Abstract
The objective of the present study was to analyses technological and textural properties of pork thawed by low intensity ultrasound compared to meat thawed conventionally in air (control) or by immersion in water. The pork thawing was done by means of a generator of constant frequency, with adjusting ultrasound intensity, coupled with a transducer plate and a water bath. The frequency of 25 kHz and the intensity of 0.6 W/cm2 allowed reducing by 87 % the time required for thawing from −5 °C to −1 °C as well as the overall thawing time as compared to thawing in air. Using intensity of 0.2, 0.4, and 0.6 W/cm2 thawing rates were 0.62, 0.73, 1 °C/min, versus 0.16 °C/min in the control. The textural and technological properties of meat thawed by ultrasound are not impaired by the significant lowering of thawing time; there were no large mass loss or modification of meat ultrastructure as compared to control.
Keywords: Ultrasound, Pork, Thawing rate, DSC, Ultrastructure, Texture
Introduction
In recent years, there has been an increasing interest in developing new food processing technologies which leads to a decrease in the processing time and the production costs without affecting the quality of the product. As frozen meat is widely used in meat industries, the process of thawing must be given considerable attention. Accordingly, besides the attention paid to improving the freezing process, there likewise is due concern for the efficiency of thawing. Different scientific studies showed that the ultrasound power has proven to be very attractive for rapid meat thawing. In this respect, Kissam (1984) and Miles et al. (1999) obtained a decrease in thawing time. A patent filed by Kissam in 1984 demonstrated that shortening the thawing process by means of low-level ultrasound frequencies and powers could be practiced with fish blocks immersed in water. The use of 1500 Hz frequency and 60 W power stimulated thermal transfer so that the reduction of time held at phase change temperatures from −5 °C to −1 °C was 82 % (Kissam 1984). Miles et al. (1999) noted that heating rate in thawed areas rises with intensity and frequency and it is higher when transmission is parallel with muscle fiber. It has been assessed that thawing occurs without excessive heating of the surface when combining a frequency of 500 kHz and 0.5 W/cm2 intensities.
Furthermore, the use of ultrasounds in meat industry has been the subject of many research papers. The effect of ultrasounds power on the physical properties of chilled meat was extensively studied, but the reported results are inconsistent (Pohlman et al. 1997a, b; Jayasooriya et al. 2007; Got et al. 1999; Lyng et al. 1997; Lyng et al. 1998a, b; Stadnik et al. 2008). Lyng et al.(1997) tested the ultrasonic treatments on Longissimus, Semitendinosus, and Biceps femoris muscles and showed that, when using ultrasonic water baths with intensities of 0.29–0.62Wcm2 and frequency of 30–47 kHz, no significant effects appeared on the tenderness of beef, collagen solubility and proteolysis of myofibrillar proteins. On the other hand, Jayasooriya et al. (2007) reported that the ultrasound treatment significantly reduced hardness, cook and total loss of beef muscle.
Although many studies have shown changes that may occur as a result of ultrasound treatment on chilled meat, to our knowledge there is no data available in the scientific literature on the quality of pork meat thawed by ultrasounds.
On the other hand, the increasing demand for frozen meat in industry and the lack of reports regarding the effects of ultrasounds assisted thawing on technological and textural properties of meat indicate that further research is needed to clarify the applicability of ultrasound for meat thawing.
Unlike other experiments (Kissam 1984; Miles et al. 1999) in the present study we used ultrasound for thawing pork not only for shortening the time of thawing but also for assessing the extent to which the technological properties of thawed meat are affected. Furthermore, the technological and textural properties of meat thawed by ultrasound and of conventionally thawed meat were compared. Consequently, the main purpose of this study was to investigate the technological and textural properties of meat thawed by low intensity ultrasounds.
Material and methods
Preparation of samples
Eight samples of Longissimus dorsi were extracted from pork carcasses of the same weight and stored for 24 h at 4 °C after slaughtering. The refrigerated meat was taken to the meat pilot station located at the “Dunarea de Jos” University of Galati, Romania. Each sample of Longissimus dorsi was cut into pieces of the same thickness (120 X 60 X 35 mm) and weight (160 g). Each sample was wrapped with polyethylene film and then frozen for 24 h at −18 °C by conventional method in an Electrolux freezer until the temperature reached −15 °C at the core. The core body temperature was measured by a Sika Electronics thermocouple thermometer. Afterwards three types of thawing were used: (1) thawing in air at 16 °C considered as control; (2) thawing by immersion in water at 15 °C; (3) ultrasound-assisted thawing at intensities of 0.2 W/cm2, 0.4 W/cm2 and 0.6 W/cm2 in water bath at 15 °C.
