Abstract
Garden cress (Lepidium sativum L) seed oil (GCO) is a rich source of α-linolenic acid (ALA, 33.6 %) and the oil has a fairly balanced SFA, MUFA and PUFA ratio. In this study we have investigated the effect of GCO and its blends with n-6 PUFA rich edible vegetable oils sunflower oil (SFO), rice bran oil (RBO) and sesame oil (SESO) on antioxidant status of oils and antioxidative enzymes in Wistar rats. Physical blending of GCO with n-6 PUFA rich vegetable oils (SFO, RBO and SESO) increased content of natural antioxidants such as tocopherols, oryzanol and lignans, decreased the n-6/n-3 PUFA ratio and improved the radical scavenging activity of blended oils. Dietary feeding of GCO and its blended oils for 60 days, increased the tocopherols levels (12.2–21.6 %) and activity of antioxidant enzymes namely catalase, glutathione peroxidase (GPx), but did not affect the activity of glutathione reductase (GR), superoxide dismutase (SOD) and glutathione S-transferase (GST) in liver compared to native oil fed rats. Thus, blending of GCO with other vegetable oil decreased n-6/n-3 PUFA ratio (>2.0) and dietary feeding of GCO blended oils increased the antioxidant status and activity of antioxidant enzymes (catalase and GPx) in experimental rats.
Keywords: Garden cress seed oil, Tocopherols, Lipid peroxides, Antioxidant enzymes
Introduction
Dietary lipids play an important role in human health by influencing physiopathological processes (Schmidt et al. 2005). Several studies have demonstrated that n-3 PUFAs have beneficial effects of lowering blood lipids, platelet aggregation and inflammation (Simopoulos 1991; Calder 2004). Most of the vegetable oils that are being consumed are rich in n-6 PUFAs and deficient in n-3 PUFAs with the n-6 to n-3 PUFA ratio of 50:1. In order to increase n-3 PUFA levels in diet, linseed oil and fish oil are supplemented in milk based formulations (Ramaprasad et al. 2004) and blending of α-linolenic acid (ALA 18:3) rich oils with edible vegetable oils are being attempted (Umesha and Naidu 2012). However, n-3 PUFAs are highly unsaturated, susceptible to autoxidation under aerobic conditions (Fritsche and Johnston 1988), cause an increase in oxidative stress through ROS production (Costabile et al. 2005) and also ameliorate antioxidant defense system (Venkatraman and Pinnavaia 1998).
Garden cress (Lepidium sativum L.) is an edible, herb belonging to Cruciferae family. Garden cress seeds contain 24 % of oil in which 32–34 % is ALA. Garden cress seed oil (GCO) is relatively stable oil due to the presence of a high concentration of antioxidants and phytosterols (Moser et al. 2009; Diwakar et al. 2010). Garden cress seeds have not been commercially exploited as an alternate source of ALA rich oil. In our earlier study, we have shown that dietary feeding of GCO and its blended oils significantly decreased cholesterol, triglyceride and LDL levels in serum and liver. GCO and its blended oil fed rats showed significant accumulation of ALA in all the tissue and also it was metabolized to LCPUFAs viz., EPA and DHA in serum, liver heart and brain(Umesha and Naidu 2012). This study was undertaken to assess the effect of n-3 rich GCO and its vegetables oils blend on antioxidant enzymes, antioxidant status in Wistar rats.
Materials and methods
Garden cress seeds were procured from local commercial suppliers, refined sunflower oil (SFO) was purchased from M/s M.K. Agro tek Industries, Sreerangapatna, India. Sesame seed oil (SESO), refined rice bran oil (RBO), flax seed oil (FLAX) and starch were procured from the local supermarket. 2,2-diphenyl-1-picryl hydrazyl free radical (DPPH),α, γ and δ tocopherols, glutathione reductase (GR), glutathione, β-nicotinamide adenine dinucleotide phosphate (reduced form) (β-NADPH), 30 % hydrogen peroxide, and t-butyl hydro peroxide were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Mineral mix was purchased from Sisco research Laboratories Ltd (SRL, India). Choline chloride, DL-methionine, vitamins and cellulose were purchased from Himedia Laboratories (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India). All other chemicals and solvents used in the experiments were of analytical grade.
Extraction of GCO
Garden cress seeds were identified and authenticated at the Department of Horticultural Sciences, University of Agriculture Sciences, Bangalore, Karnataka (India). The seeds were air dried, flaked in a roller flaker, powdered and extracted using a hydraulic press at the pilot plant facility of the institute to obtain fresh cold pressed oil. The oil was weighed and stored in dark container under nitrogen at −20 °C until further use (Diwakar, et al. 2010).
Preparation of oil blends with GCO
GCO was blended with SFO, RBO and SESO in the following ratios of SFO + GCO (50:50), RBO + GCO (60:40), SESO + GCO (60:40) and SFO + Flax (65:35) (w/w). The blended oils were stirred in a magnetic stirrer at 35 °C for 1 h under nitrogen and stored at 4 °C. The blending efficiency was monitored by determining fatty acid composition of the blended oils (Umesha and Naidu 2012).
Fatty acid composition of oil by GC
Fatty acid composition of blended oils was analyzed by GC (Shimadzu, GC-14B, Shimadzu Corporation, Japan). The oils were saponified with 0.5 M KOH and methylated with 40 % boron-trifluoride in methanol (Morrison and Smith 1960). The fatty acid methyl esters were separated on a fused silica capillary column (BP 21: 30 m length, 0.30 mm i.d., 0.50 mm film thickness). The GC was equipped with a flame ionization detector and Clarity Lite 420 integrator. The operating conditions were as follows: initial column temperature 220 °C, injection temperature 230 °C, and detector temperature 240 °C. Nitrogen was used as the carrier gas. Individual fatty acid were identified by comparing with the retention times of reference standard FAME mix (Supelco Inc.) and expressed as relative area percentage. The determinations were carried out in triplicate (n = 3).
Estimation of total natural antioxidants in blended oils
Estimation of tocopherols
Tocopherols in GCO and blended oils were estimated according to the method of Rogers et al. (1993). GCO and blended oils (1 g) were extracted with 10 ml of mobile phase, centrifuged at 3,000 rpm at 4 °C and the upper layer was used for HPLC analysis. Shimadzu LC-10A (Shimadzu Corporation, Tokyo Japan) HPLC system fitted with C18 column (25 cm × 4 mm length, 5 μm Supleco, USA) and fluorescence detector was used. The mobile phase consisted of acetonitrile /methanol/ isopropanol/ water (48:45:5:2) in isocratic condition at a flow rate of 1.0 ml/min. Individual tocopherol peaks were identified and quantified with respective standard tocopherols and tocotrienols elution pattern of palm oil tocotrienols.
