 91 | 40 | 83 |
| Cardiovascular risk factors | HL, O | HT, HL | DM, HL | HT, DM, HL, O, S | HT, DM, HL, O | HT | HT, DM, O, CHD | HT |
aPatient with dilative arteriopathy (* and vertebrobasilar dolichoectasia)
bPatient with punctate white matter lesions
CHD coronary heart disease, DM diabetes mellitus, ERT enzyme replacement therapy, F female, HT arterial hypertension, HL hyperlipidaemia, M male, NVS noninvasive ventilation support, O obesity, S smoking, WGMS Walton Gardner Medwin scale
The diameter of extra- and intracranial arteries (MCA, distal ICA, BA, V4 and V2) was enlarged compared to age- and sex-matched controls (Table 2). Dilative arteriopathy was shown in three patients mostly affecting the vertebrobasilar arteries (P4 at BA, P9 at V4, P10 at V4, BA, distal ICA and MCA). Vertebrobasilar dolichoectasia was apparent in two of these patients (P9, P10) (Fig. 1c). The diameter of distal ICA, BA and V4 increased with the duration of disease (distal ICA, Pearson r = 0.65, p < 0.05; BA, Pearson r = 0.91, p < 0.0005; V4, Pearson r = 0.77, p < 0.05). MRA and sonography of the cerebral arteries showed no stenosis. The intima–media complex of the distal CCA was elevated in three patients (P2 1.4 mm, P5 1.1 mm and P7 1.5 mm).
Table 2.
Diameter, mean flow velocities and cerebral blood flow in adult patients with Pompe disease (n = 10) and controls (n = 20)
| Pompe patients | Control group | Mann–Whitney Test | |
|---|---|---|---|
| Mean ± SD | Mean ± SD (+3SD) | ||
| Diameter (D) in mm | |||
| MCAa | 2.3 ± 0.5 | 1.9 ± 0.4 (3.2) | p < 0.005 |
| ACAa | 1.6 ± 0.3 | 1.4 ± 0.3 (2.3) | n.s. (p = 0.07) |
| Distal ICAa | 3.4 ± 0.6 | 2.7 ± 0.5 (4.1) | p < 0.00005 |
| BAa | 3.7 ± 0.9 | 2.7 ± 0.5 (4.2) | p < 0.005 |
| V4a | 2.7 ± 1.0 | 1.8 ± 0.4 (3.1) | p < 0.0005 |
| V2b | 3.8 ± 0.7 | 3.1 ± 0.5 (4.6) | p < 0.005 |
| Proximal ICAb | 5.1 ± 0.6 | 4.9 ± 0.6 (6.6) | n.s. (p = 0.07) |
| CCAb | 6.2 ± 0.6 | 6.2 ± 0.8 (8.5) | n.s. (p = 0.89) |
| Mean flow velocity (MFV) in cm/s | |||
| MCAc | 50 ± 12 | 53 ± 16 | n.s. (p = 0.54) |
| ACAc | 43 ± 19 | 38 ± 16 | n.s. (p = 0.29) |
| BAc | 31 ± 8 | 36 ± 10 | n.s. (p = 0.73) |
| V4c | 27 ± 11 | 32 ± 7 | p < 0.05 |
| V2b | 14 ± 6 | 13 ± 6 | n.s. (p = 0.62) |
| Proximal ICAb | 30 ± 10 | 28 ± 11 | n.s. (p = 0.47) |
| CCAb | 30 ± 10 | 23 ± 8 | p < 0.05 |
| Cerebral blood flow (CBF) in mL/min | |||
| MCA | 120 ± 49 | 98 ± 51 | n.s. (p = 0.14) |
| ACA | 50 ± 27 | 39 ± 23 | n.s. (p = 0.18) |
| BA | 201 ± 57 | 127 ± 59 | p < 0.05 |
| V4 | 94 ± 57 | 52 ± 24 | p < 0.05 |
| V2 | 97 ± 66 | 60 ± 30 | p < 0.05 |
| Proximal ICA | 364 ± 111 | 311 ± 119 | n.s. (p = 0.06) |
| CCA | 516 ± 150 | 417 ± 142 | p < 0.05 |
aMeasured by gadolinium-enhanced MR angiography
bMeasured by extracranial duplex sonography
cMeasured by transcranial duplex sonography
ACA anterior cerebral artery, BA basilar artery, CCA common carotid artery, Distal ICA distal internal carotid artery, MCA middle cerebral artery, n.s. not significant, Proximal ICA proximal internal carotid artery, SD standard deviation, V2 vertebral artery at proximal V2 segment, V4 vertebral artery at distal V4 segment
The MFV was elevated in the CCA and decreased in V4 (Table 2). The CBF in the CCA, BA, V4 and V2 were elevated; proximal ICA, MCA and ACA revealed a trend towards increased CBF as well (Table 2). The RI was normal in all arteries but showed a trend towards decreased values in MCA, ACA and BA (0.09 > p > 0.06). CVR was not different between patients and controls (CVRhyperventilation −30.3 ± 19.8 vs. −27.2 ± 8.3%, CVRacetazolamide 36.5 ± 22.0 vs 37.9 ± 14.1%). CVRhyperventilation was unilaterally decreased in patient P9 and elevated in patient P5.