Ultrasound assisted thawing procedure
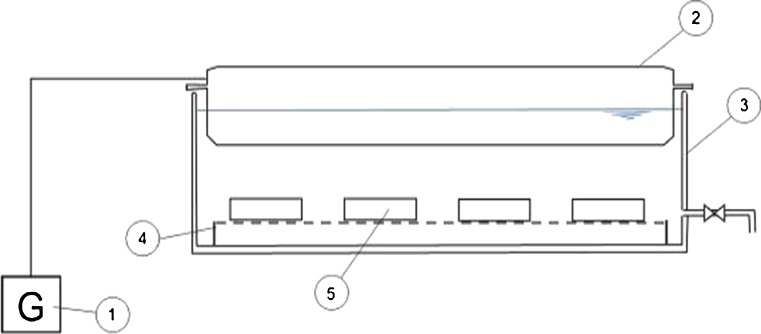
The appliance used for ultrasound assisted thawing consists of an ultrasound generator (Clangsonic), a transducer and a water bath (Fig. 1). The ultrasound is transmitted through the liquid medium from the generator (1), by means of the plate transducer (2) located above the water bath (3). As plate transducers mounted out of the bath proper are built up into the desired size, they allow for saving space, thus securing good sonic efficiency. Furthermore, a great disadvantage is avoided by selecting this design since, by using the water bath supplied with built-in generator (located on the bottom), additional heating of samples might occur. The transducer located above the water bath allows for the uniform ultrasound transmission through the entire mass of water thus uniform thawing of the entire meat sample is made possible. To avoid contact with the vessel bottom the samples (5) were placed on a grating (4). In our study, the parameters of the thawing process by use of ultrasounds were carefully selected as to ensure proper conditions for creating a transient acoustic cavity in the liquid submitted to ultrasound impact, in order to accelerate the thawing process. An acoustic cavity occurs in the liquid subjected to ultrasounds treatment, consisting of the appearance, growth and collapse of bubbles. The instant the cavity arise in a liquid close to a solid surface, the dynamics of cavity collapse change, the cavitational collapse turns asymmetric and generates high speed jets (Suslick 2001). This jet oriented directly toward the solid surface heightens thermal transfer (Mason and Bernal 2012) thus playing a significant role in thawing. Therefore one should consider that bubble collapse caused by cavity produces hot spots and increased pressure for brief periods. Such hot spots are the source of sonochemical reactions (Suslick et al. 1999a; Suslick et al. 1999b). It has been established that, in aqueous solutions submitted to sonication, hydrogen atoms and hydroxyl radicals are generated as a consequence of transient cavitation; their occurrence was proved experimentally in water sonicated by “spin trapping” technique (Riesz et al. 1985). These species can afterwards recombine to generate hydrogen peroxide, while the frequency used is consequential in minimizing the amount of primary radicals generated by the bubble. Therefore we consider that selection of very low frequencies might reduce the amount of hydroxyl radicals generated (Ashokkumar et al. 2008). Taking into account the above-mentioned considerations, a frequency of 25 kHz was selected for our study so as to lower the amount of generated hydroxyls and thus to avoid phonic pollution along with the use of an acoustic environment during thawing. Lower intensities in the range of 0.2–0.6 W/cm2 were selected to ensure uniform thawing throughout the mass of meat sample, thus avoiding temperature differences between the surface of the sample and its geometrical center.
Fig. 1.
Schematic drawing of thawing device. 1) generator; 2) plate transducer; 3) water bath; 4) grating; 5) meat samples
The ultrasound used in our experiment was of constant frequency (25 kHz) while the applied intensity was variable. The initial temperature of water in the bath was 15 °C while thawing lasted up to 2–4 °C at sample core. Temperature at core sample was monitored with a Sika Electronics thermocouple to ensure efficient thawing control along with securing maximum food security.
pH value
The suspensions (10 %) were prepared with homogenized minced meat samples and distilled water in order to quantify pH value. The measurements were carried out in duplicate by means of a Methrom pH meter
Thawing loss
The samples were weighed after freezing (W0) and then packed in vacuum bags for thawing. After thawing, the samples were taken from the vacuum bags, blotted dry and weighed (W1). The thawing loss was calculated according to the following equations:
where W0 is the weight of frozen meat, and W1 is the weight of thawed meat.
Expressible moisture
The expressible moisture was determined by centrifugal method described by Fernandez et al. (2007). Accordingly, three meat discs of known weight were placed in 250 ml tubes and centrifuged for 20 min at 3500rot/min and 4 °C in a centrifuge (Refrigerated Centrifuge TGL-16 M). The expressible moisture was calculated as percentage of moisture lost from the initial weight of the sample after centrifugation:
where P0 is the weight of sample before centrifugation, and P1 is the weight of sample after centrifugation.
Cooking loss
The samples were weighed previously, laid individually in plastic bags and thermally treated by immersion in water bath at 85 °C till inner sample temperature reached 70 °C. A thermometer inserted into sample core monitored meat temperature. After boiling, the samples were cooled in tap water jet for 30 min, dried by paper dabbing and weighed. Cooking loss was calculated as follows:
where M0 is the initial sample weight after thawing, and M1 is the final sample weight after boiling.
Texture measurement
A texturometer (TA.XT. Plus Texture Analyser) was used to measure meat hardness. Thawed samples were cut into cubes of 35 mm side. Measurements were made along the muscle fiber at 1.5 m/s penetration speed. Texture measurements were made after boiling and cooling of samples. The measurements were repeated 8 times on two opposite sides, the result being the arithmetic mean of all determinations.
Differential scanning calorimetry (DSC)
The Differential scanning calorimetry (DSC) was used to assess the response of myofibrillar proteins to thermal treatment. This method enables the use of samples with complex composition containing large quantities of proteins (Tornberg 2005). Measurements were made with a Mettler Toledo DSC1 device. At least four measurements were performed on each meat sample weighting in the range of 15 and 20 mg. The samples were weighed on high precision analytical scales, put into special aluminum pans of 40 μl and were hermetically sealed. The samples were warmed from 30 to 95 °C at a rate of 10 °C/min by using a reference empty capsule for measuring transition temperature (Tmax).
