Abstract
In the present study, refined dark chocolate mix was conched with the addition of finely powdered cinnamon in a laboratory-style conching machine to evaluate its aroma profile both analytically and sensorially. The analytical determinations were carried out by a combination of solid phase micro extraction (SPME)-gas chromatography (GC)-mass spectroscopy (MS) and-olfactometry(O), while the sensory evaluation was made with trained panelists. The optimum conditions for the SPME were found to be CAR/PDMS as the fiber, 60 °C as the temperature, and 60 min as the time. SPME analyses were carried out at 60 °C for 60 min with toluene as an internal standard. 26 compounds were monitored before and after conching. The unconched sample had a significantly higher fruity odor value than the conched sample. This new product was highly acceptable according to the overall inclination test. However some of textural properties, such as coarseness, and hardness were below the general preference.
Keywords: Chocolate, Cinnamon, Conching, Gas chromotography mass spectrometry, Olfactometry, SPME
Introduction
Development of new food products with improved quality and health benefits have been gaining increased attention in recent years. (Nebesny et al. 2007). A growing consumer interest in more differentiated food products has been observed in the recent years, herein traditional local foods. Traditional local foods have been described as foods from a certain local area produced in a traditional way and having specific sensory properties (Guerrero et al. 2009). However, food product development is necessary for survival of the product in a competitive global market (Stewart-Knox and Mitchell 2003). The concept of new product is susceptible to various definitions. A definition considered basic describes a new product to cover original products, improved products, modified products and new brands developed through an organization’s research and development efforts (Kotler 1991).
Chocolate flavoured with spices in this study falls under category of “Invention” meaning it has not been previously marketed or produced but inique and untried or unfamilar (Segall et al. 2000). The main intention of development of this product is to obtain a product wich enriched in terms of aroma.
Chocolate is unique food due to its attractive flavour. The chocolate manufacturing process of roasting and conching play an important part in determining its flavour aside the genotype/origin of the cocoa and the fermentation/drying methods. Roasting and conching involves high temperature that ensure the evaporation of undesirable compounds and at the same time the development of desirable ones that impact on the flavour of the finished product. The temperature and duration of two processes influence the type and levels of aroma compound in the finished chocolate. And thereby determine its flavour quality (Ramli et al. 2008; Counet et al. 2002).
The present study was carried out to optimize the process parameters of dark chocolate analyses in GC-MS with SPME, to identify the volatile before and after conching of dark chocolate and to elucidate the flavor interaction between the natural aroma of cocoa mass and that of the spices added as powder to the refined dark chocolate mix.
Material and methods
Conching process
The refined chocolate mix, cocoa butter, and other ingredients such as lecithin were supplied from a local chocolate plant whenever needed. The spices were purchased from a local store and were powdered and screened to 20 μm mesh prior to conching. Cinnamon powder was added at the begining of conching process like the addition of milk powder as in the case of the milk chocolate.
A 5 kg-capacity, laboratory-scale conching machine (ELK’olino single-shaft conche, Bühler, AG, Switzerland) with temperature and speed control was used. The process was composed of three phases; dry phase: 2 h at 50 °C, pasty phase: 4 h at 80 °C and final phase: 1 h with linear decrease in temperature from 80 °C to 45 °C. The conching machine was equipped with a double-jacket water circulation system for either heating or cooling. It had two internal axes with impellers, which rotated in opposing directions. The rotation speed of the machine was in the range of 600–5,000 rpm however, 1,500 rpm was selected throughout of this study. The formulation of cinnamon chocolate is form of 40.87 % cocoa mass (cocoa solid and cocoa butter, 39.14 % sugar, 15.45 % cocoa butter added, 0.34 lecithin and 4.2 % percent cinnamon powder. Difference between the formulation of cinnamon and dark chocolate is percent of cocoa mass. 45.07 % cocoa mass was used for dark chocolate.
Composition analysis
The moisture, fat, protein and ash contents were analyzed according to the standard methods (AOAC 1990). The percent carbohydrate was then determined by difference. The analyses were carried out in triplicate and the values were averaged. The composition of cinnamon chocolate consists of 0.54 % moisture, 8.73 % protein, 2.15 % ash, 57.80 % carbohydrate and 30.70 % fat.
SPME
Four types of fibers were used: 100 μm polydimethylsiloxane coating (PDMS), 65 μm polydimethylsiloxane/divinybenzene coating (PDMS/DVB), 75 μm carboxen/polydimethylsiloxane coating (CAR/PDMS) and 50/30 μm divinybenzene/carboxen on polydimethylsiloxane on a stableflex fiber (DVB/CAR/PDMS). These fibers were purchased from Supelco (Bellefonte PA, USA).
Statistical analysis
An experimental design was performed in accordance with response surface methodology. The central composite design feature of Design-Expert version 6.01.0 (Stat-Ease, Inc Minneapolis, MN) was used to observe the effects of the process variables over change in the number of organic acids, alcohols, other compounds (all components except acids and alcohols) and total (all volatile components detected by GC-MS) as shown in Table 1.
Table 1.
Desing matrix of experiment of cinnamon chocolate in desing expert
| Study Type | Response Surface | ||||
|---|---|---|---|---|---|
| Initial Design | Central Composite | ||||
| Design Model | Quadratic | ||||
| Factor | Name | Units | Tpye | Low Actual | High Actual |
| X | Temperature | °C | Numeric | 40 | 70 |
| Y | Time | Minute | Numeric | 15 | 60 |
| Z | Fiber type | Categoric | A* | D* | |
| Response | Name | Units | Analysis | Minimum | Maximum |
| Y1 | Acids | Number | Polynomial | 2.0 | 10 |
| Y2 | Alcohols | Number | Polynomial | 0.0 | 8 |
| Y3 | Others | Number | Polynomial | 1.0 | 24 |
| Y4 | Total | Number | Polynomial | 6.0 | 41 |
The data were fitted to a second order polynomial. The significance of terms in the model was found by analysis of variance (ANOVA) for each response. Significance was judged by determining the probability level. Response surface methodology was used to determine optimum condition that yield maximum acids, alcohols, other compounds and total number of compounds. In this study, temperature, time and fiber type were selected in range (shown in Table 2).
