Abstract
Age-related muscle atrophy is characterized by decreases in muscle mass and is thought be mediated, at least in part, by increases in myocyte apoptosis. Recent data has demonstrated that the degree of muscle loss with aging may differ between males and females while other work has suggested that apoptosis as indicated by DNA fragmentation may be regulated differently in fast- and slow-twitch muscles. Herein, we investigate how aging affects the regulation of muscle apoptosis in the fast-twitch extensor digitorum longus (EDL) and slow-twitch soleus muscles of young (6-month), aged (26-month), and very aged (30-month) female Fischer 344/NNiaHSD × Brown Norway/BiNia (F344BN) rats. Tissue sections were stained with hydroethidium for ROS and protein extract was subjected to immunoblotting for assessing apoptotic markers. Our data suggest that decreases in muscle mass were associated with increased DNA fragmentation (TUNEL positive) and increases in reactive oxygen species (ROS) as determined by hydroethidium staining in both the EDL and soleus. Similar to our previous work using aged male animals, we observed that the time course and magnitude of changes in Bax, Bcl-2, caspase-3, caspase-9, and cleavage of α-fodrin protein were regulated differently between muscles. These data suggest that aging in the female F344BN rat is associated with decreases in muscle mass, elevations in ROS level, increased muscle cell DNA fragmentation, and alterations in cell membrane integrity and that apoptotic mechanisms may differ between fiber types.
Keywords: Aging, Female, Skeletal muscle, Apoptosis, ROS, Caspase
Introduction
Aging in the elderly is characterized by losses in muscle mass and strength known as sarcopenia that can impair the ability of the aged to perform every day activities. Animal survivability curves developed by the National Institutes on Aging based on large, long-term studies examining Fischer 344/NNiaHSD × Brown Norway/BiNia (F344BN) mortality rates indicate that 6-, 26-, and 30-month-old female rats roughly correspond to the third, seventh, and eighth decade of life in humans [1–3]. This latter time point is considered significant by the World Health Organization as it has defined this age group as “elderly” [4]. The degree of muscle atrophy with aging appears to be greater in men than in women and may vary between fiber types [5–7]. The role that myofiber loss plays in age-related muscle atrophy is not fully understood; however, recent data has suggested that the elderly may experience significant losses in total fiber number (~30–40 % of total fibers) between the second and eighth decade of life [8]. More recently, other work has demonstrated that apoptosis may play a considerable role in mediating age-related muscle atrophy in both rats and humans [9–11].
Muscle apoptosis has been shown to occur in both caspase-dependent and independent manner [12]. Whether differences exist in signaling processes involved in controlling apoptosis between males and females is not fully understood. Recent work has suggested that skeletal muscle in males may exhibit increased expression of the anti-apoptotic proteins FLIP and Bcl-2 compared to that observed in females [13]. Other data has demonstrated that age-related apoptotic signaling in male F344BN rats may differ between different muscle types [14]. Whether a similar mechanism is present in aging female muscle is to our knowledge not known.
On the basis of previous findings from our laboratory [15, 16] and others [17] indicating that the F344BN rats exhibits a similar level of sarcopenia to that seen in aging humans, we examined the time course and regulation of apoptotic signaling in the fast-twitch muscle extensor digitorum longus (EDL) and the slow-twitch soleus muscles of adult, aged, and very aged female F344BN rats. Consistent with previous reports that have employed male rats [14], our findings demonstrate that the age-related apoptotic signaling may differ across muscle fiber type in female animals.
Material and methods
Animals
All procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” approved by the Council of the American Physiological Society and the Animal Use Review Board of Marshall University. The animals and tissues used in this study have been previously examined [3]. Fully mature adult (6 months; n = 4), post-menopausal aged (26 months; n = 4), and very aged (30 months; n = 4) female F344BN rats were obtained from the National Institute of Aging (Bethesda, MD). Rats were housed two per cage in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-approved vivarium. Rats were maintained in housing conditions consisting of a 12:12-h dark-light cycle and temperature of 22 ± 2 °C with food and water ad libitum. Rats were allowed to acclimatize to the new environment for a 2-week period before the experimentation began.
Materials
Primary antibodies against Bax [no. 2772], Bcl-2 [no. 2876], caspase-3 [no. 9662], caspase-9 [no. 9506], caspase-12 [no. 2202], α-fodrin [no. 2122], glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [no. 2118], HRP-linked anti-rabbit IgG [no. 7074], and NIH-3 T3 control cell extracts [no. 9203] were obtained from Cell Signaling Technology (Beverely, MA). The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assay kit was purchased from Roche Diagnostics Corporations (Indianapolis, IN). Antibody against dystrophin (C-terminus) was from Novocastra Laboratories Ltd. (Newcastle, UK). Texas Red anti-mouse secondary antibody [no. TI-2000] and mounting medium with DAPI [no. H-1500] were acquired from Vector Laboratories (Burlingame, CA). Precast 10 and 15 % SDS-PAGE gels were procured from Lonza (Rockland, ME) while the Enhanced Chemiluminescence (ECL) Western Blot Detection Reagents, Hyperfilm, and Hybond nitrocellulose membranes were from Amersham Biosciences (Piscataway, NG). Tissue protein extraction reagent (T-PER) was obtained from Pierce (Rockford, IL) and Dual Color Molecular Weight Markers were from Bio-Rad (Hercules, CA). All other chemicals were purchased form Sigma (St. Louis, MO).
Tissue Isolation
Rats were anesthetized with ketamine-xylazine cocktail (40 mg ketamine and 10 mg xylazine/kg body weight, I.P.) and supplemented as necessary for reflexive response. Soleus and EDL muscles were quickly removed, trimmed of connective tissue, weighed, and snap frozen in liquid nitrogen. Muscles were stored at −80 °C until further use.
In situ TUNEL, dystrophin, and DAPI triple-staining
Cross-sections (8 μm) were obtained from the mid-belly of the soleus and EDL muscles using an IEC Minotome Cryostat. DNA fragmentation was detected by TUNEL as described previously [18]. Briefly, after fixing with 4 % paraformaldehyde, sections were permeabilized with 0.1 % Triton X-100 in 0.1 % sodium citrate for 2 min at 4 °C. The TUNEL reaction mixture (50 μl) containing terminal deoxynucleotidyl transferase (TdT) and fluorescein-dUTP was added to the sections and incubated for 60 min at 37 °C in a dark humidified chamber. After washing with phosphate-buffered saline (PBS, pH 7.4), tissue sections were blocked with 3 % BSA and incubated with anti-dystrophin antibody (1:500) to visualize the cell membrane for 30 min, washed, and then incubated with secondary antibody for 30 min at room temperature. After rinsing with PBS, sections were mounted and counterstained with DAPI (4, 6-diamidino-2-phenylindole) to visualize nuclei. At least three regions from each cross-section were randomly selected and visualized under fluorescence (Olympus BX51, Melville, NY) using a ×20 objective. Control experiments performed in parallel using DNase 1 or the omission of TdT was used to verify specificity of labeling. Images were digitally recorded using a CCD camera (Olympus, Melville, NY). Differences in number of TUNEL-positive nuclei across various age groups were quantified by counting the number of TUNEL-positive nuclei and dividing it by total number of DAPI-stained nuclei in at least three different fields from each muscle section.
