summary
Objective
We investigated the relationship between the molecular weight (MW) distribution of hyaluronan (HA) in synovial fluid (SF) and risk of knee osteoarthritis (OA) progression.
Methods
HA MW was analyzed for 65 baseline knee SFs. At 3-year follow-up, knees were scored for change in joint space narrowing (JSN), osteophyte (OST) progression, or occurrence of total knee arthroplasty (TKA). HA MW distribution was analyzed using agarose gel electrophoresis (AGE), and its relationship to OA progression was evaluated using logistic regression. The association between HA MW and self-reported baseline knee pain was analyzed using Pearson's correlation coefficients.
Results
Knee OA was categorized as non-progressing (OST−/JSN−, 26 knees, 40%), or progressing based on OST (OST+/JSN−, 24 knees, 37%), OST and JSN (OST+/JSN+, 7 knees, 11%) or total knee arthroplasty (TKA, 8 knees, 12%). The MW distribution of HA in baseline SFs was significantly associated with the odds of OA progression, particularly for index knees. After adjusting for age, gender, BMI, baseline X-ray grade and pain, each increase of one percentage point in %HA below 1 million significantly increased the odds of JSN (odds ratios (OR) = 1.45, 95% CI 1.02–2.07), TKA or JSN (OR = 1.24, 95%CI 1.01–1.53) and the odds of any progression (OR = 1.16, 95% CI 1.01–1.32). HA MW distribution significantly correlated with pain.
Conclusion
These data suggest that the odds of knee OA progression increases as HA MW distribution shifts lower and highlight the value of reporting MW distribution rather than just average MW values for HA.
Keywords: Hyaluronan, Osteoarthritis progression, Molecular weight, Synovial fluid
Introduction
Hyaluronan (commonly referred to as hyaluronic acid or HA) is the polysaccharide responsible for the viscous and elastic properties of synovial fluid (SF)1,2. Since the mid-twentieth century, a reduction in the viscosity of SF has been associated with synovial joint pathologies. Ropes et al. suggested that the reduction in the viscosity of SF resulted from reduced polymerization of HA, and was useful for the differential diagnosis of joint disease3. Shortly thereafter, the severity of inflammatory joint disease was reported to be associated with a decrease in the concentration and molecular weight (MW) of HA in SF4. Sundblad demonstrated that degenerative joint disease (arthrosis deformans), commonly referred to as osteoarthritis (OA), was likewise associated with a decrease in the average MW of HA in SF based on intrinsic viscosity measurements5. During the subsequent decades, the differences between normal and OA SFs were characterized using multiple methods. Generalizable relationships were described between the concentration and average MW of HA, SF viscosity and elasticity, and the effect of these parameters on biological processes relevant to joint pathology6,7,8,9,10. In all of the above described relationships between HA MW and its biological activities, average values were used for the MW of HA. Few prior studies have reported the MW distribution of HA in SF and its change in patients with knee OA11. In addition, none to our knowledge have specifically examined how the MW distribution of HA in SF is related to risk of knee OA progression.
The concentration of HA in blood has likewise been reported to change in patients with joint disease. Rheumatoid arthritis is associated with a significant and sustained increase in the plasma concentration of HA, which increased significantly with physical activity and was sensitive to the total body load of inflamed joints12,13. Plasma HA levels have also been suggested as an index of the overall body burden of OA14. Several studies have reported that elevation of the serum HA concentration is associated with the risk of knee OA progression15,16,17. None of these prior studies have clarified the molecular basis for the reported relationship between serum HA concentration and knee OA progression.
We therefore analyzed the MW distribution of HA in SF samples available from the NIH-sponsored POP study (Prediction of OA Progression), for which 3-year follow-up radiological data on knee OA progression are available, including data on interval knee joint replacement during the 3-year study period. We hypothesized that the preponderance of low MW HA in SF would be associated with the risk of OA progression.
Materials and methods
Patients, SF samples and radiographic progression status
As previously described18, patients enrolled in the POP study had to have at least one knee with confirmed symptomatic and radiographic OA (the index knee), and to have consented to SF withdrawal from both the index and contralateral knees. Baseline SFs were available for 65 patient-knees from the 40 patients involved in the NIH-sponsored POP study18; 40 of the 65 SF samples analyzed were from the index knee used for inclusion in the original POP study. The remaining 25 SF samples were from the contralateral knees for 25 of the 40 patients. Samples were included if there was sufficient volume of SF, collected without lavage, to analyze the MW distribution of HA. Therefore, the study populations on whom we report here were enriched in patients with effusion and clinical symptoms of inflammation at baseline. Exclusion criteria included corticosteroid or hyaluronan injection within the previous 3 months, history of avascular necrosis, rheumatoid or other inflammatory arthropathy, periarticular fracture, Paget's disease, villonodular synovitis, joint infection, ochronosis, neuropathic arthropathy, acromegaly, hemochromatosis, Wilson's disease osteochondromatosis, arthroscopic knee surgery within the previous 12 months, and bilateral knee replacement. Participants were allowed to take all of their usual medications, including nonsteroidal anti-inflammatory drugs and dietary supplements such as vitamins and glucosamine and/or chondroitin sulfate.
