Abstract
In black tea manufacturing, one of the most important steps is fermentation which influences the quality of tea. The macerated tea leaves were fermented at various temperatures (20, 25, 30, 35 °C) for different duration. Changes in polyphenoloxidase and peroxidase activities, depletion patterns of individual catechins, differences in individual theaflavin levels and formation of thearubigins were measured in leaves during fermentation. Higher stability of polyphenoloxidase and peroxidase enzymes was observed at lower temperature (20 °C), and increase in temperature, led to enzyme instability. The rate of degradation of all the catechins was found to be fastest at 35 °C and slowest at 20 °C. The formation and depletion of individual theaflavins varied with temperature and fermentation duration. The time required for the formation of maximum total theaflavins (TF) was highest at lower temperature and this time duration also varied for different theaflavins formation. Maximum amount of thearubigins (TR) content and liquor colour development was observed at 35 °C, and decrease in temperature reduced thearubigins accumulation. However, maximum brightness as well as TF/TR ratio was obtained at 20 °C, which suggests that fermentation at lower temperature is suitable for manufacturing quality black tea.
Keywords: Black tea, Fermentation, Polyphenoloxidase, Peroxidase, Theaflavins, Thearubigins
Introduction
Tea, one of the most popular drinks in the world, is processed from young tender shoots (two leaves and a bud) of Camellia sinensis (L.) O. Kuntze. To produce black tea, the plucked biomass are withered, macerated, fermented and dried. In this process, fermentation plays a vital role in determining black tea quality. The fermentation step actually represents enzymatic oxidation of the polyphenols present in the leaves in presence of atmospheric oxygen to produce various oxidized pigments. Young tea shoots are rich in different kinds of polyphenols (ca. 15–35 % w/w) of which, the flavon-3-ols (catechins) are the most abundant (ca. 80 % w/w) compounds (Chunlei and Liang 2007). The major tea leaf catechins are (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)- epigallocatechin (EGC), (−)-epigallocatechin gallate (EGCG), (+)-catechin (C), and (+)- gallocatechin (GC) (Hara et al. 1995; Liang et al. 2003). As the cellular integrities of the leaves are disrupted by maceration, the catechins and the enzymes (e.g., polyphenoloxidase and peroxidase) that are present in different compartments within the cell are mixed up. This results in fermentation process where a series of biochemical reactions lead to the formation of oxidized polyphenolic compounds such as theaflavins and thearubigins. Catechins along with their oxidation products are responsible for the sensory characteristics of black tea (Roberts 1962; Sanderson et al. 1972).
The catechins with different chemical structures and reduction potentials contribute to the astringent taste of tea (Bajaj et al. 1987; Ding et al. 1992). During fermentation, initially catechins are acted upon by the enzyme polyphenol oxidase (PPO) to form quinones in presence of molecular oxygen. Different theaflavins are formed during fermentation by oxidative polymerization of the quinones produced from a simple (dihydroxy) catechin and a gallo (trihydroxy) catechin. Theaflavins are composed of simple theaflavin (TF), theaflavin-3-gallate (TF3G), theaflavin-3′-gallate (TF3′G) and theaflavin-3,3′-digallate (TF33′DG). Theaflavins are responsible for the astringency, brightness, colour (yellowish) and briskness of the black tea. Thearubigins (TR) formed during fermentation contribute to the mouthfeel (thickness) and colour (reddish brown) of the tea (Biswas and Sarkar 1973). Therefore fermentation time is very important in maintaining the TF/TR ratio.
The enzymes PPO and peroxidase (PO) are of considerable importance in black tea processing. Tasters’ score of the tea liquor correlates positively with PPO activity in leaf. It was shown that high PPO activity produces larger amount of oxidized pigments, and the changes in enzyme activity in various processing steps have been studied (Ravichandran and Parthiban 1998), however, how fermentation temperatures affects these enzymes activities are yet to be studied.
Control of fermentation conditions is necessary for production of superior quality tea. Fermentation temperature and duration are the main determining factors to produce good quality black tea. Optimum fermentation temperature and duration vary from cultivar to cultivar (Owuor and Obanda 1995). This manuscript reports the biochemical changes occurring during fermentation of a ‘Crush, Tear and Curl’ (CTC) macerated Assam cultivar TV25, aiming at producing superior black tea by manipulating the fermentation time and temperature.
