Abstract
Withania somnifera (Ashwagandha, WS) or Indian ginseng possesses multiple pharmacological properties which are mainly attributed to the active constituents, withanolides. Despite its extensive usage as a memory enhancer and a nerve tonic, few attempts have been made to ascertain its usage in the management of Parkinson’s disease. In the present study, we investigated the neuroameliorative effects of WS in a rotenone (ROT) model of Drosophilamelanogaster (Oregon-K). Initially, we ascertained the ability of WS-enriched diet (0–0.05 %) to protect against ROT induced lethality and locomotor phenotype in adult male flies. Further, employing a co-exposure paradigm, we investigated the propensity of WS to offset ROT-induced oxidative stress, mitochondrial dysfunctions and neurotoxicity. WS conferred significant protection against ROT-induced lethality, while the survivor flies exhibited improved locomotor phenotype. Biochemical investigations revealed that ROT-induced oxidative stress was significantly diminished by WS enrichment. WS caused significant elevation in the levels of reduced GSH/non-protein thiols. Furthermore, the altered activity levels of succinate dehydrogenase, MTT, membrane bound enzymes viz., NADH-cytochrome-c reductase and succinate-cytochrome-c reductase were markedly restored to normalcy. Interestingly, ROT-induced perturbations in cholinergic function and depletion in dopamine levels were normalized by WS. Taken together these data suggests that the neuromodulatory effect of WS against ROT- induced neurotoxicity is probably mediated via suppression of oxidative stress and its potential to attenuate mitochondrial dysfunctions. Our further studies aim to understand the underlying neuroprotective mechanisms of WS and withanolides employing neuronal cell models.
Keywords: Withania somnifera, Drosophila melanogaster, Rotenone, Oxidative stress, Mitochondrial dysfunctions, Parkinson’s disease
Introduction
Withania somnifera (WS), commonly known as Ashwagandha belonging to the family Solanaceae, is known for its varied therapeutic uses in Ayurvedic and Unani practices for the past 5,000 years in India (Kulkarni and Dhir 2008; Gokul et al. 2012). WS has been held in high esteem in Ayurveda because of its rejuvenative and tonic effects that are reminiscent of Asian ginseng (Chulet and Pradhan 2009). WS has been widely employed to treat variety of diseases owing to its anti-inflammatory, antitumor, antioxidant and immunomodulatory properties (Patwardhan and Gautam 2005). Different parts of the plant have been in use for centuries for the remedy of several human ailments and constantly its new biological properties are being discovered (Gupta and Rana 2007; Kulkarni and Dhir 2008; Bhatnagar et al. 2009; Alam et al. 2012). The pharmacological effect of the roots of WS is attributed to its active ingredients, withanolides which has a wide range of therapeutic applications.
WS root extracts and withanolides have been shown to stimulate growth of new dendrites in human neuroblastoma cells (Tohda et al. 2000; Zhao et al. 2002). WS root extract and withanoside VI were shown to possess inhibitory action on acetylcholinesterase activity in both in vivo and in vitro (Choudhary et al. 2005). Further, evidences suggest the protective effect of WS root extract and its constituents on pre-synaptic and post-synaptic neurons in animal models of dementia and spinal cord injury (Kuboyama et al. 2005). A poly-herbal preparation, BR-16 (Mentat®), which includes WS as one of the major component exhibited significant protective effect against reserpine-induced catalepsy in mice (Kumar and Kulkarni 2006). Few studies have demonstrated the protective efficacy of WS root extracts, against oxidative stress and degeneration of hippocampal cells in vivo under stress conditions (Parihar and Hemnani 2003; Sankar et al. 2007; Ahmad et al. 2005; Kumar and Kumar 2009). However, not many studies have demonstrated the neuroprotective efficacy of WS in Parkinson’s disease (PD) models (Manjunath and Muralidhara 2013).
Rotenone (ROT), a naturally occurring common pesticide which specifically inhibits mitochondrial complex-I activity, is capable of inducing various mitochondrial dysfunctions that phencopies PD in various invertebrate (eg. Drosophila, Caenorhabditis. elegans) and rodent models (Coulom and Birman 2004; Cannon and Greenamyre 2010). Due to its lipophilic nature, it is shown to cross the blood brain barrier and accumulate in mitochondria and elicit oxidative stress response. ROT is demonstrated to cause mitochondrial dysfunctions, oxidative stress and resulting in neurodegenerative disorders (Swarnkar et al. 2010; Santiago et al. 2010).
In the last decade, numerous studies have emphasized the advantages of employing Drosophila as an in vivo model for several neurodegenerative diseases including PD (Feany and Bender 2000; Hirth 2010). Exposure of flies to sub-lethal concentrations of ROT in the medium over 7 days has been demonstrated to result in a concentration–related locomotor dysfunction, specific dopaminergic neuronal loss and depletion in dopamine levels in adult flies (Coulom and Birman 2004). Subsequently this system has been widely employed to screen and assess a large number of therapeutic drugs and plant extracts (Chaudhuri et al. 2007; Sudati et al. 2013). We have also recapitulated these characteristic features of PD in the wild Drosophila melanogaster strains in our laboratory (Hosamani and Muralidhara 2009; Hosamani et al. 2010).
Previously, we have successfully employed Drosophila as a model to understand the neuromodulatory properties of medicinal plants (Hosamani and Muralidhara 2010), spice bioactives (Prasad and Muralidhara 2012) and the Pteridophyte, Selaginella (Girish and Muralidhara 2012). Although several studies describe the various beneficial effects of WS such as the anticancer, anti-venom, anti-stress and extension of longevity, not many attempts have been made to understand its neuromodulatory potential against chemical neurotoxin models such as ROT. In this regard, we have earlier shown the neuroprotective effect of standardized WS root powder against ROT in a mice model. In the present communication, we describe the efficacy of WS in a ROT model of neurotoxicity in Drosophila system. The study comprised of initial assessment of the potency of WS- enriched diet to modulate the endogenous levels of oxidative markers in flies and subsequently its efficacy to abrogate ROT-induced mortality response, locomotor phenotype, oxidative impairments, mitochondrial dysfunctions and neurotoxicity in a short- term co-exposure paradigm.
Materials and methods
Chemicals
Rotenone (EC No. 201-501-9; Lot: 034 K1573), 2,7-dichlorofluorescein (DCF), 2,7-dichlorofluorescein diacetate (DCF-DA), Thiobarbituric acid (TBA), Hydrogen peroxide, 1,1,3,3- tetramethoxypropane (TMP), Nicotinamide adenine di-nucleotide phosphate (reduced), CDNB, Reduced glutathione, Iodonitrotetrzolium chloride, Acetylthiocholine iodide, butyrylthiocholine iodide and Bovine serum albumin were procured from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade and were purchased from M/s Sisco Research Laboratories, (Mumbai, India).
Standardized Withania somnifera extract
Withania somnifera (WS) root extract standard powder, WSP (Withanolides, 2.57 %; Withaferin A, 2.38 %) procured from M/s Sami Labs Ltd., Bengaluru, India was used. The major active constituents of WS are withanolides (i.e., steroidal alkaloids and steroidal lactones). The withanolides are the class of compounds occurring naturally with C28-steroidal lactones built on modified ergostane framework in which C-22 and C-26 are approximately lose their configuration by oxidizing to form a six-membered lactone ring. Various other alkaloid constituents and chemical constituents such as glycosides, starch, and variety of amino acids including aspartic acid, proline, tyrosine, alanine, glycine, glutamic acid, cysteine and tryptophan are also present in trace quantities.