Posteroanterior fixed-flexion knee radiographs were obtained at baseline and 3 years with the SynaFlexer lower limb positioning frame (Synarc, San Francisco, California, USA) with a 10° caudal X-ray beam angle. X-rays were scored with high inter-rater reliability, as previously reported19 for individual radiographic features of OA in the medial and lateral compartments for joint space narrowing (JSN) and osteophytes (OST) on a scale of 0–3 using the OARSI standardized atlas20. Total scores were 0–6 for JSN and 0–12 for OST as all four margins on the knee joint were scored for this feature. Knees were scored as either positive (+) or negative (−) for JSN and OST separately, with an at least one-unit change defined as positive for progression. Kellgren–Lawrence radiographic scores were assigned to each knee at baseline21. Knee symptoms were ascertained by the National Health and Nutrition Examination Survey (NHANES) I criterion22 of pain, aching, or stiffness on most days of any 1 month in the last year; for subjects answering yes, symptoms were quantified as none, mild, moderate, or severe, yielding a total score of 0–3 for each knee.
Analytic methods
SF HA concentration was determined using a commercially available kit (Corgenix, Westminster, CO, USA). Prior to electrophoretic analysis, SF samples were treated with 50 µg pronase per µg HA to minimize interference due to contaminating proteins. The MW distribution of HA in each SF sample was determined by agarose gel electrophoresis (AGE)23, using MW standards for HA that were synthesized to provide a narrow MW distribution, and hence discreet bands on AGE. Three sets of standards were used to cover a broad MW range: Super-mega ladder standards (SL, 2–8 million); Mega ladder standards (ML, MW 1.5–6.1 million); and High ladder standards (HL, MW 0.51–1.5 million) (Hyalose, Inc. Oklahoma City, OK USA). SF samples were diluted to a final HA concentration of 0.3mg/ml, so that 1.5 µg of HA (5 µl) was equally applied to all lanes. Gels were stained in 0.005% Stains-All (3,3′-dimethyl-9-methyl-4,5,4′5′-dibenzothiacarbocyanine) in 50% ethanol for 16–24 h, destained in 10% ethanol for 4–6 h, and scanned using a UMAX Powerlook III scanner. The optical density of bound dye was analyzed using Image Master 1D Elite, version 4.10, Amersham Biosciences, and plotted as the weight fraction (percent) of the total HA-bound dye relative to the MW standards. For each SF sample, we calculated the weight average molecular weight (Mw) of the HA, and the weight fraction (weight-%) of the total HA-bound dye below an MW of 1 million. The arbitrary cutoff of 1 million was selected to focus on the preponderance of HA molecules with below-normal MW. All analyses were performed with the investigator blinded to the progression status of the patient-knee associated with a given SF sample.
Statistical methods
Results are presented both for the set index knees, where independence between knees justifies straightforward analyses, and for the set of all knees, where the correlation between index and contralateral knees in the same patient must be addressed.
Logistic regression was used to model the relationship between the MW distribution of HA and OA progression over a 3-year period. Covariates included in the model were BMI, age, gender, baseline pain and radiographic grade. Generalized estimating equations (GEE)24 were used to adjust for the correlation between same-patient knees in the all-knee sample. In order to study the association between baseline HA parameters and baseline pain, Pearson's correlations were used and Fisher's Z-tests of significance were performed.
Descriptive statistics were used to compare the population in whom HA MW distribution was analyzed to the rest of the POP population (i.e., those knees from the POP population for which sufficient SF sample was not available for HA analysis). This was done for both the index knee sample analyzed (40 knees vs 119 knees) and the full sample of study knees analyzed for HA (65 knees vs 253 knees). Descriptive comparison of these cohorts was done using Student's t-test (with clustered errors for the all-knee sample).
Results
Patient population
The 65-knee sample and the 40 index-knee-only sample analyzed in this study were generally typical of OA study populations on most of the available characteristics. Although the SFs analyzed for HA came from knees yielding somewhat more SF than the rest of the POP population, these patients were similar to the total POP population, with the exception of slightly greater mean BMI at baseline in the 40-knee sample and slightly greater mean knee pain at baseline in the 65-knee sample (Table I).
Table I.
