Abstract
Beta thalassemia is an important health problem in Nineveh province, a large province in Northwestern Iraq. No previous study of significance had focused on the spectrum of β-thalassemia mutations in this part of the country. A total of 94 unrelated β-thalassemia minor subjects from the latter province were recruited. Their carrier status was confirmed by full blood count, Hb A2 and F estimation. Thereafter their DNA was subjected to multiplex polymerase chain reaction and reverse hybridization to detect 20 β-thalassemia mutations. A total of eleven different β-thalassemia mutations were documented. The most frequent mutation was IVS-I-110 (G>A) documented in 34 %, followed by IVS-I-6 (T>C) in 9.6 %, IVS-I-5(G>C) in 8.5 %, codon 39 (C>T) and codon 44 (−C) in 7.4 % each, while IVS-I-1(G>A) and IVS-II-1(G>A) were encountered in 6.4 % each. Other mutations were less frequent including codon 8 (−AA), IVS-I-130 (G>C), codon 5 (−CT) and IVS-II-745(C>G). The current study revealed notable differences in the relative frequencies of several β-thalassemia mutations in Nineveh province as compared to other parts of Northern Iraq. Such an observation may be reflective of different ethnic backgrounds and varying historical population interactions. It is believed that these findings complement those of earlier studies on β-thalassemia mutations from the country, and are quite essential in the setting of a proposed national preventive program.
Keywords: Beta thalassemia, Nineveh, Iraq, Molecular basis
Introduction
Beta thalassemia is an inherited disorder of hemoglobin synthesis due to reduced (β+) or absent (βo) synthesis of the β-globin chains. More than 200 different mutations have been implicated, most being point mutations leading to defective expression of the β-globin gene [1, 2]. The distribution of these mutations varies in various parts of the world and in different ethnic groups, although generally a hand full of mutations constitute the bulk of these mutations in each particular population [1]. β-thalassemia is an important health problem in Iraq, with an estimated 15,000 registered patients with thalassemia major and intermedia, and an average of 4 % heterozygous carrier rate [3]. The problem is further aggravated by around 30 % consanguineous marriage rate [4]. Such a situation would make initiating a preventive program for this disorder a necessity. The determination of the mutations responsible for β-thalassemia in particular populations is an important prerequisite in establishing such a program in that population.
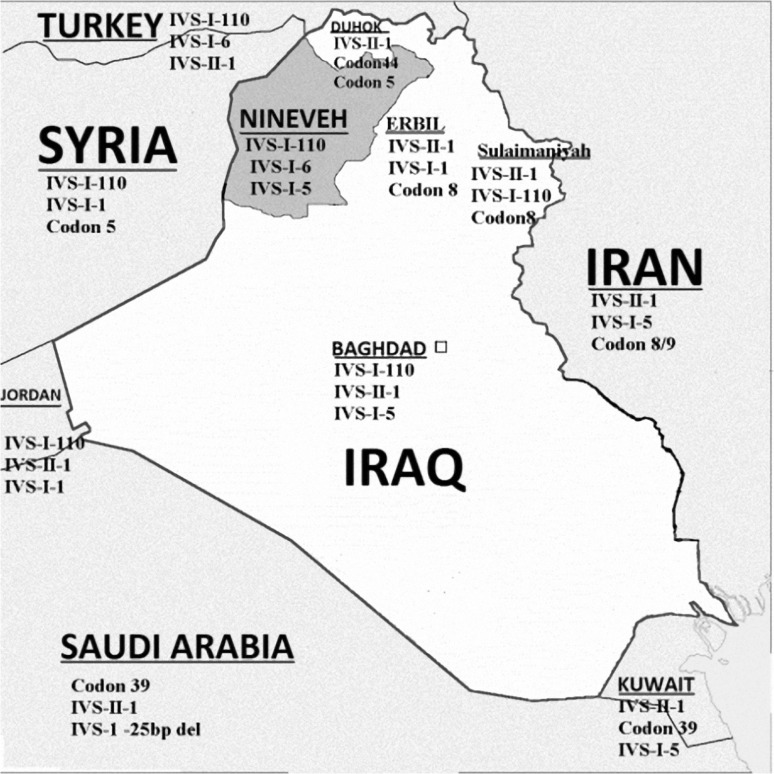
Nineveh Province is a large Northwestern Iraqi province, with Syria and Turkey to its West, and the three Northern Kurdish Iraqi provinces of Duhok, Erbil and Sulaimaniyah to its East (Fig. 1). It has an area of 37,323 km2 and an estimated population of more than 2.5 million. Nineveh has been inhabited since 6000 bc and saw the rise of the Assyrian Empire, which was one of the early civilizations that flourished in ancient Mesopotamia. After the fall of Assyrian