Lignans in SESO blended oil
Analysis of lignans in SESO and its blends was performed by HPLC (model LC-10A Shimadzu corporation, Kyoto, Japan) equipped with a UV-detector (290 nm) using 70 % methanol as the mobile phase according to Mohamed and Awatif (1998). Standard sesamol and sesamin were used for the quantification of lignans in the sample.
Oryzanol in RBO blended oil
Oryzanol content in RBO and its blends was determined according to Gopalakrishna et al. (2006) by a spectrophotometric method (UV-1601, Shimadzu Corporation, Kyoto, Japan). The oryzanol content was expressed as %.
Radical scavenging activity of GOC and blended oils
Radical scavenging activity (RSA) of GCO and its blended oils was determined by DPPH radicals scavenging method described by Bhatnagar et al. (2009). A toluenic solution of DPPH radicals (10−4 M) was freshly prepared according to Ramadan et al. (2003), with minor modifications. Known weight of the oil sample was dissolved (0.01–005 g) in 10 ml of toluene, fraction of this solution was placed in test tubes and an aliquot of DPPH toluenic solution was added (final volume was 3 ml and DPPH concentration 14.4 μM) and vortexed for 20 s at ambient temperature. The decrease in the absorption of DPPH radicals was measured at 515 nm against a blank of pure toluene without DPPH radicals after exactly 20 min of mixing, using a UV-visible spectrophotometer (model UV-1601, Shimadzu corporation, Kyoto, Japan). RSA toward DPPH radicals was estimated from the differences in absorbance of toluenic DPPH solution with or without sample(control) and inhibition percent was calculated using the following equation % inhibition = [(absorbance of control − absorbance of sample)/absorbance of control] × 100.
Experimental animals and diet
Male Wistar rats (OUTB—Wistar, IND-cft (2c)) weighing 43 ± 1.7 g, were used in this study. The experimental protocol adopted was approved by the Institute’s Animal Ethical Committee. Rats were divided into eight groups (8 rats/group) by random distribution, housed in individual cages under a 12 h light/dark cycle in animal house facility maintained at 25 ± 2 °C and 40–60 % relative humidity. Rats were fed AIN-76 diet containing native oils and GCO blended oils of SFO, RBO, FLAX and SESO. Diets were prepared every week and stored at 4 °C. Rats were given fresh diet ad libitum every day and left over diet were weighed and discarded. Animals were fed for a period of 60 days. Rats had free access to respective diets and water at all times throughout the study. The gains in body weight of rats were recorded every week. After 60 days on specified diets, rats were fasted overnight and sacrificed under diethyl ether anesthesia. Blood was drawn by cardiac puncture and serum was separated by centrifugation at 700 g for 10 min. Liver was removed and rinsed in ice-cold saline, blotted, weighed and homogenized (1 g/10 ml)in 20 mM phosphate buffer, pH 7.4, using a glass homogenizer. The homogenate was centrifuged at 600 g for 15 min and used for the analysis of antioxidant enzyme activities.
Serum and in liver lipid peroxides
Serum and liver homogenate lipid peroxide was measured by the thiobarbituric acid (TBA) method described by Yagi (1984). By adding 0.05 % BHT solution prior to reaction on a heating block. The reaction mixture was then extracted with 4 mL of n-butanol and fluorescence measurement of butanol extract carried out at an excitation wavelength of 515 nm and emission wavelength using spectro-fluorometer. Following the above procedure the standard was prepared with 1,1,3,3-tetraethoxy propane (Sigma Chemical Co., St. Louis, MO).quantified using an extinction coefficient of 1.56 × 10−5 M−1 cm−1 The lipid peroxides were expressed as MDA nmol/dL in serum and nMol/ of /mg protein in liver.
Antioxidant enzyme activities
Catalase activity
Catalase in the serum and liver was determined by measuring the decomposition of hydrogen peroxide at 240 nm as described by Aebi (1984). The reaction mixture of 1 ml consisted of liver cytosol or serum in 50 mM phosphate buffer pH 7.0 and 1.0 ml of hydrogen peroxide (30 mM). The absorbance was measured for 2 min at 240 nm immediately after adding hydrogen peroxide. Catalase activity was expressed as nmoles of hydrogen peroxide reduced/min/mg protein.
Glutathione peroxidase (GPx)
GPx activity was determined by NADPH oxidation in a coupled enzyme reaction system containing oxidized glutathione according to the method of Floche and Gunzler (1984). The reaction mixture of 1.0 ml consisted of 0.5 ml potassium 0.1 M phosphate buffer pH 7.0 with 1 m M EDTA, 0.1 ml of 10 mM glutathione, 0.1 ml of glutathione reductase and 0.1 ml of liver cytostol. The reaction mixture was incubated with 0.1 ml of 1.5 mM NADPH for 4 min at 22 °C and the absorbance was recorded at 340 nm for 5 min. Glutathione peroxidase activity was expressed as μmoles of NDAPH oxidized/min/mg protein.
Superoxide dismutase (SOD)
SOD activity was determined by quercetin oxidation method of Kostyuk and Potapovich (1989). One milliliter of the reaction mixture contained0.016 M phosphate buffer pH 7.8, TEMED 8 mM and 50 μl of quercetin (0.15 % in dimethyl formaldehyde) and the rate of quercetin autoxidation was monitored for 3 min at 406 nm following the addition of 2–10 μg liver cytosol protein SOD activity was expressed as unit/mg protein, where one unit of SOD was defined as the amount required to half-maximal inhibition of quercetin reduction.
Glutathione transferase (GST)
GST activity was measured with 1-chloro 2,4-dinitrobenzene (CDNB) as the substrate according to the method of Guthenberg et al. (1985). In brief, the final reaction mixture contained 0.5 M phosphate buffer pH 7.4, 3.0 mM reduced glutathione, 0.3 mM CDNB and 10 μg liver cytosol protein. The reaction mixture was incubated for 15 min at 37 °C, the increase in absorbance was recorded at 340 nm. GST activity is expressed as μmoles CDNB-GSH conjugate formed/min/mg protein.
Glutathione reductase(GR)
GR activity was measured according to method of Carlberg et al. (1985). The assay mixture in final volume of 1 ml contained 0.2 M phosphate buffer (pH 7.0, EDTA 2 mM), 0.2–0.3 mg cytosol protein and 50 μl NADPH (2 mM) and the decrease in absorbance was monitored at 340 nm for 3 min. The activity was expressed as μmol NADPH oxidized/min/mg protein.
