Abstract
Detection of milk fat adulteration with foreign fats/oils continues to be a challenge for the dairy industry as well as food testing laboratories, especially in the present scenario of rampant adulteration using the scientific knowledge by unscrupulous persons involved in the trade. In the present investigation a rapid reversed-phase thin layer chromatographic (RP-TLC) protocol was standardized to ascertain the purity of milk fat. RP-TLC protocol did not show any false positive results in the genuine ghee (clarified butter fat) samples of known origin. Adulteration of ghee with coconut oil up to 7. 5 %, soybean oil, sunflower oil and groundnut oil up to 1 %, while, designer oil up to 2 % level could be detected using the standardized RP-TLC protocol. The protocol standardized is rapid and convenient to use.
Keywords: Milk fat, Ghee, RP-TLC, Adulteration, Vegetable oils, Designer oils, Thin layer chromatography
Introduction
Milk fat, which has the highest price among all the edible fats, plays a significant role in the economics, nutrition and physico-chemical properties of milk and milk products. Milk fat is the most premium and preferred fat in many of the Asian countries e.g. India, Pakistan, Bangladesh, Sri Lanka and Nepal. It is mostly consumed in the form of ghee. A very complex situation arises especially during lean season in the summer months, when the supply of milk and ghee falls short of demand. Due to the premium nature, high price and demand—supply gap, it attracts the attention of unscrupulous traders to adulterate it with low-priced fats/oils, such as vegetable oils, animal body fat, concoction of these fats, inedible mineral oils and most recently some designer fats/oils. In most of the cases the adulteration is done in such a way that the quality parameters of such adulterated milk fat comply with the standards of genuine milk fat. The techniques recommended for the detection of similar adulteration are either cumbersome or require sophisticated equipment like Gas Liquid chromatography, Differential Scanning Colorimetric, High performance liquid chromatography etc. (Patel and Frede 1991; Precht 1990, 1992; Coni et al. 1994; Fontech et al. 1998; Povolo et al. 1999; Aktas and Kaya 2001; Arun et al. 2005; Zachariah et al. 2010; Kala 2013). Physico-chemical parameters based methodologies developed so far have their own limitations in detecting the type and level of adulteration. However, these are also being manipulated by the unscrupulous traders. Normal phase thin layer chromatography has the limitation of resolution of cholesterol and ß-sitosterol an index sterol in plant oils/fats (Arun et al. 2005; Patel et al. 2011). Reversed-phase thin layer chromatographic (RP-TLC) methods using silica gel –G plates impregnated with undecane (IDF 1966; Mathew and Kamath 1978). and thin layers of CaCO3 and soluble starch (10 g + 4 g) impregnated with liquid paraffin (Ramamurthy et al. 1967), are time consuming and has reproducibility and sensitivity issues. Similarly, thin layer chromatographic methods based on chromanols and tocopherols as indicator compounds could help only to some extent in detecting the addition of vegetable oils to ghee (Bailey 1951; Mahon and Chapman 1954; Nazir and Magar 1959; Markuze-Zofia 1962; Keeney et al. 1971; Chourasia et al. 1994; Arun et al. 2002, 2005). The basis of the present investigation was the fact that cholesterol is the characteristic sterol of milk fat while phytosterols especially β-sitosterol is in abundance in all vegetable oils. Keeping this in mind, an RP-TLC protocol to resolve the ß-sitosterol and cholesterol (Jarusiewics et al. 2005) has been developed, wherein new generation readymade RP-18 Silica gel-G F254S TLC were used. These plates are easy to use and have good reproducibility unlike the reverse phase TLC plates prepared in the laboratory by impregnating different stationary phases. Moreover, saponification of the milk fat itself is a time consuming process and requires washing steps, hence in the present study a rapid saponification protocol (Sharma et al. 2009) has been used to collect the unsaponifiable matter required for TLC application. The protocol developed in the present investigation is based on the tracer component of plant oils/fats i.e. ß-sitosterol, easy to perform in any milk testing laboratory, having better or/equally effective level of detection. At the same time the developed protocol does not require any sophisticated equipment unlike methods reported earlier (Zachariah et al. 2010; Ntakatsane et al. 2013) .
Materials and methods
β-sitosterol (Sigma Aldrich, USA)
Cholesterol (Sigma Aldrich, USA)
RPTLC (reversed-phase thin layer chromatography) plates
TLC silica gel 60 RP-18 F 254S (Merck Specialities Private Ltd., Mumbai, India).