empire, this area exchanged hands between Median, Seleucid, Parthian, Persian and Islamic Empires, while it fell to the Mongols in the thirteenth century, and Turkmens in the sixteenth century and then and for the next four centuries it became part of Othman Empire, until the establishment of modern day Iraq in the early twentieth century [5]. Its population currently includes, in addition to a majority of Arabs, Kurdish and Turkmen ethnic groups. Previous studies have focused on β-thalassemia mutations in Northern and Northeastern Iraq where Kurds predominate, while none of significance have studied these mutations in this Northwestern province where other ethnic groups predominate [6–8]. Accordingly, this study was initiated to determine the spectrum of these mutations in this part of the country, in the setting of a proposed national preventive program for hemoglobinopathies.
Fig. 1.
A map of Iraq showing the most frequent mutations in Nineveh province and other provinces in Northern Iraq, as well as surrounding countries
Materials and Methods
A total of 94 unrelated β-thalassemia minor subjects were recruited for the purposes of this study. These subjects were parents of known patients with thalassemia major/intermedia, registered at the thalassemia centre in Nineveh province—Northwestern Iraq. The diagnosis of β-thalassemia minor was confirmed based on a combination of reduced MCV (<80 fL) and an increased Hb A2 (>3.5 %) [9]. The red cell indices were determined using a hematology analyzer (Beckman Coulter, Fullerton, CA, USA) and Hb A2 and F by high performance liquid chromatography (VARIANTTM, Bio-Rad Laboratories, Hercules, CA, USA). Informed consent was obtained from all subjects, and the study was approved by the colleges of Medicine, universities of Duhok and Mousel, Iraq. All enrollees had their DNA extracted by a phenol–chloroform based method. The DNA was amplified in a multiplex reaction mixture, followed by hybridization to specific wild and mutant oligoprobes designed to detect 20 β-thalassemia mutations which are encountered in Mediterranean countries (β-Globin StripAssay MED® kit, Vienna Labordiagnostica GmbH, Vienna, Austria). The choice of this kit was based on previous studies from Iraq and surrounding countries [6–8, 10–13]. The β-thalassemia mutations that were screened for, included: −101 (C>T), −87 (C>G); −30 (T>A); codon 5 (−CT); codon 6 (−A); codon 8 (−AA); codon 8/9 (+G); codon 15 (TGG>TGA); codon 27 (G>T); IVS-I-1 (G>A); IVS-I-5(G>C); IVS-I-6 (T>C); IVS-I-110 (G>A); IVS-I-116(T>G); IVS-I-130 (G>C); codon 39 (C>T); codon 44 (−C); IVS-II-1 (G>A); IVS-II-745 (C>G) and IVS-II-848 (C>A). The amplification, hybridization and detection procedures were performed as recommended by the manufacturer.
Statistical analysis utilized the Mann–Whitney U test. p value <0.05 was considered significant.
Results
The enrolled thalassemia minor patients had a median age of 34 years (range 19–58 years) and included 35 males and 59 females. Seventy-one (75.5 %) were Arabs, 19 (20.2 %) were Turkmen, and 4 (4.3 %) were Kurds.