Materials and methods
Plucking of leaves
Green tea leaves of Tocklai Vegetative clone TV25 (Camellia sinensis var. assamica) were plucked from the tea garden at IIT Kharagpur, India. Flushing shoots consisting of the apical bud and first two expanded leaves were plucked early in the morning and used for manufacturing of black tea. About 5–6 kg leaves were plucked for each trial.
Manufacturing of CTC black tea
To study the effect of fermentation parameters on the physical and chemical changes in leaf, all the other processing steps viz. withering, maceration and drying were carried out under the same conditions for each experiment. An environment control chamber developed at the institute was used for withering and fermentation of the tea leaves under controlled conditions. This device can control air temperature within ± 0.1 °C and relative humidity within ± 2 % of the desired level for withering and fermentation.
After plucking, the fresh shoots were withered over a period of 12 h in an air blower-fitted trough of the environment control chamber, where temperature and relative humidity were set at 30 °C and 75 %, respectively, to achieve the moisture content of withered leaves within 69–70 %. Withered leaves were macerated in a compact single-cut maceration device. Macerated leaves were allowed to ferment in the environment control chamber in a perforated tray and air was passed through the macerated leaf from beneath at a velocity of approximately 3 m/min.
For fermentation of the macerated leaves, air temperature was maintained at one of four predetermined levels: 20, 25, 30 and 35 °C and the relative humidity was maintained at 92–95 %. The fermenting ‘dhool’ (macerated tea leaves after withering in black tea processing) was taken at 20 min interval for up to 2 h and dried with hot air at 90–95 °C for 25–30 min to bring down the moisture content to 2–4 %. The black tea samples were sorted using different sieve size. Mixture of equal amount of Broken Pekoe and Pekoe Fannings grades was used for biochemical analysis. Each treatment was replicated thrice and results were statistically analyzed.
Determination of moisture content
The moisture content of withered leaves, fermenting ‘dhool’ and dried black tea samples were measured using a Halogen moisture analyzer (Mettler Toledo), and expressed in terms of percentage dry matter (DM).
Estimation of enzyme activity
The fermenting ‘dhool’ collected at each 20 min interval was analyzed for PPO and PO enzyme activities. The activities of PPO (EC 1.14.18.1) and PO (EC 1.11.1) in the fermenting ‘dhool’ were measured as described earlier (Mahanta et al. 1993).
Analysis of individual catechins and individual theaflavins by HPLC
Aqueous extract of black tea was used as the sample for estimation of individual catechins and individual theaflavins by HPLC analysis. The extract was prepared by adding 75 ml boiling deionized water in 1 g of tea sample and brewing it for 5 min. After brewing, aqueous infusions were centrifuged at 8,000 g for 10 min. The supernatants were filtered through 0.45 μm membrane filter before analysis through HPLC. HPLC analysis was done according to Yao et al. (2004) using gradient elution system with slight modifications. The analysis were performed using a HPLC Waters 600 Separation Module (quaternary solvent delivery pumps, in-line degasser), with a photodiode array detector (Model Waters 2998), and the results were processed by Empower software (Waters Corporation, USA). Separations were performed in an Xterra RP C18 5 μm (250 mm × 4.6 mm i.d.) column. The composition of mobile phase was initially set at 8 % of acetonitrile (B) and 92 % of 0.2 % acetic acid in water (A). Solvent B then gradually increased to 31 % at 25 min, to 40 % at 35 min, to 100 % at 37 min and hold for 3 min. The photodiode array detector was set at 200–600 nm wavelengths and the chromatogram was detected at 274 nm. The separations were performed at room temperature with a 1 ml/min flow rate and the injection volume was 20 μl. Authentic standards were used to identify peaks and calculate the concentration of components in tea samples. Each peak was confirmed by comparing the retention time and absorption spectra of tea samples to those of the standard compounds.
Analysis of total theaflavins, total thearubigins, brightness and total liquor colour
The method described by Roberts and Smith (1963) was used for estimation of total theaflavins (TF), total thearubigins (TR) and brightness. Total liquor colour of tea brew was measured by the method of Thanaraj and Seshadri (1990).