Transmission electron microscopy (TEM)
The Transmission electron microscopy (TEM) was used to assess the microstructure of Longissimus dorsi muscle thawed in air and by ultrasound at intensities of 0.2 W/cm2, 0.4 W/cm2 and 0.6 W/cm2. The samples were previously prepared to this purpose by means of electron microscope using specific technique consisting of fixing and rinsing followed by immersing in Epon 812 resin. Accordingly, the samples were fixed in 2.5 % glutaraldehyde, in cacodylate buffer 0.1 N, postfixed in 2 % osmium tetroxide, rinsed, dehydrated with acetone, and finally embedded in Epon 812. Cutting operations were made with a Reichert type Ultracut-R ultramicrotome to acquire fine sections of 400–600 Å. Electron micrographs of X14000, X18000 were obtained with a Philips Model 301 electron microscope.
Statistical analysis
The statistical analysis of results was operated by using Anova Single Factor. Analysis of variance was done to determine the significance of the main effects. The results are displayed as average values together with standard deviations. Significance of differences between samples was determined using Tukey’s test.
Results and discussions
Thawing time
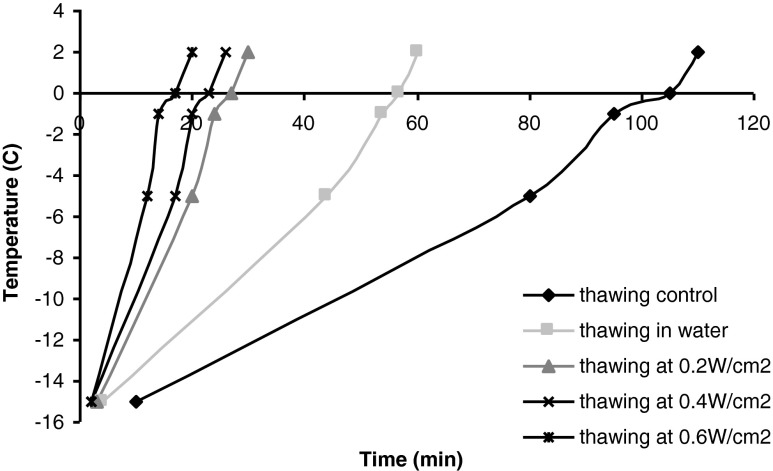
Thawing time was measured at temperature intervals: from −15 °C to −5 °C; from −5 °C to −1 °C; and from -1 °C to 2 °C. Accordingly, in the interval −15 °C to −5 °C, thawing by immersion in water and ultrasound assisted thawing occurred within a shorter interval than in air (control); yet all thawing types occur more rapidly than in -5 °C to −1 °C interval (Fig. 2). Comparison has been made between ultrasound assisted and conventional methods at phase change in the interval −5 °C to −1 °C where 73 % of the water is frozen (Gonzalez-Sanguinetti et al. 1985). Accordingly, as regards stimulating thermal transfer at phase change (from −5 °C to −1 °C), a time reduction of 80 % is noticed in case of ultrasound assisted thawing (0.6 W/cm2) compared to the immersion in water and 87 % compared to air thawing. These results are similar to those communicated by Kissam (1984) evidencing reduction in thawing time by 82 % as compared to the water immersion thawing. The lowest thawing rate (0.05 °C/min) measured for the temperature range −5 °C to −1 °C was registered in case of the control meat sample (thawing in air) and increased to 0.23, 0.26 and 0.4 °C/min for thawing by means of ultrasounds at 0.2, 0.4 and 0.6 W/cm2, respectively.
Fig. 2.
Thawing curve of pork Longissimus dorsi thawed by various methods
Concerning the overall thawing time, the results presented in Fig. 2 indicate the reduction of 76 % to 84 % compared to thawing in air, the thawing rate was influenced by the intensity of applied ultrasound: the thawing time decreases as the ultrasound intensity increases. Therefore, thawing rates for this study were 0.16 °C/min and 0.29 °C/min for thawing in air (control) and for water immersion, respectively compared to 0.62, 0.73, 1 °C/min, for thawing at intensities of 0.2, 0.4 and 0.6 W/cm2.
In case of thawing assisted by ultrasound, the water temperature raised by 2–3 °C at 0.2–0.4 W/cm2 intensity and by 4–5 °C at 0.6 W/cm2. Increasing the water temperature as a result of the physical effects of ultrasound and the high speed jets that occurs with asymmetric bubble collapse has been shown to be effective in improving heat transfer. Studies have shown that the bubble structures in acoustic cavitation depend on the sound frequencies and type of transducer used. The acoustic cavitation structure has been observed in case of using submerged transducers at 25 kHz (Mettin 2005), when the bubbles are ejected into the liquid like a flare and travel a distance into the bulk liquid, whereupon it disappears.
In our experiment, the submerged transducer was located few centimeters away from the meat sample generating a low accelerated cavitation jet that gradually lost the intensity once removed from the transducer surface. Moreover, even if an increase of the water temperature occurred during the ultrasound treatment, no excessive heating of the meat sample surface was registered.
pH measurement
There are no significant differences between control and ultrasound thawed samples in terms of pH values (Table 1, p > 0.05). These results are consistent with other reports concerning meat treated with ultrasound. Experiments conducted by Dolatowski et al. (2000), Stadnik et al. (2008) and Stadnik and Dolatowski (2011) detected no alteration of this parameter. Hence, Jayasooriya et al. (2007) detected alterations of pH in applying ultrasound of higher intensity (12 W/cm2; 24 kHz) to beef, case in which the pH increased according to ageing time (3–7 days). This progressive growth during ageing might be explained by conformational changes associated to denaturation of proteins through ultrasound treatment and proteolytic degradation of muscle fiber. However, the results of Jayasooriya et al. (2007), bear no comparison to ours since there were no similar conditions as regards parameters used: the ultrasound intensity we used was low while meat was not aged (24 h post mortem).