A total of eleven mutations were identified using StripAssay method (ViennaLab-Austria). Of the latter mutations, eight were the most frequent, seen in 85.1 % of the carriers screened. Of these eight mutations, the most frequent was IVS-I-110 (G>A), seen in 34.0 %, followed by IVS-I-6 (T>C), IVS-I-5(G>C), codon 39(C>T), codon 44(−C), IVS-I–I (G>A), IVS-II-1 (G>A) and codon 8 (−AA) (Table 1). Other mutations were less frequent, and included: IVS-I-130 (G>C), codon 5 (−CT) and IVS-II-745 (C>G).While only six subjects (6.4 %) remained uncharacterized by the employed procedures. Table 1 outlines the number and relative proportion of each of the characterized mutations.
Table 1.
The number and the relative proportion of different β-thalassemia mutations identified in the current study
| Mutation | Phenotype | No. (%) | Origin |
|---|---|---|---|
| IVS-I-110 (G>A) | β+ | 32 (34.0) | Mediterranean |
| IVS-I-6 (T>C) | β+ | 9 (9.6) | Mediterranean |
| IVS-I- 5 (G>C) | β+ | 8 (8.5) | Asian Indian |
| Codon 44 (−C) | βo | 7 (7.4) | Kurdish |
| Codon 39 (C>T) | βo | 7 (7.4) | Mediterranean |
| IVS-I-1 (G>A) | βo | 6 (6.4) | Mediterranean |
| IVS-II-1(G>A) | βo | 6 (6.4) | Mediterranean |
| Codon 8 (−AA) | βo | 5 (5.3) | Turkish |
| IVS-I-130 (G>C) | βo | 3 (3.2) | Turkish |
| Codon 5 (−CT) | βo | 3 (3.2) | Mediterranean |
| IVS-II-745 (C>G) | β+ | 2 (2.1) | Mediterranean |
| Uncharacterized | 6 (6.4) |
The main hematological parameters for the carriers of the eight most frequent mutations are outlined in Table 2. The latter table demonstrates an evident overlap in the distribution of these parameters among the carriers of various mutations. However, one notable observation was that carriers of IVS-I-6 (T>C) had the least reductions in the MCV and the MCH, as well as the least Hb A2 and F values. The significance of these differences, as well as other significant differences between various genotypes are outlined in Table 2.
Table 2.
The means ± standard deviations of the main hematological parameters in the eight main mutations in the current study
| Mutation | No. | MCV (fL) | MCH (pg) | Hb A2 (%) | Hb F (%) |
|---|---|---|---|---|---|
| IVS-I-110 (G>A)1 | 32 | 63.85 ± 3.92 | 19.31 + 1.72 | 4.85 ± 0.71 | 1.1 ± 1.06 |
| IVS-1-6 (T>C)2 | 9 | 68.79 ± 2.88 | 21.78 ± 1.28 | 4.53 ± 0.65 | 0.3 ± 0.42 |
| IVS-1-5 (G>C)3 | 8 | 65.79 ± 5.85 | 20.26 ± 2.19 | 4.79 ± 0.58 | 1.38 ± 1.02 |
| Codon 44 (−C)4 | 7 | 63.46 ± 2.81 | 20.64 ± 4.33 | 5.03 ± 0.47 | 1.64 ± 1.02 |
| Codon 39 (C>T)5 | 7 | 62.8 ± 2.22 | 19.72 ± 0.95 | 4.68 ± 0.74 | 1.3 ± 1.04 |
| IVS-1-1 (G>A)6 | 6 | 64.55 ± 7.09 | 20.20 ± 2.41 | 4.72 ± 0.67 | 1.23 + 0.81 |
| IVS-II-1 (G>A)7 | 6 | 62.8 ± 1.94 | 18.64 ± 1.16 | 5.54 ± 0.23 | 1.54 ± 0.81 |
| Codon 8 (−AA)8 | 5 | 63.58 ± 4.24 | 18.90 ± 1.68 | 5.42 ± 0.63 | 0.8 ± 1.03 |
| Comparisons (p value if significant) | 1 versus 2 (0.0014) | 1 versus 2 (0.014) | 1 versus 7 (0.009) | 1 versus 2 (0.002) | |
| 2 versus 4 (0.004) | 2 versus 7 (0.0009) | 2 versus 8 (0.045) | 2 versus 3 (0.012) | ||
| 2 versus 5 (0.005) | 2 versus 5 (0.003) | 2 versus 7 (0.025) | 2 versus 4 (0.005) | ||
| 2 versus 7 (0.006) | 2 versus 8 (0.01) | 5 versus 7 (0.036) | 2 versus 5 (0.018) | ||
| 2 versus 8 (0.046) | 6 versus 7 (0.011) | 2 versus 7 (0.006) | |||
| 3 versus 6 (0.005) |
The last row outlines the p value of significant differences between various carrier states. Non-listed comparisons are not significant (p > 0.05)
Discussion