Results and discussion
Effect of fermentation temperature on enzymes stability
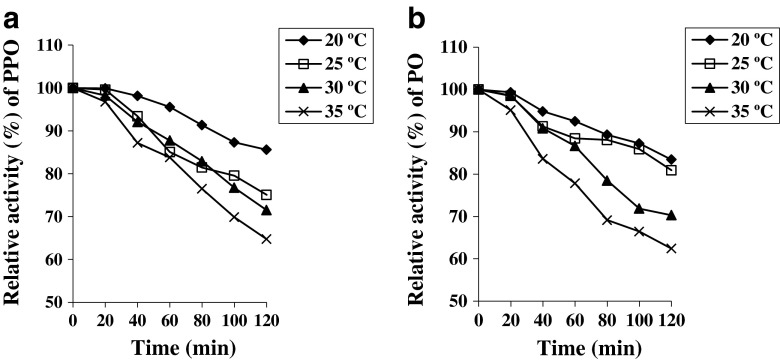
The results revealed that temperature and duration of fermentation played key role in formation and depletion of various biochemical compounds in tea leaves. Variation of PPO and PO activities with fermentation temperature and duration has been shown in Fig. 1. PPO, which mainly oxidizes polyphenol to theaflavins, exhibited maximum activity at 20 °C during fermentation because of its higher stability at lower temperature. At 35 °C, 30 °C and 25 °C, the PPO enzyme showed around 90 % stability up to 40 min while at 20 °C it showed above 90 % stability up to 80 min. Similar trend was observed in case of PO which is responsible for the production of thearubigins. With increase in temperature, the stability of enzyme decreased during fermentation. PO exhibited 90 % stability at 35 °C up to 20 min fermentation time while it exhibited 90 % stability at lower temperature (20 °C) up to 80 min. This decrease in enzyme activity during fermentation can be explained in two possible ways. First, enzyme, being a protein, gets denatured due to rise in temperature. Secondly, there may be a feedback inhibition (Ravichandran and Parthiban 1998).
Fig. 1.
Effect of different fermentation temperatures and durations on polyphenoloxidase (a) and peroxidase (b) activities. All values represented as mean and SD were less than 10 % (n = 3)
Fermentation temperature affects catechin contents
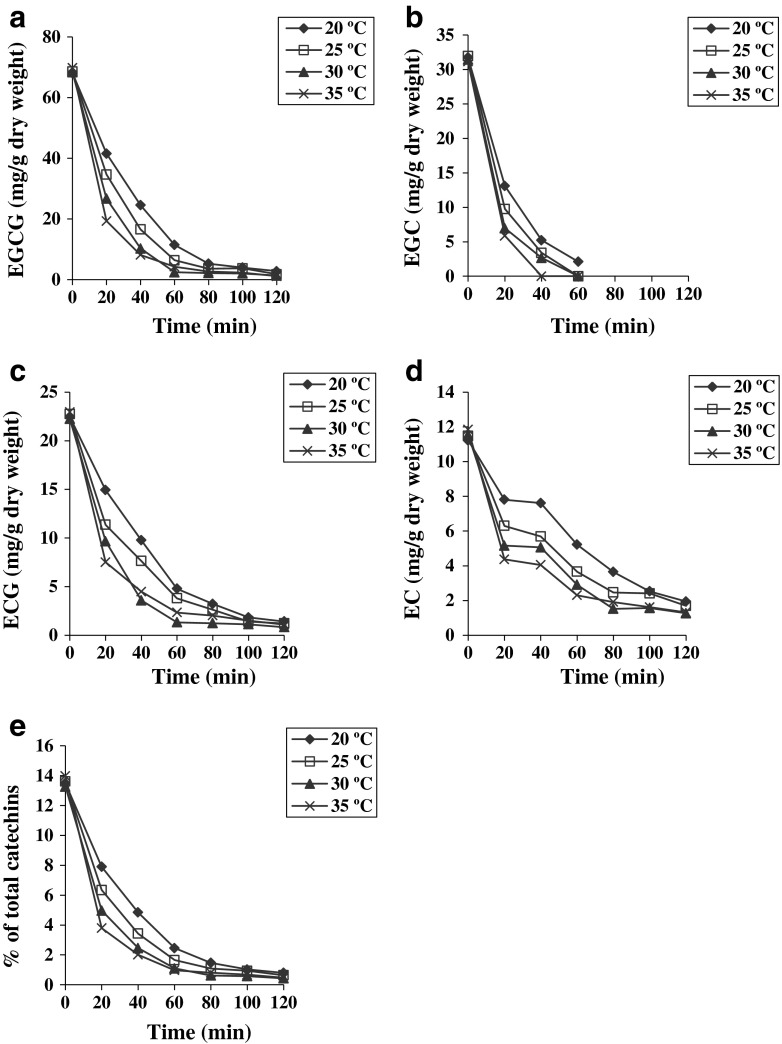
Depletion of catechins is the most important feature of fermentation as they get converted to theaflavins and further to thearubigins. The effect of fermentation temperature and time on individual and total catechins content has been shown in Fig. 2. The degradation rates of total catechin compounds increased with increase in temperatures. This is possibly due to higher activity of PPO and PO at high temperature. At the beginning of fermentation, EGCG was found in maximum amount in leaves as compared to other catechins. Rate of EGCG degradation increased with increase in fermentation temperature and degradation was most rapid at 35 °C and slowest at 20 °C. Degradation of EGC, EC and ECG showed a similar trend as EGCG. The levels of EGC were not detectable approximately after 60 min of fermentation time at all sets of experiments, as reported in the literature (Obanda et al. 2001). Residual levels of different catechins declined in the order of EGCG > EGC > ECG > EC at all fermentation temperatures considered in this study.