Table 2.
Range of process parameters
| Name | Goal | Lower limit | Upper limit | Lower weight | Upper weight |
|---|---|---|---|---|---|
| Temperature | in range | 40 | 60 | 1.0 | 1.0 |
| Time | in range | 15 | 60 | 1.0 | 1.0 |
| Fiber type | in range | A | D | 1.0 | 1.0 |
| Acid | maximize | 2.0 | 10 | 1.0 | 1.0 |
| Alcohol | maximize | 0.0 | 8.0 | 1.0 | 1.0 |
| Other | maximize | 1.0 | 24 | 1.0 | 1.0 |
| Total | maximize | 6.0 | 41 | 1.0 | 1.0 |
SPME extraction
Volatiles from samples were extracted by using 75 μm divinylbenzene/carboxen on polydimethylsiloxane on a Stable/Flex fiber (CAR/PDMS). Extractions were carried out in the vials. A 2 g-sample together with 1 μL toluene, as internal standard, was placed in a 20-ml vial. After tightly plugging its lid and inserting the SPME fiber, it was equilibrated for 60 min at 60 °C. The desorption time was 5 min and the temperature in the GC liner was 250 ° C
GC/MS
The volatiles extracted by fibers were thermally desorped and introduced in the capillary column(EQUITY™-5 FUSED SILICA Capillary Column 30 m × 0.32 mm × 0.25 μm film thickness Supelco). The GC (Perkin Elmer Clarus 500)-MS (Clarus 500 MS Perkinelmer) was set up with constant flow of 2 ml/min (helium), the oven temperature was programmed starting at 80 °C (5 min)–(10 °C/min) 150 °C–150 °C(10 min)–(10 °C/min) 200 °C-200 °C(5 min). The injector temperature was 250 °C. The analysis was carried out by using gas chromatography coupled with mass spectrometry. The ionization voltage was 70 eV, mass range m/z 40–300.
Odour identification by using olfactometry
Two trained panellists sniffed the outflowing gas from the olfactometer’s detection port. The sniffing was carried out simultaneously during GC analysis. Panelists described their perception of the sniffed odour.
Sensory evaluation
Chocolate samples were evaluated by twelve untrained panellists in terms of taste, odour and texture. Analysis was carried out under blue light in order to reduce the impact of apperance. Samples were evaluated in duplicate by each judge. Each judge was provided with a spit cup and a cup of water at room temperature to rinse their mouths prior sample tasting. Results were evaluated statistically in SPSS.
Differential scanning calorimetry
Differential scanning calorimeter (Perkin Elmer FC100 ped2 27603) was used for the determination of melting point of cinnamon chocolate. A-9.36 mg sample was placed into a pan, which was sealed with lids using a sample press. The pans were heated from −5 °C to 65 °C in a N2 stream. The onset temperature (Tonset), peak temperature (Tpeak), and the end temperature (Tend) were calculated automatically by the software (Pyris software for windows version 7).
Polarized light microscope
Microstructure of the sample and particle size was observed by Polarized Light Microscopy (Olympus BX51TF Tokyo Japan). The microstructure of samples was imaged by using a digital camera (Viewfinder version 2.1.1. Pixera). Slides were prepared by melting the chocolate at 45 °C for 10 min. A capillary pipet was used to deposit a small droplet of chocolate onto a glass slide. One drop of vegetable oil was used to solve chocolate. Glass cover slip was then placed on the surface of the droplet. The sample was observed under the microscope for up to 1 h. Images were captured every 10 min. Counting method was used to determine the dimension of particle by using the software (BS Image system BS 200 Pro Plus version 3.0).
Results and discussion
Selection of SPME fiber
Response surface methodology was used to optimize process parameters time, temperature and SPME fiber for the GC-MS analysis. It was reported that (Pawliszyn 1997) there are various parameters that determine the precision of the SPME method developed. Parameters like sample volume, exposure time, temperature, and so forth have an influence on the extraction efficiency of the method. The selection of conditions for analysis is very important in terms of correct identification of the compound in sample with GC-MS. Therefore, an experimental design in Design-Expert was used. The experimental results were statistically evaluated and results obtained are given below.
Response surface methodology was used for qualitative investigation of change in the number of acids, alcohols, others and total. Linear and quadratic regression equations describing the effects of temperature, and time on change in the number of acids, alcohols, others and total were developed and regression equation coefficients are given in Table 5. It is evident that time and temperature has a positive effect on the number of acids, alcohols, other and total as can be seen from Table 3. Fibers absorbed more acids, alcohols, other and totals with increasing process temperature and time.
Table 5.