The most frequent mutation encountered in the enrolled patients was IVS-I-110 (G>A), a severe Mediterranean β+ mutation, which is also the most frequent mutation in neighboring Turkey, Syria as well as Lebanon, Jordan and upper Egypt (Fig. 1) [10–12, 14–18]. Haplotype studies have revealed that this mutation has the highest haplotype diversity in Turkey, and there were suggestions that it may have originated there in Neolithic period, and thereafter it was spread to other parts of the Eastern Mediterranean and Eastern Europe through gene flow, where its frequency had increased by Malaria selection in these fertile agricultural lands [19]. Interestingly, the predominance of IVS1-110 mutation in Nineveh province (NW Iraq) is in contrast to its sporadic presence in neighboring Iraqi Kurdish provinces of Erbil and Duhok [7], but is consistent with that recently reported in Central Iraq [20]. In the latter study and similar to the current study, Arabs constitute the majority. Thus it appears that the reason for the latter observation is likely to be related to differences in ethnic backgrounds, and variable admixture and interactions with surrounding populations.
The second most frequent mutation was IVS-I-6 (T>C), and this is a mild Mediterranean β+ mutation, first described in a Portuguese patient, with one of its highest frequencies worldwide among Palestinians [21, 22]. Furthermore, it is also the second most common mutation in Turkey, and is frequent in Lebanon, but less frequent in Jordan, Syria and central Iraq [10–12, 15–20].
The third most common mutation encountered in the current study was IVS-I-5 (G>C). This mutation is the most common mutation in the Indian subcontinent [23, 24], and its presence in relatively high frequency is also shared by other parts of Northern as well as central Iraq [6–8, 20]. The latter is most likely related to the fact that Nineveh and Mesopotamia in general, and at various periods of history, served as important trade routes between India and the Mediterranean [5].
One notable observation of the current study was that the Mediterranean IVS-II-1 (G>A) mutation was encountered in a mere 6.4 %, in contrast to frequencies of 18.3–28.7 % in the three neighboring Kurdish provinces in Northern and northeastern Iraq, where it was the most frequently encountered mutation [6, 7]. This mutation has its highest frequency in Iran, where it may have originated, and decreases in frequency as we go west, and its frequencies in Turkey, Syria and Lebanon are comparable to some extent to our figures [10–13].
Codon 44 (−C) is another frequent mutation in the current study, it is a Kurdish mutation with a high frequency in the nearby Duhok province just to the north of Nineveh [8], but is quite sporadic in the two other Kurdish provinces to the East [6, 7]. It was suggested that this mutation may have originated in the Duhok province more than 2,000 years ago [8]. The presence of this Kurdish mutation in Nineveh is not unexpected, because of the inevitable and close interaction between the populations living in these two provinces throughout their mutual history.
The overlap in hematologic parameters among carriers of different β-thalassemia mutations as demonstrated by the current study has been also documented by previous investigators [25]. The most notable relevant observation, was the association of the mild β+IVS-I-6 with lesser reduction of MCV and MCH, and lower Hb A2, and this is consistent with the bulk of the literature [18, 25].
The choice of the technique employed in the current study, which is multiplex polymerase chain reaction (PCR) with reverse hybridization using a commercial kit (β-Globin StripAssay MED® kit), proved to be quite justified, as it managed to pick up 93.6 % of all β-thal mutations. Thus such a technique may be employed in the settings of a future prenatal diagnostic program because of its ability to detect the bulk of mutations, leaving only a small proportion of patients requiring sequencing. Furthermore, the findings may be the basis for introducing more cost-effective molecular diagnostic techniques, like amplification refractory mutation system (ARMS), since it identified the most common mutations in this region [24, 26].