Table 1.
Influence of thawing treatment on pH, expressible moisture, thawing loss and cooking loss values
| Parameter | Thawing methods | ||||
|---|---|---|---|---|---|
| Air | Water immersion | 0.2 W/ cm2 | 0.4 W/ cm2 | 0.6 W/ cm2 | |
| pH | 5.80 ± 0.023 | 5.72 ± 0.027 | 5.82 ± 0.025 | 5.79 ± 0.02 | 5.81 ± 0.021 |
| EM (%) | 17.71 ± 1.26 | 16.05 ± 1.39 | 18.15 ± 1.11 | 17.29 ± 1.15 | 16.44 ± 1.80 |
| TL (%) | 2.95 ± 0.11 | 2.48 ± 0.13 | 2.86 ± 0.12 | 2.92 ± 0.09 | 2.78 ± 0.21 |
| CL (%) | 22.11 ± 0.51 | 23.10 ± 2.55 | 23.87 ± 1.9 | 22.01 ± 2.95 | 23.93 ± 1.44 |
pH, expressible moisture (EM), thawing loss (TL) and cooking loss (CL) of Longissimus dorsi samples thawed by air (control), water immersion, ultrasound at 0.2 W/ cm2, 0.4 W/ cm2, 0.6 W/ cm2. Means with different letters in the same row are not significantly different (P > 0.05). Error bars represent ± standard deviation
Expressible moisture and thawing loss
Our study compares meat thawed through different methods. Accordingly we notice that expressible moisture was not significantly affected by ultrasound treatment (Table 1p > 0.05). Similar results were derived in thawing loss instances, where no significant differences occurred with the various thawing methods used.
On the one hand, in point of thawing time, such results do not confirm theories by Gonzalez-Sanguinetti et al. (1985) evidencing that, by lowering the thawing time the exudate productions are higher. Our results do not confirm the theories of Ngapo et al. (1999) demonstrating that drip losses are lower, proportionally to shorter thawing time period. Possible causes of such differences may lie in the species of animal, muscle type, sample size, ageing degree of meat, or drip loss calculation method included. As with research by Gonzalez-Sanguinetti et al. (1985), meat samples to be thawed were Semimembranosus muscle beef, cylindrical in shape (5 cm in diameter and 8–9 cm in length) with ageing period of 2–3 days after slaughtering and drip loss measured by centrifugation at 2000xg/10 min. As for Ngapo et al. (1999) meat samples were pork (biceps femoris) cylindrical in shape with 2-day ageing period before freezing and drip loss measured by centrifugation at 40xg for 90 min.
On the other hand, in point of using ultrasound, these results are similar to those of Pohlman et al. (1997a), Pohlman et al. (1997b) and Jayasooriya et al. (2007) who detect no drip loss differences in sonicated versus nonsonicated samples. These results are however different from those of Dolatowski et al. (2000) who, by sonicating samples (2 W/cm2) before freezing, obtains significantly lower drip loss in sonicated as against control samples both in refrigeration 48 h after sonication and after storing frozen samples for 1 month. Hence, the manner ultrasound is applied (sonication of meat before freezing), as well as other parameters can trigger the occurrence of different results.
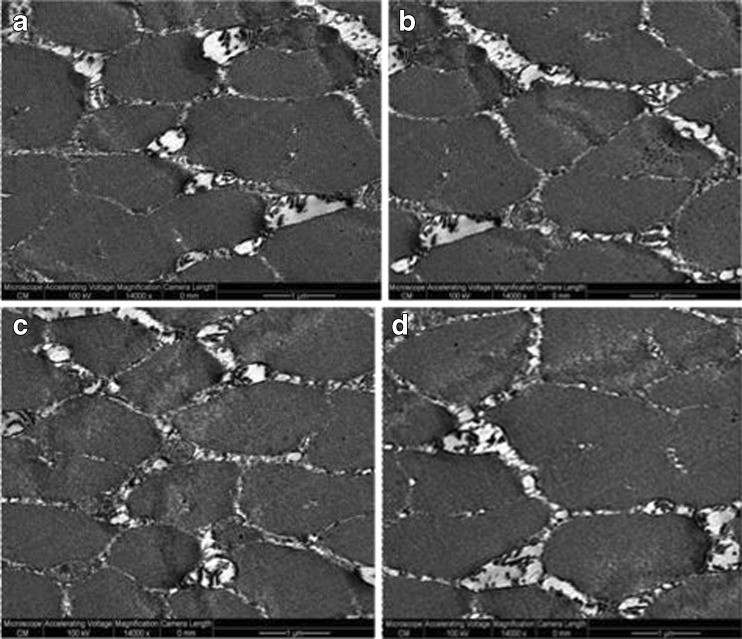
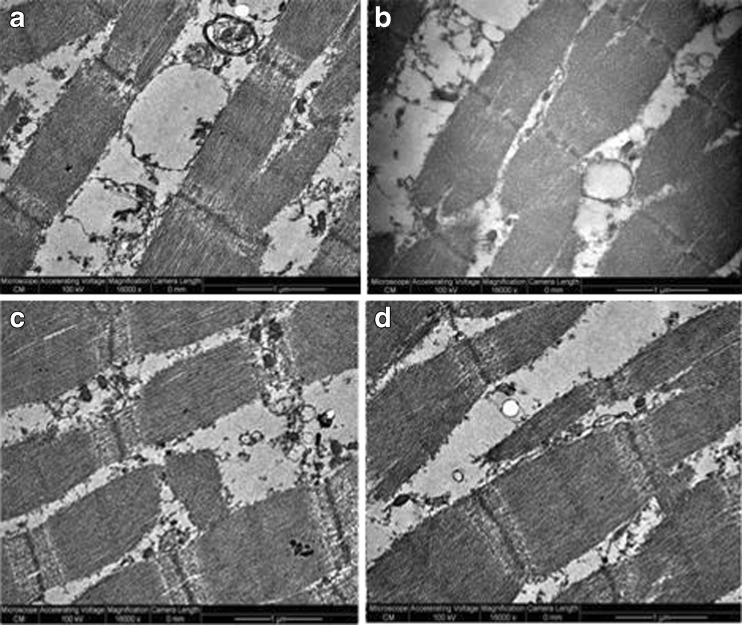