Change in the volatiles of cinnamon chocolate before and after conching (SPME -GC-MS) using fiber C
| Retention time (min.) | Compounds (before conching) | Odour description | Odour activity |
|---|---|---|---|
| 0.79 | Butanal, 3-methyl | Chocolate | 1.74 |
| 0.47 | 1-propen-2-ol-acetate | Fatty | 0.09 |
| 4.78 | Pyrazine, 2,5,dimethyl | Nutty, green | 1.94 |
| 5.10 | Pyrazine, 2,3, dimethyl | Cooked, nutty | 0.59 |
| 5.20 | 2-Isopropyl-5-methyhex-2-enal | Unknown | 0.42 |
| 5.61 | Nonanal | Unknown | 4.99 |
| 5.82 | Pyrazine, trimethyl | Cocoa, roasted | 3.83 |
| 6.41 | Acetic acid | Sour | 4.79 |
| 6.69 | Pyrazine, tetramethyl | Bean like | 32.16 |
| 7.37 | Benzaldehyde | Nutty | 9.25 |
| 7.44 | 1,6-octadien-3-ol,3,7-dimethyl-acetate | Flowery | 0.28 |
| 7.59 | Cyclobutane, 1,2,diethyl-trans | Like laurel | 0.21 |
| 7.70 | 2-butanone,3-hydroxy | Fruity | 0.34 |
| 7.83 | Propanoic acid, 2-methyl | Rancid | 0.38 |
| 7.97 | 2-Furancarboxaldehyde, 5-methyl | Sweet,caramel like | 0.23 |
| 8.11 | 2-Decanone | No smell | 0.41 |
| 8.15 | Cyclohexanol, 5-methyl-2-(1methylethy) | Like laurel | 0.80 |
| 8.70 | Butanoic acid, 4-hydroxy | Fruity | 0.17 |
| 8.75 | Benzeneacetaldehyde | Pungent | 1.84 |
| 8.99 | Butanoic acid, 3-methyl | Cheese | 1.75 |
| 10.26 | Carboxylic acid, benzyl | Sweety | 0.14 |
| 10.60 | Acetic acid, 2-phenylethyl ester | Vinegar | 1.90 |
| 10.82 | 1-butanol, 3-methyl-benzoate | Fruity | 1.11 |
| 10.90 | Hexanoic acid | No smell | 0.30 |
| 11.11 | Phenol, 2-methoxy | Woody | 0.39 |
| 11.27 | Benzyl alcohol | Almond like | 0.37 |
| 11.46 | Phenylethanol | Sweet,honey | 6.62 |
| 12.99 | Phenol, 2-methoxy | No smell | 0.17 |
| 13.12 | 4-Propyl-2-hydroxycyclopent-2en-1-one | Unknown | 0.34 |
| 13.38 | Carbonic acid, decyl pheny ester | sour | 1.81 |
| 13.66 | 2-propenal, 3-phenyl | Potato like | 1.02 |
| 13.80 | Hexanoic acid | No smell | 0.29 |
| 14.10 | 5-methyl-2-phenyl-2-hexenal | Cocoa | 0.68 |
| 16.12 | Nonanoic acid | Rancid | 0.44 |
| 5.58 | Decane, 6-ethyl-2-methyl | Unknown | 0.42 |
| 6.48 | Acetic acid | Sour, vinegar | 3.79 |
| 6.87 | Copaene | Woody | 1.38 |
| 7.37 | Benzaldehyde | Nutty | 0.79 |
| 7.76 | Bicyclo (3.1.1.heptane,6-methyl) | Unknown | 0.09 |
| 7.93 | 6,8-Nonadien-2-one, 8-methyl | No smell | 0.05 |
| 8.04 | 2-Octanone | Green | 1.05 |
| 8.17 | Bicyclo (3.1. hexan-2-ol, 2-methyl) | Floral, fruity | 0.06 |
| 10.98 | Phenylethylalcohol | Sweet, honey | 1.01 |
| 11.11 | Phenol, 2-methoxy | Smoky | 0.17 |
| 11.55 | 2-propenal, 3-phenyl | Potato like | 1.02 |
| 11.67 | Phenylethyalcohol | Sweet, honey | 0.19 |
| 13.92 | Cinnamaldehyde | Cinnamon | 56.5 |
| 16.12 | Propanedioic acid, propyl | Apple | 0.06 |
| 17.46 | Phenol, 4- (1-phenylethyl) | No smell | 0.05 |
| 19.42 | Propanedioic acid, propyl | Apple | 0.06 |
| 24.27 | 2H-1- Benzopyran-2-one | Green | 4.18 |
| 9.21 | Pentane, 2,3,3-trimethyl | Smoky | 0.17 |
| 9.26 | Cyclohexene, 4-methylene | Citrus | 0.62 |
| 9.92 | Copaene | Woody | 1.38 |
| 10.26 | Benezenepropanal | No smell | 1.84 |
| 10.45 | Pentanal, 5-methyl | Green | 0.88 |
| 10.75 | Ethanone | Fruity | 0.42 |
| 10.81 | 1-butanol, 3-methyl, benzoate | Floral, fruity | 1.44 |
| 0.23 | 3-methylpentane | No smell | 1.93 |
| 0.47 | 2-methyl-1-propanol (isobutanol) | Sweet | 3.46 |
| 0.77 | Butanal, 3-methyl | Fruity | 1.74 |
| 1.14 | Decane | Unknown | 1.19 |
| 3.16 | Cyclohexene, 1-methyl | Spicy | 0.80 |
| 6.40 | Acetic acid | Sour, vinegar | 4.77 |
| 6.66 | Pyrazine, tetramethyl | Beanlike | 32.16 |
| 7.37 | Benzaldehyde | Nutty | 9.25 |
| 7.48 | Isopropanol | No smell | 1.73 |
| 7.69 | 2-butanone, 3-hydroxy | Creamy | 0.34 |
| 7.83 | Propanoic acid, 2-methyl | Fruity | 0.38 |
| 8.68 | Butanoic acid, 3-methyl | Banana | 1.75 |
| 10.90 | Pentanoic acid | Rancid | 0.12 |
| 11.11 | Phenol, 2-methoxy | Smoky | 0.39 |
| 11.37 | Phenylethanol | Sweet, honey | 6.62 |
| 12.49 | Ethanone | Fruity | 1.56 |
| 13.65 | 2-propenol, 3-phenyl | Potato like | 1.02 |
| 16.10 | Hexanoic acid | No smell | 0.29 |
Table 3.