Preparation of pure ghee (clarified butter fat) samples
Cow and buffalo ghee samples were prepared by direct cream method (De 2011). Milks used for the preparation of respective ghee samples were collected from the cattle yard of the National Dairy Research Institute, Karnal and also from local dairy farms situated in Karnal. These samples were stored for further analysis. Homemade cow and buffalo ghee samples were procured from cotton tract area of Gujarat, Amreli (India).
Vegetable oils/fats
Refined vegetable oils (ground nut oil, soybean oil, coconut oil and sunflower oil) were collected from local market. Designer oil, which was used as an adulterant for milk fat was supplied by an undisclosed source.
Preparation of adulterated milk fat samples
Vegetable oils (Ground nut, soybean, sunflower and coconut) were added to cow and buffalo ghee at the rate of 1, 2, 5 and 10% levels. These adulterated samples were stored in amber colour bottles and used in the study.
Extraction of unsaponifiable matter (USM) from ghee samples
Unsaponifiable matter from fat samples was isolated essentially as per the method standardized by Sharma et al. (2009) for cholesterol estimation in ghee. To extract the total sterols 0.2 g molten fat sample was taken in a 15 ml capacity screw capped tube followed by the addition of 5 ml of 5 % methanolic KOH . The tube was incubated in a water bath maintained at 90 °C with intermittent shaking after every 5 min., for about 20 min. After 20 min. of incubation, the tube was cooled to room temperature under tap water. One ml water and 5 ml hexane were added in the tube and tube was vortexed for 1–2 min. followed by centrifugation at 2,000 rpm for about 2 min. The upper hexane layer was pipetted out and in a small beaker of about 10 ml capacity and hexane was evaporated to get dried unsaponifiable matter. The dried unsaponifiable matter was redissolved in chloroform and volume was made to 500 μl in an eppendorf tube.
Conditions for reversed—phase thin layer chromatography (RP-TLC) of unsaponifiable matter (USM)
Method of sterols separation on C18 stationary phase as described by Jarusiewicz et al. (2005) was adopted in the study. Developing solvent consisting of Petroleum ether: Acetonitrile: Methanol (20:40:40 v/v) was added to a TLC glass chamber lined with filter paper on the three sides. Chamber was saturated for about 15 min. 6 μl of the unsaponifiable matter solution (500 μl solution in chloroform) was spotted on TLC silica gel 60 RP-18 F 254S plate at a distance of about 1 cm from the bottom along with solutions of standard cholesterol, β-sitosterol and mixture (β-sitosterol + cholesterol) as different spots and allowed to air dry. TLC plate was then developed in the developing chamber saturated with developing solvent till the solvent front had travelled about three-quarters of the length of the plate. The plate was then removed, dried, and sprayed with phosphomolybdic acid solution (20 % solution in ethanol) and kept at 90–95 °C/3 min and position of distinct blueish bands was compared with reference standards.
Results and discussion
Separation of standard sterols and their mixture
It is evident from chromatogram (Fig. 1) that standard cholesterol and ß-sitosterol showed difference in their mobility on RP-18 TLC silica gel G F254 S plate and even the mixture of cholesterol and ß-sitosterol was also resolved into two different bands corresponding to cholesterol and ß-sitosterol. The Rf value of cholesterol standard was calculated as 0.19, whereas that of β-sitosterol as 0.16. This indicated that the standardised conditions had the potential to be used for resolving sterols, especially cholesterol and ß-sitosterol in ghee samples adulterated with vegetable oils.
Fig. 1.