In situ nitrotyrosine staining
Cross-sections (8 μm) were obtained from the mid-belly of the soleus and EDL muscles using an IEC Minotome Cryostat. Frozen tissue sections were washed with PBS for 5 min and incubated with anti-nitrotyrosine antibody (Abcam ab7048) for 1 h at room temperature. After washing (3 × 5 min with PBS), fluorescence secondary antibody was incubated for 1 h and was visualized using an Olympus BX51 microscope (Olympus America, Melville, NY, USA) equipped with Olympus WH ×10 eyepieces and an Olympus UPlanF1 ×40/0.75 objective lens.
Quantification of muscle fiber cross-sectional area and determination of myocyte superoxide levels
Muscle fiber cross-sectional area of soleus and EDL muscle sections (8 μm) was determined by tracing the outline as visualized by dystrophin-staining using the ImageJ program. The distribution of muscle fiber cross-sectional area was plotted with Vertical Box and Whisker Plots as outlined previously [18].
Dihydroethidium (HE) staining was used to evaluate superoxide levels as outlined elsewhere [18]. Briefly, dihydroethidium is oxidized and intercalates within DNA exhibiting a bright fluorescent red color. Frozen tissue sections were washed with PBS for 5 min and incubated with 10 μM of dihydroethidium (Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. After washing (3 × 5 min with PBS), ethidium fluorescence was visualized using an Olympus BX51 microscope (Olympus America, Melville, NY, USA) equipped with Olympus WH ×10 eyepieces and an Olympus UPlanF1 ×40/0.75 objective lens.
Immunoblotting
EDL and soleus muscles were homogenized 2 × 30 s using ice-cold T-PER (1 ml/100 mg tissue weight) supplemented with 1 mM PMSF, 1 mM Na3VO4, and 1 mM NaF. Samples were centrifuged (10,000×g for 15 min at 4 °C) and the supernatant removed for the determination of protein content using the Pierce 660-nm protein assay (Pierce, Rockford, IL). SDS-loading buffer was used to dilute each sample to a final concentration of 2 mg/ml. After heating for 5 min at 100 °C, 40 μg of protein was separated using SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blocked in 5 % milk in Tris-buffered saline with 0.05 % Tween 20 (TBS-T) for 1 h at room temperature and incubated with the appropriate primary antibody for overnight at 4 °C. Membranes were washed 3 × 5 min with TBS-T and exposed to horseradish peroxidase-labeled IgG secondary antibody for 1 h at room temperature. Protein bands were visualized with ECL (Amersham Biosciences), and the exposure time was adjusted to keep the integrated optical densities (IODs) within a linear and non-saturated range. Band signal intensity was quantified by densitometry using Imaging software (Alpha Ease FC) and normalized to GAPDH to verify equal loading of protein.
In situ rat IgG and dystrophin counter-staining
Cross-sections (8 μm) were obtained from the mid-belly of the soleus and EDL muscles using an IEC Minotome Cryostat. Frozen tissue sections were washed with PBS for 5 min; tissue sections were blocked with 3 % BSA, incubated with anti-dystrophin antibody (1:500) to visualize the cell membrane for 30 min, washed, and then incubated with secondary antibody for 30 min at room temperature. After washing (3 × 5 min with PBS), sections were incubated with Texas red-labeled anti-rat IgG antibody for 30 min at room temperature. After washing (3 × 5 min with PBS), samples were visualized using an Olympus BX51 microscope (Olympus America, Melville, NY, USA) equipped with Olympus WH ×10 eyepieces and an Olympus UPlanF1 ×40/0.75 objective lens.
Data analysis
Results are presented as mean ± SEM. Differences among age groups were evaluated by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test using Sigma Stat 3.5 statistical program. The level of significance accepted for differences was set at P ≤ 0.05.
Results
Age-related muscle atrophy is associated with increases in muscle ROS and in the percentage of TUNEL-positive myonuclei
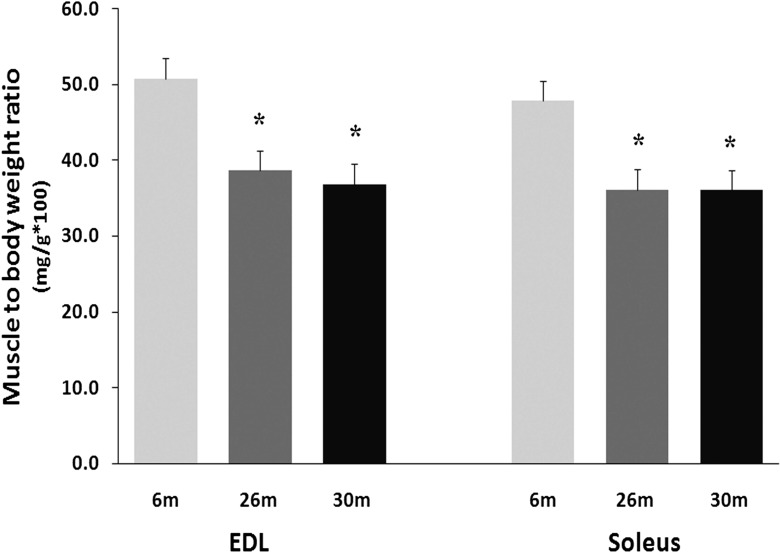
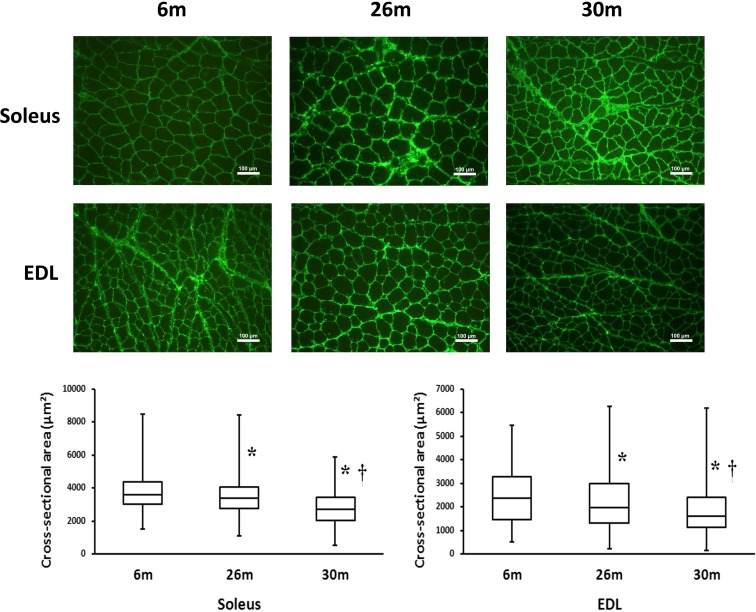
As detailed previously, the soleus muscle to body weight ratio was 25 % lower at 26 and 30 months (0.36 ± 0.04 vs. 0.36 ± 0.01 g, respectively (P < 0.05) when compared to that observed in the 6-month-old animals (0.48 ± 0.02 g) (Fig. 1). Similarly, the EDL muscle-to-body weight ratio was 24 % (0.39 ± 0.03 g) and 28 % (0.37 ± 0.02 g) lower in the 26- and 30-month-old animals (0.51 ± 0.02 g; (P < 0.05)), respectively [3]. Compared to 6-month-old animals, EDL muscle fiber cross-sectional area was 8 and 22 % lower in the 26- and 30-month animals (P < 0.05) (Fig. 2). Similarly, soleus muscle fiber cross-sectional area was 7 and 30 % less at 26- and 30 months of age (P < 0.05) (Fig. 2).