Discussion
We prospectively investigated the abnormalities of small and large cerebral arteries in adults with Pompe disease but without any cerebrovascular symptoms. We showed that the diameter of large cerebral arteries including the distal ICA and the MCA was enlarged leading to dilative arteriopathy in three patients. Vertebrobasilar dolichoectasia was found in the two oldest patients. This is a higher incidence that is reported for the general population, where the incidence of vertebrobasilar dolichoectasia was between 0.3 and 4.4% (Yu et al. 1982; Ubogu and Zaidat 2004). The predominance of dolichoectasia in the vertebrobasilar circulation has already been reported in adult Pompe patients (Laforêt et al. 2008; Sacconi et al. 2010). In our patients, however, the MCA and the distal ICA were also enlarged although the diameters of the proximal ICA and CCA were normal, implicating a more widespread, but not generalised, arterial dilation. Previous studies revealed similar findings: in adults with Pompe disease, the ascending aorta was found to be enlarged (El-Gharbawy et al. 2011), and recently a reduced diameter in the CCA was reported (Wens et al. 2014).
The pathological basis of dilative arteriopathy and dolichoectasia in the general population is unknown, but both conditions are usually associated with severe atherosclerosis. Dilative arteriopathy is combined with chronic kidney dysfunction, old age, male sex and cardiovascular risk factors as hypertension, smoking and coronary heart disease (Yu et al. 1982; Ichikawa et al. 2009). Old age and hypertension could have caused the dolichoectasia in P9 and P10. However, our adult Pompe patients had no to mild atherosclerosis and normal kidney function, and, as reported, most were younger (Yu et al. 1982; Ubogu and Zaidat 2004; Ichikawa et al. 2009). In some families with Pompe disease, the dilative arteriopathy accumulates at a premature age (Makos et al. 1987; Matsuoka et al. 1988; Cipullo et al. 2013), indicating the importance of hereditary factors. The presence of cardiovascular risk factors was distributed evenly among patients and controls. Compared with age- and sex-matched controls, the adult Pompe patients had substantially enlarged cerebral arteries. The diameters of distal ICA, BA and V4 increase with the duration of disease. In adult Pompe patients, dilative arteriopathy was histopathologically associated with glycogen accumulations, extensive vacuolar degeneration and necrosis within the vessel wall (Makos et al. 1987; Matsuoka et al. 1988; Kretzschmar et al. 1990).
Additionally, vasodilation, elongation and tortuosity of arteries supplying the brain are reported as consequences of increased CBF (Sho et al. 2004; Hoi et al. 2008). CBF is strongly related to the cerebral metabolism and controlled by the cross-sectional area of resistance vessels (Heistad and Kontos 1983): an increase of cross-sectional area due to dilation of resistance vessels leads to increased CBF. In the present study, CBF was increased in CCA, BA, V4 and V2. Therefore, temporarily or permanently dilated resistance vessels could cause dilative arteriopathy and dolichoectasia in adult Pompe patients. Histopathologically, dilation of numerous resistance vessels has been described in an adult with Pompe disease (Kretzschmar et al. 1990). Furthermore, dilation of resistance vessels would decrease the RI. In our patients, a clear trend towards reduced RI in MCA, ACA and BA was shown as compared to controls.