Protein was estimated by the method of Lowry et al. (1951), using bovine serum albumin as the reference standard. All above spctrophotometric measurements were carried out in a Shimadzu ultraviolet spectrophotometer (Shimadzu UV–VIS 1601) with 1.0 mL quartz cuvettes.
Estimation of tocopherols in serum and liver
Tocopherols in serum and liver were estimated according to the method of Sundram and Nor (2002). Serum was de-proteinized with ethanol containing 0.01 % butylated hydroxy toluene(BHT). The sample was centrifuged, the supernatant was extracted with five volumes of hexane, evaporated under nitrogen, re-dissolved in known volume of mobile phase, and used for HPLC separationas described above.
Liver samples (500 mg) were homogenized in 4 mL of ethanol, extracted with 4 mL of chloroform and repeated the extraction step twice with the addition of 3 mL chloroform: ethanol (1:1). The pooled solvent samples were evaporated under nitrogen, reconstituted in 500 μL mobile phase and used it for separation of tocopherols by HPLC method as described above.
Statistical analysis
Data are expressed as mean ± SD and analyzed by one way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test for significance at P < 0.05 using Graph Pad statistical software (GraphpadInstat).
Results
Animal diet
The composition of AIN-76 diet fed to rats is presented in Table 1. The isocaloric AIN-76 diet contained 50 % starch, 10 % sucrose, 20 % casein, 5 % cellulose, 10 % oil as fat, 3.5 % mineral mix, 1.0 % vitamin mix, 0.2 % choline chloride, and 0.3 % L-cystine. Native oils viz., GCO, RBO, SFO, SESO and GCO blended oils were supplemented at10% in the diet.
Table 1.
Diet composition
| Diet components (g/kg) | Diet groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GCO | SFO | SFO + GCO | RBO | RBO + GCO | SESO | SESO + GCO | SFO + FLAX | ||
| Casein | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | |
| Corn starch | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | |
| Sucrose | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Cellulose | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | |
| Mineral mixa | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | |
| Methionine | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Choline chloride | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| Vitamin mixb | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Oil | GCO | 100 | – | 50 | – | 40 | – | 40 | – |
| SFO | – | 100 | 50 | – | – | – | – | 65 | |
| RBO | – | – | – | 100 | 60 | – | – | – | |
| SESO | – | – | – | – | – | 100 | 60 | – | |
| FLAX | – | – | – | – | – | – | – | 35 | |
aBernheart tommerali salt mixture
bAIN-76A vitamin mix
Fatty acid composition, tocopherol and other minor components in GCO and blended oils
Diet fatty acid composition of GCO and GCO blended oils is presented in Table 2. GCO contained 33.6 % of ALA, 11.9 % LA and the LA/ALA ratio was 0.35. LA content was 64.3, 34.4 and 45.5 % respectively, in SFO, RBO and SESO oils with n-6/n-3 ratios of 160, 43, and 91 respectively. Blended oils of GCO viz., SFO + GCO, RBO + GCO, SESO + GCO and SFO + FLAX had ALA content of 16.8, 12.5, 12.7 and 20.1 % respectively and their n-6/n-3 ratio was 2.3, 2.2, 2.4 and 2.4 respectively.
Table 2.
Fatty acid composition of GCO and GCO blended oils
| Fatty acid | Dietary groups | |||||||
|---|---|---|---|---|---|---|---|---|
| GCO | SFO | SFO + GCO | RBO | RBO + GCO | SESO | SESO + GCO | SFO + FLAX | |
| 14:0 | 0.9 ± 0.1 | 0.1 ± 0.05 | 0.5 ± 0.05 | 0.5 ± 0.1 | 0.5 ± 0.0.4 | 0.3 ± 0.02 | 0.3 ± 0.03 | 0.1 ± 0.05 |
| 16:0 | 11.1 ± 0.4 | 6.6 ± 0.3 | 8.6 ± 0.3 | 20.8 ± 0.8 | 16 ± 1.0 | 10.7 ± 0.4 | 11.2 ± 0.4 | 6.3 ± 0.6 |
| 18:0 | 2.4 ± 0.1 | 2.6 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.0 ± 0.1 | 2.8 ± 0.1 | 2.1 ± 0.1 | 2.4 ± 0.1 |
| 20:0 | 2.2 ± 0.1 | Nd | 1.4 ± 0.1 | Nd | 1.5 ± 0.1 | Nd | 1.1 ± 0.1 | nd |
| 24:0 | 0.9 ± 0.05 | Nd | 0.5 ± 0.05 | Nd | 0.5 ± 0.04 | Nd | 0.4 ± 0.05 | Nd |
| 18:1 | 20.7 ± 0.5 | 26.1 ± 0.9 | 23.8 ± 1.1 | 41.2 ± 1.5 | 33 ± 1.2 | 40.2 ± 1.3 | 33 ± 0.9 | 24 ± 0.9 |
| 18:2 | 11.9 ± 0.4 | 64.3 ± 1.4 | 38.2 ± 1.4 | 34.4 ± 1.0 | 27.3 ± 1.1 | 45.5 ± 1.3 | 31.3 ± 1.2 | 47.2 ± 1.5 |
| 18:3 | 33.6 ± 1.2 | 0.4 ± 0.1 | 16.8 ± 0.4 | 0.8 ± 0.1 | 12.5 ± 0.7 | 0.5 ± 0.05 | 12.7 ± 0.4 | 20.1 ± 0.8 |
| 20:1 | 12.8 ± 0.4 | nd | 6.2 ± 0.3 | nd | 5.1 ± 0.2 | nd | 6.1 ± 0.3 | nd |
| 22:1 | 3.5 ± 0.2 | Nd | 1.7 ± 0.1 | Nd | 1.7 ± 0.1 | Nd | 1.9 ± 0.1 | Nd |
| ∑SFA | 17.5 | 9.3 | 13.3 | 23.6 | 20.5 | 13.8 | 15.1 | 8.8 |
| ∑MUFA | 37 | 26.1 | 31.4 | 41.2 | 39.8 | 40.2 | 41 | 24 |
| ∑PUFA | 45.5 | 64.7 | 55.3 | 35.2 | 39.8 | 46 | 44 | 67.3 |
| n-6/n-3 | 0.35 | 160 | 2.3 | 43 | 2.2 | 91 | 2.4 | 2.4 |
Values are mean ± SD (n = 3)
Tocopherols and other minor components of blended oil are presented in Table 3. GCO showed highest content of total tocopherols at 1,150 mg/kg of oil in which γ-tocopherol was the major one (1,055 mg/kg). Total tocols content of native oil was found to be 435, 823 and 479 mg/kg, respectively for SFO, RBO and SESO. Oryzanol, the important minor component and the antioxidant was estimated to be at 1.41 %, while SESO contained 7,236 mg/kg of lignans, in which the major lignan namely sesamin was 4,118 mg/kg and sesamolin was 2,988 mg/kg. Blended oils had higher total tocopherol content of 740, 926, and 719 mg/kg oil after blending with GCO compared to their respectively native oils. In addition to tocopherol, RBO blended oil (RBO + GCO) contained 0.8 % of oryzanol and SESO blended oil (SESO + GCO) contained 4,890 mg/kg of lignans. Thus blending of GCO with SFO, RBO and SESO increased the content of minor components in blended oils.