Fig. 1.
Changes in the muscle to body weight ratio with age in the soleus and EDL of female F344BN rats. Values are mean ± SEM, n = 4 each group. *P < 0.05 indicates significant difference from young adult (6-month) age group
Fig. 2.
Changes in muscle fiber cross-sectional area with age. Upper panel: Representative images of dystrophin-stained soleus and EDL muscle sections from 6-, 26-, and 30-month rats. Lower panel: Vertical box and whisker plots showing the distribution of muscle fiber cross-sectional area: median values (horizontal line in the box), the 25th and 75th percentile (the bottom and top of box, respectively), and the minimum and maximum (the bottom and top end of whisker, respectively). Number of fibers measured for 6-, 26-, and 30-month rats were n = 1977, 1751, and 2466 for the soleus and n = 2530, 2273, and 3207 for the EDL, respectively. *P < 0.05 indicates significant difference from young adult (6-month) age group. †P < 0.05 indicates significant difference from aged (26-month) group. n = 3 each group
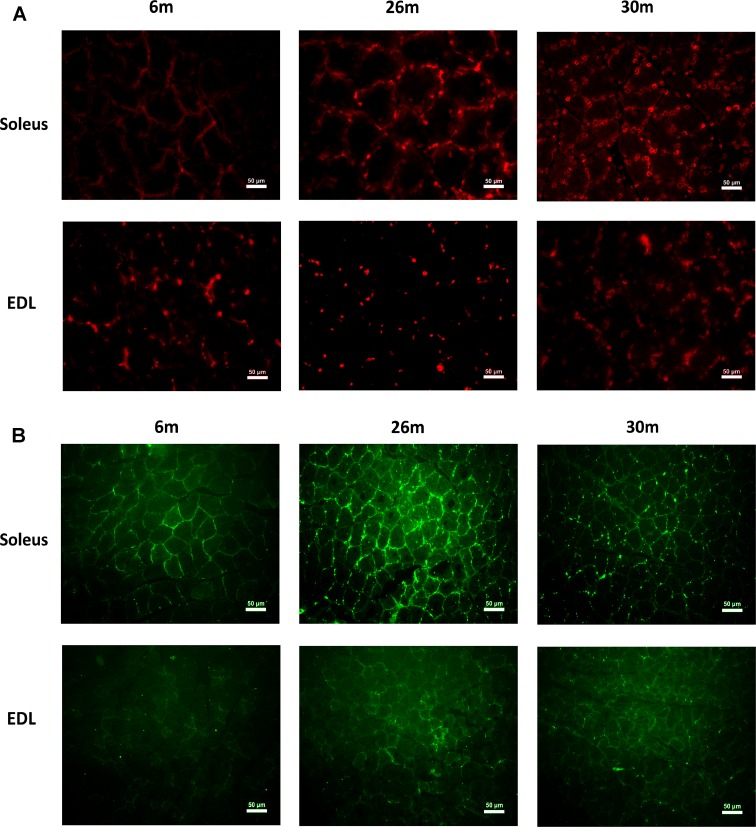
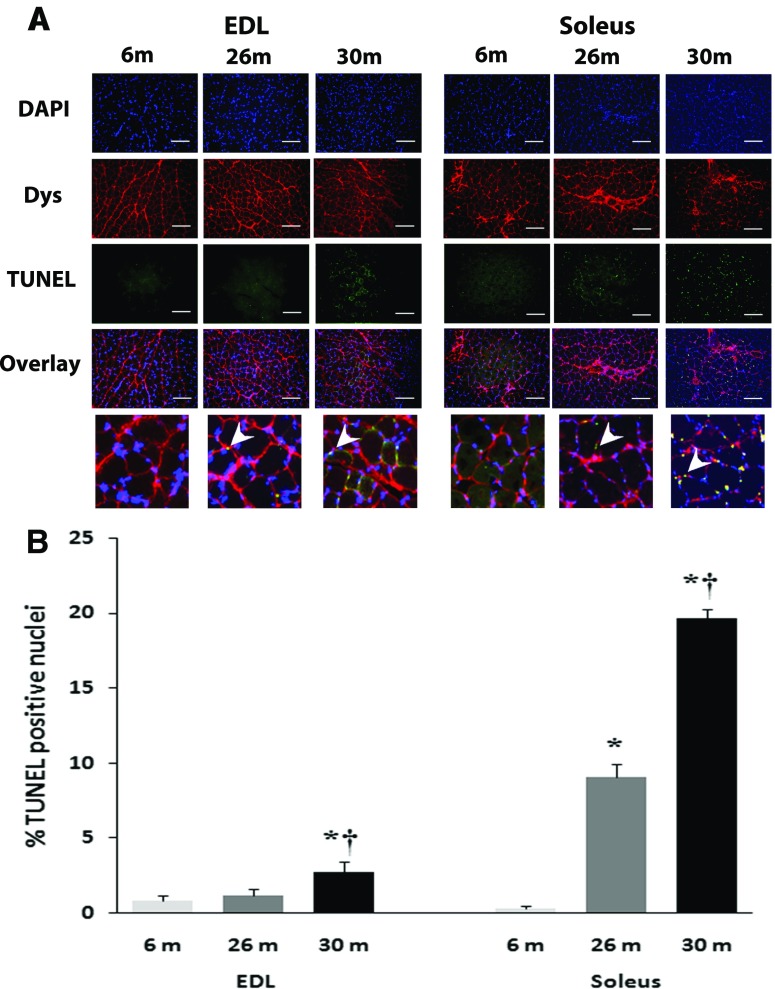
In comparison with 6-month animals, dihydroethidium reactivity appeared to be visibly higher in 26- and 30-month soleus muscles and in the 26-month-old EDL muscle (Fig. 3a). In comparison with 6-month animals, nitrotyrosine reactivity appeared to be visibly higher in 26- and 30-month soleus muscles and in the 26-month-old EDL muscle (Fig. 3b). Compared to 6-month-old animals, the percentage of TUNEL-positive nuclei was increased in the 26- and 30-month soleus (Fig. 4) but only in the 30-month EDL muscle (P < 0.05; Fig. 4).
