Abstract
Zinc is a nutritionally essential truce element, and thus zinc deficiency (ZD) severely affects human health. More than 25% of the world’s population is at risk of ZD. This study was initiated to examine the use of the vacuum impregnation (VI) technique for enriching zinc content of whole potatoes; the effect of vacuum time, restoration time, steam-cooking and storage at 4 °C on the zinc content of VI whole potatoes was evaluated. Whole potato tubers were immersed in a 9 g/100 g zinc (zinc gluconate) solution. Vacuum pressure of 1,000 Pa was applied for 0–120 min, and atmospheric pressure restoration for 0–4 h. Experimental results showed that the zinc content of VI potatoes increased with vacuum and restoration time. Moreover, VI-cooked unpeeled or peeled potatoes had 63–94 times and 47–75 times higher zinc contents than un-VI-cooked unpeeled or peeled potatoes, respectively. The world daily potato consumption (86 g) of the VI-cooked unpeeled and peeled potatoes provided adult men with 130–148% and 100–135% of the recommended daily allowance (RDA) of zinc, respectively. Also, the daily potato consumption of the unpeeled and peeled potatoes supplied adult women with 178–203% and 137–185% of the RDA level, respectively. In addition, the VI potatoes had 40 times higher zinc contents through 30 days of storage at 4 °C, compared with un-VI-treated potatoes. This study indicated that VI treatment of whole potatoes was useful for enriching the zinc content.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-013-1194-5) contains supplementary material, which is available to authorized users.
Keywords: Whole potatoes, Vacuum impregnation, Zinc enrichment, Steam-cooking, Storage
Introduction
Health benefits are one of the specific issues that will greatly influence the food industry in the coming years (Mazza 1998). Food industry companies have rather high expectations for food products that meet consumer demands for a healthy lifestyle (Menrad et al. 2000). Functional foods have been defined as those that provide an additional physiological benefit that may prevent disease or promote health and well-being (Stauffer 1999). These functional food products have been mainly launched in the dairy-, confectionery-, soft-drinks-, bakery- and baby-food market (Menrad 2003). Experts like Sloan (2000, 2002) have reckoned the global functional food market to be 47.6 billion US$; the United States is the largest market segment, followed by Europe and Japan. Moreover, functional foods represent an important growth category for the commercial sector in many countries around the world (Sibbel 2007). In addition, the functional food industry is currently striving to provide functional new products to rapidly growing markets (Bistrom and Nordstrom 2002).
Potato (Solanum tuberosum, L.) is one of the most important tuber crops widely consumed in the world (Burgos et al. 2009). It is ranked fifth in terms of human consumption and fourth in worldwide production (Burlingame et al. 2009). Apart from supply of energy and high quality protein, the potato has also been known to be an important source of vitamins and minerals (Abong et al. 2009); of the minerals, potatoes have a zinc content of 0.41 mg per 100 g on fresh weight (Burlingame et al. 2009).
Zinc is involved in many biochemical processes supporting life. The most important of these processes are cellular respiration, cellular utilization of oxygen, DNA and RNA, reproduction, maintenance of cell membrane integrity, and sequestration of free radicals (Chan et al. 1998). However, conservative estimates suggest that more than 25% of the world’s population is at risk of zinc deficiency (ZD) (Maret and Sandstead 2006). Especially, ZD may increase oxidative stress, which may directly cause DNA damage. Moreover, ZD may impair DNA damage repair responses (Ho 2004). It leads to several chronic degenerative diseases, such as cancer (Castro and Freeman 2001). The Food and Nutrition Board (FNB 2001) indicated that the recommended daily allowance (RDA) of zinc for adult men and women amounted to 11 and 8 mg/day, respectively.
One of the alternatives for the development of new products in the food industry is the use of vacuum impregnation (VI) (Igual et al. 2008). VI is the application of low pressure to a solid-liquid system, followed by the restoration of atmospheric pressure (Fito 1994; Fito and Chiralt 1997). Different applications of food VI have been developed recently in numerous studies (Gras et al. 2002; Chiralt et al. 1999; Fito and Chiralt 2000; Fito et al. 2000). These studies have validated a model which describes the coupling of the hydrodynamic mechanism (HDM) and the deformation-relaxation phenomena (DRP) of viscoelastic products (Fito et al. 1996) when they are immersed in an external liquid phase and submitted to pressure changes. These studies are mainly devoted to fruit product development (Fito et al. 2000; Fito and Chiralt 2000; Tapia et al. 1999) through osmotic treatments, and to improve salting processes of cheese (Chiralt and Fito 1997; González et al. 1999), meat and fish (Chiralt et al. 2001).
Gras et al. (2002) studied the response of several sliced vegetables (beetroot, carrot, eggplant, zucchini, mushroom and oyster mushroom) to vacuum impregnation treatments, in terms of sample volume deformation and impregnation levels. They evaluated changes in the microstructure of different vegetables by cryo-scanning electron microscopy observation, and found that VI could be used to fill intercellular air spaces (ICAS) in the vegetable matrix. Sanzana et al. (2011) studied Aloe vera fortification of vegetables (endives, cauliflower, broccoli and carrots) by VI technique.