Table 3.
Natural antioxidants in GCO and its blended oils
| Dietary groups | Tocopherols (mg/kg) | Oryzanol (%) | Total lignans (mg/kg) |
|---|---|---|---|
| GCO | 1150 ± 49 | – | – |
| SFO | 435 ± 16 | – | – |
| SFO + GCO | 740 ± 22 | – | – |
| RBO | 823 ± 29 | 1.41 ± 0.1 | – |
| RBO + GCO | 926 ± 38 | 0.8 ± 0.06 | – |
| SESO | 479 ± 18 | – | 7236 ± 76 |
| SESO + GCO | 719 ± 23 | – | 4890 ± 51 |
| SFO + FLAX | 432 ± 15 | – |
Values are means ± SD (n = 3)
DPPH radical scavenging activity GCO and blended oils
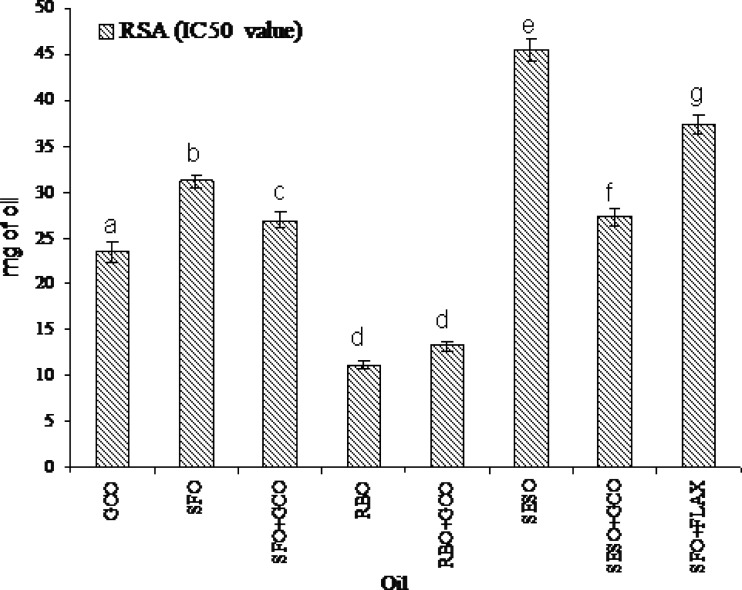
The antioxidant activity of GCO and its blended oils was determined by DPPH radical scavenging method. Dose dependent scavenging of DPPH was observed with IC50 value of 23.5, 31.2, 11.1, 45.5 mg respectively for GCO, SFO, RBO, and SESO as shown in Fig. 1. GCO and RBO showed very good scavenging activity compared to other native oils. GCO blended oils also showed good radical scavenging activity (IC50) compared to respective native oils 26.9, 13.2, and 27.3 mg for SFO + GCO, RBO + GCO and SESO + GCO respectively.
Fig. 1.
Radical scavenging (IC50) value of GCO and its blended oils. Values are means ± SD (n = 3); value sharing on bar graph same superscript a–g are not statistically significant at p < 0.05
Effect GCO and blended oils on food intake, body weight gain and food efficiency ratio
Effect GCO and its blended diet on food intake, body weight gain and food efficiency ratio (FER) are presented in the Table 4 Rats fed GCO blended oils diet didn’t show any significant change in the feed intake, body weight gain and food efficiency ratio with respect to n-6 native oils fed group.
Table 4.
Feed intake, weight gain and food efficiency ratio of rats fed GCO and its blended oils for 60 days
| Dietary groups | Food intake (g) | Weight gain (g) | FER |
|---|---|---|---|
| GCO | 11.3 ± 1.1a | 291.5 ± 20.9a | 0.34 ± 0.02a |
| SFO | 11.1 ± 0.6a | 310.0 ± 26.0a | 0.37 ± 0.03a |
| SFO + GCO | 12.0 ± 0.8a | 289.5 ± 16.7a | 0.33 ± 0.02a |
| RBO | 10.9 ± 1.1a | 297.5 ± 18.0a | 0.35 ± 0.02a |
| RBO + GCO | 11.2 ± 1.0a | 305.0 ± 15.0a | 0.36 ± 0.02a |
| SESO | 11.4 ± 0.6a | 278.0 ± 22.6a | 0.32 ± 0.02a |
| SESO + GCO | 11.8 ± 0.8a | 312.0 ± 18.8a | 0.35 ± 0.02a |
| SFO + LSO | 11.1 ± 0.5a | 293.8 ± 27.5a | 0.35 ± 0.03a |
Values are means ± SD (n = 6 rats); values sharing same superscript in column are not statistically significant at p < 0.05
Effect of GCO and its blended oil diet on serum and liver lipid peroxide levels
Serum and liver lipid peroxide of rat fed GCO and its blended oil was presented in the Tables 5 and 7 respectively. Dietary lipids have influenced the lipid peroxide content of serum and liver of rats. Rat fed diet containing oil group GCO, SFO and SFO + GCO has significantly higher lipid peroxide levels in serum when compare to RBO, SESO, RBO + GCO and SESO + GCO. However, the blends of GCO with RBO and SESO didn’t show any difference compare to their respective native oils. Similar trends also has been observed in the liver lipid peroxide levels of GCO, SFO SFO + GCO has increased 16–41 % compare to RBO, SESO RBO + GCO and SESO + GCO blended oil groups. SFO + GCO, RBO + GCO, SESO + GCO did not show any significant change compared to their native oil fed SFO, RBO and SESO respectively. SFO + FLAX diet group has more susceptible for lipid peroxidation it has increased 33.6–75 % in serum and 33.7–99 % in liver when compared to rest of diet groups.