Drosophila culture and husbandry
Synchronized adult Drosophila melanogaster flies (8–10 d) were obtained from the stock culture facility at CFTRI, Mysore, Karnataka. Flies were maintained on a standard wheat flour-agar media containing yeast granules at temperature (22 ± 1 °C) and relative humidity (70–80 %) as described earlier (Hosamani and Muralidhara 2009). Male adult flies were separated by a standardized protocol which comprised of exposing a given number of adult flies to mild diethyl ether in a small airtight glass container for less than 1 min and then separated.
Typical exposure procedure
For mortality experiments, a minimum number of 50 adult male flies per replicate (3 replicates) were exposed to either WS or ROT or a combination of WS + ROT in the medium for 7 days. Mortality was monitored on a daily basis and the data expressed as percent mortality
Rotenone exposure
Initially, flies were exposed to varying concentrations of Rotenone (ROT) for 7 days in order to assess the lethality response and locomotor phenotype as described previously (Girish and Muralidhara 2012).
Treatment of flies with Withania somnifera (WS) and Rotenone
Initially, with an objective to determine the effect of WS on endogenous levels of oxidative markers, adult male flies were exposed to three concentration of WS standardized powder (0.005, 0.01, 0.05, %). Further, employing a co-exposure paradigm, the efficacy of WS to modulate ROT-induced lethality, locomotor phenotype, oxidative stress and neurotoxicity was determined in separate experiments. We used only one concentration (IC50: 500 μM, 7 d) of ROT for these studies. The selection criteria was based on our previous findings (Hosamani and Muralidhara 2009; Hosamani et al. 2010). Several workers have also employed similar concentrations of ROT in their studies in Drosophila system (Saini et al. 2010; Sudati et al. 2013). Flies were regularly monitored for lethality, and locomotor phenotype (negative geotaxis assay).
Negative geotaxis assay in flies
A known number of mildly anesthetized flies were placed in a vertical glass column (standard length, 25 cm; diameter, 1.5 cm). After a brief improvement in the movement of the flies, were gently tapped to the bottom of the glass column. Following 30 s interval, flies were allowed to reach top of the column, number of flies which reached the top and those which remained at the bottom of the glass column were separately counted. Data was expressed as percent flies escaped beyond minimum distance of 10 cm in 30 s of interval. Fifty adult flies per replication were used for each assay and the assay was repeated for three times and the score for each replication was an average of three such trials for each group of flies including control.
Preparation of cytosol and mitochondria in flies
Control and treatment group flies were separately transferred in to a clean dried and air tighten glass container following mild anesthetization using diethyl ether approximately for 1 min. All anesthetized flies were collected in homogenization vial according their respective groups. Cytosol of whole body was prepared by using sodium-phosphate buffer (0.1 M, pH 7.4). After homogenization, samples were subjected to centrifugation at 2,500 × g for 10 min at 4 °C, supernatant filtered via nylon mesh (pore size, 10 μm) and used as cytosol for biochemical investigation, stored at −20 °C until future use.
For mitochondrial preparation, homogenates of flies (4 %) were prepared in Tris–sucrose ice-cold buffer (Tris buffer, 2 mM and Sucrose, 250 mM) of 0.25 M, pH 7.4 using a Teflon glass grinder. The filtrate was centrifuged (2,500 × g for 10 min), pellets were discarded and obtained supernatant was further subjected to centrifugation at 7,800 × g for 10 min to collect the nuclear pellets. The post-nuclear supernatant was again subjected to centrifugation at 10,000 × g for 10 min to obtain mitochondrial pellets (Trounce et al. 1996). The pellets were washed with mannitol-sucrose-HEPES buffer (Mannitol, 200 mM; Sucrose, 70 mM; EDTA, 0.1 mM and HEPES, 10 mM) and resuspended in the buffer, stored at −20 °C until future use.
Biochemical determinations
Determination of lipid peroxidation (LPO)
Induction of oxidative impairments was ascertained by measuring the extent of LPO and was quantified by measuring the formation of thiobarbituric acid reactive substances (TBARS) following the method described earlier (Ohakawa et al. 1979).
Measurement of reactive oxygen species (ROS) generation
ROS generation was assayed using dihydrodichlorofluorescein diacetate (H2 DCFH-DA), a non-polar compound that, after conversion to a polar derivative by intracellular esterases, can rapidly react with ROS to form the highly fluorescent compound dichlorofluorescein (Shinomol and Muralidhara, 2008). ROS formation was calculated from a DCF- standard curve and the data was expressed as pmol DCF formed/min/mg protein.
Measurement of hydroperoxide (HP) levels
The hydroperoxide levels were determined in cytosolic fractions following the method described previously (Wolf 1994) and the data was expressed as μmoles hydroperoxides/mg protein.
Determination of reduced glutathione
The estimation of reduced glutathione (GSH) was based on a fluorimetric method by employing o-phthalaldehyde (OPT). Aliquots of whole body homogenates were added to 0.1 M formic acid and spun at 5,200 × g for 10 min to precipitate protein, subsequently the supernatant was allowed to react with OPT (1 mg/ml in methanol) at room temperature for 30c and fluorescence was measured at excitation of 345 nm and emission at 425 nm (Mokrasch and Teschke 1984).
Activities of antioxidant enzymes
The activity of SOD was determined by monitoring the inhibition of quercetin auto oxidation. Briefly, to 1 mL reaction mixture containing 2–5 μg protein, 0.016 M sodium phosphate buffer (pH 7.8); N,N,N,N tetramethyl ethylenediamine (TEMED), 8 mM and ethylene diamine tetra acetic acid (EDTA, 0.08 mM) and the reaction was initiated by adding 0.15 % quercetin (dissolved in dimethyl formamide DMF). The kinetic reaction was monitored for 3′ at 406 nm and expressed in terms of amount of protein required to inhibit 50 % of quercetin auto oxidation (Kostyuk and Potapovich 1989). The activity of catalase was measured according to a standard method described previously (Aebi 1984). In brief, 1 mL of reaction mixture contained 8.8 mM H2O2 (3 %), 0.1 M sodium phosphate buffer, pH 7.0 and the enzymatic reaction was initiated by adding an aliquot (equivalent to 10 μg protein) of sample. The H2O2 decomposition was monitored for 3 min at 240 nm and expressed in terms of μmol of H2O2 decomposed/min/mg protein (MEC-H2O2 44.1 mM−1 cm−1). The activity of GST enzyme was measured by following an increase in absorbance at 340 nm. The reaction mixture consisted of 50 μL of homogenate, sodium phosphate buffer (pH 6.5, 0.1 M), 1 mM EDTA, 20 mM reduced glutathione and 20 mM CDNB. Rate of formation of conjugates between reduced glutathione (GSH) and 1-chloro-2, 4-dinitrobenzene (CDNB) (Guthenberg et al. 1985) was monitored. The non-protein thiols were determined using dithio-bis-nitrobenzoic acid and expressed in terms of μmol thiols/min/mg protein (Ellman 1959).
Activity of acetylcholinesterase (AChE)
Acetylcholine esterase activity levels were determined according to a previously described method (Ellman et al. 1961) by taking 1 mL reaction mixture containing phosphate buffer (0.1 M, pH 8.0), 5,5-dithiobis-2-nitrobenzoic acid (DTNB, 10 mM), an aliquot of sample and acetylthiocholine iodide (78 mM) and the change in absorbance was monitored for 3 min at 412 nm. The enzyme activities were expressed in terms of nmoles of substrate hydrolyzed/min/mg protein.