Regarding VI operation, Zhao and Xie (2004) reported that the qualities of finished products by VI are determined by processing conditions such as vacuum time and restoration time. Concerning vacuum time, and restoration time of VI treatment for vegetables or fruits, Mújica-Paz et al. (2003) used a 13.5–67.4 kPa of vacuum pressure for 10 min, and 10-min restoration time for VI of the sucrose solution (41–60 Brix) for slices (3.5 × 2.5 × 1.2 cm) of mango, apple and melon. Gras et al. (2003) used a vacuum pressure of 5 kPa for 10 min, and a 10-min restoration time for impregnation of isotonic solutions of sucrose and calcium lactate mixtures for eggplants, carrots and oyster mushroom. Salvatori et al. (1998) reported that in plant tissue samples of about 2 cm in diameter, with relatively large intercellular spaces and elastic cellular arrangement, the necessary length of vacuum period in VI operations is on the order of 5 min and impregnation times with sugar syrups are on the order of the time required to achieve a stationary pressure in the tank after the valve is opened to restore atmospheric pressure.
In addition to the above-mentioned vacuum time and restoration time, changes in nutritional value mainly occur during storage and cooking such as steaming for potato tubers (Murniece et al. 2011). Faller and Fialho (2009) reported that steaming led to reduction in the antioxidant activity of vegetables.
In the present study, we attempted to enrich zinc content of whole potato tubers by VI in order to supply nutritious whole potatoes to consumers, and to investigate effects of vacuum time, restoration time, steam cooking and storage on zinc content of VI potatoes. Concerning VI of potato, only one report was found regarding enrichment of ascorbic acid content of whole potatoes (Hironaka et al. 2011). However, no studies exist on vacuum impregnation of zinc for whole potatoes.
In this paper, we first evaluated the effect of vacuum time (t1) and restoration time (t2) on the zinc content of VI potato tubers. The effects of steam-cooking and storage at 4 °C on zinc content of VI potato tubers were also evaluated.
Materials and methods
Materials
Two cultivars of processing potatoes were used: Toyoshiro and Snowden. They were harvested in September, late in 2011. After harvesting, tubers approximately 150–200 g in size, and with three characteristic diameters, 7.4–8.8, 5.1–6.8 and 3.8–4.7 cm, respectively, were selected, and then washed with tap water to remove the attached soil. Tubers were then dried with tissue papers, and provided for the VI treatment.
Zinc gluconate and hydrochloric acid were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
VI treatment of potatoes
The zinc gluconate used for zinc fortification in this study is recognized as a food additive by the US Food and Drug Administration (FDA) (Whittaker 1998).
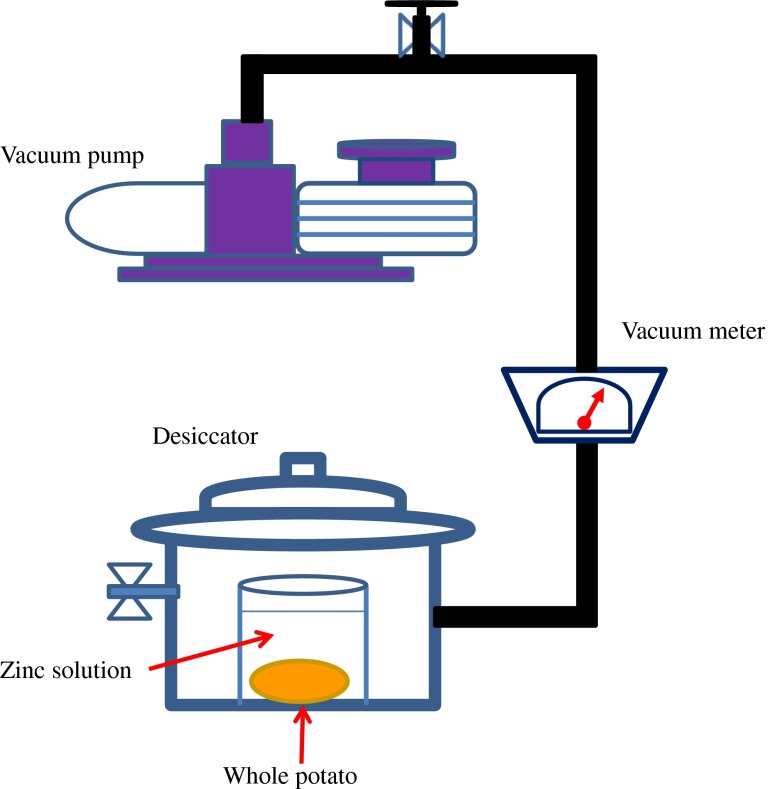
VI treatment of whole potato was carried out in a saturated solution of zinc gluconate solution (concentration of 9 g/100 g). A mass ratio, of potato to the solution, of 1/33 (W/W) was used to ensure adequate immersion and minimize the dilution effect (leaching of intercellular sap) on the concentration of VI solution (Sormani et al. 1999). Also, a vacuum meter (PM-32A, Shimadzu, Kyoto, Japan) placed between the desiccator and vacuum pump (GDH-362, Shimadzu, Kyoto, Japan) was used to measure pressure values (Fig. 1).
Fig. 1.
Schematic representation of the vacuum impregnation system
The experimental designs of VI treatment for vacuum time and restoration time experiments were expressed in Table 1. For design of vacuum time, a vacuum pressure of 1,000 Pa was applied to the system for 0, 15, 30, 60 and 120 min (t1). After the vacuum period, the atmospheric pressure was restored, and the potato tuber was maintained in the zinc solution for a restoration time (t2) of 3 h. For design of restoration time, a vacuum pressure of 1,000 Pa was applied for 1 h (t1), and afterwards the atmospheric pressure was restored and the system remained at this pressure condition for 0, 1, 2, 3 and 4 h (t2).
Table 1.