RP-TLC of standard sterols A: mixture (cholesterol + ß-sitosterol); B: mixture (cholesterol + ß-sitosterol + campasterol); C: Cholesterol; D: ß-sitosterol
RP-TLC profile of sterols in USM of pure ghee samples
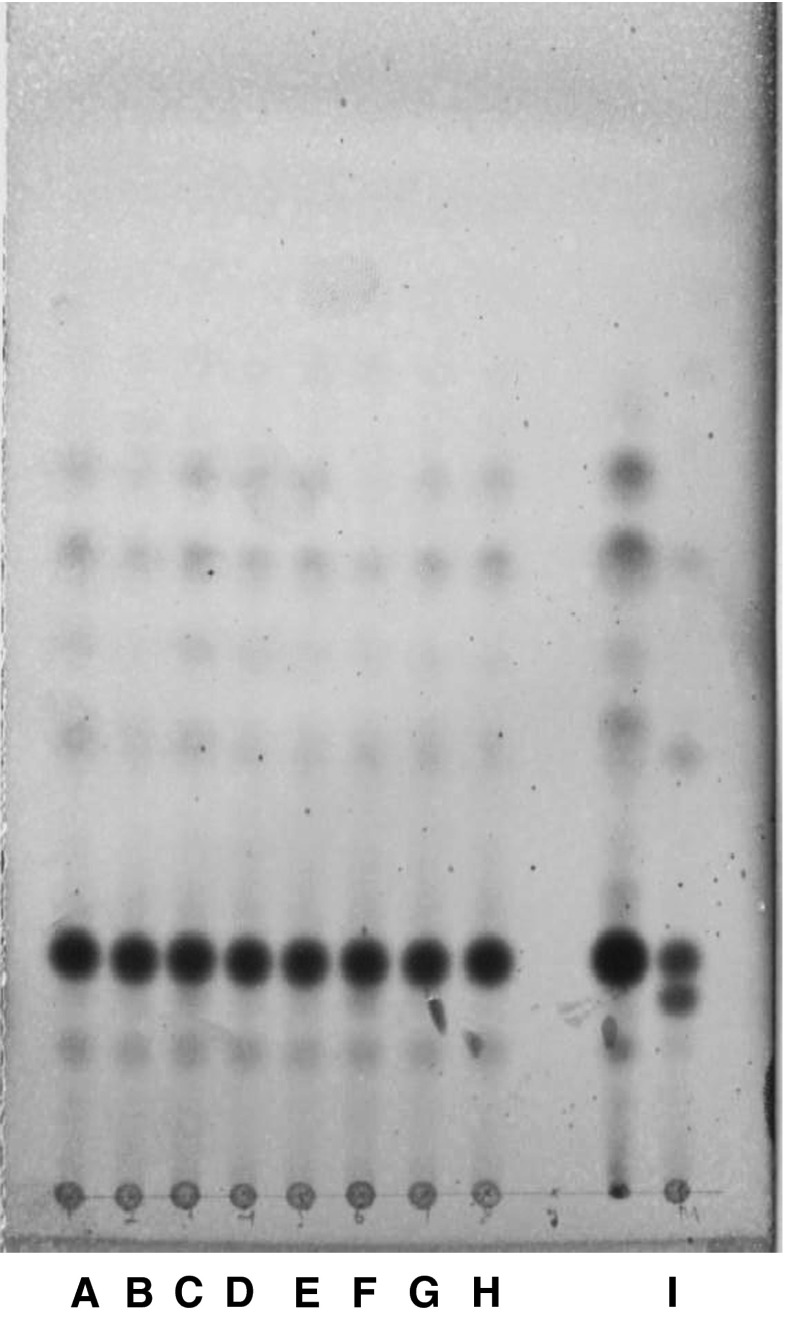
It can be seen from the chromatogram that in case of pure ghee samples (cow and buffalo) the position of prominent band was corresponding to the Rf value of cholesterol and there was no band corresponding to the Rf value of ß-sitosterol (Fig. 2). However, in case of pure adulterant oils a prominent band corresponding to the Rf value of ß-sitosterol appeared as evident in Fig. 3. These observations clearly indicated that ß-sitosterol was the prominent sterol in all the oils selected for the study, thereby the appearance of any ß-sitosterol band in ghee sample could be considered as an indicator of adulteration of ghee with vegetable oils and/or designer oil.
Fig. 2.
RP-TLC of pure ghee samples: A: Pure cow ghee; B: Pure buffalo ghee
Fig. 3.