Mitochondrial assays: activities of complex I–III (NADH-Cytochrome C Reductase) and complex II–III (Succinate-Cytochrome C Reductase)
Mitochondria (50 μg) fractions were added to phosphate buffer (0.1 M, pH 7.4) containing NADH (0.2 mM) and KCN (1 mM). The reaction was initiated by the addition of cytochrome C (0.1 mM) to the reaction mixture and the decrease in absorbance was monitored for 3 min at 550 nm. The activity was expressed in terms of μmol cytochrome C reduced/min/mg protein (molar extinction coefficient (MEC) −19.6 mM/cm) (Navarro et al. 2004). Likewise the activity of Complex II–III was also determined in aliquots mitochondrial homogenate which were added to phosphate buffer (0.1 M, pH 7.4, 2 mM EDTA) containing KCN (1 mM) and succinate (20 mM). The reaction was initiated by the addition of cytochrome C (0.1 mM) to the reaction mixture and change in absorbance was recorded for 3 min at 550 nm. The activity was expressed in terms of μmol cytochrome C reduced/min/mg protein (molar extinction coefficient (MEC) −19.6 mM/cm) (Navarro et al. 2002).
Analysis of dopamine concentrations by HPLC
Known number of adult male flies were taken and homogenized in 500 μL of 0.1 M phosphate buffer (ice cold, pH, 7.4) containing 1 mM EDTA. Followed by centrifugation at 2,500 × g for 10 min, the supernatant of the homogenization was filtered via tissue sieve and known volume will be injected directly into HPLC column (Discovery C-18, 25 cm × 4.6 mm, 5 μm, Supelco Sigma-Aldrich) equipped with ultraviolet detector set at 280 nm. The flow rate of a mobile phase consisting of 0.2 % aqueous trifluoroacetic acid and methanol (70:30 v/v) was maintained flow rate of 1 ml/min (Dalpiaz et al. 2007).
Determination of protein
Concentrations of protein in the whole body homogenates were estimated as described previously (Lowry et al. 1951) using bovine serum albumin as the standard.
Statistical analysis
Data are represented as the group means ± standard error (SE) for each experimental group. The data was analyzed using one-way analysis of variance (ANOVA) followed by post hoc ‘Tukey’ test for comparison of means to determine the significance of differences among the groups. P-value ≤ 0.05 were considered as statistically significance. All statistical analysis was performed using SPSS statistical package version 17.0.
Results
Per se effects of WS enrichment
Effect of WS on endogenous levels of oxidative markers, glutathione (GSH) and antioxidant enzymes
Whole body homogenates of adult male Drosophila flies fed with WS -enriched diet for 5 days exhibited significant decrease in the endogenous levels of oxidative stress markers viz., reactive oxygen species (ROS, 19 %), lipid peroxidation (LPO, 24 %) and hydroperoxides (HP, 24 %). The effect was more pronounced only at the higher concentration (Table 1).
Table 1.
Effect of Withania somnifera (WS) enriched diet on the endogenous oxidative markers, activities of antioxidant enzyme and cholinergic enzymes in whole body homogenates of the adult male Drosophila flies
| Withania somnifera | ||||
|---|---|---|---|---|
| 0 | 0.005 % | 0.01 % | 0.05 % | |
| Oxidative markers | ||||
| ROSa | 22.6 ± 1.200 | 19.5 ± 0.400 | 19.4 ± 0.800 | 18.2 ± 1.600* |
| LPOb | 15.6 ± 1.010 | 13.4 ± 0.810 | 13.4 ± 0.120 | 11.9 ± 0.190* |
| HPc | 0.68 ± 0.004 | 0.62 ± 0.006 | 0.61 ± 0.005 | 0.57 ± 0.004* |
| Antioxidant enzymes | ||||
| CATd | 0.12 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.04 | 0.15 ± 0.02 |
| SODe | 239.0 ± 3.7 | 257.3 ± 5.4 | 269.0 ± 3.2 | 296.2 ± 6.9* |
| GSTf | 0.079 ± 0.004 | 0.085 ± 0.005 | 0.090 ± 0.002 | 0.093 ± 0.003 |
| Cholinergic assays | ||||
| AChEg | 0.067 ± 0.002 | 0.063 ± 0.003 | 0.064 ± 0.002 | 0.055 ± 0.002* |
| BChEh | 0.029 ± 0.002 | 0.028 ± 0.002 | 0.026 ± 0.001 | 0.022 ± 0.001 |
Values are mean ± SE (in triplicates): Data was analysed by one way ANOVA followed by post hoc Dunnette test (*p < 0.05). Significances were determined by making comparisons between CTR vs. WSP (n = 50 flies per replicate, three such replication used for assay)
aReactive oxygen species – pmol DCF/min/mg protein
bMalondialdehyde – nmol malondialdehyde/mg protein
cHydroperoxides – nmol hydroperoxides/mg protein
dCatalase – nmol Hydroperoxides/min/mg protein
eSuperoxide dismutase – Units/mg protein
fGlutathione-s-transferase – nmol conjugate formed/min/mg protein
gAcetylcholinesterase – nmol substrate hydrolysed/min/mg protein
hButrylcholinesterase – nmol substrate hydrolysed/min/mg protein
The levels of GSH (25 %), NPT (23 %) and GST (18 %) were markedly enhanced in the whole body homogenates of flies which were exposed to WS enriched media. In addition, WS also significantly elevated the activity levels of antioxidant enzymes such as SOD (24 %), CAT (25 %) and GST (18 %) (Table 1). Flies administered with WS enriched diet, exhibited diminished activities of acetylcholinesterase (19 %) and butrylcholinesterase (24 %) enzymes suggesting the effect of WS on cholinergic function.
Rotenone induced lethality and locomotor phenotype
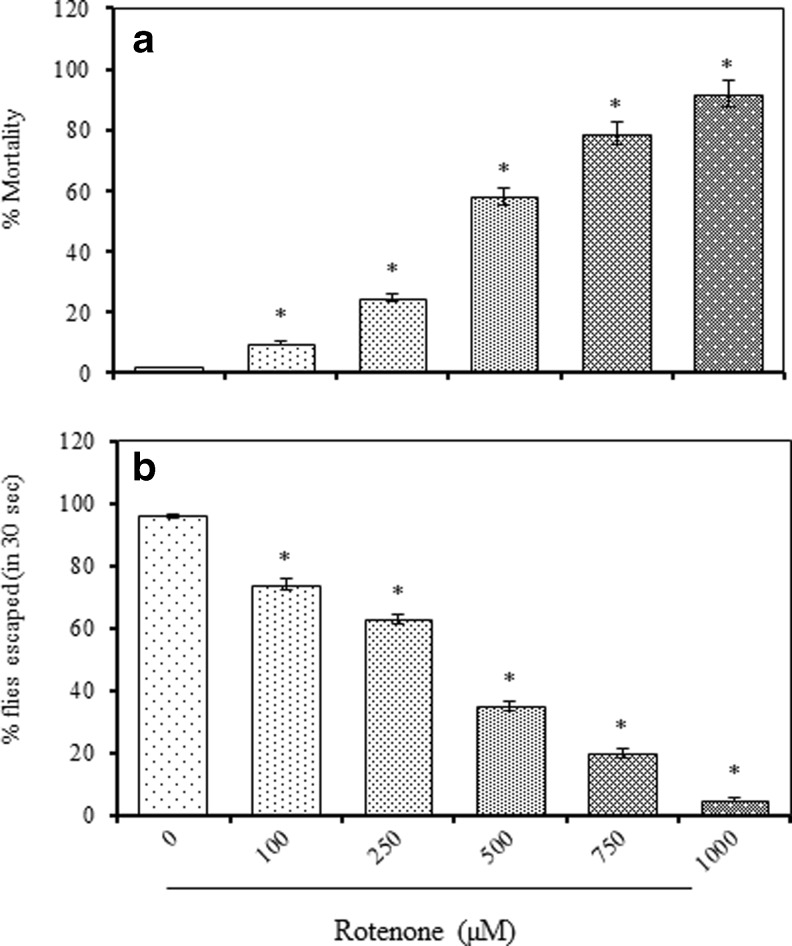
Exposure of adult male flies to ROT resulted in a concentration-dependent lethality and locomotor phenotype during a 7 day experimental period. The mortality occurred between 4 and 7 days and terminally a concentration of 500 μM resulted in nearly 50 % mortality, while a concentration of 1,000 μM caused 92 % mortality (Fig. 1a).