Table 5.
Serum lipid peroxide and tocopherol content of rat fed GCO and its blended oils for 60 days
| Dietary groups | Lipid peroxide (nM of MDA/dL | Tocopherols (μg/dL) |
|---|---|---|
| GCO | 68.6 ± 2.3a | 48.7 ± 1.4a |
| SFO | 75.1 ± 1.8a | 32.1 ± 1.5b |
| SFO + GCO | 70.7 ± 1.9a | 40.8 ± 1.5c |
| RBO | 56.0 ± 1.9b | 41.3 ± 1.6c |
| RBO + GCO | 57.2 ± 2.2b | 43.5 ± 1.7cd |
| SESO | 60.4 ± 2.5b | 38.8 ± 1.2c |
| SESO + GCO | 61.1 ± 2.3b | 44.2 ± 1.1d |
| SFO + FLAX | 98.5 ± 2.9d | 20.1 ± 0.9e |
Values are means ± SD (n = 6 rats); values sharing same superscript in row are not statistically significant at p < 0.05
Table 7.
Liver lipid peroxide, tocopherol, GSH content and antioxidant enzyme activates of rat fed GCO and its blended oils for 60 days
| Antioxidants/antioxidant enzymes | GCO | SFO | SFO + GCO | RBO | RBO + GCO | SESO | SESO + GCO | SFO + FLAX |
|---|---|---|---|---|---|---|---|---|
| Lipid peroxide (nmoles MDA/g) | 27.7 ± 1.2a | 31.7 ± 0.9a | 28.7 ± 0.8a | 20.0 ± 0.7b | 23.1 ± 1.1b | 24.4 ± 0.9b | 23.9 ± 1.3b | 39.7 ± 1.1c |
| Tocopherols (μg/g) | 40.2 ± 2.2a | 23.1 ± 0.7b | 27.7 ± 0.8c | 30.2 ± 1.1cd | 33.3 ± 0.9d | 27.9 ± 0.7c | 34.4 ± 1.2d | 18.1 ± 0.7e |
| GSH (nmoles/mg of protein) | 3.1 ± 0.2ae | 3.2 ± 0.1ae | 3.2 ± 0.2a | 4.1 ± 0.2b | 3.8 ± 0.2b | 3.5 ± 0.3a | 3.4 ± 0.2a | 2.9 ± 0.2ae |
| Catalase (μmoles /min/mg protein) | 66.1 ± 1.9a | 63.2 ± 1.8ab | 58.1 ± 1.0b | 48.7 ± 0.8c | 54.5 ± 0.7b | 50.2 ± 0.5c | 56.4 ± 0.8b | 67.3 ± 1.9a |
| GPX (μmoles/min/mg protein) | 285.2 ± 8.2a | 278.2 ± 8.0a | 269.4 ± 6.4ab | 255.1 ± 6.9b | 263.1 ± 7.1b | 251.5 ± 5.8b | 260.8 ± 8.7b | 296.6 ± 8.1a |
| GR (μmoles /min/mg protein) | 174.1 ± 11.8a | 168.7 ± 10.3ab | 167.5 ± 9.4ab | 150.1 ± 7.8b | 156.4 ± 10.5b | 160.4 ± 8.1b | 166.8 ± 7.5b | 177.3 ± 9.1a |
| SOD (U/mg of protein) | 5.9 ± 0.9a | 5.7 ± 1.0a | 5.5 ± 1.3a | 5.4 ± 0.9a | 5.7 ± 0.7a | 4.9 ± 0.8a | 5.80 ± 1.0a | 8.6 ± 1.3b |
| GST(μmoles/min/mg protein) | 489.1 ± 21.8a | 499.7 ± 19.0a | 495.0 ± 18.3a | 485.8 ± 23.1a | 501.0 ± 17.6a | 503.0 ± 20.6a | 499.7 ± 18.1a | 535.2 ± 21.4b |
Values are means ± SD (n = 6 rats); values sharing same superscript (a–e) in row are not statistically significant at p < 0.05
Effect of GCO and its blended oil diet on serum and liver tocopherol levels
Tocopherol (Vitamin E) content of serum and liver presented in the Tables 5 and 7 respectively. The tocopherol content of GCO and its blended oil fed group showed almost similar trends in serum and liver. In serum GCO oil fed group registered significantly highest tocopherol content 48.7 μg/dL and followed by 44.3 38.8, and 32.1 μg/dL for RBO, SESO and SFO respectively. GCO group in liver has 40.2 μg/g and it is significantly higher (P < 0.001) compared to all other groups. GCO blended oil groups have significantly higher (P < 0.005) content of tocopherol in SFO + GCO, and SESO + GCO compare to native oil group SFO and SESO respectively groups in serum as well as in liver. SFO + FLAX blended oil fed rats recorded significantly lower levels (P < 0.001) of tocopherol compared to all other groups.
Effect of GCO and its blended oil diet on liver GSSH levels
Liver GSSH content was presented in Table 7. RBO and RBO + GCO fed groups showed significantly elevated GSSH compared to other blended oil fed groups.
Effect of GCO blended oil diet on antioxidant enzyme activities of liver
Antioxidant activities of liver enzymes are presented in Table 5. GCO fed rats registered increased catalase activity (31.8–36 % P < 0.001) compared to RBO and SESO fed group. Further, GCO blended oil fed groups viz., RBO + GCO (12 %) and SESO + GCO (12.3 %) showed significantly elevated catalase activity in compared to native fed groups. However, SFO v/s GCO or SFO + GCO didn’t show any significant change. SFO + FLAX group having significantly higher (P < 0.001) catalase activity when compared to RBO, SESO and its blends of GCO groups.
GPx activities in liver significantly higher for GCO group 21.1 %, 14.2 %, 18.2 % and 16.7%when compared to RBO, SESO, RBO + GCO and SESO + GCO respectively. GCO v/s SFO or SFO + GCO didn’t show any significant changes. SFO + FLAX (P > 0.05) has also showed higher activities compared to RBO, SESO and its blends of GCO. Liver glutathione reductase activity (GR) significantly higher only in GCO and SFO + FLAX, however it didn’t change in GCO blended oil fed groups. SOD activity of GCO and its blended oil didn’t show any significant changes compare to native oils fed groups except SFO + FLAX (P < 0.01) which is significantly higher compare to all other groups. GCO and GCO blended oils didn’t show any difference in glutathione transferee’s activity.