RP-TLC of USM of adulterant oils: A: Refined sunflower oil; B: Refined ground nut oil; C: Refined safflower oil; D: Refined soybean oil; E: Coconut oil; F: Cholesterol standard; G: ß-sitosterol standard; H: Designer oil-1; I: Designer oil-II; J: Designer oil-III
Validity and specificity of RP-TLC method
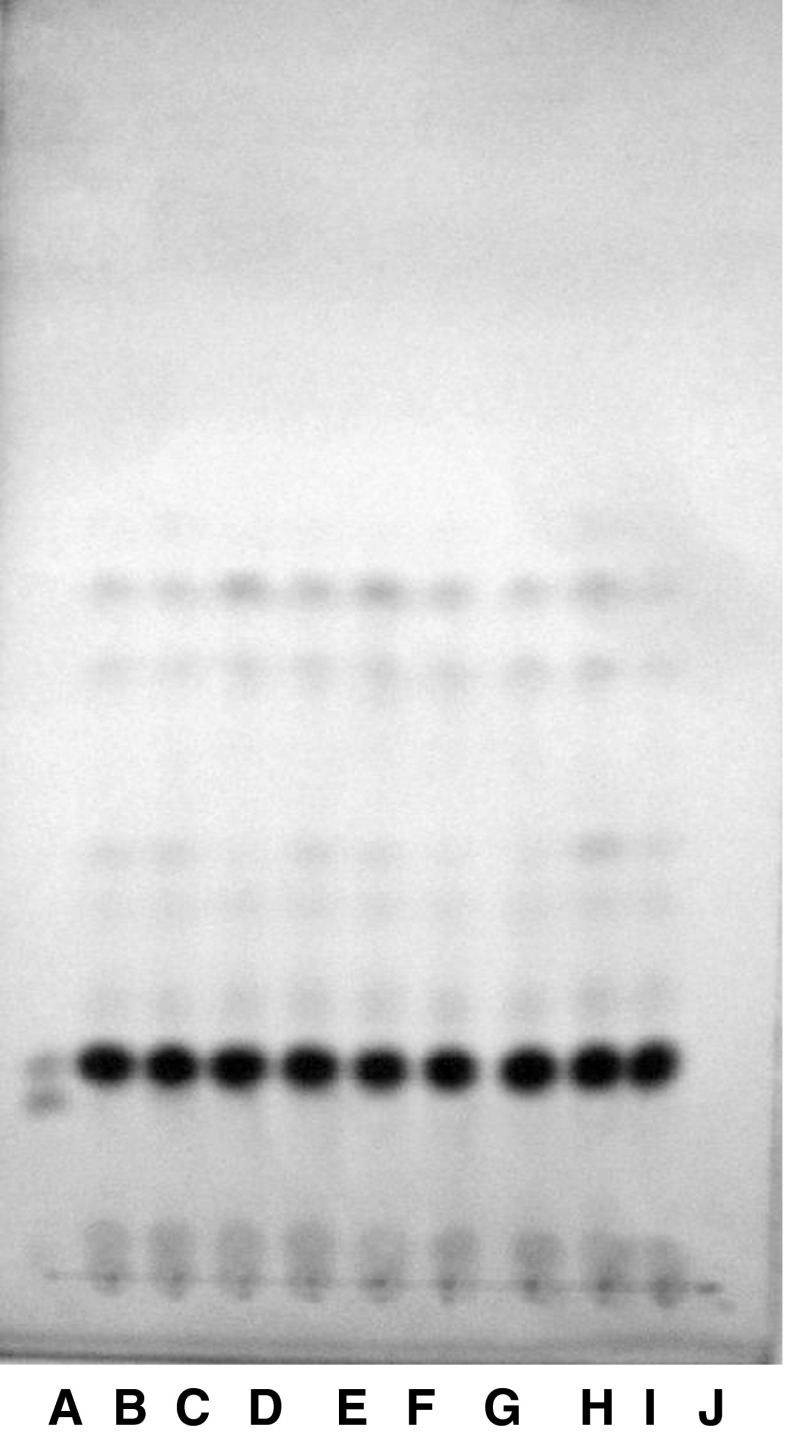
To validate the standardised RP-TLC protocol and to rule out the false positive results expected due to variation in management of the animals under different conditions, genuine ghee samples from cotton tract area as well as samples prepared from milk of local dairy farms were also used in the study. The unsaponifiable matter from these samples was subjected to RP-TLC analysis to obtain the profile of sterols. It is evident from the RP-TLC chromatogram (Fig. 4) that none of the genuine ghee samples showed any band corresponding to the band of ß-sitosterol. This clearly indicated that standardized protocol was very specific in detecting the added refined vegetable oils and designer oil in ghee and method could be used to detect the adulteration of ghee with vegetable oils without showing any false positive results.
Fig. 4.