Experimental design for vacuum time and restoration time
| Design of vacuum time | Design of restoration time | ||
|---|---|---|---|
| Vacuum period t1 (min) | Restoration period t2 (h) | Vacuum period t1 (h) | Restoration period t2 (h) |
| 0 | 3 | 1 | 0 |
| 15 | 3 | 1 | 1 |
| 30 | 3 | 1 | 2 |
| 60 | 3 | 1 | 3 |
| 120 | 3 | 1 | 4 |
VI-treated whole potatoes were drained, rinsed with distilled water to remove the attached solution, and gently wiped with tissue papers. Zinc contents of VI-treated samples, and non-VI (NV) treated samples (control; immersion in the zinc solution without vacuum treatment) were analyzed immediately.
Cooking
Cooking experiments were done to investigate the effect of steam-cooking on zinc content of VI potatoes. VI potatoes were divided into two sections: (1) unpeeled, and (2) peeled. Peeled potatoes were obtained by peeling to a depth of 0.5 mm using a hand peeler (Mondy et al. 1992). Unpeeled or peeled whole potatoes were placed in a stainless steam cooker, which was covered with a lid and steamed over boiling water at atmospheric pressure until easily penetrated with a fork (25 min) (Faller and Fialho 2009; Weaver et al. 1983).
Storage
Wustman and Struik (2007) reported that proper storage temperature is 4–5 °C for table stock potato. Thus, the VI treatment potatoes in the present study were stored also at 4 °C and 90% relative humidity for up to 30 days. The zinc contents of the samples were measured at 5, 10, 20 and 30 days.
Zinc determination
Potato skin is considered to be inedible (Fierens et al. 2012). Thus, zinc determination in this study was performed after removal of the potato skin; one potato tuber was washed, peeled (except already peeled) and diced into approximately 5-mm cubes. After mixing well whole cubes, approximately 10 g of potato cubes was placed in a drying oven (DPS-41, Yamato Scientific Co., Ltd., Tokyo, Japan) at 70 °C for 18 h and then put in a vacuum drying oven (DPS-48, Yamato Scientific Co., Ltd., Tokyo, Japan) under 54 kPa vacuum at 70 °C for 2 h (AOAC 1970). The dried sample was powdered with a mortar and pestle. The powdered sample of 1 g was put into a crucible, and then incinerated at 550 °C in a muffle furnace (ETR-17K, Shibata Scientific Technology Ltd., Tokyo, Japan) system for 24 h (Ruerez 2002). Subsequently, the incinerated sample was dissolved to 5 ml of 2 mol/L of hydrochloric acid at 60 °C with a hotplate. Then, the dissolved solution was diluted to 100 ml in a volumetric flask with distilled water. The zinc content was determined with an atomic absorption spectrometer (AA-6300, Shimadzu, Kyoto, Japan) system at a wavelength of 213.8 nm (Tompkins et al. 2007). A calibration curve was established, using solutions containing 0, 0.1, 0.5, 1 and 3 μg/ml.
Statistical analysis
One tuber was used per treatment. Each treatment condition was assayed 6 times. Results were averaged to obtain mean values. Duncan’s multiple range test of SPSS 9.0 (SPSS Inc., Chicago, USA) was used to determine differences between means of vacuum time, restoration time and cooking treatment.
Results and discussion
Effect of vacuum time and restoration time
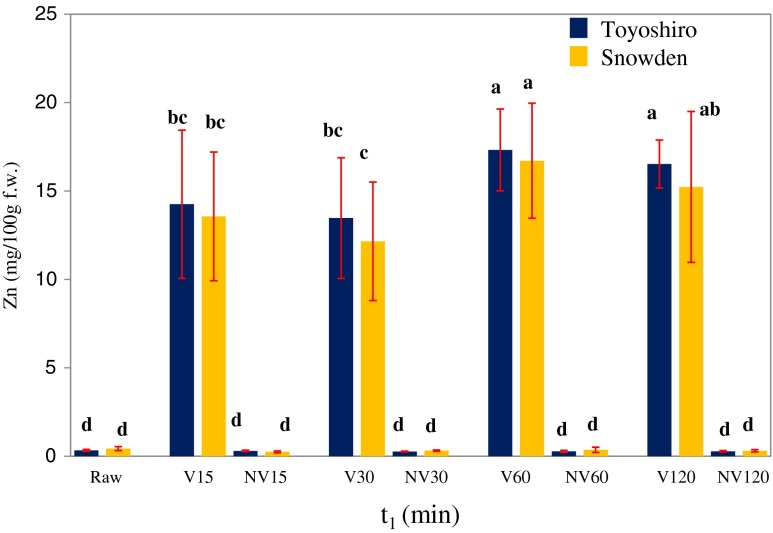
Figure 2 shows the relation between the zinc content of VI potato and vacuum time (t1). As shown in this figure, the zinc content increased with t1; especially, the 60 min VI potato of the Toyoshiro had a 61 times higher (p < 0.05) zinc content than the control (non-vacuum 60 min) potato, and contained a 17.3 mg zinc content per 100 g. In addition, the 60 min VI potatoes (per 100 g) could provide adult men and women more than 100% of the RDA values. Even the 15 min VI potatoes (per 100 g) could exceed RDA values (11 mg/day of adult man and 8 mg/day of women) (FNB 2001). For the Snowden, the 60 min VI potato had a 45 times higher (p < 0.05) zinc content than the control potato and had a 16.7 mg zinc content per 100 g. In addition, the VI potato (per 100 g), beyond 15 min of t1, had zinc contents higher than the RDA values for adult men (11 mg/day) and women (8 mg/day). Especially, 60 min VI potatoes (100 g) could provide adult men and women with 152 and 210 % of the RDA values (FNB 2001), respectively.