Fig. 1.
Concentration and time course lethality response of adult male Drosophila melanogaster following g exposure to rotenone (ROT, 0-1,000 μM, 7 days) expressed as percent mortality (a) and the incidence of locomotor deficits expressed as percent flies escaped in 30 s (as determined by negative geotaxis assay) (b); Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by one way ANOVA followed by post hoc ‘Dunnett’ test (*p < 0.05)
Locomotor phenotype as assessed by negative geotaxis assay among flies exposed to ROT enriched diet for 7 days. A large numbers of flies showed a tendency to stay at the bottom of vertical graduated glass column at the higher concentrations of ROT. Among the control flies (untreated), more than 96 % flies were able to reach the top of the glass column within 30 s. ROT exposed flies were exhibited concentration depended decrease (0: 96 %, 100 μM: 74 %, 250 μM: 52 %, 500 μM: 35 %), clearly indicating the ability ROT to induce locomotor deficits (Fig. 1b).
Modulatory effect of WS against rotenone (ROT) induced neurotoxicity
Effect on ROT induced lethality and locomotor deficits
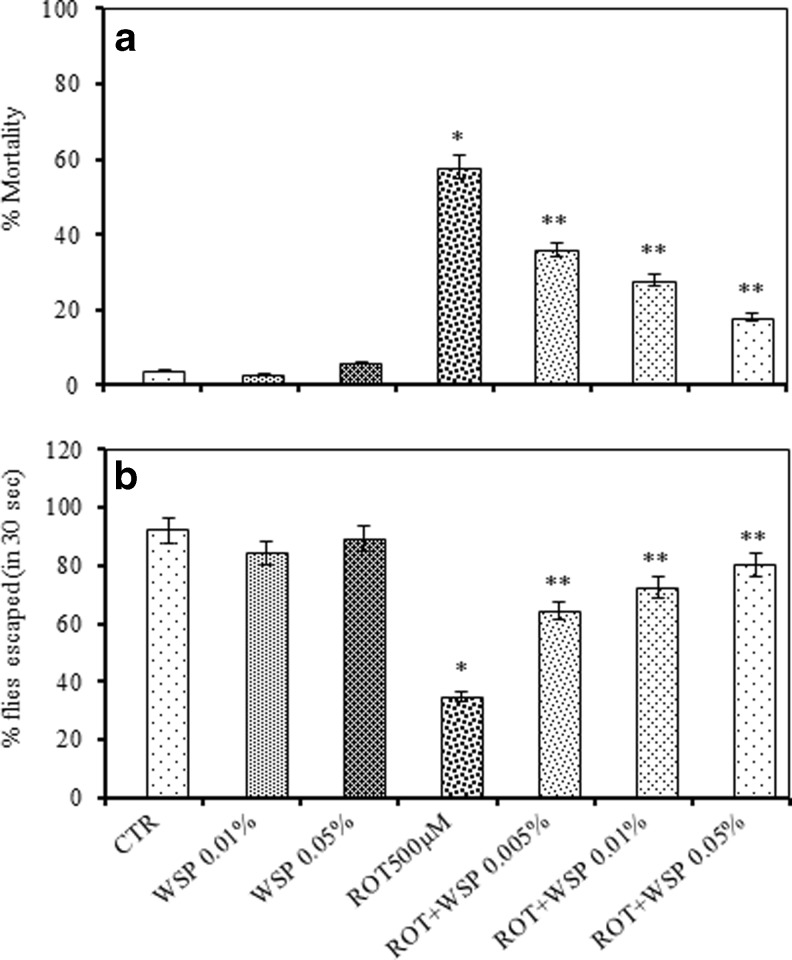
For the modulation study, only one concentration (LC50) of ROT was chosen. Exposure of ROT (500 μM) resulted in significant induction of mortality (58 %) among flies, while co-exposure with WS (0.05 %) significantly reduced the incidence of mortality. The level of protection offered by WS against ROT induced mortality was concentration related significant (Fig. 2a). Further, we determined locomotor deficits among the flies co-treated with WS and ROT employing a similar paradigm. ROT (500 μM) exposed flies exhibited severe motor dysfunctions as evidenced by the large number of flies retained at the bottom of the glass column due to inability to co-ordinate with body movements (Fig. 2b). In contrast, flies provided with WS enriched diet, showed significant improvement in the locomotor performance
Fig. 2.
Modulatory effect of standardized extract of Withania somnifera (WS, 0.01 and 0.05 %, 7 days) on ROT (500 μM, 7 days) -induced mortality expressed as percent mortality (a) and negative geotaxis (b) among adult male Drosophila melanogaster; This was assessed in a co-exposure paradigm in which flies were exposed to ROT in a WS-enriched medium for 7 days; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by one way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Efficacy of WS in modulating ROT induced oxidative impairments
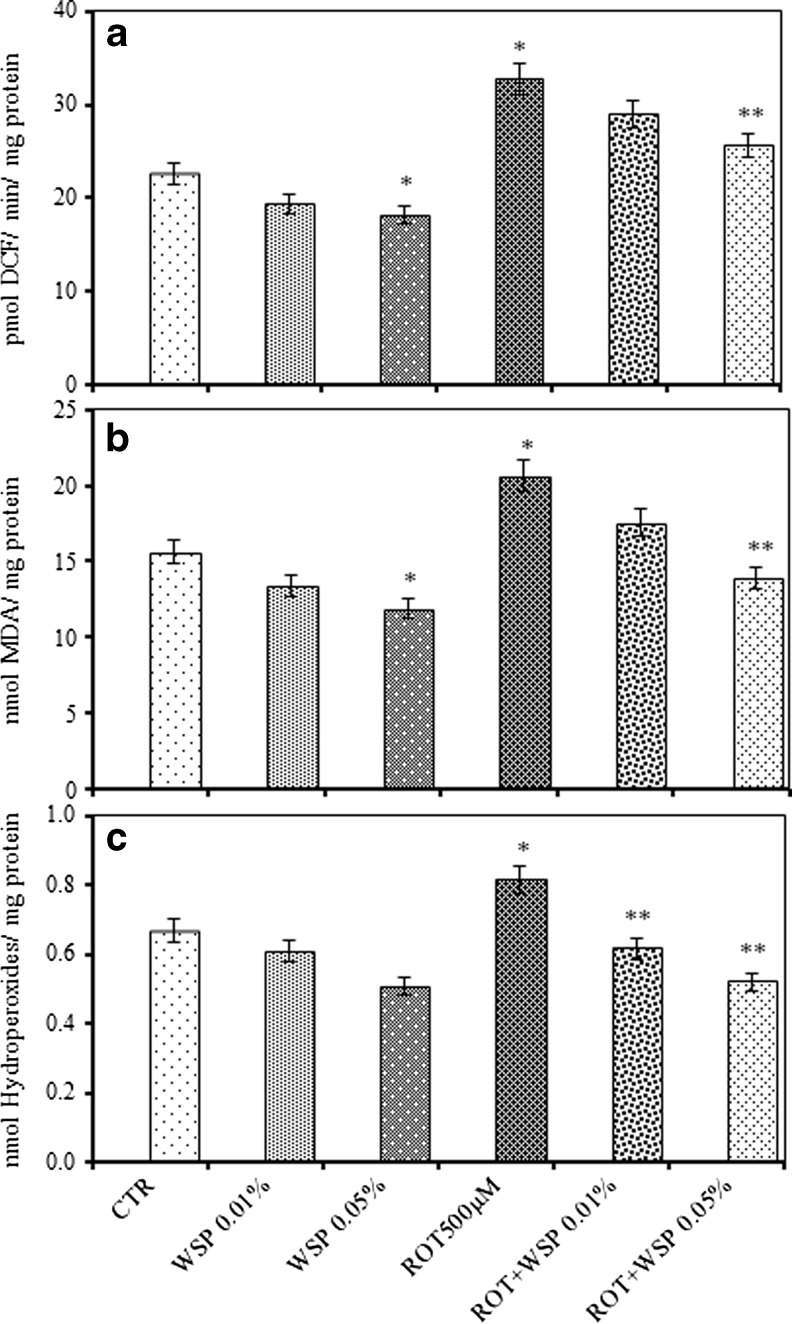
Exposure of flies to ROT induced a significant increase in the levels of ROS (45 %), LPO (32 %) and HP (22 %) when compaired to control flies. Interestingly, WS co-treatment significantly abrogated the ROT induced oxidative impairments as evidenced by decrease in the ROS (21 %), LPO (33 %) and HP (36 %) levels among the co-treated flies (Fig. 3a–c).