Fig. 2.
Effect of different fermentation temperatures and durations on the individual catechin contents and total catechins content. a, b, c, d and e are EGCG, EGC, ECG, EC and total catechins, respectively. All values represented as mean and SD were less than 10 % (n = 3)
Synthesis and depletion of theaflavins during fermentation
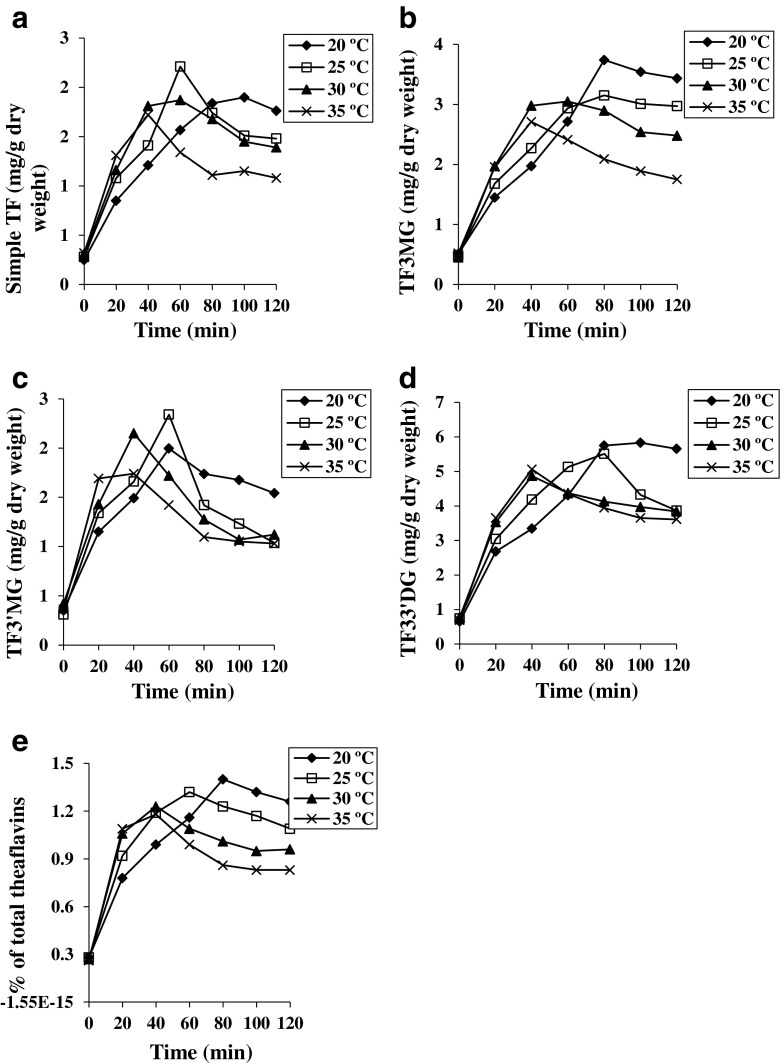
Variations in the amount of total theaflavins and individual theaflavins at different temperatures have been presented in Fig. 3. Total theaflavin levels increased up to 40 min at both 30 °C and 35 °C fermentation temperatures, and up to 80 min and 60 min at 20 °C and 25 °C temperatures, respectively. Maximum TF accumulation was observed with 20 °C fermentation temperature. This could be explained by the fact that the optimum activity of PPO as detected at 30 °C. In all the trials, theaflavins content after reaching a maximum declined with time. This declination is due to the conversion of theaflavin to polymeric thearubigins and low levels of catechins. At high temperature, TF degraded rapidly as compared to low temperature because TF converted to TR rapidly at high temperature. It was apparent that during fermentation, change in total theaflavin levels occurred in three distinct phases (as well observed at 35 °C): a linear phase, where the theaflavin formation occurs; a plateau phase, where the TF levels attain maximum and level off; and a declination or consumption phase, where TF levels decline with further increase of fermentation time. Cloughley (1980) reported that TF formation and consumption rates are dependent on temperature, as the temperature raises the rate of formation and consumption also increases and vice versa. Simple TF content attained a maximum at around 60 min of fermentation time at 25 °C and beyond that it declined. At the highest fermentation temperature (35 °C), maximum simple TF content was attained at 40 min while at the lowest temperature (20 °C), maximum amount of simple TF was formed at 100 min. Maximum amount of TF3MG was formed at 20 °C followed by 25 °C, 30 °C, and eventually 35 °C but the time taken was maximum at 20 °C which was about 80 min. Maximum TF3′MG was formed at 25 °C at around 60 min of fermentation time. Maximum amount of TF33′DG was formed at 20 °C at around 80 min of fermentation time. It was found that all theaflavins are not formed at the same rate and in same amount under the same fermentation conditions in all sets of experiment as also observed by Owuor and Reeves (1986) and Owuor and McDowell (1994).