Fig. 2.
Effect of vacuum time (t1) on zinc content of vacuum-impregnated whole potato tubers. Values are the means ± SD (n = 6). Different letters show significant differences at 0.05 probability. Vacuum impregnation (VI) conditions: VI solution, saturated solution of zinc gluconate; vacuum pressure, 1,000 Pa; restoration time, 3 h. Abbreviations: V 15: t1, 15 min; NV 15: non-vacuum 15 min-immersion in the zinc solution; V 30: t1, 30 min; NV 30: non-vacuum 30 min-immersion in the zinc solution; V 60: t1, 60 min; NV 60: non-vacuum 60 min-immersion in the zinc solution; V 120: t1, 120 min; NV 120: non-vacuum 120 min-immersion in the zinc solution
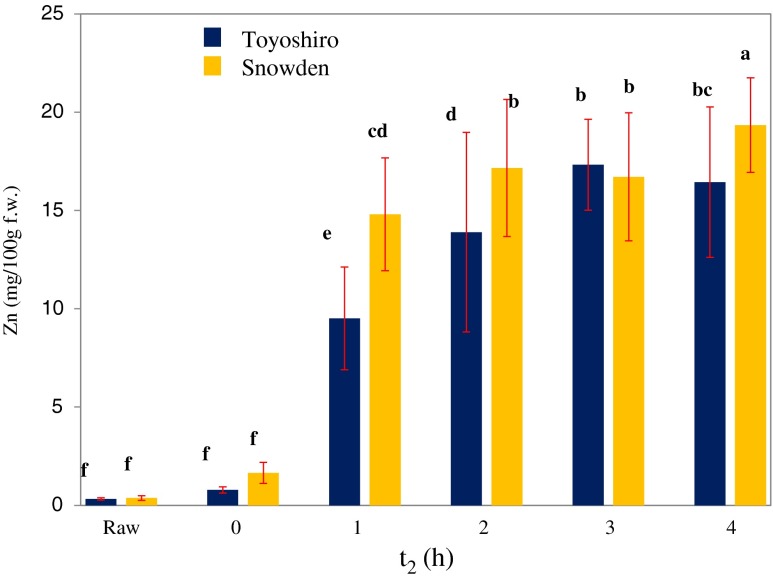
The VI potato of zinc content increased during restoration time (t2) (Fig. 3). The 3 h VI treatment potato (Toyoshiro) had a 53 times higher (p < 0.05) zinc content than the raw potato, and reached a 17.3 mg zinc content per 100 g. Similarly, the maximum zinc content of the Snowden VI potato was obtained beyond 4 h of t2, and led to 19.3 mg zinc content per 100 g.
Fig. 3.
Effect of restoration time (t2) on zinc content of vacuum-impregnated whole potato tubers. Values are the means ± SD (n = 6). Different letters show significant differences at 0.05 probability. Vacuum impregnation (VI) conditions: VI solution, saturated solution of zinc gluconate; vacuum pressure, 1,000 Pa; vacuum time, 1 h
Concerning the VI system, Fito (1994) reported that the VI processes of porous products when reduced pressures are imposed in a solid liquid system (vacuum step) followed by the restoration of atmospheric pressure. During the vacuum step (t1) the internal gas in the product pores is expanded and partially flows out. All this is coupled with the capillary penetration as a function of the interfacial tension of the liquid and the diameter of pores. In the atmospheric step (t2), the residual gas is compressed and the external liquid flows into the pores as a function of the compression ratio. Plant tissue has intercellular spaces that may contain a gas or liquid phase and are susceptible to impregnation with an external solution (Zhao and Xie 2004). Sun and Li (2003) observed that the intact cells of fresh potato tissue are in perfect contact, although some small intercellular voids exist. Their voids are ICAS which are common in parenchymous tissue. The ICAS volumes in potatoes are estimated as 1% of the total volume in potatoes (Aguilera and Stanley 1990). Therefore, in the present study, the external solution with high zinc content may gradually penetrate into the ICAS within the potato in the atmospheric step (restoration step).
In the present study, a long vacuum time (1 h) and a long restoration time (3 or 4 h) were needed for whole potatoes. As mentioned previously, Salvatori et al. (1998) reported that in plant tissue samples of about 2 cm in characteristic dimension, with the relatively large intercellular spaces and elastic cellular arrangement, the necessary lengths of vacuum time and restoration time are within 5 min. Nevertheless, in big pieces with small pores, much more time could be necessary to complete sample equilibration in mechanical terms because of difficulties for gas release. Likewise, impregnation times can be lengthened due to the greater pressure drops (Chiralt et al. 2001). In the present study, potatoes have shapes larger than 2 cm in diameter. In addition, the ICAS of potatoes is very small (1%) compared with that of apple (25%), peach (15%) and mushroom (37–45%) (Alzamora et al. 2000). Also, whole potatoes have thick periderm, which is less permeable to water and gas (Peterson et al. 1985). Thus, these long vacuum and restoration times in the present study may be due to the above reasons.