RP-TLC of USM of ghee samples of known origin A: mixture (cholesterol + ßsitosterol).; B & C: Pure cow ghee; D: Homemade cow ghee Amreli; E & F: Homemade cow ghee; G: Ghee made from collected milk-I; H: Ghee made from collected milk-II; I: Ghee made from collected milk III; J: Homemade buffalo ghee Amreli
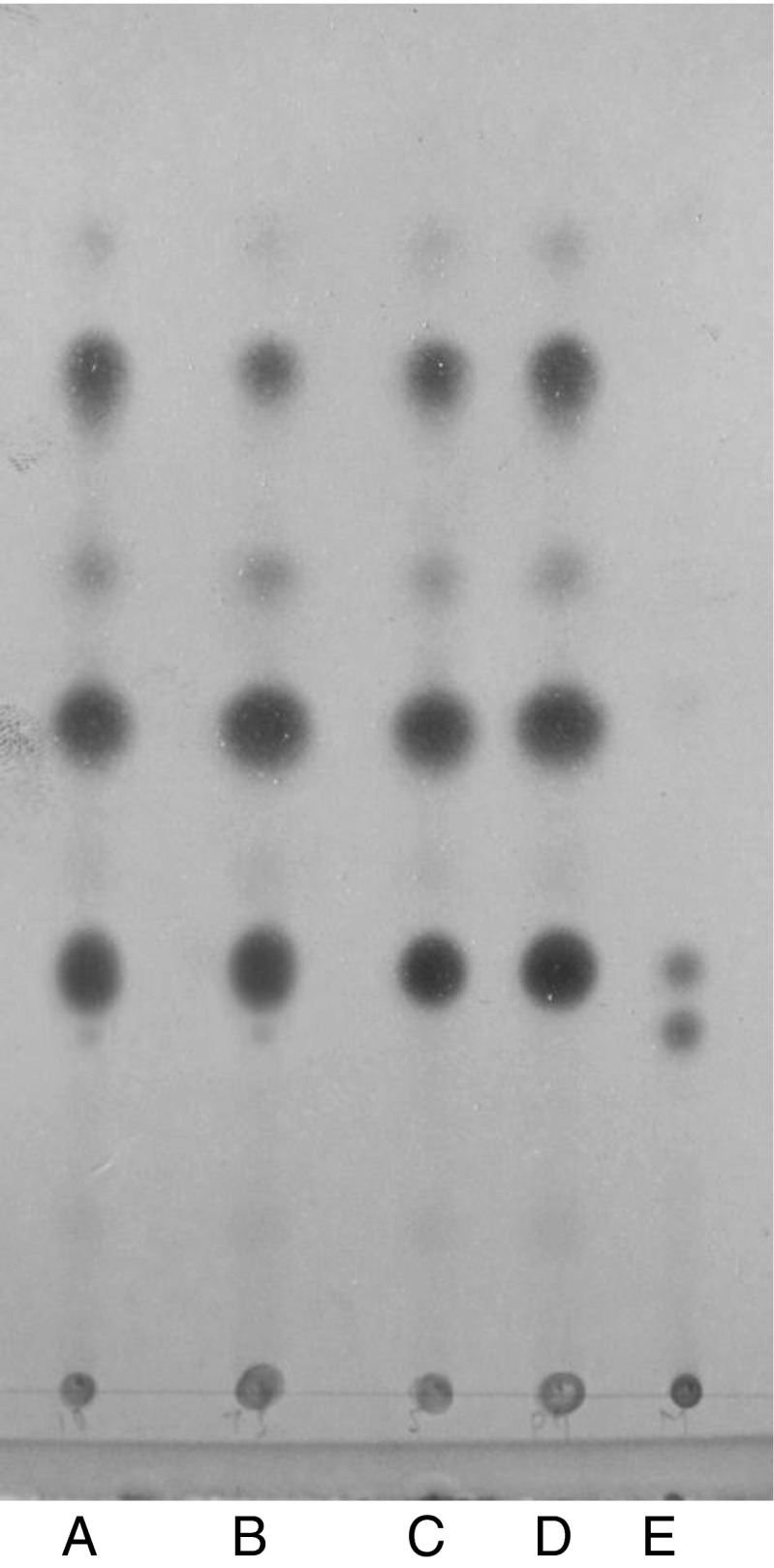
Level of detection of adulteration of ghee with refined vegetable and designer oil
The unsaponifiable matter of the ghee samples adulterated with adulterant oils at different levels was subjected to the above standardized RP-TLC method. RP-TLC chromatograms (Figs. 5 and 6), clearly showed that the adulteration of ghee with vegetable oils such as, sunflower oil and groundnut oil could be detected up to 1 % level while, soybean oil and designer oil up to 2 % level. In case of coconut oil added to ghee at 5 % level a very faint band was visible in the chromatogram, but at 7.5 % level, the visibility of band corresponding to ß-sitosterol band was more (Fig. 7). Hence, the reasonable level of detection of coconut oil in ghee was selected as 7.5 %. The RP-TLC method standardized in the present investigation has better sensitivity over the reversed phase thin layer chromatographic methods used by earlier workers (IDF 1966; Ramamurthy et al. 1967; Mathew and Kamath 1978); where the level of detection reported for coconut oil was 25 % and for other vegetable oils 2–13 %.