Fig. 3.
a Indices of cellular ROS (superoxide anion [O2•–]) change with aging and fiber type. a The fluorescent superoxide indicator dihydroethidium (HE) was used to evaluate oxidative stress in 6-, 26-, and 30-month EDL and soleus muscles. b Nitotyrosine changes with aging and fiber type. Anti-nitrotyrosine antibody was use to evaluate tyrosine nitrosylation in 6-, 26-, and 30-month EDL and soleus muscles. Bar 50 μm. n = 3 each group
Fig. 4.
Quantification of apoptosis with age is shown in soleus and EDL of female F344BN rats. a Representative images of the triple staining (DAPI, Dystrophin, TUNEL, and an overlay of the three) for EDL (left) and soleus (right) muscle sections from 6-, 26-, and 30-month female FBN rats. Apoptotic myonuclei were visualized with TUNEL staining. Muscle borders were visualized using mouse monoclonal antibody dystrophin (C-terminus), and all nuclei were stained with 4′, 6-diamidino-2-phenyllindole (DAPI). Arrows in magnified overlays indicate TUNEL-positive nuclei. b Graph representing the TUNEL-positive nuclei in EDL and soleus muscle sections. Data are presented as mean ± SEM. *P < 0.05 indicates significant difference from young adult (6-month) age group. † P < 0.05 indicates significant difference from aged (26-month) group. n = 3 each group
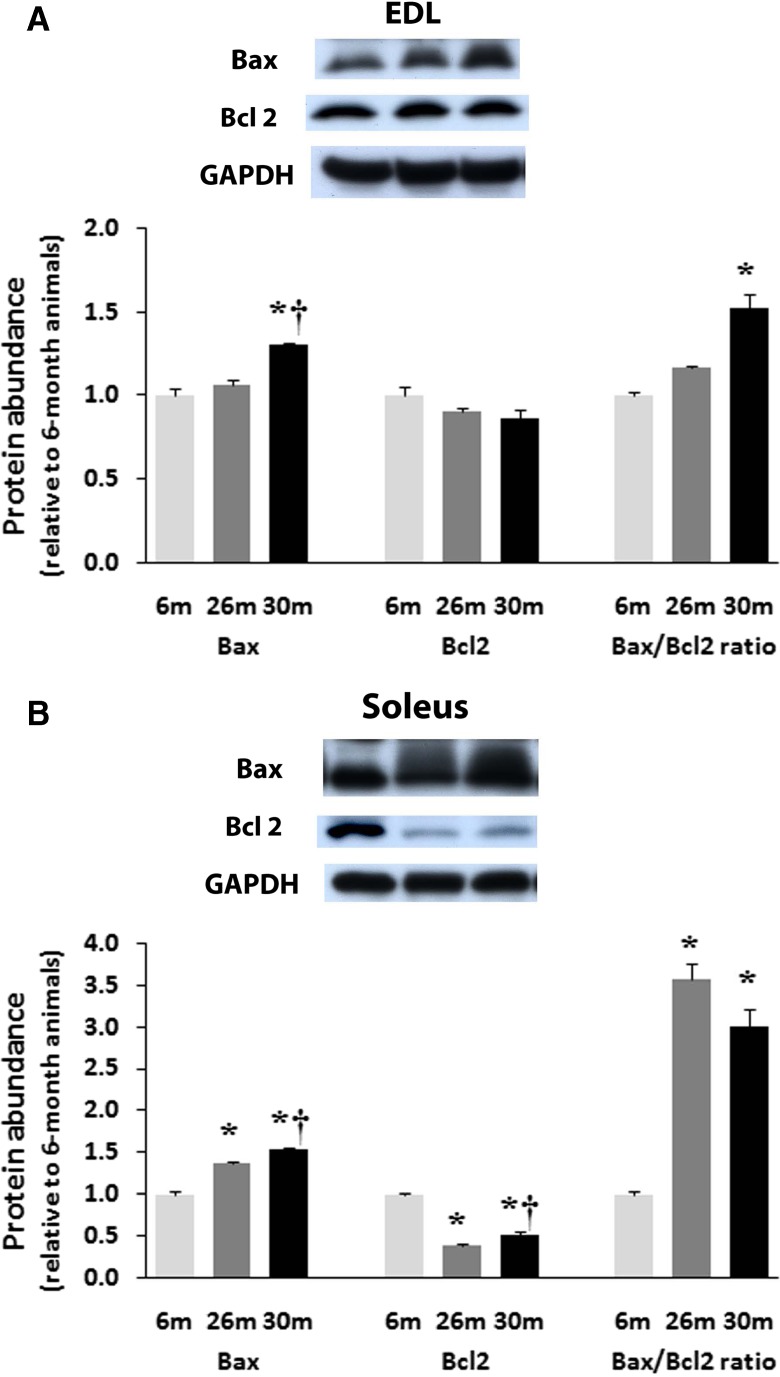
The regulation of Bax and Bcl-2 protein expression with aging differs across muscle type
In the EDL, Bax expression was 31 % higher (P < 0.05) in 30-month-old animals compared to that found in muscles from 6- and 26-month animals (Fig. 5a). Unlike the soleus, Bcl-2 expression in the EDL was not changed with aging (Fig. 5a). The ratio of Bax to Bcl-2 in the aging EDL was 31 % higher at 30 months compared to 6 months (Fig. 5a).
Fig. 5.
Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in EDL (a) and Soleus (b) of female F344BN rats with age as determined by Western blot analysis. Expression of Bax and Bcl-2 were normalized to GAPDH. Data are presented as mean ± SEM. *P < 0.05 indicates significant difference from young adult (6-month) age group. † P < 0.05 indicates significant difference from aged (26-month) group. n = 4 each group
Compared to 6-month animals, the expression of pro-apoptotic Bax was 37 and 53 % higher (P < 0.05) in the soleus muscles from 26- and 30-month-old animals (Fig. 5b). Conversely, the amount of the anti-apoptotic Bcl-2 was 61 and 48 % lower (P < 0.05) in 26- and 30-month solei compared to that observed in 6-month animals. Aging increased the ratio of Bax to Bcl-2 in the soleus by 263 and 206 % at 26- and 30-months, respectively (P < 0.05; Fig. 5b).
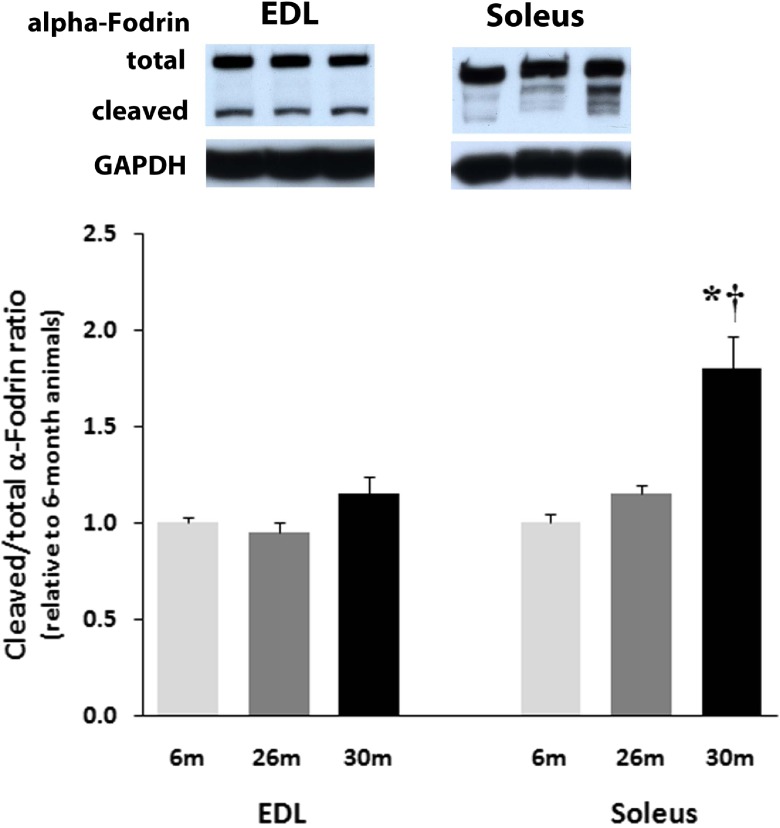
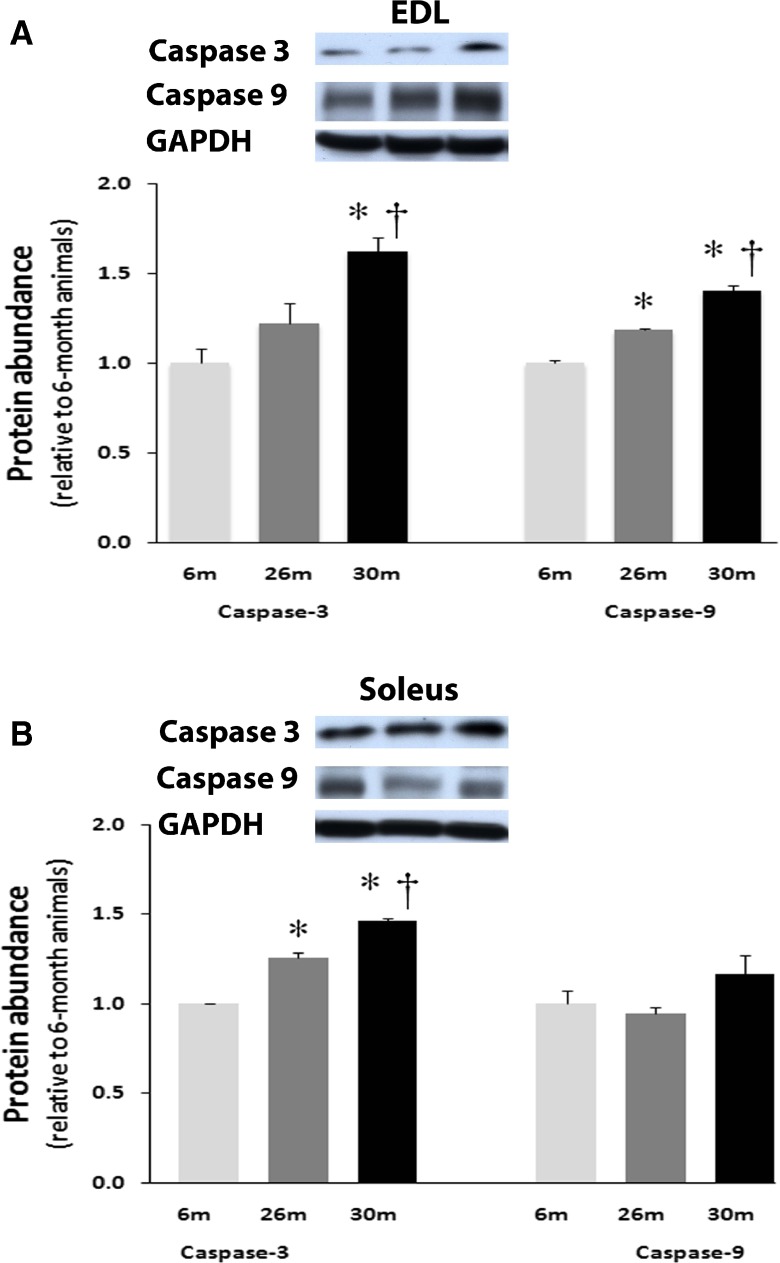
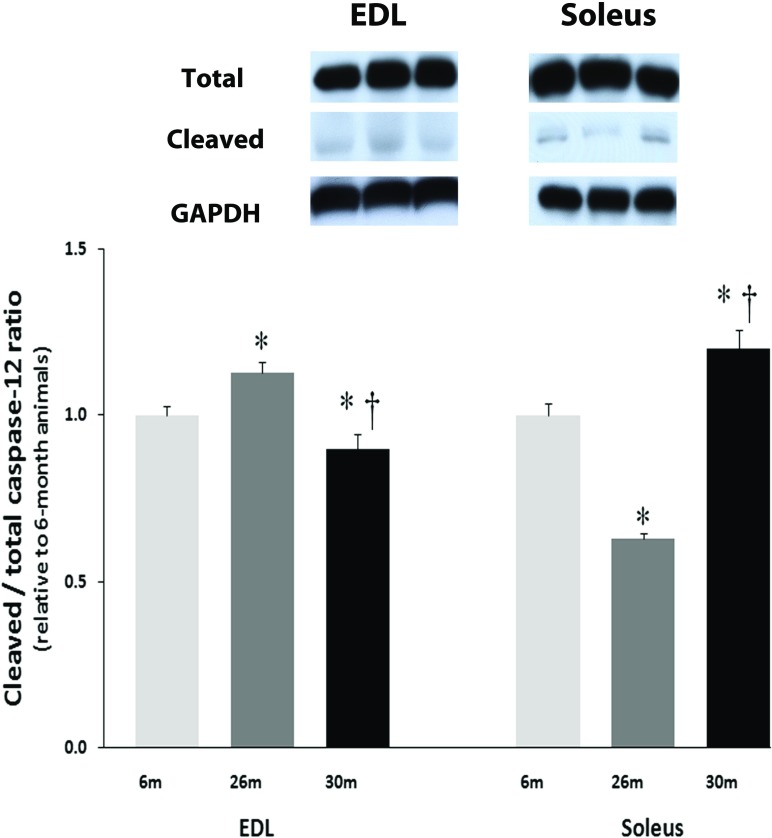
Age-related changes in α-fodrin, caspase-3, caspase-9, and caspase-12 are regulated differently across muscle type
The amount of alpha-fodrin cleavage in the EDL muscle was unchanged with aging (Fig. 6). Compared to soleus muscles from 6-month animals, the amount of cleaved α-fodrin was 83 % higher (P < 0.05) in the 30-month solei muscles (Fig. 6). Aging increased the amount of uncleaved caspase-3 in the EDL by 62 % at 30 months (P < 0.05; Fig. 7a). Compared to 6-month animals, the amount of uncleaved caspase-3 protein was 26 and 46 % higher (P < 0.05) in 26- and 30-month solei muscles (Fig. 7b). Caspase-9 levels in the EDL were 18 and 40 % higher in the 26- and 30-month EDL muscles, respectively, compared to that observed in the 6-month EDL muscles (P < 0.05; Fig. 7a) while it was unchanged with aging in the soleus muscle (Fig. 7b). However, it is to be noted that changes in caspase 3 or 9 expression do not necessarily reflect activity. Cleaved to total caspase-12, levels in the EDL were 13 % higher and 10 % lower in the 26- and 30-month EDL muscles, respectively, compared to that observed in the 6-month EDL muscles (P < 0.05; Fig. 8) while it was 37 % lower and 20 % higher in the 26- and 30-month soleus muscles, respectively, compared to that observed in the 6-month soleus muscles with aging (Fig. 8).
Fig. 6.