Effect of steam-cooking
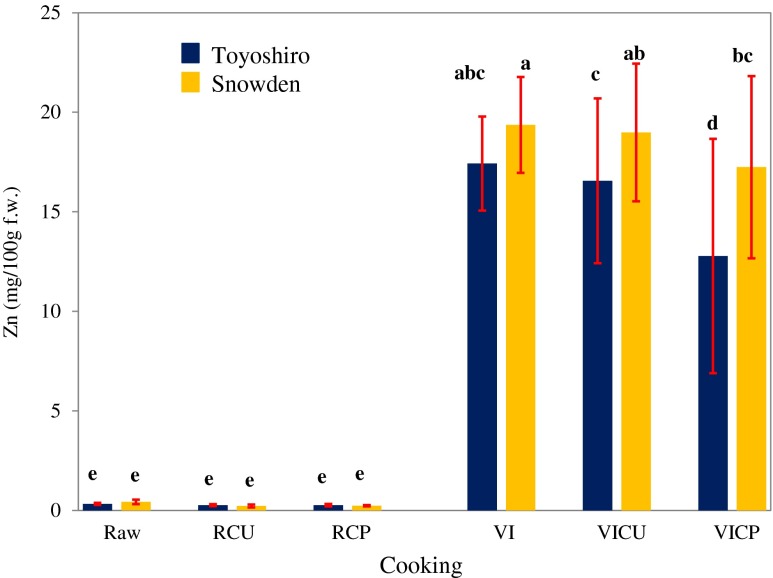
Figure 4 shows the decrease in zinc content of VI potatoes by steam-cooking. As shown in the figure, the zinc content of Toyoshiro VI-cooked unpeeled potatoes decreased by 4% whereas VI-cooked peeled potatoes declined to a greater extent (26%). Moreover, VI-cooked unpeeled potatoes had 63 times higher (p < 0.05) zinc contents than un-VI-cooked unpeeled potatoes. Also, VI-cooked peeled potatoes had 47 times more (p < 0.05) zinc contents than un-VI-cooked peeled potatoes. In addition, the zinc content of VI-cooked unpeeled and peeled potatoes of Snowden variety decreased by 2 and 11 %, respectively. However, VI-cooked unpeeled potatoes had 94 times higher (p < 0.05) zinc contents than un-VI-cooked unpeeled potatoes. Moreover, VI-cooked peeled potatoes had 75 times higher (p < 0.05) zinc contents than un-VI-cooked peeled potatoes.
Fig. 4.
Effect of steam-cooking on zinc content of vacuum-impregnated whole potato tubers. Values are the means ± SD (n = 6). Different letters show significant differences at 0.05 probability. Vacuum impregnation (VI) conditions: VI solution, saturated solution of zinc gluconate; vacuum pressure, 1,000 Pa; vacuum time, 1 h; restoration time, 3 h. Abbreviations: RCU, raw-cooking (unpeeled); RCP, raw-cooking (peeled); VICU, VI-cooking (unpeeled); VICP, VI-cooking (peeled)
Worldwide daily potato uptake per capita was 86 g in 2005 (IYP 2008). Thus, daily potato consumptions of VI-cooked unpeeled and peeled potatoes could provide adult men with 130–148 and 100–135 % of the zinc RDA values, respectively. In addition, those of the unpeeled and peeled of VI-cooked potatoes could give adult women 178–203 and 137–185 % of the zinc RDA levels (FNB 2001), respectively. Thus, the VI potatoes had a health advantage in providing foods enriched with zinc content.
As for calcium distribution in tissues of calcium VI products (eggplant, carrot and oyster mushroom), Gras et al. (2003) showed that calcium incorporation mainly occurred in the intercellular spaces using an energy dispersive X-ray microanalysis. Thus, the zinc may be also located in ICAS in the present study. Peterson et al. (1985) indicated that the lenticels of potatoes allow excess water loss from the internal tissues of the tuber. Heat by steam-cooking maybe cause an expansion of gas in the ICAS of the potato, which might lead to pushing of zinc solution from the ICAS. In Fig. 4, cooking losses in zinc of the whole potato are much smaller than those of the peeled potato. This may be due to the thick periderm of whole potatoes, which is less permeable to water and gas (Peterson et al. 1985). Further study is needed on zinc distribution in the tissue of VI potatoes.
Effect of storage
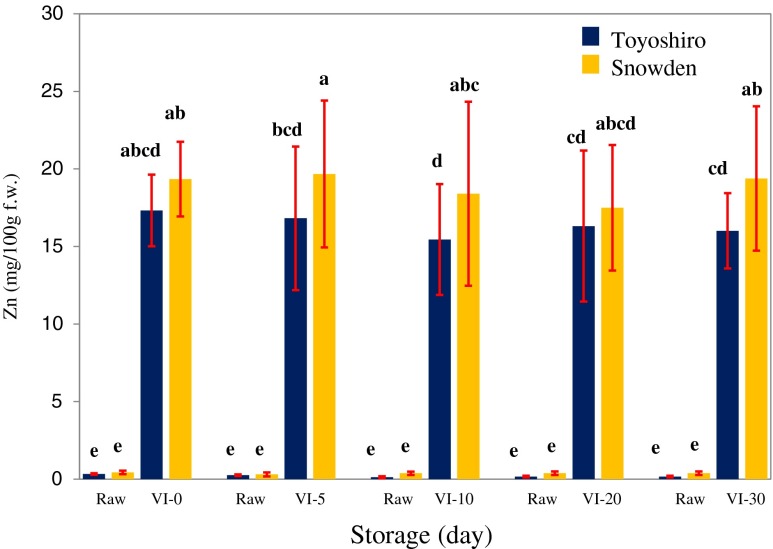
Figure 5 shows the change in zinc content of VI potatoes during storage at 4 °C. As shown in this figure, there was no significant difference in zinc content of the VI potato tuber for Toyoshiro or Snowden variety between 0 and 30 days of storage. Thus, VI potatoes of Toyoshiro retained 50 times higher zinc contents during storage compared with raw potatoes. In addition, for Snowden variety, VI potatoes also kept 40 times higher levels during storage compared with raw potatoes.