Regression equation coefficients for acids, alcohols, others and total
| Acids | ||||||
|---|---|---|---|---|---|---|
| Fiber | Intercept | Temperature | Time | |||
| A | 0.82193 | 9.838E-003 | 7.582E-003 | |||
| B | 0.60313 | 9.838E-003 | 7.582E-003 | |||
| C | 0.74148 | 9.838E-003 | 7.582E-003 | |||
| D | 0.70638 | 9.838E-003 | 7.582E-003 | |||
| Alcohols | ||||||
| Fiber | Intercept | Temperature | Time | Temp.*Time | Temp.2 | Time.2 |
| A | 1.2305 | 0.1203 | 0.1115 | −2.849E-004 | −1.175E-003 | −1.509E-003 |
| B | 7.8935 | 0.2162 | 0.1134 | −2.849E-004 | −1.175E-003 | −1.509E-003 |
| C | 6.7574 | 0.2072 | 0.1321 | −2.849E-004 | −1.175E-003 | −1.509E-003 |
| D | 1.9310 | 0.1406 | 0.1049 | −2.849E-004 | −1.175E-003 | −1.509E-003 |
| Others | ||||||
| Fiber | Intercept | Temperature | Time | Temp.*Time | Temp.2 | Time.2 |
| A | 26.7755 | 0.78690 | 0.3252 | −6.010E-003 | 6.651E-003 | 8.287E-004 |
| B | 59.5827 | 0.83076 | 0.3234 | −6.010E-003 | 6.651E-003 | 8.287E-004 |
| C | 16.6997 | 0.59560 | 0.2927 | −6.010E-003 | 6.651E-003 | 8.287E-004 |
| D | 23.7084 | 0.73880 | 0.3514 | −6.010E-003 | 6.651E-003 | 8.287E-004 |
| Total | ||||||
| Fiber | Intercept | Temperature | Time | |||
| A | 1.2748 | 0.0205 | 7.555E-003 | |||
| B | 1.1390 | 0.0205 | 7.555E | |||
| C | 1.2775 | 0.0205 | 7.555E | |||
| D | 1.1399 | 0.0205 | 7.555E | |||
An inverse correlation was observed between the process time and temperature. In other words as it is seen in Table 3, an increase in the process temperature caused the process time to decrease. Mestres et al. (2000) reported that time and temperature are parameters closely related to each other e.g., an increase in temperature enables shorter exposure time, thus accelerating the analysis time
Also Pawliszyn (2002) declared that the exposure time is also very significant. A longer time favors the occupation of more sites on the fiber by analyte molecules, but prolonged time when all sites are occupied does not affect the pre-concentration efficiency and sometimes can cause desorption. In another study by Reto et al. (2007), five different exposure times were tested in order to determine the best compromise between time and analyte response and establish extraction profiles of the vitamin K from green tea. They concluded that the HPLC peak areas of vitamin K increased with an increase in exposure time.
The results show that the number of acids, alcohols, others and total is linearly affected by temperature and time. The influence of temperature was found to be highly significant (p < 0.05). The significance of each coefficient was statistically determined. The larger F values and small p-values are thought to be significant.
The number of acids, alcohols, others and total compounds absorbed by fiber in the each run were evaluated statistically depending on extraction time and temperature. The response surface plot is presented in Fig. 1 which indicates the increase in the number of acid absorbed by fiber is directly proportional to extraction time and temperature. Also, fiber C was observed to retain more acids than other fibers. No quadratic effect of temperature and time-temperature interaction on the in the number of acids was observed. Similar results were obtained for alcohols, others and total compounds. (data not given).
Fig. 1.
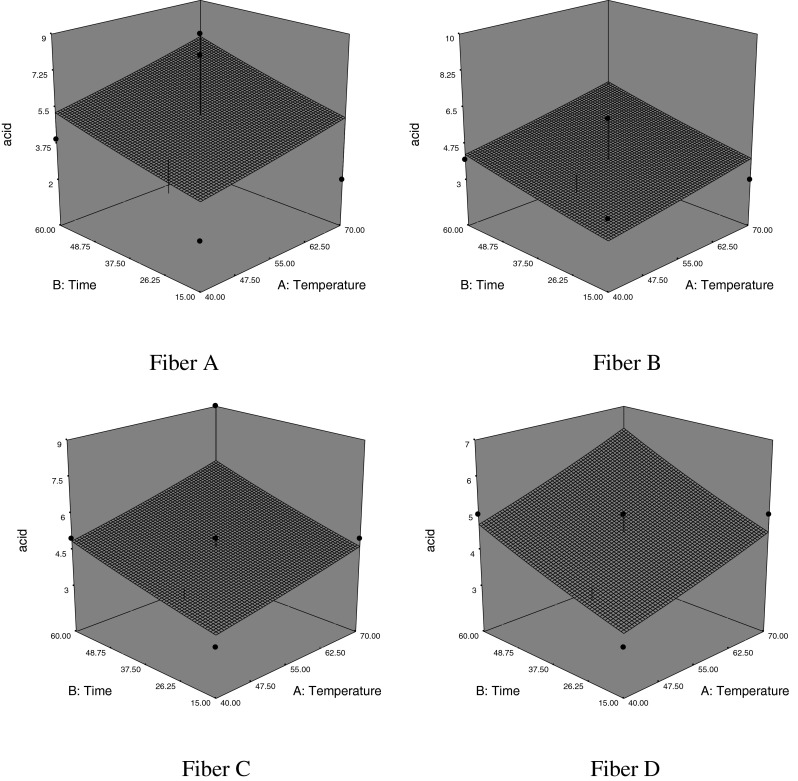
Variation in the number of acids as a function of time and temperature and fiber type
Optimization of process variables
Response surface methodology was used to determine optimum conditions that yield maximum number acids, alcohols, others and their total. King et al. (2003) reported that an increase in extraction temperature causes an increase in extraction rate but a decrease in the distribution constant. This conversely causes a decrease in the sensitivity of the extraction process. A well-balanced compromise between sensitivity and extraction rate with regard to the extraction temperature can be obtained by careful optimization.
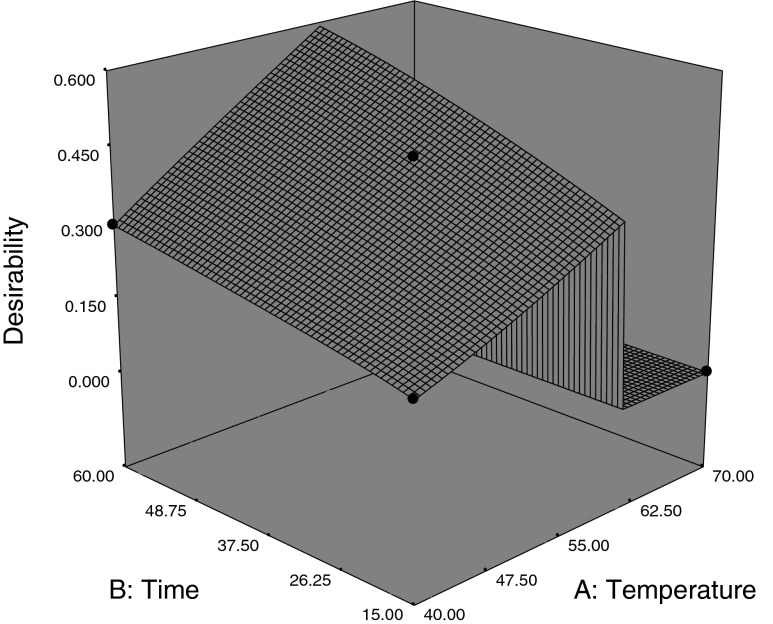
In this study, temperature, time and fiber type were selected in the range shown in Table 4. Applying desirability function method, seven solutions were obtained for optimum criteria. Also desirability value of first solution was greater than others. The optimum conditions were determined to be 60 °C, 60 min and fiber C (Fig. 2). At optimum the point, the number of acids, alcohols, others and their total were found to be 6, 5, 11 and 22 respectively.