In conclusion, this study, which is the first such report from NW Iraq, has revealed that a relatively wide spectrum of β-thal mutations including those of Mediterranean, Asian-Indian, Turkish and Kurdish origins was implicated. The latter spectrum when compared to previous reports from other parts of Northern Iraq, clearly shows an evident heterogeneity in the mutations distribution, which is likely to be a reflection of ethnic heterogeneity and variable historical interactions even within a relatively restricted geographical area. Moreover, the findings provide the health authorities with one of the essential pre-requisites before embarking on the much needed national preventive program for thalassemia, which has long been overdue.
Acknowledgments
The authors acknowledge the contributions of D. Jassim, C. Saleem and M. Riyad who have done part of the DNA extractions for this study.
Conflict of interest
The authors have no conflict of interest to report.
References
- 1.Weatherall DJ, Clegg JB. The thalassaemia syndromes. 4. Oxford: Blackwell; 2001. [Google Scholar]
- 2.Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5:11. doi: 10.1186/1750-1172-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamamy H, Al-Allawi N. The epidemiology of haemoglobinopathies in Arab countries. J Commun Genet. 2013;4:147–167. doi: 10.1007/s12687-012-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tadmouri GO, Nair P, Obeid T, Al-Ali MT, Hamamy HA. Consanguinity and reproductive health among Arabs. Reprod Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.http://www.britannica.com/EBcheched/topic/376828/history-of-Mesopotamia
- 6.Jalal S, Al-Allawi N, Bayat N, Imanian H, Najmabadi H, Faraj A. Beta thalassemia mutations in the Kurdish population of Northeastern Iraq. Hemoglobin. 2010;34:469–476. doi: 10.3109/01676830.2010.513591. [DOI] [PubMed] [Google Scholar]
- 7.Al-Allawi N, Hassan KMA, Sheikha AK, Nerweiy FF, Dawood RS, Jubrael J. Beta thalassemia mutations among transfusion dependent thalassemia major patients in Northern Iraq. Mol Biol Int. 2010 doi: 10.4061/2010/479282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Allawi N, Jubrael J, Hughson M. Molecular characterization of β thalassemias in Dohuk Region of Iraq. Hemoglobin. 2006;30:479–486. doi: 10.1080/03630260600868097. [DOI] [PubMed] [Google Scholar]
- 9.Galanello R, Eleftheriou A, Traaeger-Synedions J, Petrou M, Angastiniotis M. Prevention of thalassemia and othe hemoglobin disorders. Nicosia: TIF Publications; 2003. [Google Scholar]
- 10.Altay C. The frequency and distribution pattern of β-thalassemia mutations in turkey. Turk J Hematol. 2002;19:309–315. [PubMed] [Google Scholar]
- 11.Makhoul NJ, Wells RS, Kaspar H, Shbaklo H, Taher A, Chakar N, Zalloua PA. Genetic heterogeneity of beta thalassemia in Lebanon reflects historic and recent population migration. Ann Hum Genet. 2005;69:55–66. doi: 10.1046/j.1529-8817.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 12.Kyriacou K, Al QF, Pavlou E, Christopoulos G, Ioannou P, Kleanthous M. Molecular characterization of beta-thalassemia in Syria. Hemoglobin. 2000;24:1–13. doi: 10.3109/03630260009002268. [DOI] [PubMed] [Google Scholar]
- 13.Najmabadi H, Karimi-Nejad R, Sahebjam S, Pourfarzad F, Teimourian S, Sahebjam F, Amirizadeh N, Karimi-Nejad MH. The beta-thalassemia mutation spectrum in the Iranian population. Hemoglobin. 2001;25:285–296. doi: 10.1081/HEM-100105221. [DOI] [PubMed] [Google Scholar]