Expression of cleaved α-fodrin (150 kDa) compared to total α-fodrin (240 kDa) with aging in soleus and EDL muscles of female F344BN rats as determined by Western blot analysis and normalized to GAPDH. Data are presented as mean ± SEM. *P < 0.05 indicates significant difference from young adult (6-month) age group. † P < 0.05 indicates significant difference from aged (26 month) group. n = 4 each group
Fig. 7.
Expression of caspase-3 and caspase-9 in EDL (a) and Soleus (b) of female F344BN rats with age as determined by Western blot analysis. Expression of caspase-3 and caspase-9 were normalized to GAPDH. Data are presented as mean ± SEM. *P < 0.05 indicates significant difference from young adult (6-month) age group. † P < 0.05 indicates significant difference from aged (26 month) group. n = 4 each group
Fig. 8.
Expression of caspase-12 and cleaved caspase-12 in EDL and Soleus of female F344BN rats with age as determined by Western blot analysis. Expression of caspase-12 and cleaved caspase-12 were normalized to GAPDH. Data are presented as mean ± SEM. *P < 0.05 indicates significant difference from young adult (6-month) age group. † P < 0.05 indicates significant difference from aged (26 month) group. n = 4 each group
Age-related muscle fiber membrane de-stability
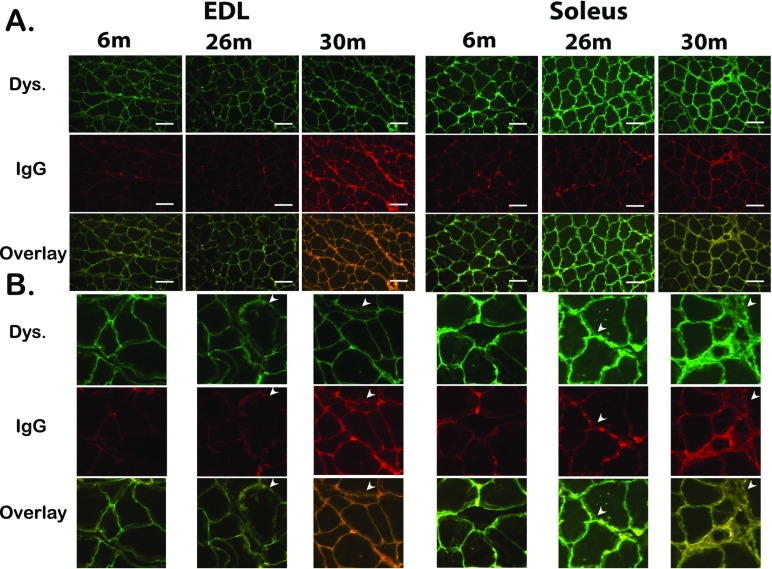
In comparison with 6-month animals, dystrophin reactivity appeared to be visibly different in 26- and 30-month soleus muscles and EDL muscle (Fig. 9). In comparison with 6-month animals, rat IgG reactivity appeared to be visibly higher in 26- and 30-month soleus muscles and in the 26-month-old EDL muscle (Fig. 9). Additionally, with advance ad in the EDL, dystrophin appears to become more diffuse and disrupted with advancing age. These alterations appear to increase the rat IgG infiltration into the myofiber area as well. In the soleus, these changes appear to be more pronounced leading to entire myofibers being infiltrated by rat IgG (Fig. 9).
Fig. 9.
Aging is associated with a loss of myocyte membrane integrity. Double immunofluorescence labeling with dystrophin (FITC) and rat IgG (Texas Red) in the 6-, 26-, and 30-month female FBN rats EDL and Soleus. Representative images of the triple staining (DAPI, Dystrophin, TUNEL, and an overlay of the three) for EDL (left) and soleus (right) muscle sections from 6-, 26-, and 30-month female FBN rats. a ×20 image. b Magnified regions of the images in panel a. Arrows indicate areas of dystrophin disruption, rat IgG infiltration, and co-localization.