Discussion
Most of the vegetable oils that are being consumed are rich in n-6 PUFA and deficient in n-3 PUFAs. A high intake of n-6 PUFAs is suggested to promote inflammatory and cardiovascular disorders (Simopoulos 1991). The deficiency of n-3 PUFAs has been implicated in number of inflammatory diseases such as atopic dermatitis, rheumatoid arthritis, asthma, ulcerative colitis and cancer. Dietary supplementation of n-3 PUFAs such as ALA and long chain fatty acids namely eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) are known to effectively alleviate many of the symptoms of the above diseases and thus has a wide range of health benefits (Calder 2004). In this study, GCO which is rich in ALA was blended with edible oils to supplement n-3 PUFAs and decrease n-6/n-3 PUFA ratio to >2.0 in oils. Dietary supplementation of GCO and GCO blended oils significant decreased total cholesterol (TC), triglyceride (TG), LDL-C levels in GCO and GCO blended oil fed rats compared to native oil fed rats (Umesha and Naidu 2012)However, the n-3 PUFAs are susceptible to auto-oxidation due to the high degree of unsaturation, further feeding of dietary oils rich in unsaturated fatty acids have been reported to increase lipid peroxidation in target tissues liver and deplete endogenous antioxidants (Porter et al. 1995; Pathasarathy et al. 1999; Venkatraman and Pinnavaia 1998). In this study, we have evaluated the effect of dietary feeding of n-3 PUFA rich GCO and its vegetable oil blends on antioxidant status and antioxidative enzyme activities in Wistar rats.
Dietary feeding of GCO, SFO, SFO + GCO and SFO + FLAX to rats significant increased the lipid peroxides in serum and liver compared to RBO and SESO and its GCO blended oil fed rats. SFO and SFO + FLAX blended oil fed rats showed higher lipid peroxides because of the presence of relatively high levels of unsaturated ALA and low levels of tocopherols (Table 6). A decrease in vitamin E content and an increase in lipid peroxidation was reported in rats fed on n-3 fatty acid supplemented diets (Chautan et al. 1990; Javouhey-Donzel et al. 1993). However, the presence of antioxidants namely oryzanol and sesaminol in RBO and SESO could have protected the lipid peroxidation in liver. Further, a significant increase in tissue tocopherol and other minor constituent levels in serum and liver could offer protection against lipid peroxidation in tissues of rats fed with vegetables blended with GCO.
Table 6.
Liver fatty acid composition (%) of GCO and GCO blended oil fed rats for 60 days
| Fatty acids | GCO | SFO | SFO + GCO | RBO | RBO + GCO | SESO | SESO + GCO | SFO + FLAX |
|---|---|---|---|---|---|---|---|---|
| 16:0 | 14.5 ± 0.8a | 13.5 ± 1.1a | 16.2 ± 2.9a | 15.5 ± 0.7a | 16.6 ± 1.9a | 15.0 ± 1.5a | 16.6 ± 1.4a | 14.5 ± 0.9a |
| 16:1 | 4.4 ± 0.9a | 3.6 ± 0.6a | 4.5 ± 1.5a | 3.0 ± 0.9a | 3.2 ± 0.2a | 3.0 ± 1.1a | 3.4 ± 1.1a | 2.5 ± 0.7a |
| 18:0 | 9.3 ± 1.5a | 10.1 ± 3.2a | 9.9 ± 2.2a | 10.0 ± 1.4a | 9.3 ± 2.3a | 10.5 ± 1.2a | 9.9 ± 1.9a | 12.2 ± 2.2a |
| 18:1 | 21.8 ± 2.9a | 20.3 ± 2.4a | 24.3 ± 4.3a | 23.0 ± 1.1a | 23.9 ± 2.2a | 22.0 ± 1.1a | 21.9 ± 5.5a | 20.8 ± 2.9a |
| 18:2 | 22.2 ± 0.8a | 30.2 ± 5.1b | 27.7 ± 3.1bc | 25.1 ± 1.1ac | 24.5 ± 1.1ac | 25.9 ± 2.8ac | 24.8 ± 2.1bc | 27.9 ± 2.2bc |
| 18:3 | 6.3 ± 1.5a | 0.2 ± 0.2b | 2.7 ± 0.2c | 0.2 ± 0.05b | 2.5 ± 0.9c | 0.1 ± 0.0b | 2.8 ± 0.5c | 3.0 ± 0.7c |
| 20:1 | 1.8 ± 0.5a | ndb | 1.4 ± 0.3a | ndb | 1.2 ± 0.2a | ndb | 2.2 ± 0.2a | ndb |
| 20:4 | 9.8 ± 1.6a | 21.8 ± 3.2bc | 10.5 ± 3.2a | 22.8 ± 1.7b | 13.4 ± 3.1a | 22.8 ± 1.7b | 14.9 ± 2.9a | 14.7 ± 3.0ac |
| 20:5 | 5.7 ± 0.5a | ndb | 0.7 ± 0.1b | ndb | 1.8 ± 0.3c | ndb | 1.3 ± 0.2c | 1.4 ± 0.4c |
| 22:6 | 4.2 ± 1.3a | 0.3 ± 0.05b | 2.2 ± 0.4c | 0.5 ± 0.1b | 3.3 ± 0.5ac | 0.7 ± 0.1b | 2.9 ± 0.7c | 3.0 ± 0.5b |
| ∑SFA | 23.8 | 23.6 | 26.1 | 26.5 | 26.2 | 26.5 | 25.9 | 26.7 |
| ∑MUFA | 28.0 | 23.9 | 30.0 | 26.0 | 28.3 | 25.0 | 27.4 | 23.3 |
| ∑PUFA | 48.2 | 52.5 | 43.6 | 48.6 | 45.5 | 49.5 | 46.7 | 50 |
| n-6/n-3 | 3.2 | 104.0 | 6.3 | 69.0 | 5.1 | 61.0 | 5.6 | 5.8 |
Values are means ± SD (n = 6 rats); values sharing same superscript (a–d) in row are not statistically significant at p < 0.05
Dietary supplementation of n-3 fatty acids rich diet is reported to increase the acyl CoA oxidase in peroxisomes and H2O2 production in liver of mice (Vamecq et al. 1993). H2O2 is a weak oxidant, but it participates in Fenton reaction and generates highly reactive hydroxyl radicals in a biological system. The increased H2O2 has to be removed by enhanced catalase and GPX activities. In the present study, dietary feeding of n-3 rich GCO and its blended oils to rats increased the catalase and GPX activity compared to SFO, RBO and SESO fed rats. N-3 PUFAs are reported to increase catalase activity in peroxisomes and also peroxisomal beta-oxidation resulting in enhanced defense against free oxygen radicals (Chen et al. 1993; Iraz et al. 2005). Venkatraman et al. (1994) reported an increase in the activities and mRNA expression of CAT, GPX and SOD in mice fed n-3 PUFA rich fish oil diet compared to n-6 PUFA rich diets. Further, the decreased GPX would lower the capacity to remove H2O2 and would lead to increased catalase activity (Schull et al. 1991). These reports support our finding on the dietary supplementation of n-3 PUFA rich GCO and its blended oils modulate the activity of antioxidant enzymes in rats (Table 7).