The expressible moisture correlates with thawing losses and ultrastructure photograms (Figs. 4 and 5) where no difference is evidenced in myofibril structure regardless of ultrasound intensity selected for thawing. Accordingly, it can be assessed that the mechanism of water migration in consequence of alterations in meat ultrastructure, due to ultrasound treatment, is not affected. Concurrently, as result of various intensities of applied ultrasound, no acceleration of meat ageing could be evidenced to have led to changing in water holding capacity, in accordance with the survey by Stadnik et al. (2008). Hence values not differing significantly might be interpreted that no major changes occurred at ultrastructure level.
Fig. 4.
TEM images of transversal section of thawed Longissimus dorsi muscle at 14000 units magnification: a thawing control; b thawing at 0.2 W/cm2; c thawing at 0.4 W/cm2; d thawing at 0.6 W/cm2
Fig. 5.
Structural modifications in meat thawed by various methods at 18000 magnification units; a thawing control; b thawing at 0.2 W/cm2; c thawing at 0.4 W/cm2; d thawing at 0.6 W/cm2
Cooking loss
Analyzing the results presented in Table 1 it can be noticed that there are no significant differences (p > 0.05) between samples thawed by conventional or ultrasound means. Insignificant differences as regards cooking losses of samples submitted to different thawing methods indicate that there was no structural weakening in proteins submitted to sonication, which conflicts with the study by Stadnik et al.(2008) pointing to an acceleration in ageing induced by ultrasound. Previous studies evidence a correlation between cooking losses and the ageing process. Shanks et al. (2002) showed that muscle loses ability to preserve humidity with ageing, while Wu et al. (2006) conclude that higher cooking losses in aged meat can be due to protein aggregation after ageing. Furthermore, Straadt et al. (2007) noticed that extended ageing associates with the growth of cooking losses while microstructural alterations occurring in ageing result in severe structural alterations. In this case, large spaces, similar to “black holes,” can be perceived while the fiber amount changing structural characteristics along with the number of “black holes” grow with ageing time. Consequently, as a result of such studies we can assert that, if ultrasound had induced this ageing acceleration, cooking loss in ultrasound thawed samples would have been higher than in samples thawed conventionally which never occurred with our survey. On the other hand, our results are consistent with Pohlman et al. (1997a) noticing no significant effect (caused by ultrasound treatment at low intensities of 1.55 W/cm2) on cooking losses, and with Pohlman et al. (1997b) evidencing that neither higher intensities applied (22 W/cm2), nor the length of ultrasound treatment bear upon cooking losses. On the other hand, Jayasooriya et al. (2007) indicate that cooking losses are only modified by ageing time rather than ultrasound treatment.
To sum up, cooking losses are consistent with other results of our survey (hardness, expressible moisture, TEM microscopy), indicating that no acceleration of the ageing process occurred at the parameters used in ultrasound thawing of meat.