Discussion
To our knowledge, this is the first report to examine the regulation of muscle apoptosis between muscle types in an aging female rat model. We utilized the F344BN aging rat which appears to model the age-related atrophy observed in humans [2] and exhibits a decreased incidence of age-related lesions compared to seen in other strains [1]. Previous reports in humans and rats have suggested that the degree and rate of muscle atrophy may differ between muscle type and sex [3, 5, 6]. Other data has demonstrated that the degree of muscle atrophy continues to increase with age in the male F344BN rat [2, 18, 19]. Conversely, here, we found that age-related muscle loss in female animals plateaued at 26 months and remained constant thereafter (Fig. 1). Although similar differences between the rates of muscle atrophy with aging between genders have been demonstrated in humans [5, 6], it is clear that muscle atrophy in human females is a progressive process that appears to continue even at advanced age. Why the loss of muscle mass appears to remain constant after a certain age in the female, F344BN is not clear; however, it is possible that the examination of animals above 30 months of age could have yielded different results. Future studies perhaps employing female animals older than the ones used in this study will no doubt be useful in clarifying this possibility.
Recent data examining the regulation of muscle apoptosis with aging has suggested that the degree of apoptosis may vary by muscle type [14, 20]. Our findings support this contention. For example, in the aging soleus muscle, the amount of TUNEL-positive nuclei increases sharply at 26 months and then again at 30 months of age (Fig. 4). Conversely, in the aging female EDL, the number of TUNEL-positive nuclei does not appear to significantly increase until the animals are 30 months of age. In addition, the incidence of apoptotic nuclei is less in the aging EDL than soleus. This latter finding is similar to our previous data when examining the incidence of apoptosis in the aging F344BN male. Why the amount of apoptosis as determined by DNA fragmentation might differ between muscle types is not entirely clear. Given that different muscle types exhibit differences in their resistance to muscle atrophy, metabolic profile, and degree of usage, it would not be surprising that fast- and slow-twitch muscles may also exhibit different proclivities to nuclei loss during aging. Additional investigation using other muscles or muscles that contain a mixture of fiber types will be useful in expanding our understanding of this finding.
It has been suggested that the mechanisms of age-related apoptosis may differ in fast- and slow-twitch muscle types [21–23]. The data of the present study are consistent with this notion. One of the main findings of the present study is that the regulation of apoptotic regulators appears to vary between muscle types with aging. For example, anti-apoptotic protein Bcl-2 content in the soleus is decreased with aging, whereas in the EDL, Bcl-2 content remained constant (Fig. 5). Similarly, in the soleus, pro-apoptotic Bax content was significantly increased at 26 and 30 months, while in the EDL, Bax levels did not change until 30 months (Fig. 5). Support for this notion is given by our analysis of the Bax-to-Bcl-2 ratio; in the aging EDL, the ratio of Bax to Bcl-2 remains constant until 30 months of age while in the soleus, this ratio is elevated significantly at 26 months (Fig. 5). Recent data indicate that enhanced production of ROS may induce a pro-apoptotic shift of the pattern of expression of Bcl-2 proteins (e.g., increased Bax-to-Bcl-2 ratio) [24]. Given the fact that the soleus muscle contains a higher concentration of mitochondria and a greater reliance on oxidative activity to produce energy than the EDL, it is possible that it also experiences a higher elevation of age-related ROS (Fig. 3a, b).
Increases in mitochondrial dysfunction with aging is considered a powerful stimulus for apoptosis [25]. Impairment of mitochondrial function has been shown to trigger the release of cytochrome C [26]. It is thought that this process is controlled, at least in part, by the ratio of Bax to Bcl-2 with the release of cytochrome C and cell death favored as the balance shifts toward Bax [27]. In the aging F344BN female EDL and soleus muscles, the alterations we observe in the ratio of Bax to Bcl-2 suggest that mitochondrial-mediated processes may play a role in regulation of age-related muscle apoptosis. Similar to our findings for Bax and Bcl-2, it appears that the temporal expression of caspase-3 with aging and the degree of caspase-3 expression with regard to the percentage of TUNEL positive nuclei could differ between the EDL and soleus. Nonetheless, a common theme in both muscles was the significant alteration in the levels of caspase-3 in both the aging EDL and soleus muscles (Fig. 7). Additionally, we failed to find evidence of caspase-3 or caspase-9 cleavage in either the EDL or soleus muscle (data not shown). Although present, the presence of caspase-12 cleavage varied with age and muscle type; it was greater in the aged soleus (Fig. 8). Care must be taken in interpreting these finding, a review by Gunderson and Bruusgaard has suggested that detection of apoptotic activity in striated skeletal muscle may reflect an increase in turnover of stromal and satellite cells rather than net loss of nuclei [28].
Age-associated apoptosis in the soleus muscle is associated with α-fodrin cleavage and loss of membrane integrity
Age-related changes in the ability of skeletal muscle to regulate intracellular calcium (calcium dyshomeostasis) are thought to be associated with increased cellular apoptosis [25]. Increases in cellular calcium, if excessive, can result in the activation of calpains. Calpains are a family of calcium-dependent, non-lysosomal cysteine proteases that can participate in the breakdown of numerous proteins and have been postulated to play a role in muscle atrophy [29, 30]. The α-fodrin protein also known as spectrin II [31] has been shown to localize in the “costameres” of skeletal muscle [32]. Costameres are structures that function to connect the sarcomere of the muscle to the cell membrane [33], and are located over the M lines and Z lines of adjacent sarcomeres on the cytoplasmic surface of the sarcolemma [34–36]. The α-fodrin protein is a 240-kDa protein that can be cleaved by activated calpains to yield a N-terminal fragment of 150 kDa [37, 38]. Similar to our findings for Bax and Bcl-2, the aged EDL and soleus appear to regulate calpain activation differently with aging. For example, we show increased calpain-dependent cleavage of α-fodrin in the soleus with aging but not in the EDL (Fig. 6). No evidence of capase-dependent cleavage of α-fodrin was present in either muscle (data not shown). This latter finding is different from what has previously been shown in the aging male F344BN where evidence of caspase-dependent cleavage was evident in both the soleus and EDL [14]. The presence of α-fodrin cleavage may be associated with lose of membrane integrity or force transduction.