GSH is an important endogenous antioxidant, plays a defensive role against oxidative insult and scavenges free radicals (Cooper and Kristal 1997). GSH is an indicator of antioxidant status in a biological system (Pastore et al. 2001). Maintenance of GSH levels in liver under conditions of increased lipid peroxidation is suggested to be a compensatory mechanism to regulate oxidative stress (Cooper and Kristal 1997; Spolarics and Meyenhofer 2000). In this study, no significant change in GSH levels of liver was observed in most of the dietary oils fed groups. However, low GSH level in liver was observed in RBO, SFO + FLAX fed group. The decreased GSH levels could be related to increased lipid peroxidation and compensatory glutathione oxidation in n-3 PUFA rich FLAX fed rats (Jenkinson et al. 1999).
The minor components namely oryzanol, sesamin, sesamolin, sesamol and tocopherols present in RBO, SESO and GCO and its blended oils may increase the antioxidant status and antioxidant properties of the blended oils. RBO contains tocopherols, tocotrienols and gamma-oryzanol in unsaponifiable matter (Khatoon and Gopalakrishna 2004). Sesame oil has tocopherols and lignans such as sesamin, sesamolin and sesamol (Hemalatha 2004). α-tocopherols and tocotrienols are lipid-soluble antioxidants and scavenge ROS such as singlet oxygen (Liebler 1993; Khanna et al. 2006). γ-oryzanol is a potent inhibitor of highly reactive hydroxyl radicals and possess antioxidant activity in stabilizing lipids (Juliano et al. 2005). Lignans in SESO are good antioxidants and the dietary sesamin and sesamanol are reported to inhibit lipid peroxidation in rats (Hemalatha and Raghunath 2004). GCO and its blended oils showed good radical scavenging activity and the enhanced radical scavenging activity of blended oils could be attributed to the presence of reported minor components such as tocopherols, oryzanol and lignans in above oils.
Conclusion
In the present study, we have demonstrated that blending of n-3 fatty acid rich GCO with edible vegetable oils significantly decreasedthen-6/n-3 PUFA ratio in oils and enhanced the antioxidants in blended oils. Dietary feeding of GCO and GCO blended oils significantly enhanced the natural antioxidant status, radical scavenging activity and also antioxidant enzymes in rats. Thus blending of n-3 rich GCO with edible vegetable oil may potentiate the antioxidant status and antioxidant activity of oils.
Acknowledgments
Mr. S. S. Umesha, gratefully acknowledges the Council of Scientific and Industrial Research (CSIR) New Delhi, for providing a Senior Research Fellowship. KAN acknowledges the financial support in the form of a Project (SR/SO/HS-0005/2010) awarded by Department of Science and Technology (DST), New Delhi, India.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar AS, Prasanth Kumar PK, Hemavathy J, GopalaKrishna AG. Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J Am Oil Chem Soc. 2009;86:991–999. doi: 10.1007/s11746-009-1435-y. [DOI] [Google Scholar]
- Calder PC. n-3 Fatty acids and cardiovascular disease, evidence explained and mechanisms explored. Clin Sci. 2004;107:1–11. doi: 10.1042/CS20040119. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervick FT, Dryle DD. Glutathione reductase. Methods Enzymol. 1985;113:489–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chautan M, Calaf R, Leonard J, Charbonnier M, Andre M, Portugal H, Pauli AM, Lafont H, Nalbone G. Inverse modifications of heart and liver-tocopherol status by various dietary n-6/n-3 polyunsaturated fatty acid ratios. J Lipid Res. 1990;31:2201–2208. [PubMed] [Google Scholar]
- Chen LC, Boissonneault G, Hayek MG, Chow CK. Dietary fat effects on hepatic lipid peroxidation and enzymes of H2O2 metabolism and NADPH generation. Lipids. 1993;28:657–662. doi: 10.1007/BF02536062. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. J Biol Chem. 1997;378:793. [PubMed] [Google Scholar]
- Costabile R, Hili CS, Melino M, Easton C, Ferrante A. The immunomodulatory effect of novel beta-oxa, beta-thia and gamma-thia polyunsaturated fatty acids on human T lymphocyte proliferation, cytokine production and activation of protein kinase C and MAPKs. J Immunol. 2005;174:233–2343. doi: 10.4049/jimmunol.174.1.233. [DOI] [PubMed] [Google Scholar]
- Diwakar BT, Dutta PK, Lokesh BR, Naidu KA. Physicochemical properties of garden cress (Lepidiumsativum L.) seed oil. J Am Oil Chem Soc. 2010;87:539–548. doi: 10.1007/s11746-009-1523-z. [DOI] [Google Scholar]
- Floche L, Gunzler WA. Assays of glutathione peroxidase in. Methods Enzymol. 1984;105:115–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Fritsche KI, Johnston PV. Rapid autooxidation of fish oil in diets without added antioxidants. J Nutr. 1988;118:425–426. doi: 10.1093/jn/118.4.425. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna AG, Hemakumar KH, Khatoon S. Study on the composition of rice bran oil and its higher free fatty acids value. J Am Oil Chem Soc. 2006;83:117–120. doi: 10.1007/s11746-006-1183-1. [DOI] [Google Scholar]
- GraphpadInstat Demo, [DATASET1.ISD]. http://www.graphpad.com, Graph Pad Software Inc., USA
- Guthenberg C, Alin P, Mannervik B. Glutathione transferase from rat testis. Methods Enzymol. 1985;113:507–510. doi: 10.1016/S0076-6879(85)13067-3. [DOI] [PubMed] [Google Scholar]
- Hemalatha S. Lignans and tocopherols in Indian sesame cultivars. J Am Oil Chem Soc. 2004;81:467–470. doi: 10.1007/s11746-004-0924-5. [DOI] [Google Scholar]
- Hemalatha S, Raghunath M. Dietary sesame (Sesamum indicum cultivar Linn) oil inhibits iron-induced oxidative stress in rats. Brit J Nutr. 2004;92:581–587. doi: 10.1079/BJN20041239. [DOI] [PubMed] [Google Scholar]