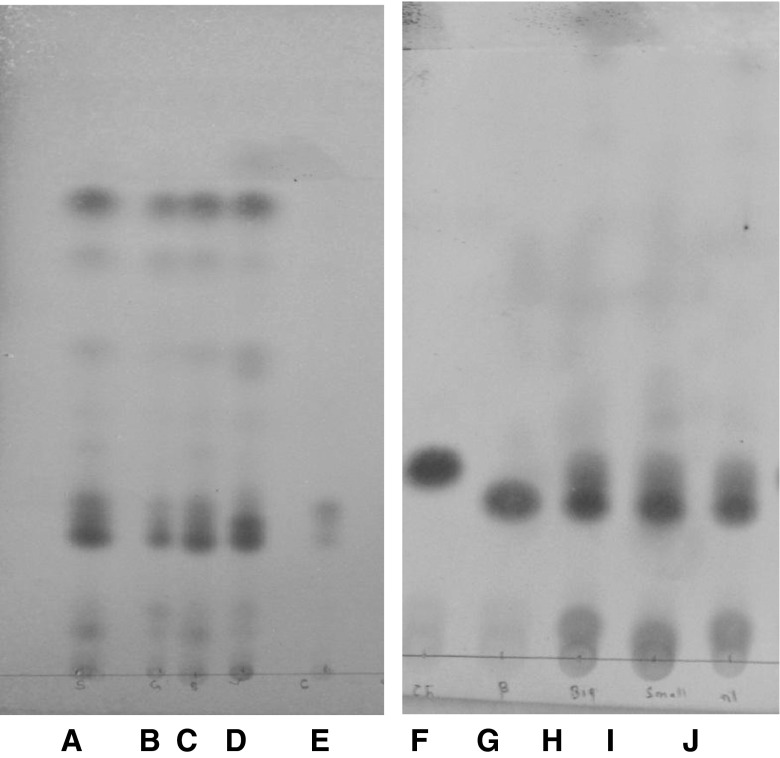
Fig. 5.
RP-TLC of USM of ghee samples adulterated with adulternt oils: A: 1 % groundnut oil adulterated cow ghee; B: 2 % groundnut oil adulterated cow ghee; C: 5 % groundnut oil adulterated cow ghee; D: 1 % sunflower oil adulterated cow ghee; E: 2 % sunflower oil adulterated cow ghee; F: 5 % sunflower oil adulterated cow ghee; G: 2 % soybean oil adulterated cow ghee; H: Pure cow ghee-1; I: mixture (cholesterol + ß-sitosterol)
Fig. 6.
RP-TLC of USM of ghee samples adulterated with designer oils A: mixture (cholesterol + ß-sitosterol).; B: Pure cow ghee; C: 1 % designer oil adulterated cow ghee; D: 2 % designer oil adulterated cow ghee; E: 5 % designer oil adulterated cow ghee; F: 10 % designer oil adulterated cow ghee; G: 1 % designer oil adulterated buffalo ghee; H: 2 % designer oil adulterated buffalo ghee; I: 5 % designer oil adulterated buffalo ghee; J: 10 % designer oil adulterated buffalo ghee
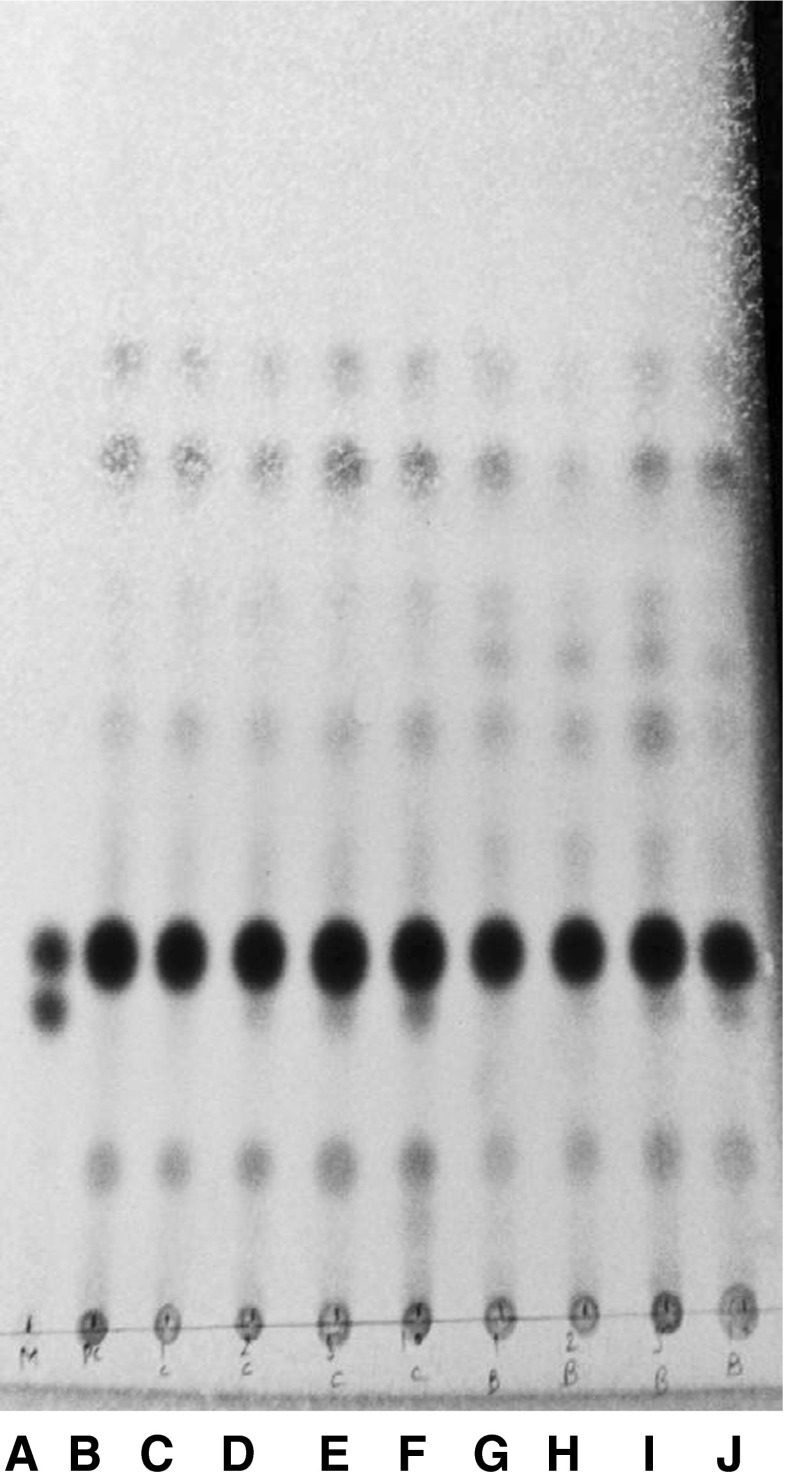
Fig. 7.
RP-TLC of USM of ghee samples adulterated with coconut oil A: 7.5 % coconut oil adulterated cow ghee.; B: 10 % coconut oil adulterated cow ghee; C: 5 % coconut oil adulterated cow ghee; D: Pure cow ghee; E: mixture (cholesterol + ß-sitosterol)
Conclusions
Using the reversed-phase thin layer chromatographic protocol standardised in the present study the adulteration of ghee with adulterant oils such as soybean oil, sunflower oil and groundnut oil could be detected up to 1 % level while, designer oil up to 2 % level. Similarly, coconut oil addition in ghee could be detected up to 7.5 % level. Validation of method showed that chances of getting false positive results are almost negligible. The protocol developed in the present study is rapid and easy to perform in any laboratory assessing the quality of milk fat and does not require sophisticated equipment, so it can be recommended to the testing laboratories of law enforcing agencies as well as of dairy/food testing laboratories.
Acknowledgments
The authors are thankful to the Director, National Dairy Research Institute, Karnal for providing necessary facilities for carrying out the research. Due acknowledgement is being paid to Indian Council for Agricultural Research, New Delhi for providing the junior fellowship to the first author.