Due to the presence of α-fodrin cleavage, we decided to look at the dystrophin-associated glycoprotein (DAG) complex protein dystrophin. Although the exact function of the DGC is not fully understood, it is known to regulate the stability of the muscle cell membrane and is believed to link the structural proteins within the extracellular matrix to the internal cytoskeletal system of individual myofibers [39]. Our data would indicate that dystrophin localization/organization is altered with aging and that these alterations vary with muscle type. Our finding of increased rat IgG infiltration with aging which varied with muscle type is consistent with this notion. This membrane destabilization had been linked to increase cellular apoptosis [40, 41] which is in agreement with our finding and could be associated with a loss of myofibril contractile machinery membrane anchorage integrity and transmembrane myofibril force transduction. As with α-fodrin cleavage, these changes appear to be greater in the soleus (Fig. 9). Whether these changes are a result of increased apoptotic-induced membrane instability (blebbling) is unclear. Taken together, the data obtained from this study and previous work suggests that the mechanism(s) responsible for age-associated muscle atrophy may differ between muscle fiber types and with sex.
Conclusion
These data suggest caspase-dependent apoptosis may play a role in the age-related loss of muscle nuclei in the skeletal muscles of the female F344BN rat. In addition, we confirm previous observations demonstrating that proteolytic and apoptotic regulatory events are regulated differently in fast- and slow-twitch muscles. Studies investigating the potential involvement of other apoptotic regulators (i.e., apoptosis inducing factor (AIF) or endonuclease G) will be useful in furthering our understanding of why the degree of muscle atrophy might vary with fiber type during advancing age.
Acknowledgments
This study was supported by DOE funding no. DE-SC0005162 to E.R.B.
References
- 1.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51(1):B54–59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6(5):335–343. doi: 10.1007/s10522-005-4808-0. [DOI] [PubMed] [Google Scholar]
- 3.Paturi S, Gutta AK, Katta A, et al. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech Ageing Dev. 2010;131(3):202–209. doi: 10.1016/j.mad.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP. Epidemiology of aging. Epidemiol Rev. 2000;22(1):95–106. doi: 10.1093/oxfordjournals.epirev.a018031. [DOI] [PubMed] [Google Scholar]
- 5.Doherty TJ. The influence of aging and sex on skeletal muscle mass and strength. Curr Opin Clin Nutr Metab Care. 2001;4(6):503–508. doi: 10.1097/00075197-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 8.Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci.;50 Spec No:11–16. [DOI] [PubMed]
- 9.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282(2):R519–527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 10.Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 2005;450(6):437–446. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- 11.Paturi S, Gutta AK, Kakarla SK, et al. Impaired overload-induced hypertrophy in obese Zucker rat slow-twitch skeletal muscle. J Appl Physiol. 2010;108(1):7–13. doi: 10.1152/japplphysiol.00330.2009. [DOI] [PubMed] [Google Scholar]
- 12.Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis: Int J Programmed Cell Death. 2006;11(12):2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast- and slow-twitch muscles of the aging F344/N x BN rat model. Mech Ageing Dev. 2006;127(8):670–679. doi: 10.1016/j.mad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 x Brown Norway rats. J Appl Physiol. 2000;88(4):1265–1270. doi: 10.1152/jappl.2000.88.4.1265. [DOI] [PubMed] [Google Scholar]
- 16.Kinnard RS, Mylabathula DB, Uddemarri S, Rice KM, Wright GL, Blough ER. Regulation of p70(S6k), GSK-3beta, and calcineurin in rat striated muscle during aging. Biogerontology. 2005;6(3):173–184. doi: 10.1007/s10522-005-7953-6. [DOI] [PubMed] [Google Scholar]
- 17.Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve. 2003;27(3):339–347. doi: 10.1002/mus.10314. [DOI] [PubMed] [Google Scholar]
- 18.Wu M, Desai DH, Kakarla SK, et al. Acetaminophen prevents aging-associated hyperglycemia in aged rats: effect of aging-associated hyperactivation of p38-MAPK and ERK1/2. Diabetes Metab Res Rev. 2009;25(3):279–286. doi: 10.1002/dmrr.932. [DOI] [PubMed] [Google Scholar]
- 19.Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci. 2008;63(9):921–927. doi: 10.1093/gerona/63.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129(9):542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003;58(8):687–697. doi: 10.1093/gerona/58.8.B687. [DOI] [PubMed] [Google Scholar]
- 22.Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283(1):C66–76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- 23.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36(1):27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Mishra OP, Randis T, Ashraf QM, Delivoria-Papadopoulos M. Hypoxia-induced Bax and Bcl-2 protein expression, caspase-9 activation, DNA fragmentation, and lipid peroxidation in mitochondria of the cerebral cortex of newborn piglets: the role of nitric oxide. Neuroscience. 2006;141(3):1339–1349. doi: 10.1016/j.neuroscience.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005;35(6):473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- 26.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304(3):437–444. doi: 10.1016/S0006-291X(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 27.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- 28.Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol. 2008;586(Pt 11):2675–2681. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartoli M, Richard I. Calpains in muscle wasting. Int J Biochem Cell Biol. 2005;37(10):2115–2133. doi: 10.1016/j.biocel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 30.von Wnuck LK, Keul P, Lucke S, et al. Degraded collagen induces calpain-mediated apoptosis and destruction of the X-chromosome-linked inhibitor of apoptosis (xIAP) in human vascular smooth muscle cells. Cardiovasc Res. 2006;69(3):697–705. doi: 10.1016/j.cardiores.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Winkelmann JC, Forget BG. Erythroid and nonerythroid spectrins. Blood. 1993;81(12):3173–3185. [PubMed] [Google Scholar]
- 32.Zhou D, Ursitti JA, Bloch RJ. Developmental expression of spectrins in rat skeletal muscle. Mol Biol Cell. 1998;9(1):47–61. doi: 10.1091/mbc.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava D, Yu S. Stretching to meet needs: integrin-linked kinase and the cardiac pump. Genes Dev. 2006;20(17):2327–2331. doi: 10.1101/gad.1472506. [DOI] [PubMed] [Google Scholar]
- 34.Repasky EA, Granger BL, Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- 35.Craig SW, Pardo JV. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motility. 1983;3(5–6):449–462. doi: 10.1002/cm.970030513. [DOI] [PubMed] [Google Scholar]
- 36.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements ("costameres") mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A. 1983;80(4):1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273(25):15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 38.Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J Biol Chem. 1996;271(49):31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Pelagio KP, Bloch RJ, Ortega A, Gonzalez-Serratos H. Biomechanics of the sarcolemma and costameres in single skeletal muscle fibers from normal and dystrophin-null mice. J Muscle Res Cell Motil. 2011;31(5–6):323–336. doi: 10.1007/s10974-011-9238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94(8):1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- 41.Danialou G, Comtois AS, Dudley R, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15(9):1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]