Measuring meat texture
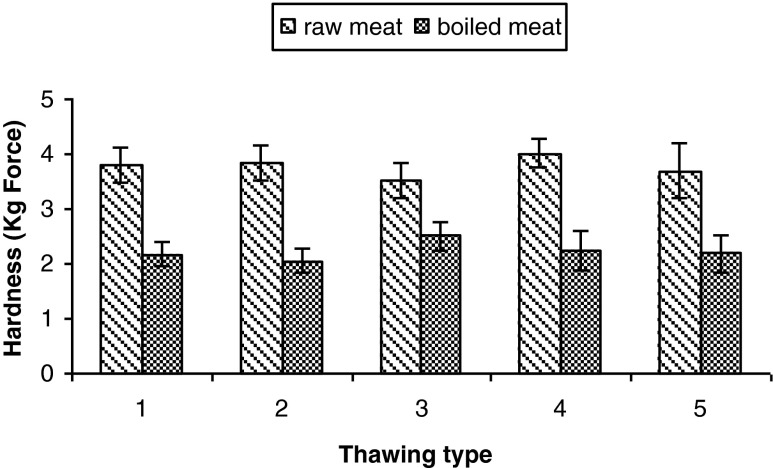
Hardness was measured both in raw and in cooked meat. In Fig. 3 presents the raw meat results, evidencing the absence of significant differences (p > 0.05) in meat samples submitted to either conventional or ultrasound thawing. As regards the influence of ultrasound on altering raw meat texture, similar results were derived in samples immersed in ultrasonic baths at low frequencies (30–40, 34–42, 47 kHz) or high intensities (62,39, 29 W/cm2) where no alteration in beef texture were detected (Lyng et al. 1997). Similarly, though at different parameters (40 kHz, 1500 W), Chang et al. (2009) observed no differences in control or sonicated samples. Likewise, no improvement was noticed in meat texture regardless of used parameters when using an ultrasonic probe either. Accordingly, Lyng et al. (1998a) and Lyng et al. (1998b) likewise evidenced no improvement in sonicated meat by applying ultrasound of high intensity (62 W/cm2) and low frequencies (20 kHz) both in beef and lamb meat. Absence of ultrasound effect on meat tenderization maintained in lamb and beef since the magnitude was insufficient to bear upon meat tenderness, leading to the conclusion that an adequate amount of energy has to be directed to meat sample to improve proteolysis. Even with Got et al. (1999) applying high frequencies (2.6 MHz) no positive affect was detected in improving the rate of tenderizing in beef. In this case, although ultrasound of high intensity induced pre-rigor phase ultrastructure, it led to no improvement in meat tenderizing.
Fig. 3.
Hardness depending on thawing type: 1) thawing control; 2) thawing in water; 3) thawing at 0.2 W/cm2; 4) thawing at 0.4 W/cm2; 5) thawing at 0.6 W/cm2
No significant differences were noticed in case of the cooked meat in terms of texture (Fig. 3) regardless of thawing methods – ultrasound or conventionally (p > 0.05). Such results are consistent with others in the literature pertaining to absence of texture improvement even though the meat was ultrasonicated in a bath at 20 kHz, and 1.55 W/cm2 (Pohlman et al. 1997a), or even though ultrasound (22 W/cm2, 20 kHz) was applied in cooled sonic environment (Pohlman et al. 1997b). Along with Pohlman et al. (1997a) we might consider such results as deriving from the low intensities applied, unable to cause significant cell damage due to sample thickness (3.5 cm) blocking low intensity waves from penetrating to the core. As to ultrasound exposing time length, we noticed it was ineffectual on meat tenderizing which is consistent with Pohlman et al. (1997b).
In our case, due to the parameters we applied, positive results were not possible as with Jayasooriya et al. (2007), likely because of the lower intensities or the low meat temperature in meat at the end of the process. Meat tenderization improvement was explained by Jayasooriya et al. (2007), as resulting from warming meat tissue (possibly leading to an increased protease action) or as a consequence of high intensity of applied ultrasound (12 W/cm2). In case of our study, the use of meat 24 h post-mortem might likewise be the cause of leading to the different results compared to those reported by Stadnik and Dolatowski (2011) who noticed an improvement in meat tenderization within the 48–72 h interval ( 2 W/cm2)). They interpreted these results through protease acceleration in ultrasound treatment, consistent with the analysis of modifications occurring in meat ultrastructure during ageing and of the values of samples capacity to retain water.
Differential scanning calorimetry (DSC)
Thermal transition curve was recorded for each tested samples identifying 3 transition zones marked by 3 peak levels between 54 and 58 °C, corresponding to myosin; between 65 and 67 °C to collagen and sarcoplasmatic proteins; between 80 and 83 °C corresponding to actin (reviewed by Tornberg 2005). Table 2 shows the results of the DSC test for meat thawed conventionally and by applying the ultrasound methods. In our study, the thermograms of each thawing method evidence specific, well defined peak levels located within the three temperature intervals presented by Tornberg (2005). Similarities are remarked pertaining to the profile of samples thawed by different methods. Accordingly, peak levels were noticed for myosin at approximately 57 °C, collagen at approximately 66 °C, actin at approximately 80 °C, while no significant differences were identified among the five thawing methods. Similarly, the total enthalpy of protein denaturation did not change significantly (Table 2, p > 0.05). DSC results are likewise consistent with the investigations of Ngapo et al. (1999), evidencing no differences between fresh, frozen and thawed meat samples, implying that freezing causes no protein denaturation. The DSC testing results when thawing the meat through various methods are consistent with the previous results in terms of texture and expressible moisture of samples. Accordingly, due to similitudes in thermogram profiles and to previous results pertaining to meat characteristics thawed by different methods, we might consider that both conventional (air, immersion in water) and ultrasound thawing evidenced no modification in proteins structure. Low intensity applied in ultrasound-thawed samples of 3.5 cm in thickness may explain the absence of protein denaturation.
Table 2.