Fig. 3.
Modulatory effect of Withania somnifera (WS, 0.01 and 0.05 %, 5 days) on ROT (500 μM, 5 days) induced impairments in endogenous oxidative markers: reactive oxygen species (a); lipid peroxidation (b); and hydroperoxides (c) in whole body homogenates of adult male Drosophila melanogaster; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by one way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Modulatory effect of WS on GSH, non-protein thiols and enzymic antioxidant defenses
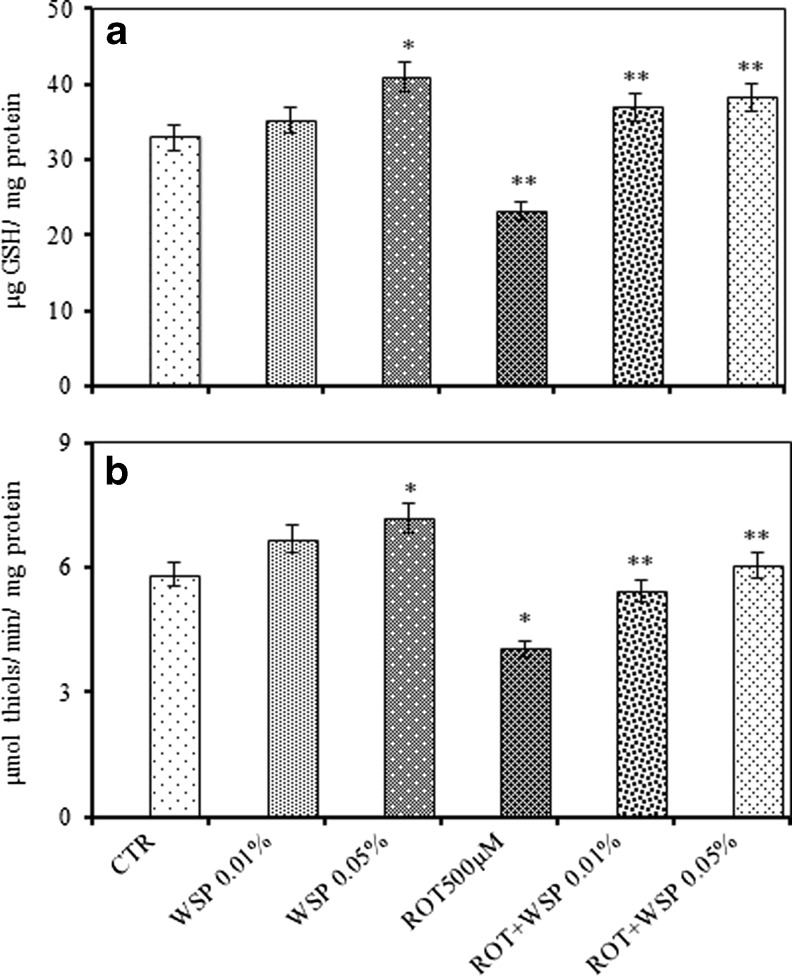
WS per se treatment for 7 days enhanced the levels of GSH (25 %), non-protein thiols (23 %); and the activity levels of SOD (24 %), CAT (25 %) and GST (18 %) in the whole body homogenates of flies. Although, ROT exposure with similar paradigm caused significant depletion in the levels of GSH (29 %) and non-protein thiols (NPT, 31 %), on co-exposure with the WS, the levels were completely restored (Fig. 4a and b). Further, ROT exposure significantly enhanced the activity levels of antioxidant enzymes (SOD: 28 %; CAT: 19 % and GST: 47 %). However, WS enrichment markedly diminished the ROT induced perturbations with respect to the activities of GST and AChE (Table 2).
Fig. 4.
Modulatory effect of Withania somnifera powder (WS, 0.01 and 0.05 %, 5 days) on ROT (500 μM, 5 days) induced alteration in reduced glutathione (GSH) (a) and non-protein thiols (NPT) (b) in whole body homogenates of adult male Drosophila melanogaster; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by One way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Table 2.