In patients with Pompe disease, two additional factors may lead to dilation of resistance vessels: partial pressure of blood carbon dioxide and glycogen-associated changes of vessel walls. The common occurrence of respiratory insufficiency leads to elevated partial pressures of carbon dioxide, causing vasodilation. Glycogen-filled vacuoles in the wall of resistance vessels (Kretzschmar et al. 1990) could disturb the production of extracellular matrix proteins like collagen and elastin (Dobrin 1978), of matrix metalloproteinases (Loftus and Thompson 2002), or of vasoactive substances like nitric oxide (McCarron et al. 2006). We suppose that confounding cardiovascular risk factors leading to cerebral microangiopathy diminish the effect of dilated resistance vessels on RI. The vasoconstriction and vasodilation function of resistance vessels is thus upheld, as is indicated in our patients by normal CVR.
In adult patients with Pompe disease, the abnormalities of cerebral arteries will probably increase with the duration of disease, resulting in increased risk of adjacent structures compression and cerebral ischemic and hemorrhagic stroke. We visualise the cerebral vessels in a newly diagnosed Pompe patient and suggest a follow-up every 5 years. We screen patients with siblings affected by dilative arteriopathy in shorter intervals. The changes of cerebral arteries and CBF should be examined in further studies.
Our study is limited by the small number of patients, the limited resolution of MR angiography, and the fact that MRA and Doppler examinations occurred at different dates. The cross-sectional area of resistance vessels can’t be measured directly; therefore, we had to use the CBF and RI as surrogates.
In conclusion, dilation of small and large cerebral arteries seems to be a common feature in adults with Pompe disease. Our data suggest CBF increase due to dilated resistance vessels. In contrast, dysfunction of resistance vessels was rarely seen.
Acknowledgements
We thank P. R. Joshi, S. Demuth and Z. Lukacs for the genetic and enzymatic analysis, K. Birch and A. C. F. van Maanen for copyediting the manuscript and O. Kuß for the statistical support. We also gratefully acknowledge the effort of all the patients who participated in the study.
Compliance with Ethics Guidelines
Conflict of Interest
F. Hanisch has received lecturer honoraria from Genzyme Corporation.
M. Deschauer has received payment for lectures and manuscript preparation and a grant from Genzyme Corporation.
T. Müller has received speaking fees from Boehringer Ingelheim.
O. Hensel, D. Stoevesandt and K. Stock report no disclosure.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients prior to inclusion.
Individual Contributions
O. Hensel: acquisition, analysis and interpretation of sonography data; drafting and revising the manuscript content, including the medical content; corresponding author
F. Hanisch: study concept and design; recruitment of patients; drafting and revising the manuscript content, including the medical content; interpretation of data
K. Stock: acquisition, analysis and interpretation of radiological data, drafting and revising the manuscript content
D. Stoevesandt: acquisition, analysis and interpretation of radiological data, drafting and revising the manuscript content
M. Deschauer: study concept and design, revising the manuscript content
T. Müller: study concept and design; drafting and revising the manuscript content, including medical writing of content; interpretation of data; study supervision and coordination; principal investigator
Footnotes
Competing interests: None declared
Ole Hensel and F. Hanisch have contributed equally.
Contributor Information
Ole Hensel, Email: ole.hensel@medizin.uni-halle.de.