Fig. 3.
Effect of fermentation temperatures and durations on the individual theaflavins content and total TF content. a, b, c, d and e are simple TF, TFM3G, TF3′MG, TF33′DG and total theaflavins, respectively. All values represented as mean and SD were less than 10 % (n = 3)
Brightness, colour development and thearubigins formation during fermentation
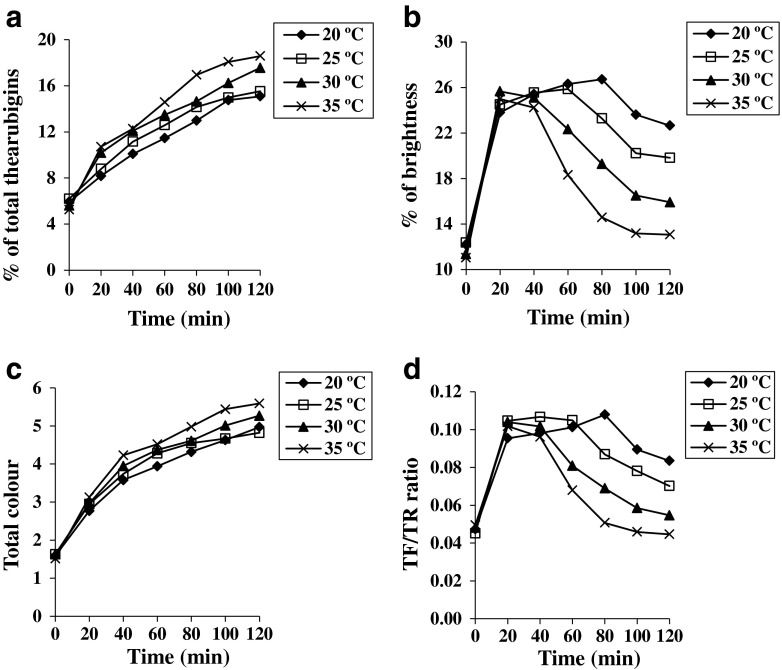
Variation of thearubigins (TR) content with temperature is shown in Fig. 4. TR content clearly increased with increase in fermentation temperature and duration. Owour and Obanda (2001) and Cloughley (1980) reported similar results. At high temperature TR formation increased may be due to high activity of peroxidase at high temperature. Initially brightness increased with increased in temperature but it declined with fermentation duration (Fig. 4). Theaflavin primarily contributes to the brightness of tea (Hilton and Ellis 1972; Roberts and Smith 1963) and Ngure et al. (2009) reported that TF content have positive effect on brightness of black tea infusion but total TR levels have negative effect on it. As maximum amount of TF and relatively low amount of TR was formed at 20 °C of fermentation temperature, brightness was found to be the highest at 20 °C at around 80 min of fermentation time. As a strong correlation exists between the quality of black tea and the coloured TF content, the TF value is considered to be an important measurable parameter for assessing the quality of tea (Owuor and Reeves 1986; Owuor and McDowell 1994). Depletion of different catechins and formation of TFs and TR depend on air temperature and duration of fermentation. With extended fermentation time and rise in temperature, a decline in the levels of total theaflavins, liquor brightness and briskness have been reported (Owuor and McDowell 1994; Sanderson et al. 1976). The TF/TR ratio varies with changes in fermentation times (Robertson 1983; Tufekci and Guner 1997). With increase in temperature, TF/TR ratio increased for low range of fermentation time, and beyond that TF/TR ratio decreased with increased in temperature (Fig. 4). Black teas containing higher ratios of theaflavins to thearubigins are always in higher demand in the auction market (Roberts and Smith 1963). Variation of total liquor colour with temperature (Fig. 4) showed the same trend as that of TR because TR content mainly determines the colour and body of tea (Hilton and Ellis 1972; Roberts and Smith 1963). Maximum liquor colour was obtained at 35 °C, and it decreased with decrease in temperature.