Fig. 5.
Change in zinc content of vacuum-impregnated whole potato tubers during storage at 4 °C. Values are the means ± SD (n = 6). Different letters show significant differences at 0.05 probability. Vacuum impregnation (VI) conditions: VI solution, saturated solution of zinc gluconate; vacuum pressure, 1,000 Pa; vacuum time, 1 h; restoration time, 3 h. Abbreviations: VI-0, 0 day storage of VI potato; VI-5, 5 days storage of VI potato; VI-10, 10 days storage of VI potato; VI-20, 20 days storage of VI potato; VI-30, 30 days storage of VI potato
VI can be considered as a tool in the development of fruit or vegetable products without disrupting their cellular structure, while conveniently modifying their original composition (Chiralt et al. 1999). Moreover, Gras et al. (2003) showed that many calcium ions of VI products existed only in the ICAS, not inside of cells. In the present study, the zinc solution may be also kept in the ICAS without disrupting internal cells, and leaching of the zinc solution from the tuber during storage.
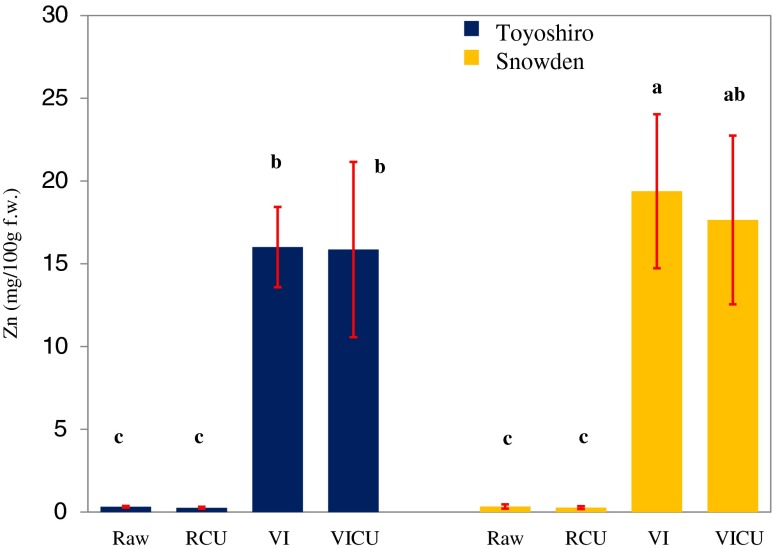
Figure 6 shows changes in zinc content of 30-day-storage VI-unpeeled potato tubers by steam cooking. In Toyoshiro cultivar, there were no significant differences in zinc content between the VI potatoes and VI-cooked potatoes. Although the zinc contents of 30-day-storage VI-unpeeled potatoes of Snowden variety declined by 9%, there were no significant differences in zinc content between the VI potatoes and VI-cooked potatoes. Zinc contents of the VI-cooked potatoes were 65 times higher than those of raw-cooking potatoes.
Fig. 6.
Zinc content of vacuum-impregnated whole potato tubers after 30 days of storage at 4 °C and cooked. Values are the means ± SD (n = 6). Different letters show significant differences at 0.05 probability. Vacuum impregnation (VI) conditions: VI solution, saturated solution of zinc gluconate; vacuum pressure, 1,000 Pa; vacuum time, 1 h; restoration time, 3 h. Abbreviations: RCU, raw-cooking (unpeeled); VICU, VI-cooking (unpeeled)
Finally, this study indicates that VI treatment with zinc solution of whole potatoes was useful for enriching the zinc content of peeled, unpeeled or stored cooked-potatoes. The daily worldwide uptake (86 g) of VI potatoes by this method will be beneficial for the health of adult men because it exceeds the RDA levels.
Conclusions
The results of the present study indicate that the zinc content of VI potatoes increased with vacuum time and restoration time. Moreover, VI-cooked unpeeled or peeled potatoes had 63–94 times and 47–75 times higher zinc contents than un-VI-cooked unpeeled or peeled potatoes, respectively. Daily worldwide potato consumption (86 g) of the VI-cooked unpeeled and peeled potatoes provided adult men with 130–148% and 100–135% of the RDA of zinc, respectively. Also, the daily potato consumption of the unpeeled and peeled potatoes supplied adult women with 178–203% and 137–185% of the RDA levels, respectively. The high zinc content of VI potatoes was kept during 4 °C-storage for 30 days. Our study therefore indicated that VI treatment with zinc solution of whole potato was useful for enriching the zinc content of peeled or unpeeled cooked potatoes.
Electronic supplementary material
(DOCX 15 kb)
Acknowledgments
We acknowledge the Shimadzu Corporation for kindly providing the rotary oil vacuum pump and the vacuum meter.