Collaborators: Johannes Zschocke
References
- Cipullo F, Sampaolo S, Farina O, Simonetti M, Cirillo M, Di Iorio G. Cerebral vascular anomalies in a large Italian family with late-onset glycogenosis II. BMC Musculoskelet Disord. 2013;14(Suppl 2):P10. doi: 10.1186/1471-2474-14-S2-P10. [DOI] [Google Scholar]
- Dobrin PB. Mechanical properties of arteries. Physiol Rev. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- Eicke BM, Buss E, Bähr RR, Hajak G, Paulus W. Influence of acetazolamide and CO2 on extracranial flow volume and intracranial blood flow velocity. Stroke. 1999;30:76–80. doi: 10.1161/01.STR.30.1.76. [DOI] [PubMed] [Google Scholar]
- El-Gharbawy AH, Bhat G, Murillo JE, et al. Expanding the clinical spectrum of late-onset Pompe disease: dilated arteriopathy involving the thoracic aorta, a novel vascular phenotype uncovered. Mol Genet Metab. 2011;103:362–366. doi: 10.1016/j.ymgme.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Garancis JC. Type II glycogenosis. Biochemical and electron microscopic study. Am J Med. 1968;44:289–300. doi: 10.1016/0002-9343(68)90160-5. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Kontos HA. Cerebral Circulation. In: Shepherd JT, Abboud FM, editors. Handbook of physiology, Section 2: The Cardiovascular System. Washington DC: American Physiological Society; 1983. pp. 137–182. [Google Scholar]
- Hoi Y, Gao L, Tremmel M, et al. In vivo assessment of rapid cerebrovascular morphological adaptation following acute blood flow increase. J Neurosurg. 2008;109:1141–1147. doi: 10.3171/JNS.2008.109.12.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Takahashi N, Mukai M, Katoh H, Akizawa T, Kawamura M. Intracranial dilative arteriopathy is associated with chronic kidney disease and small vessel diseases in the elderly. J Stroke Cerebrovasc Dis. 2009;18:435–442. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Gläser D, Schmidt S, et al. Molecular diagnosis of German patients with late-onset glycogen storage disease type II. J Inherit Metab Dis. 2008;31(Suppl 2):S261–S265. doi: 10.1007/s10545-008-0820-2. [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Wagner H, Hübner G, Danek A, Witt TN, Mehraein P. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J Neurol Sci. 1990;98:169–183. doi: 10.1016/0022-510X(90)90258-O. [DOI] [PubMed] [Google Scholar]
- Laforêt P, Petiot P, Nicolino M, et al. Dilative arteriopathy and basilar artery dolichoectasia complicating late-onset Pompe disease. Neurology. 2008;70:2063–2066. doi: 10.1212/01.wnl.0000313367.09469.13. [DOI] [PubMed] [Google Scholar]
- Loftus IM, Thompson MM. The role of matrix metalloproteinases in vascular disease. Vasc Med. 2002;7:117–133. doi: 10.1191/1358863x02vm420ra. [DOI] [PubMed] [Google Scholar]
- Makos MM, McComb RD, Hart MN, Bennett DR. Alpha-glucosidase deficiency and basilar artery aneurysm: report of a sibship. Ann Neurol. 1987;22:629–633. doi: 10.1002/ana.410220512. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Senda Y, Hirayama M, Matsui T, Takahashi A. Late-onset acid maltase deficiency associated with intracranial aneurysm. J Neurol. 1988;235:371–373. doi: 10.1007/BF00314237. [DOI] [PubMed] [Google Scholar]
- McCarron RM, Chen Y, Tomori T, et al. Endothelial-mediated regulation of cerebral microcirculation. J Physiol Pharmacol. 2006;57(Suppl 11):133–144. [PubMed] [Google Scholar]
- Pompe JC. Over idiopathische hypertrophic van het hart. Ned Tijdschr Geneeskd. 1932;76:304–311. [Google Scholar]
- Sacconi S, Bocquet JD, Chanalet S, Tanant V, Salviati L, Desnuelle C. Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease. J Neurol. 2010;257:1730–1733. doi: 10.1007/s00415-010-5618-0. [DOI] [PubMed] [Google Scholar]
- Sho E, Nanjo H, Sho M, et al. Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress. J Vasc Surg. 2004;39:601–612. doi: 10.1016/j.jvs.2003.10.058. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Zaidat OO. Vertebrobasilar dolichoectasia diagnosed by magnetic resonance angiography and risk of stroke and death: a cohort study. J Neurol Neurosurg Psychiatry. 2004;75:22–26. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362:1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]
- Wens SCA, Kuperus E, Mattace-Raso FUS, et al. Increased aortic stiffness and blood pressure in non-classic Pompe disease. J Inherit Metab Dis. 2014;37:391–397. doi: 10.1007/s10545-013-9667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YL, Moseley IF, Pullicino P, McDonald WI. The clinical picture of ectasia of the intracerebral arteries. J Neurol Neurosurg Psychiatry. 1982;45:29–36. doi: 10.1136/jnnp.45.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]