Table 4.
Range of process parameters in the design expert response surface methodology
| Parameter | Goal | Lower limit | Upper limit | Lower weight | Upper weight |
|---|---|---|---|---|---|
| Temperature | in range | 40 | 60 | 1.0 | 1.0 |
| Time | in range | 15 | 60 | 1.0 | 1.0 |
| Fiber type | in range | A | D | 1.0 | 1.0 |
| Acid | maximize | 2.0 | 10 | 1.0 | 1.0 |
| Alcohol | maximize | 0.0 | 8.0 | 1.0 | 1.0 |
| Other | maximize | 1.0 | 24 | 1.0 | 1.0 |
| Total | maximize | 6.0 | 41 | 1.0 | 1.0 |
Fig. 2.
Optimum conditions as obtained by the design expert
Aroma evaluation
There are three basic operations affecting the formation of the characteristic chocolate flavour: fermentation, roasting and conching. Concentration of volatile acids, alcohols, aldehydes and other components cause unpleasant flavors diminishing during conching whereas interaction between cocoa mass, sugar and cocoa butter develop the flavour of chocolate. In this study, finely powdered cinnamon was added at the begining of the conching process and this was observed to cause a flavor interaction between sugar and cocoa mass. The newly formed aroma was evaluated both instrumentally using GC-MS-O, and sensorially by an untrained panel. Thus, the components originating from cinnamon, and those formed as a result of interaction between cinnamon and cocoa mix was identified.
As shown in Table 5, 36 volatile compounds were identified before conching of dark chocolate, meanwhile 26 compounds were identified after conching of cinnamon chocolate and 19 compounds were identified after conching of dark chocolate. Compared to dark chocolate, cinnamon chocolate was found to be more aromatic. Cinnamon chocolate had more flowery and fruity aroma than that of dark chocolate. It was considered that bicycle (3.1.1. heptanes, 6-methyl), 8-methyl-6,8-Nonadien-2-one, bicycle(3.1.hexan-2-ol, 2-methyl), copaene and 1-butanol, 3-methyl-benzoate occurred as a result of interaction between cinnamon and cocoa mass during conching. Because when GC-MS data of dark chocolate with and without cinnamon was examined, these compound was absent GC-MS date of dark chocolate. These compounds were an indication of interaction between cocoa mass and cinnamon powder. M. Owusu et al. (2008) investigated different types of chocolates in GC-MS and GC-O. They identified 58 volatile compounds in the dark chocolate. Also, Emmanuel Ohene Afoakwa et al. (2009) studied the effect of particle size on volatiles of dark chocolate and they examined these volatiles by GC-MS. They identified 48 volatile compounds. Another study related to this subject, was carried out by Sorel Muresan et al. (2000). They identified 41 volatile compounds in dark chocolate.
In the present study, as a result of GC-MS analysis, some of the volatile compounds were observed to disappear during the conching process while new volatiles was formed as a result of interaction between ingredients. For example, compounds such as 1-propen-2-ol-acetate, 3-hydroxy-2-butanon, 2-furan carboxyaldehyde and 2-decanone disappeared while bicyclo (3.1.1.heptane,6-methyl), 6,8-nonadien-2-one, 8-methyl and cinnamaldehyde formed after conching. As shown in Table 8 and 9, the cinnamon chocolate volatiles contained components such as cinemaldehyde, copaene, bornyl acetate, and caryophyllene which are some of the common volatiles appearing both in the cinnamon and cinnamon chocolate after conching.
It is understood from panel test that cinnamon has a highly acceptable effect on the chocolate flavor. Reineccius (2006) declared that, components like acetic acid, benzaldehyde and butanoic acid disappear during conching. When Table 5 is examined, these components might be thought to have been present after conching of cinnamon chocolate. However, as seen in Table 6, these components originate from cinnamon and therefore they appear in the cinnamon chocolate aroma.
Table 6.
Identified volatile compounds of cinnamon powder by SPME-GC –MS
| Retention time (min.) | Compounds | Odour description | Method of identification |
|---|---|---|---|
| 5.22 | Cyclobutene, 1,3 dissopropenyl trans | Woody | MS |
| 5.82 | Eucalyptol | Camphor | MS |
| 6.24 | 1,4 cyclohexadiene, 1-methyl-4-(1-methylethyl) | Citrus | MS |
| 6.56 | Benzene, 1-methyl-2-(1-methylethyl) | Flowery | MS |
| 8.04 | Nonanal | Floral | MS |
| 8.67 | Benzene, 2-methyl-1-propenyl | Green | MS |
| 9.43 | Copaene | Spicy | MS |
| 9.88 | Benzaldehyde | Nuty | MS |
| 9.95 | Bicyclo (2,2,1) Heptane, 7,7 dimethyl-2-methylene | Sweet | MS |
| 10.35 | 1,3 bis(2-cyclopropyl, 2-methylcyclopropyl) | Unknown | MS |
| 10.47 | Bornyl acetate | Pine | MS |
| 10.73 | Bicyclo(3,1,0) Hexan-2-ol-2-methyl-5-(1-methylethyl) | Odourless | MS |
| 10.76 | Caryophyllene | Fruity | MS |
| 11.55 | Benzoic acid | Sour | MS |
| 11.60 | Alpha-caryophyllene | Fruity | MS |
| 11.88 | 3-cyclohexene-1-methanol, alpha-4-trimethyl | Lilac | MS |
| 11.96 | Borneol,heptafluorobutyrate | Flowery | MS |
| 12.14 | Propanal, 2-methyl-3-phenyl | Unknown | MS |
| 12.40 | 1,3-cyclooctadiene, 5-bromo | Smoky | MS |
| 12.58 | 2-propenal, 3-phenyl | Potato like | MS |
| 12.93 | Cinnamaldehyde | Cinnamon | MS |
| 13.05 | 2-propenoic acid, 3-phenyl ester | Cinnamon | MS |
| 13.53 | 1H-cycloproplelazulene 1A,2,3,4A,5,6,7B-octahhydro1,1,4,7 tetram | Odourless | MS |
| 13.70 | Phenol, 2-methoxy-4-(1-propenyl) | Spicy | MS |
| 14.27 | Phenol, 2-methyl-5-(1-methylethy) | Nuty | MS |
| 15.91 | 2H-1-Benzopyran-2-one | Green | MS |
Not all odorants produced specific smell; some of them were odorless as a result of analysis carried out in an olfactometer. The corresponding odorants of cocoa mass and chocolate volatiles are shown in Table 5.