Demographic comparison of the patient-knee samples at baseline
| Index knee | All knees | |||||
|---|---|---|---|---|---|---|
| Non-HA index cohort | HA index cohort | P-value† | Non-HA cohort | HA cohort | P-value‡ | |
| Patient-knees (n) | 119 | 40 | – | 253 | 65 | – |
| Mean Age (SD) | 63.3 (11.5) | 64.8 (12.7) | 0.53 | 63.3 (11.7) | 65.3 (12.0) | 0.41 |
| Sex (% Female) | 77 | 68 | 0.29 | 77 | 65 | 0.21 |
| Baseline Pain (0–3 scale) (SD) | 1.7 (0.68) | 1.8 (0.59) | 0.16 | 1.4 (0.75) | 1.8 (0.65) | 0.01* |
| BMI | 30.5 (6.5) | 33.2 (7.0) | 0.03* | 30.7 (6.5) | 33.0 (7.3) | 0.12 |
| KL grade distribution | ||||||
| 0 | 3% | 0% | 0.08 | 2% | 2% | 0.78 |
| 1 | 24% | 15% | 0.18 | 24% | 12% | 0.16 |
| 2 | 14% | 10% | 0.46 | 22% | 11% | 0.22 |
| 3 | 53% | 68% | 0.1 | 41% | 57% | 0.03* |
| 4 | 6% | 7% | 0.73 | 7% | 19% | 0.07 |
Indicates statistically significant difference, P < 0.05.
Comparison between 40 index knees and the rest of the index knees in the HA POP population.
Comparison between 65 knees in HA cohort to the rest of the POP study population. Clustered errors used to account for within-patient correlations.
HA analysis
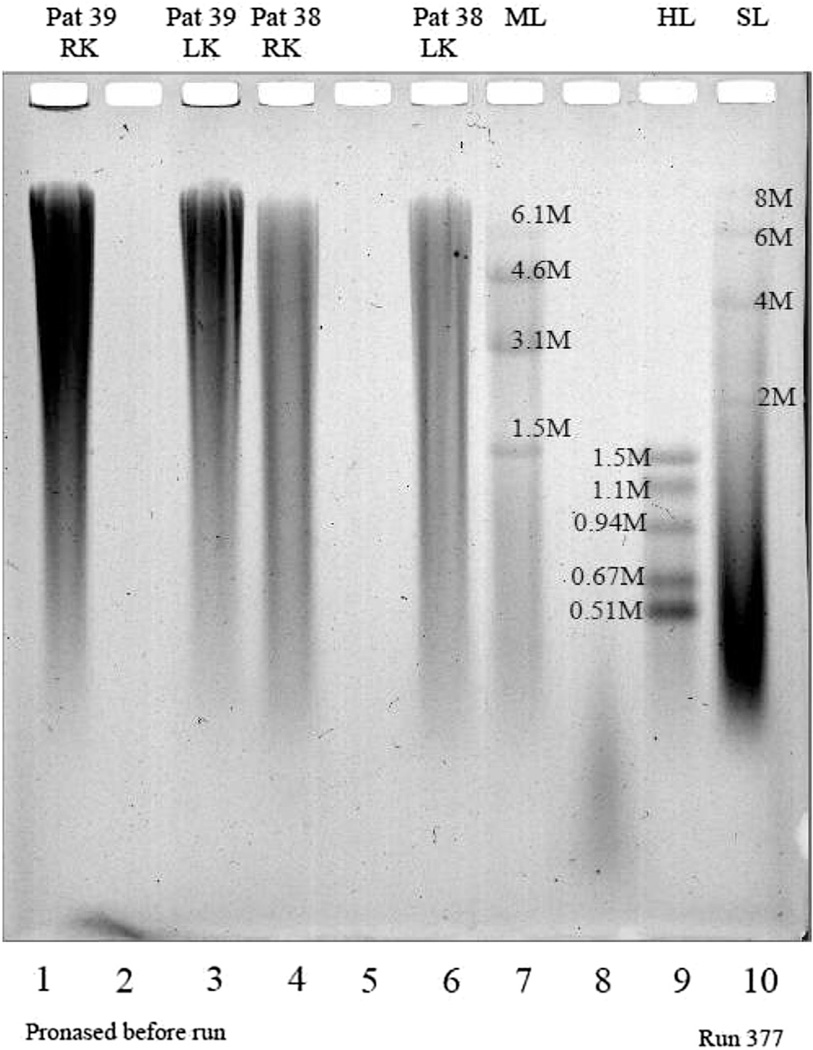
Figure 1 illustrates the MW distribution of HA on agarose gels from two representative SF samples. Patients #38 and #39 were chosen as examples because both had SF available for both the right knee (RK) and left knee (LK), and they covered an interesting range of baseline Kellgren–Lawrence (K-L) grades and 3-year outcomes; patient #38 had bilateral knee K-L grade 2 at baseline and total knee arthroplasty (TKA) outcomes for both knees; patient #39 had bilateral knee K-L grade 3 at baseline and progression of osteophytes (OST+/JSN) for both knees. The size distribution of HA was determined by comparison with MW standards. Unlike the standards, SF HA did not run as discrete MW bands. This is because naturally occurring HA is very polydisperse with respect to MW compared to the HA standards, which are synthesized using carefully timed enzymatic reactions that enable production of a very narrow MW distribution and hence sharp electrophoretic bands. Visible differences in the MW distribution of HA are apparent between the patient-knees that progressed to TKA and those that only progressed with respect to OST but not JSN (OST+/JSN−); namely, the SF of the knees of patient #38 (that progressed to TKA) had a larger proportion of lower MW HA compared with SF from the knees of patient #39. It is also interesting to note that the two knees of each patient had a similar MW distribution, and likewise, had the same 3-year outcome.