The modulatory effect of Withania somnifera (WS, 0.05 %) on rotenone (500 μM) induced alteration in the activity levels of antioxidant and cholinergic functions in the whole body homogenates of adult male Drosophila melanogaster
| Groups | Enzyme activity | Cholinergic functions | |||
|---|---|---|---|---|---|
| Catalasea | SODb | GSTc | AChEd | BChEe | |
| CTR | 0.118 ± 0.02 | 239.0 ± 3.7 | 0.079 ± 0.004 | 0.067 ± 0.002 | 0.029 ± 0.002 |
| WS (0.05 %) | 0.147 ± 0.02 | 296.2 ± 6.9 | 0.093 ± 0.003 | 0.055 ± 0.002 | 0.022 ± 0.001 |
| ROT (500 μM) | 0.140 ± 0.03 | 285.1 ± 4.2 | 0.116 ± 0.007 | 0.089 ± 0.003 | 0.033 ± 0.002 |
| WS + ROT | 0.155 ± 0.02* | 312.3 ± 5.16* | 0.068 ± 0.001* | 0.070 ± 0.003* | 0.025 ± 0.002 |
Values are mean ± SE (in triplicates); Data was analysed by one way ANOVA followed by post hoc Tukey test (*p < 0.05). Significances were determined by making comparisons between CTR vs. WSP and ROT vs ROT + WSP (n = 50 flies per replicate, three such replication used for assay)
aCatalase – nmol Hydroperoxides/min/mg protein
bSuperoxide dismutase – Units/mg protein
cGlutathione-s-transferase – nmol conjugate formed/min/mg protein
dAcetylcholinesterase – nmol substrate hydrolysed/min/mg protein
eButrylcholinesterase – nmol substrate hydrolysed/min/mg protein
Modulatory effect of WS on mitochondrial functions
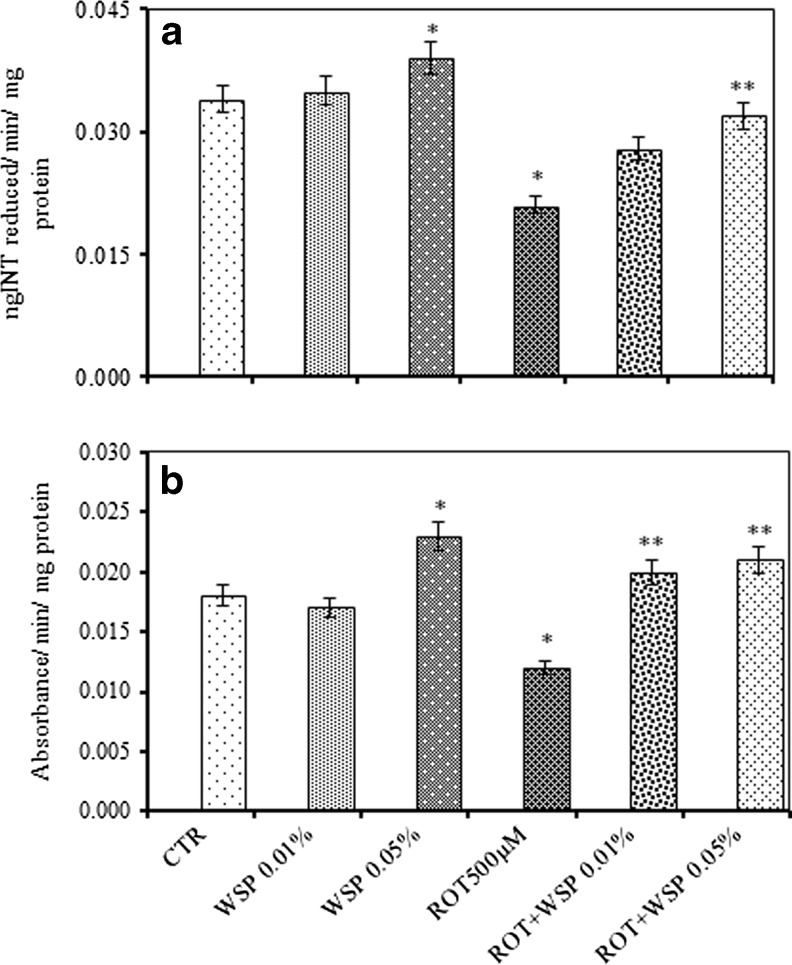
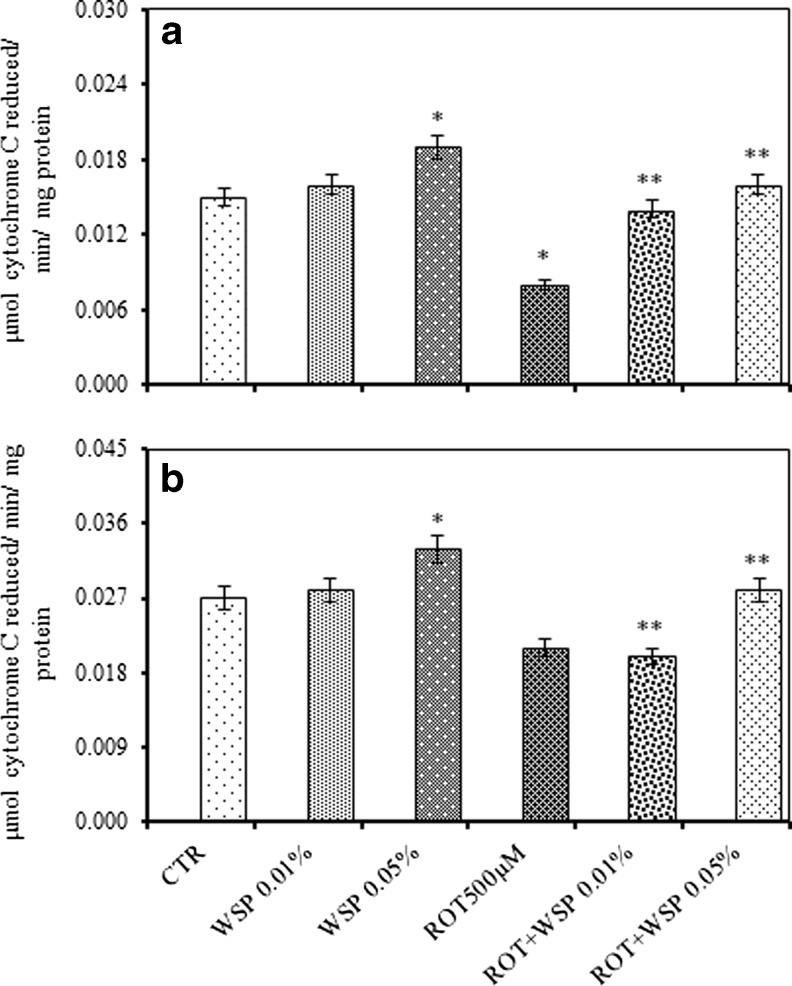
ROT exposure caused significant decrease in the activity levels of SDH (38 %) and MTT (33 %). However, co-exposure with WS resulted in significant restoration of the activity levels (Fig. 5a and b). Further, ROT exposure also caused significant inhibition in the activity levels of complex I–III (47 %) and complex II–III (22 %). However, co-exposure with WS attenuated ROT induced alterations and offered significant protection (Fig. 6a and b).
Fig. 5.
Modulatory effect of Withania somnifera powder (WS, 0.01 and 0.05 %, 5 days) on ROT (500 μM, 5 days) altered impairments in the activity level of succinic dehydrogenase (SDH) (a) and MTT (b) assay in whole body homogenates of adult male Drosophila melanogaster; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by One way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Fig. 6.
Modulatory effect of Withania somnifera powder (WS, 0.01 and 0.05 %, 5 days) on ROT (500 μM, 5 days) induced alteration in the activity levels of complex-I-III (a), complex-II-III (b) in the whole body homogenates of adult male Drosophila melanogaster; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by One way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Effect of WS on cholinergic function and dopamine levels
Interestingly, WS enriched diet significantly diminished the basal activity levels of acetylcholine esterase (23 %) and butrylcholinesterase (17 %). While ROT exposure caused significant enhancement in the activity levels of AChE (33 %) and BChE (14 %), WS enrichment resulted in marked restoration of the altered levels (Table 2).
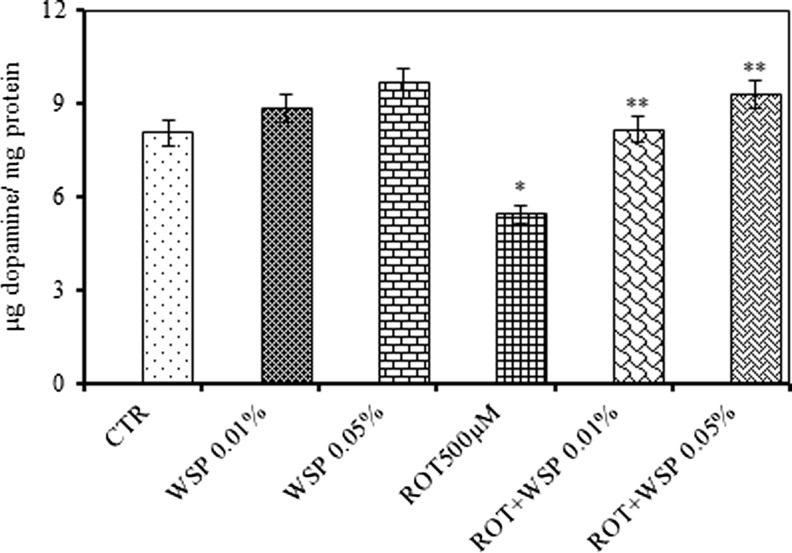
Exposure of WS enriched diet caused marked elevation (20 %) in the dopamine levels. ROT exposure caused marked decrease in the dopamine (32 %) levels and co-exposure with WS caused rendered protection against the ROT induced depletion in the dopamine levels (Fig. 7).