26 attributes were used to describe the aroma and flavour of cinnamon chocolate and cocoa mass. The unconched sample had a significantly higher fruity odor value than the conched sample. Volatiles such as phenylethyl alcohol, butanic acid, 4 hydroxy pentanal, 2,3 butanediol and phenol had sweet odors. Aldeydes are a significant fraction of cocoa flavour, contributing to the flowery, spicy and pungent flavours of chocolate (Afoakwa et al. 2009). Volatiles like 6,8-nonadien-2-one, phenol and benzenepropanal have no smell whereas 2-octanone, 2H-1-Benzopyran-2-one, 5-methly-pentanal and 2-methyl-1-propenyl were characterized as green odours.
Conching decreased the amount of volatiles by about 57 %. Table 10 shows that there is a drastic change in the aroma profile of cinnamon chocolate compared to dark chocolate. The amount of total acid volatiles change from 5,862 ppb to 1,448 ppb for dark chocolate meanwhile from 5,862 ppb to 1,147 ppb for cinnamon chocolate. This shows that dark chocolate are more acidic than cinnamon chocolate. The greatest impact of conching was observed on the pyrazine. Total amount of pyrazine reduced from 19,887 to 1,402 ppb for dark chocolate whereas from 5,862 ppb to 0 ppb dor cinnamon chocolate. In contrast to pyrazine, total amount aldehyde inceased after concing from 9,643 to 10,846 ppb for dark chocolate and from 9,643 to 30,364 ppb for cinnamon chocolate.
As seen in Table 5, there was no remarkable chance in the total amount of alcohol. 75 % and 60 % decrease in the amount of aldehyde and the alcohol was observed respectively after conching. Although 26 volatile components were determined after conching of cinnamon chocolate, only 11 of them were observed to be odor-active while 9 compounds out of 19 of dark chocolate were odor-active. This is means that only 11 components contribute to the odor of cinnamon chocolate after conching. Benzaldeyde, 2-Octanone, copaene, benzenepropanal and cinnamaldehyde were some of the odor active components.
Sensory evaluation
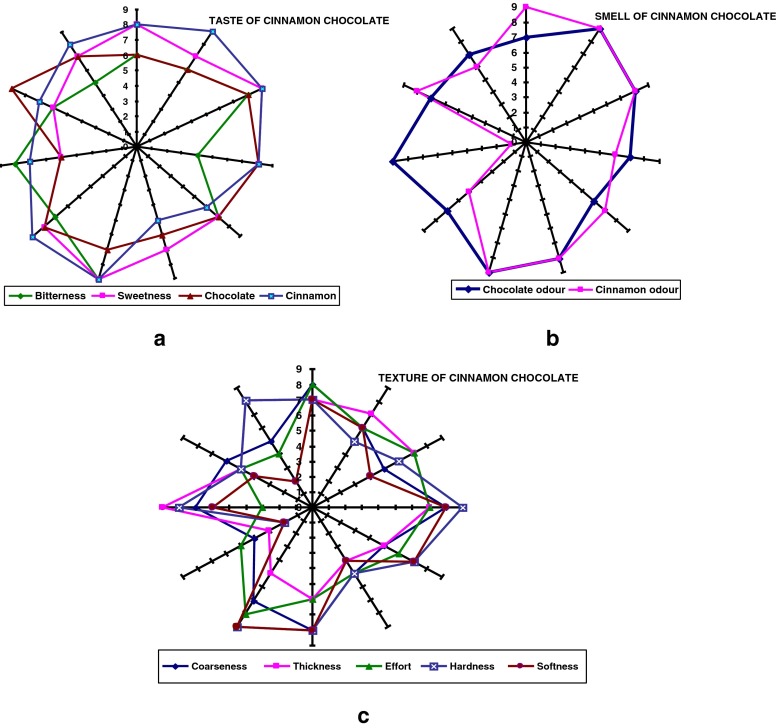
The sensory evaluation of cinnamon chocolate was performed out by rating of different characteristics: odour, taste and texture as shown in the Fig. 3a, b and c. In this figure sweetness, chocolate, effort, coarseness and thickness: refer to the taste associated with sucrose, aromatic taste associated with chocolate liquor, work required to melt, manipulate and swallow the sample, perception of particles against the roof of the mouth, perception of the viscosity of the melted chocolate sample in the mouth respectively.
Fig. 3.
Flavor profile of cinnamon chocolate as assessed by the panel
A 12-non-trained sensory panel evaluated the chocolate sample using the above scaling procedure. The results obtained were evaluated statistically. The high scores of all examined sensory characteristics gave an indication of high quality of chocolate sample.
T-test was used for evaluating the panel test results. As seen in Table 8, there is no significant difference statistically for sweetness, cinnamon, chocolate odour and cinnamon odour (p > 0.05). However, there is a significant difference statistically for bitterness, chocolate, coarseness, thickness, hardness and softness (p < 0.05). This means that panel evaluation for these parameters is not too satisfactory.