Fig. 1.
Illustration of a representative result of hyaluronan (HA) electrophoresed on an agarose gel to analyze the MW distribution of HA. Patients #38 and #39 were chosen as examples because both had SF available for both the RK and LK. Patient #38 had K-L grade 2 for both knees at baseline and TKA outcome for both knees. Patient #39 had K-L grade 3 in both knees at baseline and OST+/JSN− outcome for both knees. Patient samples are in lanes 1 (#39 LK), 3 (#39, RK), 4 (#38, LK) and 6 (#38, RK). Lanes 7, 9 and 10 represent three sets of HA standards to cover a broad MW range: lane 7 Mega ladder (ML) standards are 1.5, 3.1, 4.4 and 6.1 million MW; lane 9 High ladder (HL) standards are 0.51, 0.67, 0.94, 1.14 and 1.5 million MW; and lane 10 Super-mega ladder (SL) standards are 2, 4, 6 and 8 million MW.
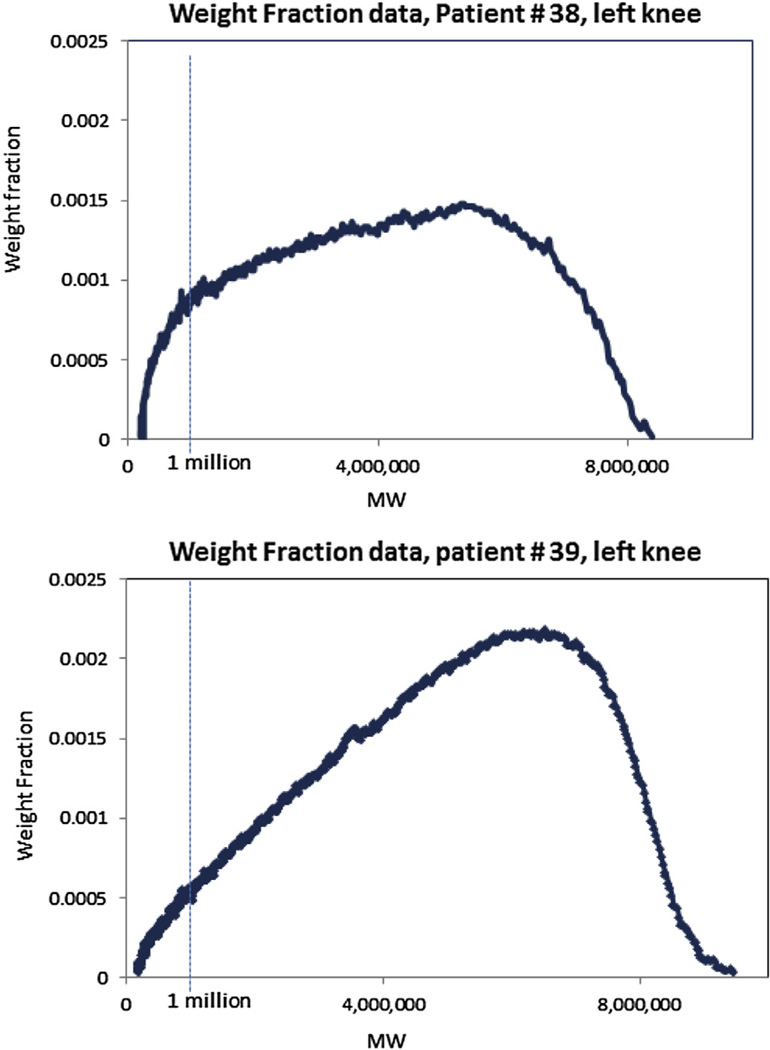
Figure 2 plots the optical absorption of the HA-bound dye as a function of MW, from laser scans of the agarose gel in Fig. 1, for Patient 38, LK (TKA outcome) and patient 39, LK (OST+/JSN− outcome). The dye absorption value is expressed as the weight fraction – i.e., the percent of the total amount of HA-bound dye in the gel, at each MW interval. These plots are used to calculate the samples' average MW (Mw), and the weight % of HA below 1 million, as illustrated by the dotted line at the MW corresponding to 1 million. The latter is used as an index of the preponderance of lower MW species in the SF sample. These plots highlight the difference in MW distribution between the two samples better than the agarose gels themselves, and enable quantification of between-patient differences. In these examples, patient #38 has a weight % below 1 million of 26% and 28% for the left and RK samples respectively, while patient #39 has a weight % below 1 million of 14% and 11% for left and RK samples respectively.
Fig. 2.