- Iraz M, Erdogan H, Ozyurt B, Ozugurlu F, Ozgocmen S, Fadillioglu E. Omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Ann Clin Lab Sci. 2005;35:169–173. [PubMed] [Google Scholar]
- Javouhey-Donzel A, Guenot L, Maupoil V, Rochette L, Roequelin G. Rat vitamin E status and heart lipid peroxidation, effect of dietary alpha-linolenic acid and marine n-3 fatty acids. Lipids. 1993;28:651–655. doi: 10.1007/BF02536061. [DOI] [PubMed] [Google Scholar]
- Jenkinson A, Franklin MF, Whale K, Duthie GG. Dietary intake of polyunsaturated fatty acids and indices of oxidative stress in human volunteers. Eur J Clin Nutr. 1999;53:528–533. doi: 10.1038/sj.ejcn.1600783. [DOI] [PubMed] [Google Scholar]
- Juliano C, Cossu M, Alamanni MC, Piu L. Antioxidant activity of gamma-oryzanol, mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int J Pharm. 2005;299:146–154. doi: 10.1016/j.ijpharm.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the potent neuroprotective properties of the natural vitamin E alpha tocotrienol. J Neurochem. 2006;98:1474–1486. doi: 10.1111/j.1471-4159.2006.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon S, Gopalakrishna AG. Fat soluble nutraceuticals and fatty acid composition of selected Indian rice varieties. J Am Oil Chem Soc. 2004;81:939–943. doi: 10.1007/s11746-004-1005-5. [DOI] [Google Scholar]
- Liebler DC. The role of metabolism in the antioxidant function of vitamin E. Crit Rev Toxicol. 1993;23:147–169. doi: 10.3109/10408449309117115. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein estimation with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mohamed HMA, Awatif II. The use of sesame oil unsaponifiable matter as a natural antioxidants. Food Chem. 1998;62:3269–3276. doi: 10.1016/S0308-8146(97)00193-3. [DOI] [Google Scholar]
- Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron trifluoride methanol. J Lipid Res. 1960;5:600–608. [PubMed] [Google Scholar]
- Moser BR, Shah SN, Winkler-Moser JK, Vaughn SF, Evangelista RL. Composition and physical properties of cress (Lepidiumsativum L.) and field pennycress (Thlaspiarvense L) oils. Ind Crop Prod. 2009;30:199–205. doi: 10.1016/j.indcrop.2009.03.007. [DOI] [Google Scholar]
- Pastore A, Piemonte F, Locatelli M, Russo AL, Gaeta LM, Tozzi G, Federici G. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin Chem. 2001;47:1467–1469. [PubMed] [Google Scholar]
- Pathasarathy S, Santanam N, Auge N. Antioxidants and low density lipoprotein oxidation. In: Papas AM, editor. Antioxidant status, diet, nutrition, and health. Boca Raton: CRC Press; 1999. [Google Scholar]
- Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Ramadan MF, Kroh LW, Mörsel JT. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotiaabyssinica Cass.) crude seed oils and oil fractions. J Agric Food Chem. 2003;51:6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- Ramaprasad TR, Baskaran V, Sambaiah K, Lokesh BR. Supplementation and delivery of n-3 fatty acids through spray-dried milk reduce serum and liver lipids in rats. Lipids. 2004;39:627–632. doi: 10.1007/s11745-004-1275-6. [DOI] [PubMed] [Google Scholar]
- Rogers EJ, Rice SM, Nicolosi RJ, Carpenter DR, McClelland CA, Romanczky LJ. Identification and quantification of γ-Oryzanol components and simultaneous assessment of tocols in rice bran oil. J Am Oil Chem Soc. 1993;70:301–307. doi: 10.1007/BF02545312. [DOI] [Google Scholar]
- Schmidt EB, Arnesen H, de Caterina R, Rasmussen LH, Kristensen SD. Marine n-3 polyunsaturated fatty acid and coronary disease: Part I. Background, epidemiology, animal data, effects on risk factors and safety. Thromb Res. 2005;115:163–170. doi: 10.1016/j.thromres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Schull S, Herntz NH, Perisamy M, Manohar M, Janssen YMW, Marsh IP, Mossman BT. Differential regulation of antioxidative enzymes in response to oxidation. J Biol Chem. 1991;266:24398–24403. [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Spolarics Z, Meyenhofer M. Augmented resistance to oxidative stress in fatty rat livers induced by a short-term sucrose-rich diet. Biochim Biophys Acta. 2000;1487:190–200. doi: 10.1016/S1388-1981(00)00093-7. [DOI] [PubMed] [Google Scholar]
- Sundram K, Nor RM (2002) Analysis of tocotrienols in different sample matrixes by HPLC. In: Oxidative stress biomarkers and antioxidant protocols. pp. 221–232. Humana Press [DOI] [PubMed]
- Umesha SS, Naidu KA. Vegetable oil blends with α-linolenic acid rich Garden cress oil modulate lipid metabolism in experimental rats. Food Chem. 2012;135:2845–2851. doi: 10.1016/j.foodchem.2012.05.118. [DOI] [PubMed] [Google Scholar]
- Vamecq J, Vallee L, DeLaPorte P, Fontaine M, DeCraemer D, Van den Branden C, LaFont H, Grataroli R, Nalbone G. Effects of various n-3/n-6 fatty acid ratio contents of high fat diets on rat liver and heart peroxisomal and mitochondrial superoxide dismutase in trained mice. Arch Biochem Biophys. 1993;291:147–153. doi: 10.1016/0005-2760(93)90065-h. [DOI] [PubMed] [Google Scholar]
- Venkatraman JT, Pinnavaia L. Effects of saturated, ω-6 and ω-3 lipids on activities of enzymes involved in antioxidant defense in normal rats. Nutr Res. 1998;18:341–350. doi: 10.1016/S0271-5317(98)00026-8. [DOI] [Google Scholar]
- Venkatraman JT, Chandrasekar B, Kim JD, Fernandes G. Effects of n-3 and n-6 fatty acids on the activities and expression on hepatic antioxidant enzymes in autoimmune-prone NZBxNZW F mice. Lipids. 1994;29:561–568. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- Kostyuk VA, Potapovich AI. Superoxide driven oxidation of quercetin and a simple sensitive for determination of superoxide dismutase. Biochem Int. 1989;19:1117–1124. [PubMed] [Google Scholar]
- Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–331. doi: 10.1016/S0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]