Fig. 7.
Modulatory effect of Withania somnifera powder (WS, 0.01 and 0.05 %, 5 days) on ROT (500 μM, 5 days) induced depletion in the levels of dopamine in the whole body homogenates of adult male Drosophila melanogaster; Values are mean ± SE (n = 50 flies per replicate, three such replication used for assay); Data was analyzed by One way ANOVA followed by post hoc ‘Tukey’ test (*p < 0.05)
Discussion
In recent times, Drosophila system is extensively employed not only to obtain basic mechanistic data on the pathophysiology of several neurodegenerative diseases, but also as a primary platform to screen various putative phytochemicals for their neuromodulatory potency against experimentally-induced neurodegeneration (Laurent et al. 2013; Sudati et al. 2013). Rotenone (ROT), a well-established mitochondrial toxin is often used as a model chemical, since it reproduces some important aspects of PD pathology both in Drosophila and rodent models (Coulom and Birman, 2004; Canon and Greenamyere 2010). In this regard, the use of Drosophila as a PD model is rather well accepted by numerous researchers (Hirth 2010; Rand, 2010). Earlier our findings have shown the advantages and reproducibility of the ROT model and the efficacy of select medicinal plant extracts and spice active principles to attenuate oxidative stress and neurotoxicity in the Drosophila system (Hosamani and Muralidhara, 2009; Girish and Muralidhara, 2012; Prasad and Muralidhara, 2012). Accordingly, we have investigated the propensity of WS supplements to offset rotenone –induced oxidative stress, locomotor phenotype and mitochondrial dysfunctions in wild Drosophila.
In our experimental design, exposure of male flies to ROT reproduced the key aspect of PD viz. induction of a temporal and concentration dependent locomotor phenotype (as evident by significant impaired climbing ability). These data are consistent with findings of several other researchers (Sudati et al. 2013; Laurent et al. 2013) and our findings in the fly model (Girish and Muralidhara, 2012). Interestingly, we demonstrate substantial improvement of ROT-induced early mortality as well as locomotor impairment with WS -enrichment. This WS mediated rescue of flies was associated with diminished oxidative stress, enhanced antioxidant defenses and reduced cholinergic function and elevated dopamine depletion.
Rotenone has been demonstrated to cause dose-dependent ATP depletion, oxidative damage and early mortality (Spivey 2011; Sherer et al. 2003). Several evidences suggests that exposure of flies to chemical neurotoxins such as rotenone, paraquat, MPTP (1-methyl-4 phenyl-1,2,3,6-tetrahydropyridine) can result in PD like manifestations and pathology. While the underlying mechanism by which ROT induces these PD symptoms following chronic exposure to ROT is not clearly known, the involvement of oxidative stress linked with excessive generation of ROS and mitochondrial dysfunctions (eg. inhibition of Complex I) is well appreciated and supported by numerous experimental evidences (Cichetti et al. 2009; Goldman et al. 2012) . More importantly, the ROT-induced Drosophila model of PD has been considered quite useful since it parallels the human response to L-dopa therapy (Coulum and Birman 2004; Cannon and Greenamyre 2010). Hence during the last decade, many workers have accepted the antioxidant hypothesis related to PD and demonstrated the potency of various neuroprotective agents in neurotoxin based models.
In the present study, we found that flies maintained on WS –enriched diet exhibited enhanced survivorship against ROT in the co-exposure paradigm clearly suggesting the ability of WS to promote survival pathways. While flies maintained on ROT alone displayed a robust phenotype in terms of locomotor deficits, WS –enrichment resulted in significant improvement in the locomotor performance clearly suggesting its specific ability to abrogate oxidative stress mediated effects. Previous studies have shown that Withania somnifera contains caffeic acid, chlorogenic acid, ellagic acid, ferulic acid, gallic acid, catechin, tannic acid, kaempferol, quercetin and rutin which are powerful antioxidants (Gokul et al., 2012; Bhatnagar et al., 2009). Owing to the presence of cock-tail of antioxidants, we speculate that the neuroprotective effect of WS in this model may be attributed to its ability to abrogate oxidative stress. These findings are consistent with previous reports on the antioxidant and neuroprotective effect of WS in mammalian models (Kulkarni and Dhir, 2008; Bhatnagar et al., 2009).
Significant diminution in the levels of endogenous oxidative markers (such as ROS, HP and malondialdehyde) evident among flies maintained on WS –enriched diet indicated its potential to regulate the basal levels. Further, enhanced GSH levels and the upregulated activity levels of antioxidant enzymes also may be significantly contributing towards the observed protective effect. Interestingly in the present study, co-exposure of ROT with WS resulted in significant reduction in the levels of ROS generation, MDA and HP levels clearly suggesting the antioxidative effect of WS. GSH, a ubiquitous thiol tripeptide, provides protection from oxidative stress –induced damage through the reduction of ROS (Zeevalk et al., 2008; Smeyne and Smeyne, 2013). GSH is known to either act alone or in concert with other enzymes to scavenge free radicals such as superoxide, hydroxyl radicals and peroxynitirtes (Dringen 2000). In the co-exposure paradigm, WS also caused complete restoration of GSH, non-protein thiols along with higher activity levels of antioxidant enzymes further strengthening the notion that the protective effects of WS may at-least in part be mediated through abrogation of ROT induced oxidative stress in the fly model. This observation in the fly model corroborates our recent findings on the efficacy of WS to modulate ROT induced neurotoxicity in the mice model (Manjunatha and Muralidhara 2013). It is pertinent to note that depletion of cytosolic GSH in the substantia nigra dopaminergic neurons is one of the vital events which occur in human PD subjects. Hence replenishment of GSH pool has been an alternative option to alleviate the problem under such conditions. In view of this, it is speculated that GSH-mediated enhancement may play a major role in the observed neuroprotective effect of WS. Further studies in cell models may provide better explanation in this regard.
With regard to PD, postmortem studies have revealed a loss of mitochondrial function, especially the activity levels of Complex I with concomitant depletion in the GSH levels (Heales et al. 2011). It is well established that generation of free radicals is one of the vital mechanism by which ROT exposure results in impaired activity of ETC enzymes. In the present model, we observed significantly diminished activity levels of SDH and MTT reduction among ROT exposed flies. These perturbations in SDH and MTT were restored significantly among flies provided WS-enriched diet. Likewise, the protective effect of WS was also evident in terms of restoration of complex I–III and complex II–III enzymes suggesting the specific effect of WS in modulating the mitochondrial enzyme activities. We have previously evidenced similar protective effects of WS in a mice model of ROT. These findings add further credence to the thinking that it is possible to obtain basic insights on the underlying mechanism of action of phytochemicals employing Drosophila system.
In the present model, another important observation made by us is related to the attenuation of cholinergic function by WS per se which corroborates previous findings in which the inhibitory potential of withanolides were demonstrated in vitro (Choudhary et al. 2005) and in vivo (Kumar and Kumar 2009). Interestingly, in the co-exposure paradigm, ROT induced enhancement in the activity levels of AChE and BChE were restored to normal levels by WS. This finding is consistent with our recent findings in the ROT model of neurotoxicity in mice (Manjunatha and Muralidhara 2013). Diminished AChE activity suggests enhanced acetylcholine levels in the synaptic cleft and can significantly enhance cognitive functions. Hence it would be interesting to assess the cognitive enhancing ability of WS in the fly model. It appears highly relevant since WS has been employed in the clinical management of AD and other dementia disorders in humans.