- 14.Jiffri EH, Bogari N, Zidan KH, Teama S, Elhawary NA. Molecular updating of β-thalassemia mutations in the upper Egyptian population. Hemoglobin. 2010;34:538–547. doi: 10.3109/03630269.2010.526440. [DOI] [PubMed] [Google Scholar]
- 15.Sadiq MF, Eigel A, Horst J. Spectrum of β-thalassemia in Jordan: identification of two novel mutations. Am J Hematol. 2001;68:16–22. doi: 10.1002/ajh.1143. [DOI] [PubMed] [Google Scholar]
- 16.Tadmouri GO, Tüzmen S, Özçelik H, Özer A, Baig SM, Senga EB, Başak AN. Molecular and population genetic analyses of β-thalassemias in Turkey. Am J Hematol. 1998;57:215–220. doi: 10.1002/(SICI)1096-8652(199803)57:3<215::AID-AJH6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Başak AN. The molecular pathology of β-thalassemia in Turkey: the Boģaziçi university experience. Hemoglobin. 2007;31:233–241. doi: 10.1080/03630260701296735. [DOI] [PubMed] [Google Scholar]
- 18.Őner R, Altay C, Gurgey A, Aksoy M, Kilinc Y, Stoming TA, Reese AL, Kutlar A, Kutlar F, Huisman THJ. β-Thalassemia in Turkey. Hemoglobin. 1990;14:1–13. doi: 10.3109/03630269009002250. [DOI] [PubMed] [Google Scholar]
- 19.Tadmouri GO (1999) β-thalassemia in Turkey: distribution, diversity, evaluation and phenotype-genotype correlations. PhD thesis. Boǵaziçi University. Turkey
- 20.Al-Allawi NAS, Al-Mousawi BMS, Badi AIA, Jalal SD. The spectrum of β-thalassemia mutations in Baghdad, Central Iraq. Hemoglobin. 2013;37:444–453. doi: 10.3109/03630269.2013.810641. [DOI] [PubMed] [Google Scholar]
- 21.Tamagnini GP, Lopes MC, Castanheira ME, Wainscot JS, Wood WG (1983) β+Thalassaemia—Portuguese type: clinical, haematological and molecular studies of a newly defined form of β-thalassaemia. Br J Haematol 54:189–200 [DOI] [PubMed]
- 22.El-Latif MA, Filon D, Rund D, Oppenheim A, Kanaan M. The β+-IVS-I-6 (T→C) mutation accounts for half of the thalassemia chromosomes in the Palestinian populations of the mountain regions. Hemoglobin. 2002;26:33–40. doi: 10.1081/HEM-120002938. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Sarwai S, Pathak N, Agarwal S. Beta-globin gene mutations in India and their linkage to β-haplotypes. Int J Hum Genet. 2008;8:237–241. [Google Scholar]
- 24.Old JM, Khan SN, Verma I, Fucharoen S, Kleanthous M, Ioannou P, Kotea N, Fisher C, Riazuddin S, Saxena R, Winichagoon P, Kyriacou K, Al-Quobaili F, Khan B. A multi-center study in order to further define the molecular basis of β-thalassemia in Thailand, Pakistan, Sri Lanka, Mauritius, Syria, and India, and to develop a simple molecular diagnostic strategy by amplification refractpry mutation system—polymerase chain reaction. Hemoglobin. 2001;25:397–407. doi: 10.1081/HEM-100107877. [DOI] [PubMed] [Google Scholar]
- 25.Patrinos GP, Giardine B, Riemer C, Miller W, Chui DKH, Anagnou NP, Wajcman H, Hardison RC (2004) Improvement in HbVar Database of human hemoglobin variants and thalassemia mutations for population and sequence variation studies. Nucleic Acids Res 32 (Database issue): D537–D541 (http://globin.sce.psu.edu) [DOI] [PMC free article] [PubMed]
- 26.Nerweiy FF, Al-Allawi NA, Jubrael J, Dawood RS. The application of the amplification refractory mutation system (ARMS) for characterization β-thalassaemia mutations in Duhok. Duhok Med J. 2010;4:8–20. [Google Scholar]