Laser scan of selected lanes from the agarose gel in Fig. 1 for SF from patient # 38, LK, TKA outcome (top panel); and patient # 39, LK OST+/JSN− outcome (bottom panel). The area under the curve to the left of the vertical 1 million MW line represents the weight %, preponderance of lower MW species in a sample.. In these examples, patient #38 has a weight % below 1 million of 26% for the LK sample, while patient #39 has a weight % below 1 million of 14% for LK sample.
Knee OA progression over the 3-year study period
Table II reports the means and SDs for each HA MW distribution parameter for the patient subgroups by OA progression status. Overall, approximately 12% (eight patient-knees) underwent TKA surgery within the 3-year study period (exact timing is unknown), 11% experienced radiological progression with respect to both OST and JSN, 37% experienced only OST progression and 40% experienced no detectable radiologic deterioration. A similar distribution was observed for the index-knee-only cohort (8%, 10%, 30%, and 52%, respectively). Notably, no patients fell into the JSN+/OST− group, indicating that all patients who experienced progressive JSN also experienced progressive OST, but the converse was not true.
Table II.
Mean (SD) values for the HA MW distribution parameters according to OA progression status, for the index knee sample (N = 40), and the sample of all available knees (N = 65)
| OA progression categories | N (%) | HA-weight average MW |
HA-weight percent < 1 mil |
|---|---|---|---|
| Index-knee only (N = 40) | |||
| Non-progression (JSN−/OST−) | 21 (53) | 3.9 (0.55) | 11.9 (5.09) |
| OST progression only (JSN−/OST+) | 12 (30) | 3.86 (0.62) | 13.42 (5.78) |
| Progression for both OST and joint space width (JSN+/OST+) | 4 (10) | 3.17 (0.59) | 21.5 (8.19) |
| TKA patients | 3 (8) | 3.4 (0.53) | 19 (4.36) |
| All available knees (N = 65) | |||
| Non-progression (JSN−/OST−) | 26 (40) | 3.92 (0.56) | 11.5 (5.22) |
| OST Progression only (JSN−/OST+) | 24 (37) | 3.75 (0.87) | 14.58 (5.76) |
| Progression for both OST and joint space width (JSN+/OST+) | 7 (11) | 3.66 (0.95) | 17 (9.5) |
| TKA patients | 8 (12) | 3.51 (0.5) | 16.75 (7.07) |
Association between HA MW distribution and the risk of OA progression
Table III provides the results of logistic regression analyses performed to evaluate the relationship between the MW distribution of SF HA and the risk of knee OA progression, reported as odds ratios (OR) and 95% confidence intervals. For Mw, the odds of TKA or JSN progression decreased (OR less than 1) as Mw increased. For the weight-percent of HA below 1 million, the odds of TKA or JSN progression increased (OR greater than 1) as the percent of the HA below an MW of 1 million increased. The relationship between the MW distribution of HA and the risk of OA progression was most apparent for analyses that only included the index knees (N = 40). The weight-percent of HA below an MW of 1 million was the MW distribution parameter that most consistently predicted the odds of knee OA progression.
Table III.
Association between HA MW distribution parameters and the risk of OA progression
| Index Knees (40) | ||||
| TKA (N = 3) | +/+ (N = 4) | TKA or +/+ (N = 7) | Any progression (TKA, +/+, +/−) (N = 19) | |
| Weight Average MW Mean (OR and 95% Confidence Intervals) | ||||
| No adjustment | 0.285 (0.031, 2.6) | 0.069 (0.048, 0.999) | 0.107 (0.0151, 0.757) | 0.472 (0.154, 1.45) |
| Adjusted for BMI, Age, Gender, Pain, XR grade | NA | 0.002 (0, 1.45) | 0.104 (0.0099, 1.09) | 0.412 (0.109, 1.56) |
| Weight Fraction < 1 mil (OR and 95% Confidence Intervals) | ||||
| No adjustment | 1.11 (0.933, 1.33) | 1.27 (1.02, 1.57) | 1.25 (1.05, 1.48) | 1.12 (0.998, 1.27) |
| Adjusted for BMI, Age, Gender, Pain, XR grade | 1.24 (0.642, 2.41) | 1.45 (1.02, 2.07) | 1.24 (1.01, 1.53) | 1.16 (1.01, 1.33) |
| All Knees (65) | ||||
| TKA (8) | +/+ (7) | TKA or +/+ (15) | Any progression (TKA, +/+, +/−) (39) | |
| Weight Average MW Mean (OR and 95% Confidence Intervals) | ||||
| No adjustment | 0.502 (0.183, 1.38) | 0.676 (0.137, 3.35) | 0.416 (0.116, 1.5) | 0.573 (0.226, 1.46) |
| Adjusted for BMI, Age, Gender, Pain, XR grade | 0.716 (0.175, 2.93) | 0.959 (0.165, 5.59) | 0.833 (0.23, 3.01) | 0.776 (0.254, 2.38) |
| Weight Fraction < 1 mil (OR and 95% Confidence Intervals) | ||||
| No adjustment | 1.08 (0.966, 1.2) | 1.1 (0.951, 1.28) | 1.12 (1.01, 1.24) | 1.12 (1.04, 1.21) |
| Adjusted for BMI, Age, Gender, Pain, XR grade | 1.01 (0.89, 1.16) | 1.07 (0.889, 1.28) | 1.05 (0.931, 1.18) | 1.1 (0.981, 1.23) |
Logistic regression was used to analyze the association (OR, and 95% CI) between the baseline MW distribution parameters of HA in synovial fluid, and the risk of radiographic progression or TKA over the 3-year study period. For the all-knee analysis, GEE were used to account for within-patient correlations. For Mw, the OR is expressed per 1 million-unit increase in MW. For the weight-percent below 1 million, the OR is expressed per 1 percent increase. +/+ = joint space narrowing and OST progression; +/− = OST progression only; significant values are in bold.