There is no difference statistically for overall inclination test (p > 0.05) and its mean is 7.3. This means that panel evaluation for these parameters is satisfactory.
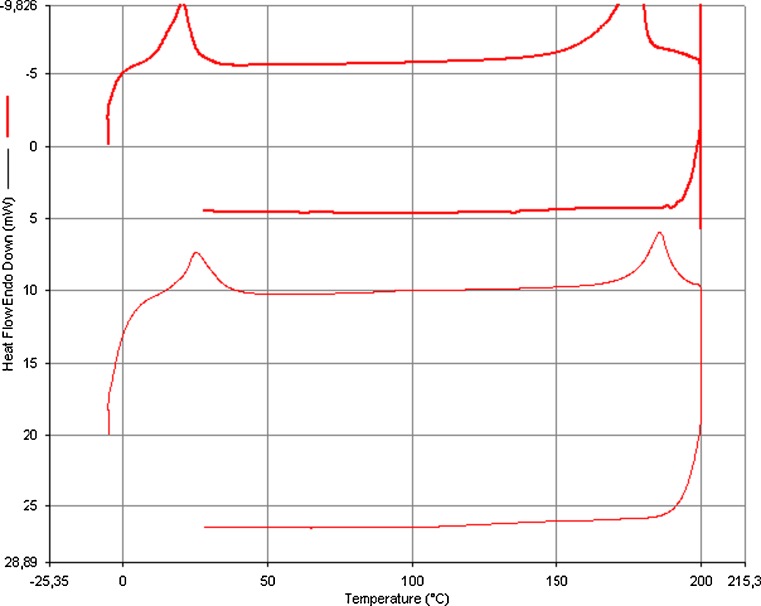
The following conclusion was obtained as a result of the statistic. Panelists liked this new product according to overall inclination test. However some of textural properties are below the general preference like coarseness. These results were clearly observed from Figure. Results obtained from the panel test were observed to be supported by DSC and polarize light microscopy: cinnamon chocolate was examined in these instruments. Figure 4 shows a typical DSC melting curve for cinnamon and dark chocolate. The peak temperature for melting is the average of melting point of chocolate. The onset of melt indicates the time when the fat just starts to melt.
Fig. 4.
DSC thermograms showing fat melting profile of cinnamon chocolate and dark chocolate
The heat of fusion and melting point can be determined from melting curve. Chocolate with and without cinnamon, whose melting curve are concave in shape and chacterized by temperature of peak maxima (shown in Fig. 4). Data from DSC indicated that cinnamon addition produce change in melting properties and observed in difference in their peak withs. Result from data Tonset (°C), Tend (°C), Tpeak (°C) and ∆Hmelt (j/g) of dark chocolate with and without cinnamon are 19.29 ±0.07−17.47 ±0.04; 34.13 ±0.08−27.89±0.05;25.26 ±0.05−24.89 ±0.06; 20.66 ±0.04−20.07 ±0.07 respectively. Dark chocolate with and without cinnamon showed significant difference for Tonset (°C), Tend (°C), Tpeak (°C) and ∆Hmelt (j/g). Addition of cinnamon caused consistent reduction in Tend (°C), from 34.13 °C to 27.89 °C representing a difference 6.24 °C. Dark chocolate with cinnamon require slightly lower temperature to complete melting than dark chocolate. DSC thermograms showed difference in melting profile, resulting from widened peak with but no difference were noted in sugar melting properties. Melting properties of chocolate is important because of taste and sensation in the mouth. As it is seen in both Table 13 and Fig. 4, melting point of chocolate increases with ∆H and T end. It is also observed that cinnamon chocolate melts at a lower temperature than that of dark chocolate. These clearly indicate the effect of cinnamon powder on the texture of chocolate Table 7.
Table 7.
One-sample T test for aroma profile panel test with a test value = 7
| 95 % Confidence interval of the difference | ||||||
|---|---|---|---|---|---|---|
| t | df | Sig. (2-tailed) | Mean difference | Lower | Upper | |
| Bitterness | −0.842 | 11 | 0.417 | −0.33333 | −1.2042 | 0.5376 |
| Sweetness | 0.432 | 11 | 0.674 | 0.16667 | −0.6829 | 1.0162 |
| Chocolate | −0.248 | 11 | 0.809 | −0.08333 | −0.8232 | 0.6566 |
| Cinnamon | 1.609 | 11 | 0.136 | 0.66667 | −0.2454 | 1.5787 |
| Chocolate odour | 2.345 | 11 | 0.039 | 0.66667 | 0.0410 | 1.2923 |
| Cinnamon odour | 0.000 | 11 | 1.000 | 0.00000 | −1.4590 | 1.4590 |
| Coarseness | −2.057 | 11 | 0.064 | −0.83333 | −1.7250 | 0.0584 |
| Thickness | −1.836 | 11 | 0.094 | −0.91667 | −2.0158 | 0.1824 |
| Effort | −2.399 | 11 | 0.035 | −1.08333 | −2.0772 | −0.0894 |
| Hardness | −0.699 | 11 | 0.499 | −0.41667 | −1.7288 | 0.8955 |
| Softness | −1.935 | 11 | 0.079 | −1.33333 | −2.8499 | 0.1832 |
According to the results, cinnamon chocolate melts at a lower temperature than that of dark chocolate and no particles over 20 μm was identified in this sample, which is supported by sensory test as shown in Fig. 5.
Fig. 5.