References
- Abong GO, Okoth MW, Karuri EG, Kabira JN, Mathooko FM. Levels of reducing sugars in eight Kenyan potato cultivars as influenced by stage of maturity and storage conditions. J Anim Plant Sci. 2009;2:76–84. [Google Scholar]
- Aguilera JM, Stanley DW (1990) Microstructural principles of food processing and engineering. Elsevier Science Publishers Ltd., Essex
- Alzamora SM, Tapia MS, Leunda A, Guerrero SN, Rojas AM, Gerschenson LN. Minimal preservation of fruits: a cited project. In: Lozano JE, Anon C, Parada-Arias E, Barbosa-Canovas GV, editors. Trends in Food Engineering. Pennsylvania: Technomic Publishing; 2000. pp. 205–225. [Google Scholar]
- AOAC (1970) Official Methods of Analysis of Association of Official Agricultural Chemists 11th edn. Washington, DC
- Bistrom M, Nordstrom K. Identification of key success factors of functional dairy foods product development. Trends Food Sci Tech. 2002;13:372–379. doi: 10.1016/S0924-2244(02)00187-5. [DOI] [Google Scholar]
- Burgos G, Auqui S, Amoros W, Salas E, Bonierbale M. Ascorbic acid concentration of native Andean potato varieties as affected by environment, cooking and storage. J Food Compos Anal. 2009;22:533–538. doi: 10.1016/j.jfca.2008.05.013. [DOI] [Google Scholar]
- Burlingame B, Mouille B, Charrondiere R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J Food Compos Anal. 2009;22:494–502. doi: 10.1016/j.jfca.2009.09.001. [DOI] [Google Scholar]
- Castro L, Freeman BA. Reactive oxygen species in human health and disease. Nutr. 2001;17(161):163–165. doi: 10.1016/s0899-9007(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Chan S, Gerson B, Subramaniam S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. J Lab Clin Med. 1998;18:673–685. [PubMed] [Google Scholar]
- Chiralt A, Fito P (1997) Salting of Manchego type cheese by vacuum impregnation. In P. Fito, E. Ortega, G. Barbosa, Food Engineering. Chapman & Hall, New York, pp 214–230
- Chiralt A, Fito P, Andrés A, Barat JM, Martínez-Monzó J, Martínez-Navarrete N. Vacuum impregnation: a tool in minimally processing of foods. In: Oliveira FAR, Oliveira JC, editors. Processing of foods: quality optimization and process assessment. Boca Raton: CRC Press; 1999. pp. 341–356. [Google Scholar]
- Chiralt A, Fito P, Barat JM, Andrés A, González-Martínez C, Escriche I, Camacho MM. Use of vacuum impregnation in food salting process. J Food Eng. 2001;49:141–151. doi: 10.1016/S0260-8774(00)00219-3. [DOI] [Google Scholar]
- Faller ALK, Fialho E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res Int. 2009;42:210–215. doi: 10.1016/j.foodres.2008.10.009. [DOI] [Google Scholar]
- Fierens T, Vanermen G, Van Holderbeke M, De Henauw S, Sioen I. Effect of cooking at home on the levels of eight phthalates in foods. Food Chem Toxicol. 2012;50:4428–4435. doi: 10.1016/j.fct.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Fito P. Modelling of vacuum osmotic dehydration of food. J Food Eng. 1994;22:313–328. doi: 10.1016/0260-8774(94)90037-X. [DOI] [Google Scholar]
- Fito P, Chiralt A. Osmotic dehydration: an approach to the modeling of solid food-liquid operations. In: Fito P, Ortega E, Barbosa-Cánovas G, editors. Food Engineering 2000. New York: Chapman & Hall; 1997. pp. 231–252. [Google Scholar]
- Fito P, Chiralt A. Vacuum impregnation of plant tissue. In: Alzamora SM, Tapia MS, López-Malo A, editors. Minimally processed fruits and vegetables. Maryland: Aspen Publishers Inc; 2000. pp. 185–205. [Google Scholar]
- Fito P, Andrés A, Chiralt A, Pardo P. Coupling of hydrodynamic mechanism and deformation-relaxation phenomena during vacuum treatments in solid porous food-liquid systems. J Food Eng. 1996;27:229–240. doi: 10.1016/0260-8774(95)00005-4. [DOI] [Google Scholar]
- Fito P, Chiralt A, Barat JM, Martínez-Monzó J. Vacuum impregnation in fruit processing. In: Lozano JE, Añón MC, Parada-Arias E, Barbosa-Cánovas GV, editors. Trends in Food Engineering. Technomic Pub Co: Lancaster; 2000. pp. 149–163. [Google Scholar]
- Food and Nutrition Board (FNB), Institute of Medicine (2001) In: Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, editor. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. National Academy Press, Washington DC, pp 422–501
- González C, Fuentes C, Andrés A, Chiralt A, Fito P. Effectiveness of vacuum impregnation brining of Manchego-type curd. Int Dairy J. 1999;9:143–148. doi: 10.1016/S0958-6946(99)00035-7. [DOI] [Google Scholar]
- Gras M, Vidal-Brotóns D, Betoret N, Chiralt A, Fito P. The response of some vegetables to vacuum impregnation. Innov Food Sci Emerg Technol. 2002;3:263–269. doi: 10.1016/S1466-8564(02)00032-2. [DOI] [Google Scholar]
- Gras ML, Vidal D, Betoret N, Chiralt A, Fito P. Calcium fortification of vegetables by vacuum impregnation interactions with cellular matrix. J Food Eng. 2003;56:279–284. doi: 10.1016/S0260-8774(02)00269-8. [DOI] [Google Scholar]