A strong association between the MW distribution of HA and OA progression was evident using logistic regression on the index knee sample. Mw was significantly associated with the odds of JSN (OR = 0.069, 95% CI (0.048–0.999) and TKA or JSN (OR = 0.107, 95% CI 0.015–0.757), in the model without covariates. Importantly, the weight-percent of HA below 1 million remained significantly associated with the odds of progression when the full set of covariates was included in the model. After adjusting for age, gender, BMI, baseline X-ray grade and baseline pain, each 1 percentage point increase in the weight-percent of HA below 1 million was significantly associated with the odds of JSN (OR = 1.45, CI 1.02–2.07), TKA or JSN (OR = 1.24, CI 1.01–1.53)) and the odds of any progression (OR = 1.16, CI 1.01–1.32). This analysis predicts that an increase of 5 percentage points in the weight-percent of HA below 1 million would correspond to an approximately 6-fold increase (=1.455) in the odds of progressive JSN in the index knee within the range of values reported here. These analyses also demonstrate the utility of objective radiologic measures, compared to complex measures such as the decision to undergo TKA, which is impacted by multiple social and psychological factors.
For the full 65-knee cohort, Mw was not significantly associated with the odds of OA progression. However, the weight-percent of HA below 1 million was significantly associated with the odds of TKA or JSN (OR = 1.12, 95% CI 1.01–1.24), and with the odds of any progression (OR = 1.12, 95% CI 1.04–1.21) when using the model that did not include any covariates, but not in the model that included all covariates.
Association between baseline pain and HA MW distribution
All of the MW distribution parameters significantly correlated with baseline pain in this patient population (Table IV). This was true for both the full 65 patient-knee sample, as well as for the index-knee only sample. Mw negatively correlated to pain, indicating that pain increased as Mw decreased. The percent of HA below 1 million positively correlated to pain, indicating that as the proportion of low MW species increased, pain increased.
Table IV.
Association between MW distribution parameters and baseline pain
| MW distribution parameters | Correlation to baseline pain* | P-value |
|---|---|---|
| Index-knee only (n = 40) | ||
| Weight Average Mw | −0.35 | 0.03 |
| Weight Percent < 1 mil | 0.41 | 0.009 |
| All available knees (n = 65) | ||
| Weight Average Mw | −0.363 | 0.003 |
| Weight Percent < 1 mil | 0.392 | 0.001 |
Pearson correlation.
Discussion
This study was motivated by two distinct lines of prior research: (1) studies reporting that the HA in SF from patients with OA of the knee has, on average, a lower concentration and MW compared to SF from normal knees6,25; and (2) studies reporting an association between the concentration of HA in serum and the risk of OA progression15–17. Our analyses specifically address previously unexplored aspects of the relationship between OA progression and the MW of HA in SF, namely, the importance of measuring MW distribution and the preponderance of lower MW HA. These analyses are intended to contribute to understanding the physiologic and pathologic mechanisms that underlie the relationship between HA and OA.
We report that a shift in the MW distribution of SF HA toward lower values is associated with an increased risk for rapid OA progression. Because HA can be cleaved by reactive oxygen species generated during inflammation26,27, this finding is consistent with the previously hypothesized relationship between inflammation and rapid OA progression28,29. It is also consistent with data reporting that high MW HA down-regulates inflammatory cell activity8,10, and that HA fragments stimulate innate immune system activity30,31. Our analyses therefore support the potential relevance of evaluating the MW distribution of HA in SF as a means for differentiating inflammatory and rapidly progressing OA phenotypes from patients having a less progressive disease phenotype32. These results also highlight the potential relevance of inflammation as a therapeutic target for disease-modifying interventions. Although not practical for clinical use as a diagnostic tool, AGE of SF reveals the utility of measuring the MW distribution of HA, and the limitations imposed when only average MW values are considered.