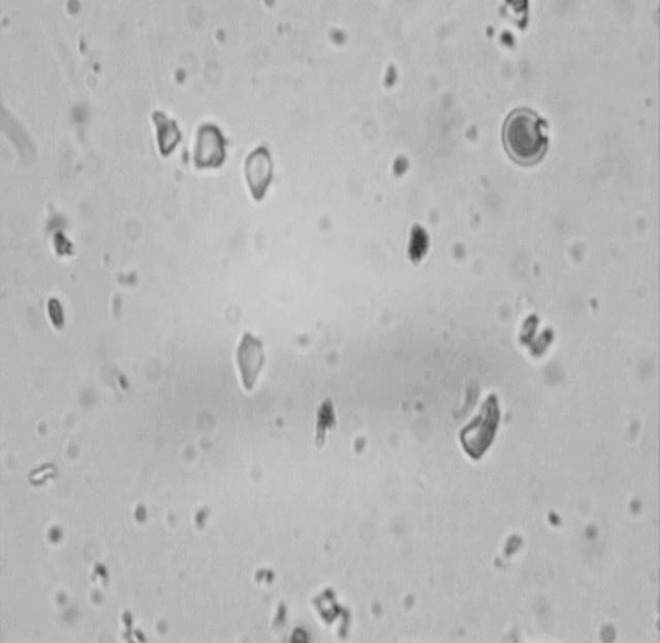
Structure of cinnamon chocolate
Conclusion
Gas chromotography-Olfactometry in combination with SPME using CAR/PDMS was able to identify most of odour active compounds of cocoa mass, dark chocolate and cinnamon chocolate. The identified cocoa mass and chocolate odour was originated from a wide range of alcohols, aldehydes, ketons, ester and pyrazines. These compounds are thought to make up the characteristic cocoa mass and chocolate aroma in terms of sweet, nutty, caramel and chocolate-like notes as well as defective cocoa aroma such as smoky and rancid. When analyzing the result of GC-MS cinnamon chocolate is more aromatic than dark chocolate. Copaene, cinnamaldehyde, 4-methylene-cyclohexene, bicyclo (3.1.1.heptane, 6-methyl) and bicyclo (3.1. hexan-2-ol, methyl) are aromatic compound of cinnamon chocolate which formed as a result of interaction between cocoa mass and cinnamon powder. After conching amount of pyrazine reduced whereas amount of aldehyde increased in both chocolate type.
Contributor Information
F. Albak, Phone: +90-342-3172359, Email: albak@gantep.edu.tr
A. R. Tekin, Email: tekin@gantep.edu.tr
References
- AOAC . Offical methods of analysis. 15. Arlington: Aspects of Official Analysis Chemistry, Inc; 1990. [Google Scholar]
- Counet C, Callemien D, Ouwerx C, Collin S. Use of gas chromotograpy-olfactometry to identify key odorant compound in dark chocolate. Comprasion of sample before and after conching. J Agric Food Chem. 2002;5:2385–2391. doi: 10.1021/jf0114177. [DOI] [PubMed] [Google Scholar]
- Afoakwa EO, Paterson A, Fowler M, Ryan A. Matrix effects on flavour volatiles release in dark chocolates varying in particle size distribution and fat content using GC-mass spectrometry and GC-olfactometry. Food Chem. 2009;113(1):208–215. doi: 10.1016/j.foodchem.2008.07.088. [DOI] [Google Scholar]
- Guerrero L, Guárdia MD, Xicola J, Verbeke W, Vanhonacker F, Zakowska-Biemans S, et al. Consumer-driven definition of traditional food products and innovation in traditional foods. A qualitative cross-cultural study. Appetite. 2009;52:345–354. doi: 10.1016/j.appet.2008.11.008. [DOI] [PubMed] [Google Scholar]
- King AJ, Readman JW, Zhou JL. The application of solid-phase micro-extraction (SPME) to the analysis of polycyclic aromatic hydrocarbons (PAHs) Environ Geochem Health. 2003;25:69–75. doi: 10.1023/A:1021248932084. [DOI] [PubMed] [Google Scholar]
- Kotler P. Marketing management: Analysis, planning, ımplementation and control. 7. New Jersey: Prentice Hall, Inc; 1991. [Google Scholar]
- Mestres M, Marti MP, Busto O, Gausch J. Analysis of low-volatility organic sulphur compounds microextraction and gas chromatography. J Chromatography. 2000;881:583–590. doi: 10.1016/S0021-9673(00)00326-5. [DOI] [PubMed] [Google Scholar]
- Nebesny E, Zyzelewicz D, Motyl I, Libudzisz Z. Dark chocolates supplemented with Lactobacillus strains. Eur Food Res Technol. 2007;225:33–42. doi: 10.1007/s00217-006-0379-9. [DOI] [Google Scholar]
- Pawliszyn J. Solid phase microextraction: Theory and practice. New York: John Wiley and Sons; 1997. pp. 25–55. [Google Scholar]
- Pawliszyn J. Solid phase microextraction. In: Issaq, editor. Acentury of separation science. New York: Marcel Dekker Inc; 2002. pp. 399–419. [Google Scholar]
- Ramli N, Hassan O, Said M, Samsudin, Idris NA. Infuence of roasting conditions on volatile favour of roasted Malaysia cocoa bean (abstract) Journal of Food Processing and Preservation. 2008;30:280–298. doi: 10.1111/j.1745-4549.2006.00065.x. [DOI] [Google Scholar]
- Reineccius G. Flavour chemistry and technology. Boca Raton: Second edition. CRC Press; 2006. [Google Scholar]
- Reto M, Figueira ME, Filipe HM, Almeida CM. Analysis of vitamin K in green tea leafs and infusion by SPME- GC-FID. Food Chemist. 2007;100:405–411. doi: 10.1016/j.foodchem.2005.09.016. [DOI] [Google Scholar]
- Segall SD, Artz WE, Raslan DS, Ferraz VP, Takahashi JA. Ouricuri (Syagrus coronata) TAG Analysis using HPLC and positive ion electrospray Tandem MS. J Am Oil Chem Soc. 2000;81:143–149. doi: 10.1007/s11746-004-0872-0. [DOI] [Google Scholar]
- Stewart-Knox B, Mitchell P. What separates the winners from the losers in new food product development? Trends Food Sci Technol. 2003;14:58–64. doi: 10.1016/S0924-2244(02)00239-X. [DOI] [Google Scholar]
- Muresan S, Eillebrecht MAJL, De Rijk TC, De Jonge HG, Legujit T, Nijhuis HH. J Food Chemist. 2000;68(2000):167–174. doi: 10.1016/S0308-8146(99)00171-5. [DOI] [Google Scholar]