Influence of different thawing type on maximum transition temperatures (Tmax) and ehthalpy (ΔH) values in pork Longissimus dorsi muscle
| Samples | Transition temperatures (°C) | ΔH(J/g) | ||
|---|---|---|---|---|
| Tmax1 | Tmax2 | Tmax3 | ||
| Air | 57.48 ± 0.19 | 66.46 ± 0.51 | 79.77 ± 0.71 | 0.42 ± 0.081 |
| Water | 60.14 ± 2.1 | 69.8 ± 3.95 | 80.12 ± 0.98 | 0.45 ± 0.934 |
| 0.2 W/ cm2 | 56.08 ± 0.83 | 65.73 ± 1.05 | 79.54 ± 0.65 | 0.4 ± 0.075 |
| 0.4 W/ cm2 | 57.11 ± 1.34 | 66.07 ± 0.88 | 79.53 ± 0.49 | 0.43 ± 0.069 |
| 0.6 W/ cm2 | 56.85 ± 0.76 | 66.32 ± 1.12 | 80.28 ± 1.23 | 0.42 ± 0.061 |
Means with different letters in the same column are not significantly different (P > 0.05)
ΔH value represent the total enthalpy change for all the transitions. Error bars represent ± standard deviation. Standard deviation obtained from three measurements
Transmission electron microscopy (TEM)
Figures 4 and 5 show the results of muscle Longissimus dorsi ultrastructure thawed in air (control sample) and by ultrasound. To completely evaluate the muscle ultrastructure we used images magnified at 14000 units (Fig. 4) and 18000 units (Fig. 5). No major differences are noticed by inspecting the cross section of Longissimus dorsi muscle (Fig. 4) in terms of muscle fiber microstructure, and the outer space among control sample (Fig. 4a) and the other samples thawed by ultrasound (Fig. 4b,c,d). Sarcomeres image seen at 18000 units magnification (Fig. 5) indicate that they maintained the initial form; no voids are noticed, no difference between ultrasonicated and control samples occurs. Sarcomeres are noticed to be well preserved after thawing and retain filamentous shape in all instances displayed. No structural modifications of myofibrils were detected, no fractures of I bands, while lines M and Z were clearly marked. As the sample texture underwent no significant modification, we conclude that no significant degradation of myofibril structure was recorded. Ultrastructure results of our study do not comply with Stadnik et al.(2008) who identified a more advanced modification of structural elements of sarcomere in sonicated as against control samples, which led to the hypothesis that sonication accelerated the process of meat tenderization. Such differences may arise from the different manner ultrasounds were used. Yet, by applying 2.6 MHz and 10 W/cm2, Got et al. (1999) demonstrated that ultrasound treatment post-rigor caused no ultrastructure alterations, while 6 days after of maturation no differences were noticed in sonicated and control samples. Our results indicate no major differences in samples regardless of the thawing procedures. We assess the parameters we used to have had no effects on meat ultrastructure which is consistent with results derived from texture testing including thawing and cooking losses.
Conclusions
The process of pork thawing was influenced positively by using low intensities of ultrasound by accelerating the thawing process, while technological and textural properties of thawed meat were not altered. Using intensity of 0.2, 0.4, and 0.6 W/cm2 thawing rates were 0.62, 0.73, 1 °C/min compared to 0.16 °C/min in the control samples. As regards the stimulating thermal transfer at phase change (from −5 °C to −1 °C), at 25 kHz and 0.6 W/cm2, the time was reduced by 87 % and thawing rate was improved to 0.4 °C/min from 0.05 °C/min when compared to control thawing. Technological and textural properties of pork were not affected by diminishing thawing time. The process had no affect on meat ultrastructure, thus the results being consistent with those derived by texture analysis, DSC and losses in thawing or cooking. Therefore we may conclude that the parameters used in the present study (25 kHz; 0.6 W/cm2) can be applied in thawing pork to acquire reduction of thawing time, without bearing upon sensorial and technological properties of meat. Unlike other similar studies, our study presents the effects of ultrasound on technological properties of thawed pork meat.
Despite many technological inconveniences, the conventional methods of thawing are still extensively used in many countries all over the world. The obtained results may contribute to the advanced in the field, demonstrating the possibility of successfully using the ultrasound treatment for thawing pork meat, without affecting its functional and technological properties. The knowledge gathered in this study can be successfully used as a basis for the optimization of processing meat by means of ultrasounds at industrial scale.
References
- Ashokkumar M, Sunartio D, Kentish S, Mawson R, Simons L, Vilkhu K, Versteeg C. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov Food Sci Emerg Technol. 2008;9(2):155–160. doi: 10.1016/j.ifset.2007.05.005. [DOI] [Google Scholar]
- Chang H, Xu X, Zhou G, Li C, Huang M. Effects of characteristics changes of collagen on meat physicochemical properties of beef semitendinosus muscle during ultrasonic processing. Food Bioprocess Technol. 2009;5:285–295. doi: 10.1007/s11947-009-0269-9. [DOI] [Google Scholar]
- Dolatowski Z, Stasiak DM, Latoch A (2000) Effect of ultrasound processing of meat before freezing on its texture after thawing. Electron J Pol Agric Univ 3(2) http://www.ejpau.media.pl
- Fernandez PP, Sanz PD, Molina-Garcıa AD, Otero L, Guignon B, Vaudagna SR. Conventional freezing plus high pressure–low temperature treatment: physical properties, microbial quality and storage stability of beef meat. Meat Sci. 2007;77:616–625. doi: 10.1016/j.meatsci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sanguinetti S, Anon MC, Calvelo A. Effect of thawing rate on the exudates production of frozen beef. J Food Sci. 1985;50:697–700. doi: 10.1111/j.1365-2621.1985.tb13775.x. [DOI] [Google Scholar]
- Got F, Culioli J, Berge P, Vignon X, Astruc T, Quideau JM, Lethiecq M. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physico-chemical properties of beef. Meat Sci. 1999;51:35–42. doi: 10.1016/S0309-1740(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Jayasooriya SD, Torley PJ, D’Arcy BR, Bhandari BR. Effect of high power ultrasound and ageing on the physical properties of bovine Semitendinosus and Longissimus muscles. Meat Sci. 2007;75:628–639. doi: 10.1016/j.meatsci.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Kissam AD (1984) Acoustic thawing of frozen food. United States Patent No. 4464 401, filed 22.04.82.