We also found that baseline pain was negatively correlated with the MW of SF HA. This is consistent with prior reports demonstrating an inverse relationship between pain, the rheological properties of SF, and the average MW of SF HA33–36. Broadly speaking, all these studies report that a decrease in SF viscoelasticity, and/or the MW and concentration of HA, is associated with increased pain. Our analyses confirm this relationship in human subjects, and additionally show that the preponderance of low MW HA is significantly correlated to knee pain. As would be predicted, pain increased as Mw decreased, while pain increased as the weight % of HA below 1 million increased. This relationship between HA and pain is consistent with an association between low MW HA and inflammation.
We performed separate analyses of the 40 index knees, and the full 65-knee cohort from which SF was available. The index knees presumably represent a more uniform and representative sample because they were used as the basis for inclusion in the original POP study. In addition, the index knees are all from different patients and are therefore independent of each other. We believe that our observing similar results in both the full sample and the index knee-only sample adds strength to our conclusions, and demonstrates the robustness of the analysis.
There are a number of important caveats to bear in mind when interpreting these results. Compared with the full POP cohort, the 65 knee samples from which SF was available for this study represent knees that yielded a greater volume of SF at baseline, and which did not require lavage to obtain a SF sample. Radiologic severity of disease was also somewhat more advanced in the sample we analyzed. This may limit the generalizability of our conclusions to other OA populations. A second important limitation of our analysis is that the statistically significant relationships we report should be interpreted as descriptive rather than conclusive, because they do not take multiplicity into account. Our results need to be confirmed by a prospective study that is appropriately powered for the multiple associations being analyzed. Strengths of our analysis include the blinding of all personnel performing laboratory measurements to progression status, the scientific plausibility of the relationships described, and the consistency of our findings with conclusions drawn in other studies of inflammatory arthritic conditions37.
In conclusion, our data suggest that baseline OA pain, and the risk of knee OA progression, are associated with the MW distribution of SF hyaluronan. The use of MW distribution parameters for HA, rather than simple MW averages, provides an important tool for probing the molecular basis of the relationship between HA and OA, and potentially between HA and inflammation.
Acknowledgments
This work was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (RO1AR48769 and P01 AR050245) and the National Institute of Aging (5P30 AG028716) at the National Institutes of Health (VBK), by a research grant from The Vilcek Foundation, Inc. (H-GW), and a research grant from the Rudin Foundation (PB).
Role of the funding source
Study sponsors did not have any role in study design, data collection or analysis.
Footnotes
Contributions
Philip A Band: conception and design, analysis and interpretation of data, drafting of article, administrative and logistic support, critical revision of manuscript, final approval of manuscript, obtaining of funding.
Julie Heeter: data acquisition, technical and logistic support, collection and assembly of data, analysis of data, critical revision of manuscript, final approval of manuscript.
Hans-Georg Wisniewski: conception and design, analysis and interpretation of data, drafting of article, final approval of manuscript.
Victoria Liublinska: analysis of data, statistical expertise, drafting of article, critical revision of manuscript, final approval of manuscript.
Cassandra W Pattanayak: statistical expertise, critical revision of manuscript, final approval of manuscript.
Raj J Karia: analysis of data, collection and assembly of data, final approval of manuscript.
Thomas V Stabler: collection and assembly of data, administrative and logistic support, provision of study materials, final approval of manuscript.
Endre A Balazs: conception and design, provision of study materials, analysis and interpretation of data, drafting of article, critical revision of manuscript, final approval of manuscript, obtaining of funding.
Virginia B Kraus: conception and design, provision of study materials, analysis and interpretation of data, drafting of article, administrative and logistic support, critical revision of manuscript, final approval of manuscript, obtaining of funding.
The authors P A Band (philip.band@nyumc.org) and H-G Wisniewski (hans-georg.wisniewski@nyumc.org), V Liublinska (vliublin@fas.harvard.edu), VB Kraus (vbk@duke.edu) take responsibility for the integrity of the work as a whole.
Conflicts of interest
No authors report any conflict of interest.
Contributor Information
P.A. Band, Email: philip.band@nyumc.org, phlp.band@gmail.com.
H.-G. Wisniewski, Email: hans-georg.wisniewski@nyumc.org.
V. Liublinska, Email: vliublin@fas.harvard.edu.
V.B. Kraus, Email: vbk@duke.edu.