Fig. 4.
Changes in total thearubigins content (a) brightness (b), total liquor colour (c) and TF/TR ratio (d) with fermentation temperatures and durations. All values represented as mean and SD were less than 10 % (n = 3)
Conclusion
The above study provided an insight on the importance of fermentation time and temperature for manufacturing of quality CTC black tea. The role of PPO and PO in transforming catechin compounds to TF and TRs have also been highlighted here, which has direct bearing on the quality of black tea. Tea PPO is reported to have optimum activity at 30 °C. This study also supports this finding as catechin degradation which is carried out by PPO is most pronounced in the temperature range of 30–35 °C. Slight variations in temperature for optimum enzyme activity may be attributed to clonal and agroclimatic variations. Optimum temperature for tea PO activity or its mechanism of action is not clear till date. The study has also showed that the formation of TR is favoured at high temperature, which suggests that PO may have optimum activity at higher temperature. However, more efficient conversion of catechins to TF was shown to occur at a lower temperature (20 °C); this temperature also favours catechins to TR conversion, but with a prolonged fermentation time. Further investigation is needed to resolve this issue.
Acknowledgments
This work was funded by the Tea Board of India (Ministry of Commerce & Industry, Government of India) in the form of a research project [no. IRL-21(20)/2007-P-I] during XI th Plan period.
References
- Bajaj KL, Anan T, Tsushida T, Ikegaya K. Effects of (−) epicatechin on oxidation of theaflavins by polyphenol oxidase from tea leaves. Agric Biol Chem. 1987;51:1767–1772. doi: 10.1271/bbb1961.51.1767. [DOI] [Google Scholar]
- Biswas AK, Sarkar AR. Biological and chemical factors affecting the valuations of North-East Indian Plains teas. II.- Statistical evaluation of the biochemical constituents and their effects on briskness, quality and cash valuations of black teas. J Sci Food Agric. 1973;24:1457–1477. doi: 10.1002/jsfa.2740241202. [DOI] [Google Scholar]
- Chunlei MA, Liang C. Research progress on isolation and cloning of functional genes in tea plants. Front Agric China. 2007;1:449–455. doi: 10.1007/s11703-007-0074-z. [DOI] [Google Scholar]
- Cloughley JB. The effect of fermentation temperature on the quality parameters and price evaluation of Central African black teas. J Sci Food Agric. 1980;31:911–919. doi: 10.1002/jsfa.2740310908. [DOI] [Google Scholar]
- Ding Z, Kuhr S, Engelhardt UH. Influence of catechins and theaflavins on the astringent taste of black tea brews. Z Lebensm Unters Forsch. 1992;195:108–111. doi: 10.1007/BF01201768. [DOI] [Google Scholar]
- Hara Y, Luo SJ, Wickremashinghe RL, Yamanishi T. Biochemistry of processing black tea. Food Rev Int. 1995;11:457–471. doi: 10.1080/87559129509541037. [DOI] [Google Scholar]
- Hilton PJ, Ellis RT. Estimation of market value of Central African Tea by Theaflavin analysis. J Sci Food Agric. 1972;23:227–232. doi: 10.1002/jsfa.2740230210. [DOI] [Google Scholar]
- Liang Y, Lu J, Zhang L, Wu S, Wu Y. Estimation of black tea quality by analysis of chemical composition and colour difference of tea infusions. Food Chem. 2003;80:283–290. doi: 10.1016/S0308-8146(02)00415-6. [DOI] [Google Scholar]