- Hironaka K, Kikuchi M, Koaze H, Sato T, Kojima M, Yamamoto K, Yasuda K, Mori M, Tsuda S. Ascorbic acid enrichment of whole potato tuber by vacuum-impregnation. Food Chem. 2011;127:1114–1118. doi: 10.1016/j.foodchem.2011.01.111. [DOI] [PubMed] [Google Scholar]
- Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Igual M, Castelló ML, Ortolá MD, Andrés A. Influence of vacuum impregnation on respiration rate, mechanical and optical properties of cut persimmon. J Food Eng. 2008;86:315–323. doi: 10.1016/j.jfoodeng.2007.06.002. [DOI] [Google Scholar]
- International Year of Potato (IYP) (2008) IYP: Potatoes, nutrition and diet. http://www.potato2008.org/en/photocontest/. Accessed 1 May 2013
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Mazza G. Functional foods: biochemical and processing aspects. Lancaster: Technomic Pub Co; 1998. [Google Scholar]
- Menrad K. Market and marketing of functional food in Europe. J Food Eng. 2003;56:181–188. doi: 10.1016/S0260-8774(02)00247-9. [DOI] [Google Scholar]
- Menrad M, Hüsing B, Menrad K, Reiβ T, Beer-Borst S, Zenger CA (2000) Functional Food. TA 37/2000. Schweizerischer Wissenschafts und Technologierat, Bern
- Mondy NI, Munshi CB, Seetharaman K. Residue levels of isopropyl N-(3-chlorophenyl) carbamate (CIPC) in potatoes as affected by level of application, storage time and temperature, and method of cooking. Food Res Int. 1992;25:375–379. doi: 10.1016/0963-9969(92)90112-I. [DOI] [Google Scholar]
- Mújica-Paz H, Valdez-Fragoso A, López-Malo A, Palou E, Welti-Chanes J. Impregnation and osmotic dehydration of some fruits: effect of the vacuum pressure and syrup concentration. J Food Eng. 2003;57:305–314. doi: 10.1016/S0260-8774(02)00344-8. [DOI] [Google Scholar]
- Murniece I, Karklina D, Caloburda R, Santare D, Skrabule I, Costa HS. Nutritional composition of freshly harvested and stored Latvian potato (Solanum tuberosum, L.) varieties depending on traditional cooking methods. J Food Compos Anal. 2011;24:699–710. doi: 10.1016/j.jfca.2010.09.005. [DOI] [Google Scholar]
- Peterson RL, Barker WG, Howarth MJ. Development and structure of tubers. In: Li PL, editor. Potato Physiology. Orlando: Florida Academic Press; 1985. [Google Scholar]
- Ruerez P. Mineral content of edible marine seaweeds. Food Chem. 2002;79:23–26. doi: 10.1016/S0308-8146(02)00171-1. [DOI] [Google Scholar]
- Salvatori D, Andrés A, Chiralt A, Fito P. The response of some properties of fruits to vacuum impregnation. J Food Process Eng. 1998;21:59–73. doi: 10.1111/j.1745-4530.1998.tb00439.x. [DOI] [Google Scholar]
- Sanzana S, Gras ML, Vidal-Brotóns D. Functional foods enriched in Aloe vera. Effects of vacuum impregnation and temperature on the respiration rate and the respiratory quotient of some vegetables. Procedia Food Sci. 2011;1:1528–1533. doi: 10.1016/j.profoo.2011.09.226. [DOI] [Google Scholar]
- Sibbel A. The sustainability of functional foods. Soc Sci Medicine. 2007;64:554–561. doi: 10.1016/j.socscimed.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Sloan AE. The top ten functional food trends. Food Technol. 2000;54:33–62. [Google Scholar]
- Sloan AE. The top ten functional food trends: the next generation. Food Technol. 2002;56:32–57. [Google Scholar]
- Sormani A, Maffi D, Bertolo G, Torreggiani D. Textural and structural changes of dehydrofreeze-thawed strawberry slices: effects of different dehydration pretreatments. Food Sci Technol Int. 1999;5:479–485. doi: 10.1177/108201329900500605. [DOI] [Google Scholar]
- Stauffer JE. Nutraceuticals. Cereal Foods World. 1999;44:115–117. [Google Scholar]
- Sun DW, Li B. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. J Food Eng. 2003;57:337–345. doi: 10.1016/S0260-8774(02)00354-0. [DOI] [Google Scholar]
- Tapia MS, López-Malo A, Consuegra R, Corte P, Welti-Chanes J. Minimally processed papaya by vacuum osmotic dehydration techniques. Food Sci Tech Int. 1999;1:43–52. [Google Scholar]
- Tompkins TA, Renard NE, Kiuchi A. Clinical evaluation of the bioavailability of zinc-enriched yeast and zinc gluconate in healthy volunteers. Biol Trace Elem Res. 2007;120:28–35. doi: 10.1007/s12011-007-0072-2. [DOI] [PubMed] [Google Scholar]
- Weaver ML, Timm H, Ng H. Changes in nutritional composition of Russet Burbank potatoes by different processing methods. Am Potato J. 1983;60:735–744. doi: 10.1007/BF02856893. [DOI] [Google Scholar]
- Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998;68:442S–446S. doi: 10.1093/ajcn/68.2.442S. [DOI] [PubMed] [Google Scholar]
- Wustman R, Struik PC. The canon of potato science: 35. Seed and ware potato storage. Potato Res. 2007;50:351–355. doi: 10.1007/s11540-008-9079-0. [DOI] [Google Scholar]
- Zhao Y, Xie J. Practical applications of vacuum impregnation in fruit and vegetable processing. Trends Food Sci Tech. 2004;15:434–451. doi: 10.1016/j.tifs.2004.01.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)