In conclusion, we have provided evidence that WS –enrichment among flies has the ability to diminish the levels of endogenous oxidative markers. Further, WS could markedly mitigate ROT- induced oxidative stress, mitochondrial dysfunction and neurotoxicity in the Drosophila system. Based on marked the attenuation of locomotor phenotype and enhanced survival of flies with WS-enrichment, and also our previous findings in the mice model, we propose that WS may be a useful therapeutic adjuvant under conditions of oxidative stress -mediated neurodegenerative conditions such as PD.
Acknowledgments
We wish to thank the Director, CFTRI for his encouragement in this study. The first author (MJM) thanks the University Grants Commission (UGC), Government of India for the award of a research fellowship under the Rajeev Gandhi National Fellowship (RGNF) scheme.
Conflicts of interest
None
Abbreviations
- WS
Withania somnifera
- ROT
Rotenone
- LPO
Lipid peroxidation
- ROS
Reactive oxygen species
- TBARS
Thiobarbituric acid reactive substances
- MDA
Malondialdehyde
- DCF
2′, 7′-dichlorofluorescein diacetate
References
- Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem Rev. 2012;11:97–112. doi: 10.1007/s11101-011-9221-5. [DOI] [Google Scholar]
- Ahmad M, Saleem S, Ahmad AS, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- Bhatnagar M, Sharma D, Salvi M (2009) Neuroprotective effects of Withania somnifera dunal: a possible mechanism. Neurochem Res 34:1975–1983. doi:10.1007/s11064-009-9987-7 [DOI] [PubMed]
- Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- Choudhary MI, Nawaz SA, Ul-Haq Z, et al. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun. 2005;334:276–287. doi: 10.1016/j.bbrc.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Chulet R, Pradhan P. A review on rasayana. Pharmacogn Rev. 2009;3:229. [Google Scholar]
- Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Coulom H, Birman S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci. 2004;24:10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpiaz A, Filosa R, de Caprariis P, et al. Molecular mechanism involved in the transport of a prodrug dopamine glycosyl conjugate. Int J Pharm. 2007;336:133–139. doi: 10.1016/j.ijpharm.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Girish C, Muralidhara Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: Implications for Parkinson’s disease. Neurotoxicology. 2012;33:444–456. doi: 10.1016/j.neuro.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Gokul K, Manjunath MJ, Muralidhara Exploring the neuroprotective efficacy of Withania somnifera: a medicinal plant with diverse biological effects. RPMP Ethnomedicine and Therapeutic Validation. 2012;32:377–402. [Google Scholar]
- Goldman JG, Stebbins GT, Bernard B, et al. Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov Disord. 2012;27:727–734. doi: 10.1002/mds.24938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GL, Rana AC. Withania somnifera (Ashwagandha): a review. Pharmacogn Rev. 2007;1:129. [Google Scholar]
- Guthenberg C, Alin P, Mannervik B. Glutathione transferase from rat testis. Meth Enzymol. 1985;113:507–510. doi: 10.1016/S0076-6879(85)13067-3. [DOI] [PubMed] [Google Scholar]
- Heales SJR, Menzes A, Davey GP. Depletion of glutathione does not affect electron transport chain complex activity in brain mitochondria: Implications for Parkinson disease and postmortem studies. Free Radic Biol Med. 2011;50:899–902. doi: 10.1016/j.freeradbiomed.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hirth F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol Disord Drug Targets. 2010;9:504–523. doi: 10.2174/187152710791556104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosamani R, Muralidhara Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology. 2009;30:977–985. doi: 10.1016/j.neuro.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Hosamani R, Muralidhara Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster. Indian J Biochem Biophys. 2010;47:75–82. [PubMed] [Google Scholar]
- Hosamani R, Ramesh SR, Muralidhara Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem Res. 2010;35:1402–1412. doi: 10.1007/s11064-010-0198-z. [DOI] [PubMed] [Google Scholar]
- Kostyuk VA, Potapovich AI. Superoxide–driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem Int. 1989;19:1117–1124. [PubMed] [Google Scholar]
- Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144:961–971. doi: 10.1038/sj.bjp.0706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kulkarni SK. Protective effect of BR-16A, a polyherbal preparation against social isolation stress: possible GABAergic mechanism. Phytother Res. 2006;20:538–541. doi: 10.1002/ptr.1873. [DOI] [PubMed] [Google Scholar]
- Kumar P, Kumar A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J Med Food. 2009;12:591–600. doi: 10.1089/jmf.2008.0028. [DOI] [PubMed] [Google Scholar]
- Laurent SR, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manjunath MJ, Muralidhara Effect of Withania somnifera supplementation on rotenone-induced oxidative damage in cerebellum and striatum of the male mice brain. Cent Nerv Syst Agents Med Chem. 2013;13:43–56. doi: 10.2174/1871524911313010007. [DOI] [PubMed] [Google Scholar]
- Mokrasch LC, Teschke EJ. Glutathione content of cultured cells and rodent brain regions: a specific fluorometric assay. Anal Biochem. 1984;140:506–509. doi: 10.1016/0003-2697(84)90201-X. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Navarro A, Sánchez Del Pino MJ, Gómez C, et al. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol. 2002;282:R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J Biosci. 2003;28:121–128. doi: 10.1007/BF02970142. [DOI] [PubMed] [Google Scholar]
- Patwardhan B, Gautam M. Botanical immunodrugs: scope and opportunities. Drug Discov Today. 2005;10:495–502. doi: 10.1016/S1359-6446(04)03357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SN, Muralidhara Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster - its amelioration with spice active enrichment: relevance to neuropathy. Neurotoxicology. 2012;33:1254–1264. doi: 10.1016/j.neuro.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Rand MD. Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol. 2010;32:74–83. doi: 10.1016/j.ntt.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N, Oelhafen S, Hua H, Georgiev O, Schaffner W, Bueler H. Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol Dis. 2010;40:82–92. doi: 10.1016/j.nbd.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Sankar SR, Manivasagam T, Krishnamurti A, Ramanathan M. The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: an analysis of behavioral and biochemical variables. Cell Mol Biol Lett. 2007;12:473–481. doi: 10.2478/s11658-007-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago RM, Barbieiro J, Lima MMS, et al. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1104–1114. doi: 10.1016/j.pnpbp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomol GK, Muralidhara Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology. 2008;29:948–957. doi: 10.1016/j.neuro.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey A. Rotenone and paraquat linked to Parkinson’s disease: human exposure study supports years of animal studies. Environ Health Perspect. 2011;119:A259. doi: 10.1289/ehp.119-a259a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudati JH, Vieira FA, Pavin SS, et al. Valeriana officinalis attenuates the rotenone-induced toxicity in Drosophila melanogaster. Neurotoxicology. 2013;37:118–126. doi: 10.1016/j.neuro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Swarnkar S, Singh S, Mathur R, et al. A study to correlate rotenone induced biochemical changes and cerebral damage in brain areas with neuromuscular co-ordination in rats. Toxicology. 2010;272:17–22. doi: 10.1016/j.tox.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Tohda C, Kuboyama T, Komatsu K. Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK-N-SH cells. Neuroreport. 2000;11:1981–1985. doi: 10.1097/00001756-200006260-00035. [DOI] [PubMed] [Google Scholar]
- Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/S0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods enzymol. 1994;233:182. doi: 10.1016/S0076-6879(94)33021-2. [DOI] [Google Scholar]
- Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Zhao J, Nakamura N, Hattori M, et al. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull. 2002;50:760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]