- Mahanta PK, Boruah SK, Boruah HK, Kalita JN. Changes of Polyphenol Oxidase and Peroxidase activities and pigment composition of some manufactured black teas (Camellia sinensis L.) J Agric Food Chem. 1993;41:272–276. doi: 10.1021/jf00026a026. [DOI] [Google Scholar]
- Ngure FM, Wanyoko JK, Mahungu SM, Shitandi AA. Catechins depletion patterns in relation to theaflavin and thearubigins formation. Food Chem. 2009;115:8–14. doi: 10.1016/j.foodchem.2008.10.006. [DOI] [Google Scholar]
- Obanda M, Owuor PO, Mangoka R. Changes in the chemical and sensory quality parameters of black tea due to variations of fermentation time and temperature. Food Chem. 2001;75:395–404. doi: 10.1016/S0308-8146(01)00223-0. [DOI] [Google Scholar]
- Owour PO, Obanda M. Comparative responses in plain black tea quality parameters of different tea cultivars to fermentation temperature and duration. Food Chem. 2001;72:319–327. doi: 10.1016/S0308-8146(00)00232-6. [DOI] [Google Scholar]
- Owuor PO, Mcdowell I. Changes in theaflavins composition and astringency during black tea fermentation. Food Chem. 1994;51:251–254. doi: 10.1016/0308-8146(94)90023-X. [DOI] [Google Scholar]
- Owuor PO, Obanda M. Clonal variation in the individual theaflavins and their impact on astringency and sensory evaluation. Food Chem. 1995;54:273–277. doi: 10.1016/0308-8146(95)00046-L. [DOI] [Google Scholar]
- Owuor PO, Reeves SG. Optimising fermentation time in black tea manufacture. Food Chem. 1986;21:195–203. doi: 10.1016/0308-8146(86)90017-8. [DOI] [Google Scholar]
- Ravichandran R, Parthiban R. Changes in enzyme activities (polyphenoloxidase and phenylalanine ammonia lyase) with type of tea leaf and during black tea manufacture and the effect of enzyme supplementation of dhool on black tea quality. Food Chem. 1998;62:277–281. doi: 10.1016/S0308-8146(97)00220-3. [DOI] [Google Scholar]
- Roberts EAH. Economic importance of flavonoid substances. In: Geissman TA, editor. Chemistry of flavonoid compounds. Oxford: Pergamon Press; 1962. pp. 468–512. [Google Scholar]
- Roberts EAH, Smith RF. Phenolic substances of manufactured tea. II. Spectrophotometric evaluation of tea liquors. J Sci Food Agric. 1963;14:689–700. doi: 10.1002/jsfa.2740141002. [DOI] [Google Scholar]
- Robertson A. Effects of physical and chemical conditions on the in vitro oxidation of tea leaf catechins. Phytochemistry. 1983;22:889–896. doi: 10.1016/0031-9422(83)85017-1. [DOI] [Google Scholar]
- Sanderson GW, Berkowitz JE, Co H, Graham HN. Biochemistry of tea fermentation: products of the oxidation of tea flavanols in a model tea fermentation system. J Food Sci. 1972;37:399–404. doi: 10.1111/j.1365-2621.1972.tb02648.x. [DOI] [Google Scholar]
- Sanderson GW, Ranadive AS, Eisenberg LS, Farrel FJ, Simon R, Manley CH, Coggon P. Contributions of polyphenolic compounds to the taste of tea. ACS Symp Ser. 1976;26:14–46. doi: 10.1021/bk-1976-0026.ch002. [DOI] [Google Scholar]
- Thanaraj SNS, Seshadri R. Influence of polyphenoloxidase activity and polyphenol content of tea shoot on quality of black tea. J Sci Food Agric. 1990;51:57–69. doi: 10.1002/jsfa.2740510107. [DOI] [Google Scholar]
- Tufekci M, Guner S. The determination of optimum fermentation time in Turkish black tea manufacture. Food Chem. 1997;60:53–56. doi: 10.1016/S0308-8146(96)00302-0. [DOI] [Google Scholar]
- Yao L, Jiang Y, Datta N, Singanusong R, Liu X, Duan J, Raymont K, Lisle A, Xu Y. HPLC analysis of flavanols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem. 2004;84:253–263. doi: 10.1016/S0308-8146(03)00209-7. [DOI] [Google Scholar]