References
- Aktas N, Kaya M. Detection of beef body fat and margarine in butter fat by differential scanning calorimetry. J Therm Anal Calorim. 2001;66:795–801. doi: 10.1023/A:1013196106365. [DOI] [Google Scholar]
- Arun K, Lal D, Seth R, Sharma R. Recent trends in detection of adulteration in milk fat—a review. Indian J Dairy Sci. 2002;55:319–330. [Google Scholar]
- Arun K, Lal D, Seth R, Sharma V. Detection of groundnut oil and vanaspati in ghee by thin layer chromatography. Indian J Dairy Sci. 2005;58:250–252. [Google Scholar]
- Bailey AE. Industrial oil and fat products. New York: Interscience Publishers Inc.; 1951. [Google Scholar]
- Chourasia LO, Patil MN, Jaiswal PK. Detection of kokum fat in ghee by thin layer chromatography. J Oil Technolgists India. 1994;26:115. [Google Scholar]
- Coni E, Di Pasquale M, Coppolelli P, Bocca A, Di Pasquale M. Detection of animal fats in butter by differential scanning calorimetry: a pilot study. J Am Oil Chem Soc. 1994;8:807–810. doi: 10.1007/BF02540453. [DOI] [Google Scholar]
- De S. Outlines of dairy technology. New Delhi: Oxford University Press; 2011. [Google Scholar]
- Fontech J, Diaz V, Fraga MJ, Juarez M. Triglyceride analysis by gas chromatography in assessment of authenticity of goat milk fat. J Am Oil Chem Soc. 1998;12:1893–1896. doi: 10.1007/s11746-998-0347-6. [DOI] [Google Scholar]
- IDF (1966) Detection of vegetable fat in milk fat by thin layer chromatography of steryl acetates. FIL-IDF 38
- Jarusiewics J, Sherm J, Fried B. Separation of sterols by reverse phase and argentation thin layer chromatography. Their identification in shail bodies. J Liq Chromatogr Relat Technol. 2005;28:2607–2617. doi: 10.1080/10826070500189869. [DOI] [Google Scholar]
- Kala Amrutha AL. Detection of possible adulteration in commercial ghee samples using low-resolution gas chromatography triglyceride profiles Int. J Dairy Technol. 2013;66:346–351. doi: 10.1111/1471-0307.12049. [DOI] [Google Scholar]
- Keeney MKC, Bachman HH, Tikriti AS, King L. Rapid vitamin E method for detecting the adulteration of dairy products with non-coconut vegetable oils. Indian J Dairy Sci. 1971;54:1702–1703. doi: 10.3168/jds.S0022-0302(71)86092-7. [DOI] [Google Scholar]
- Mahon JH, Chapman RA. Detection of adulteration of butter with vegetable oils by means of the tocopherol content. Anal Chem. 1954;26:1195. doi: 10.1021/ac60091a028. [DOI] [Google Scholar]
- Markuze-Zofia RZH. Method for the estimation of tocopherol content of butter and its use for the detection of adulteration with vegetable fats. Warsz. 1962;13:255–262. [Google Scholar]
- Mathew TV, Kamath KS. Detection of vegetable oils in ghee and vice-versa by reverse phase thin layer chromatography. Res Ind. 1978;23:168–169. [Google Scholar]
- Nazir DJ, Magar NG. Tocopherol content of Indian butter and its use in detecting adulteration of butterfat. Indian J Dairy Sci. 1959;12:125. [Google Scholar]
- Ntakatsane PM, Liu XM, Zhou P. Short communication: rapid detection of milk fat adulteration with vegetable oil by fluorescence spectroscopy. J Dairy Sci. 2013;96:2130–2136. doi: 10.3168/jds.2012-6417. [DOI] [PubMed] [Google Scholar]
- Patel AA, Frede E. Studies on thermal properties of cow and buffalo milk fats. Lebensm Wiss Technol. 1991;24:323–327. [Google Scholar]
- Patel AM, Sharma V, Lal D, Arora S. Validation of crystallization time test, apparent solidification time test and complete liquification time test for detection of adulteration in ghee. Indian J Dairy Sci. 2011;64:298–304. [Google Scholar]
- Povolo M, Bonfitto E, Contarini G, Toppino M, Daghetta A. Study on the performance of three different capillary gas chromatographic analysis in the evaluation of milk fat purity. J High Resolut Chromatogr. 1999;22:97–102. doi: 10.1002/(SICI)1521-4168(19990201)22:2<97::AID-JHRC97>3.0.CO;2-O. [DOI] [Google Scholar]
- Precht D. Quantification of animal body fats and vegetable fats in milk by triglyceride analysis. 22nd Int Dairy Cong. 1990;1:234. [Google Scholar]
- Precht D. Detection of foreign fat in milk fat. 1. Quantitative detection of triacyl-glycerol formulae. Z Lebensm Unters Forsch. 1992;194:1–8. doi: 10.1007/BF01191031. [DOI] [Google Scholar]
- Ramamurthy MK, Narayanan KM, Bhalerao VR, Dastur VN. A TLC method for detection of adulteration of ghee with vegetable fats. Indian J Dairy Sci. 1967;20:11. [Google Scholar]
- Sharma V, Sudharshana RMJ, Arora S, Kumar A, Lal D, Seth R, Wadhwa BK, Sharma GS. Applicability of enzymatic diagnostic kit for cholesterol estimation in ghee. J Food Sci Technol. 2009;46:244–246. [Google Scholar]
- Zachariah SP, Parmar SC, Bhavadasan MK, Nath BS. Detection of adulteration of ghee with coconut oil or palm oil. Indian J Dairy Sci. 2010;63:278–282. [Google Scholar]