- Lyng JG, Allen P, McKenna BM. The influence of high intensity ultrasound baths on aspects of beef tenderness. J Muscle Foods. 1997;8:237–249. doi: 10.1111/j.1745-4573.1997.tb00630.x. [DOI] [Google Scholar]
- Lyng JG, Allen P, McKenna BM. The effects of pre- and post-rigor high-intensity ultrasound treatment on aspects of lamb tenderness. Lebensm Wiss Technol. 1998;31(4):334–338. doi: 10.1006/fstl.1997.0361. [DOI] [Google Scholar]
- Lyng JG, Allen P, McKenna BM. The effect on aspects of beef tenderness of pre- and post-rigor exposure to a high intensity ultrasound probe. J Sci Food Agri. 1998;78(3):308–314. doi: 10.1002/(SICI)1097-0010(199811)78:3<308::AID-JSFA123>3.0.CO;2-F. [DOI] [Google Scholar]
- Mason TJ, Bernal VS (2012) An Introduction to Sonoelectrochemistry. In B.G. Pollet (Ed.) Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution, John Wiley & Sons, Ltd, pp. 21–45
- Mettin R. Bubble structures in acoustic cavitation. Bubble and Particle Dynamics in Acoustic Fields: Modern Trends and Applications. Kerala: Research Signpost; 2005. pp. 1–36. [Google Scholar]
- Miles CA, Morley MJ, Rendell M. High power ultrasonic thawing of frozen foods. J Food Eng. 1999;39:151–159. doi: 10.1016/S0260-8774(98)00155-1. [DOI] [Google Scholar]
- Ngapo TM, Babare IH, Reynolds J, Mawson RF. Freezing and thawing rate effects on drip loss from samples of pork. Meat Sci. 1999;53:149–158. doi: 10.1016/S0309-1740(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Pohlman FW, Dikeman ME, Zayas JF. The effect of low-intensity ultrasound treatment on shear properties, color stability and shelf life of vacuum packaged beef semitendinosus and biceps femoris muscles. Meat Sci. 1997a;45(3):329–337. doi: 10.1016/S0309-1740(96)00106-4. [DOI] [PubMed] [Google Scholar]
- Pohlman FW, Dikeman ME, Kropf DH. Effects of high intensity ultrasound treatment, storage time and cooking method on shear, sensory, instrumental color and cooking properties of packaged and unpackaged beef pectoralis muscle. Meat Sci. 1997b;46(1):89–100. doi: 10.1016/S0309-1740(96)00105-2. [DOI] [PubMed] [Google Scholar]
- Riesz P, Berdahl D, Christman CL. Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ Health Persp. 1985;64:233–252. doi: 10.1289/ehp.8564233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks BC, Wulf DM, Maddock RJ. Technical note: The effect of freezing on Warner-Bratzler shear force values of beef longissimus steaks across several postmortem aging periods. J Anim Sci. 2002;80:2122–2125. doi: 10.2527/2002.8082122x. [DOI] [PubMed] [Google Scholar]
- Stadnik J, Dolatowski ZJ, Baranowska HM. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing. Lwt-Food Sci Technol. 2008;41:2151–2158. doi: 10.1016/j.lwt.2007.12.003. [DOI] [Google Scholar]
- Stadnik J, Dolatowski ZJ. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus) Eur Food Res Tehnol. 2011;233:553–559. doi: 10.1007/s00217-011-1550-5. [DOI] [Google Scholar]
- Straadt IK, Rasmussen M, Andersen HG, Bertram HC. Aging-induced changes in microstructure and water distribution in fresh and cooked pork in relation to water-holding capacity and cooking loss – A combined confocal laser scanning microscopy (CLSM) and low-field nuclear magnetic resonance relaxation study. Meat Sci. 2007;75:687–695. doi: 10.1016/j.meatsci.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Suslick KS, McNamara WB, Didenko Y. Hot Spot Conditions During Multi-Bubble Cavitation. In: Crum LA, Mason TJ, Reisse J, Suslick KS, editors. Sonochemistry and Sonoluminescence. Dordrecht: Kluwer Publishers; 1999a. pp. 191–204. [Google Scholar]
- Suslick KS, Didenko Y, Fang MM. Acoustic cavitation and its chemical consequences. Philos Tr Soc A. 1999b;357:335–353. doi: 10.1098/rsta.1999.0330. [DOI] [Google Scholar]
- Suslick KS. Sonoluminescence and Sonochemistry. In: Meyers RA, editor. Encyclopedia of Physical Science and Technology. San Diego: Academic Press, Inc; 2001. pp. 1–22. [Google Scholar]
- Tornberg E. Effects of heat on meat proteins – Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bertram HC, Kohler A, Böcker U, Ofstad R, Andersen HJ. Influence of aging and salting on protein secondary structures and water distribution in uncooked and cooked pork. A combined FT-IR Microspectroscopy and H NMR Relaxometry study. J Agr Food Chem. 2006;54:8589–8597. doi: 10.1021/jf061576w. [DOI] [PubMed] [Google Scholar]