References
- 1.Balazs EA, Sundblad L. Viscosity of hyaluronic acid solutions containing proteins. Acta Soc Med Ups. 1959;64:137–146. [PubMed] [Google Scholar]
- 2.Balazs EA. Viscosity of hyaluronic acid solutions containing proteins. Acta Soc Med Ups. 1959;64 [PubMed] [Google Scholar]
- 3.Ropes M, Robertson WB, Rossmeisl EC, Peabody RB, Bauer W. Synovial fluid mucin. Acta Med Scand. 1947;128:700. [Google Scholar]
- 4.Ragan C, Meyer K. The hyaluronic acid of synovial fluid in rheumatoid arthritis. J Clin Invest. 1949;28:56–59. doi: 10.1172/JCI102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundblad L. Studies on hyaluronic acid in synovial fluids. Acta Soc Med Ups. 1953;58 [PubMed] [Google Scholar]
- 6.Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967;10:357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs DA, Merrill EW, Smith KA, Balazs EA. Rheology of hyaluronic acid. Biopolymers. 1968;6:777–791. doi: 10.1002/bip.1968.360060603. [DOI] [PubMed] [Google Scholar]
- 8.Darzynkiewicz Z, Balazs EA. Effect of connective tissue intercellular matrix on lymphocyte stimulation. Exp Cell Res. 1971;66:113–123. doi: 10.1016/s0014-4827(71)80018-6. [DOI] [PubMed] [Google Scholar]
- 9.Balazs EA. Viscoelastic properties of hyaluronic acid and biological lubrication. Univ Mich Med Cent J. 1968:255–259. [PubMed] [Google Scholar]
- 10.Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435–446. [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 1994;219:278–287. doi: 10.1006/abio.1994.1267. [DOI] [PubMed] [Google Scholar]
- 12.Engstrom-Laurent A, Hallgren R. Circulating hyaluronic acid levels vary with physical activity in healthy subjects and in rheumatoid arthritis patients. Relationship to synovitis mass and morning stiffness. Arthritis Rheum. 1987;30:1333–1338. doi: 10.1002/art.1780301203. [DOI] [PubMed] [Google Scholar]
- 13.Poole AR, Witter J, Roberts N, Piccolo F, Brandt R, Paquin J, et al. Inflammation and cartilage metabolism in rheumatoid arthritis. Studies of the blood markers hyaluronic acid, orosomucoid, and keratan sulfate. Arthritis Rheum. 1990;33:790–799. doi: 10.1002/art.1780330605. [DOI] [PubMed] [Google Scholar]
- 14.Kraus VB, Kepler TB, Stabler T, Renner J, Jordan J. First qualification study of serum biomarkers as indicators of total body burden of osteoarthritis. PLoS One. 2010;5:e9739. doi: 10.1371/journal.pone.0009739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharif M, George E, Shepstone L, Knudson W, Thonar EJ, Cushnaghan J, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995;38:760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- 16.Pavelka K, Forejtova S, Olejarova M, Gatterova J, Senolt L, Spacek P, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2004;12:277–283. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65:1050–1054. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, et al. Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis. 2009;68:1673–1679. doi: 10.1136/ard.2008.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniel G, Renner JB, Sloane R, Kraus VB. Association of knee and ankle osteoarthritis with physical performance. Osteoarthritis Cartilage. 2011;19:634–638. doi: 10.1016/j.joca.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MA, Ettinger WH, Neuhaus JM. Obesity and osteoarthritis of the knee: evidence from the National Health and Nutrition Examination Survey (NHANES I) Semin Arthritis Rheum. 1990;20:34–41. doi: 10.1016/0049-0172(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 23.Cowman MK, Chen CC, Pandya M, Yuan H, Ramkishun D, LoBello J, et al. Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal Biochem. 2011;417:50–56. doi: 10.1016/j.ab.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Liang KY, Zeger SL. Longitudinal analysis using generalized linear models. Biometrika. 1986;73 [Google Scholar]
- 25.Balazs EA. The Physical Proerties of Synovial Fluid and the Special Role of Hyaluronic Acid. Phialdelphia, PA: Lippincott; 1982. [Google Scholar]
- 26.Henderson EB, Grootveld M, Farrell A, Smith EC, Thompson PW, Blake DR. A pathological role for damaged hyaluronan in synovitis. Ann Rheum Dis. 1991;50:196–200. doi: 10.1136/ard.50.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995;54:505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 29.Doherty M. Synovial inflammation and osteoarthritis progression: effects of nonsteroidal antiinflammatory drugs. Osteoarthritis Cartilage. 1999;7:319–320. doi: 10.1053/joca.1998.0179. [DOI] [PubMed] [Google Scholar]
- 30.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 31.Pure E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan. Cell Signal. 2009;21:651–655. doi: 10.1016/j.cellsig.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felson D, Niu J, Sack B, Aliabadi P, McCullough C, Nevitt MC. Progression of osteoarthritis as a state of inertia. Ann Rheum Dis. 2013;72:924–929. doi: 10.1136/annrheumdis-2012-201575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3–9. doi: 10.1007/pl00005742. [DOI] [PubMed] [Google Scholar]
- 34.Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50:314–326. doi: 10.1002/art.11421. [DOI] [PubMed] [Google Scholar]
- 35.Band P, Goldman AI, Cowman MK, MOreland L, Lee HG, Balazs EA. Proceedings of the European League against Rheumatism. Glasgow, Scotland: 1999. Correlation of knee pain with synovial fluid properties in osteoartritic patients. [Google Scholar]
- 36.Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993;52:817–822. doi: 10.1136/ard.52.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engstrom-Laurent A. The Chemistry, Biology and Medical Applications of Hyaluronana and its Derivatives. Wenner-Gren International Series; Hyaluronan Analysis as a Tool in Evaluating Rheumatic Diseases. 199872. [Google Scholar]