Abstract
In this study, the effects of fat (0.5 %, 3.2 % and 5.0 %), inulin (0.0 and 1.0 %) and starter culture (0.0 %, 0.5 %, 1.0 % and 1.5 %) on the angiotensin converting enzyme (ACE)-inhibitory activity of probiotic yogurt containing non-viable bacteria were assessed. Proteolytic activities of bacteria were also investigated. Yogurts were prepared either using a sole yogurt commercial culture including Streptococcus thermophilus and Lactobacillus delbrueckii subs. bulgaricus or bifidobacterium animalis BB-12 and Lactobacillus acidophilus La5 in addition to yogurt culture. Relative degrees of proteolysis were found to be considerably higher in yogurt samples than UHT milk as the control. Both regular and probiotic yogurts showed considerable ACE-inhibitory activities. Results showed that degree of proteolysis was not influenced by different fat contents, while was increased by high concentration of starter culture (1.5 % w/w) and reduced by inulin (1 % w/w). ACE-inhibitory activities of yogurt were also negatively affected by the presence of inulin and high levels of fat (5 % w/w). Moreover, yogurt containing probiotic bacteria showed higher inhibitory against ACE in comparison to the yogurt prepared with non-probiotic strains.
Keywords: Probiotic yogurt, Fat, Inulin, Proteolysis, ACE-inhibitory activity, IC50
Introduction
High blood pressure is one of the major independent risk factors for cardio-vascular diseases (CVD) such as arteriosclerosis, stroke and myocardial infraction and also end-stage renal disease. Angiotensin I-converting enzyme (ACE) plays an important role in the regulation of blood pressure (Ramchandran and Shah 2011; Tsai et al. 2008). The activity of ACE leads to the production of angiotensin 2, a vasoconstrictor agent, and break down of bradykinin which is a vasodilating agent. Release of aldosterone is also induced by Angiotensin 2 which subsequently causes an increase in the blood pressure (Laragh 1979; Leclerc et al. 2002). Thus, factors inhibiting the activity of ACE may potentially lower blood pressure.
In recent years, biologically active peptides namely bioactive peptides from food proteins have been recognized to have ACE inhibitory activity and therefore have been the interest of many studies (Seppo et al. 2003; Sipola et al. 2001; Donkor et al. 2007). These peptides are released from parent protein by digestive enzymes, adding microbial and plant enzymes and by proteolytic activities of starter cultures and probiotic bacteria during the fermentation of milk and milk products (Moslehishad et al. 2013; Donkor et al. 2007). Lactic acid bacteria (LAB) produce proteinases which are able to hydrolyze milk proteins. Some of the resulting peptides are used by the bacteria and the most are accumulated in the medium (Leclerc et al. 2002). Bioactive peptides have been identified and characterized in many dairy products such as cheese, yogurt, and fermented sour milks (Seppo et al. 2003; Fitzgerald and Murray 2006; Pihlanto-Leppala 2001). Among these, yogurt and its peptides have been more widely studied for their effects in reducing blood pressure (Ramchandran and Shah 2011). Other than regular yogurt, probiotic yogurt and dairies have been widely studied and their antihypertensive effects have been evaluated in animal models and human volunteers. It has been reported that functionality of yogurt may be enhanced by adding probiotic bacteria (Ramchandran and Shah 2008). Bifidobacterium spp. and Lactobacillus spp. are the main selected strains incorporated into milk and fermented milk products as probiotics (Saarela et al. 2000). These organisms have the ability to colonize the gut and improve the health promoting effects of yogurt. Diastolic blood pressure has been reduced by 30 % and 44 % in spontaneously hypertensive rats (SHR) fed by diet fortified with yogurt and probiotic yogurt, respectively (Ramchandran and Shah 2011). Milk fermented by Lactobacillus helveticus LBK-16H containing bioactive peptides had a blood pressure–lowering effect in hypertensive subjects in normal daily use (Seppo et al. 2003). Compared to conventional yogurt, probiotic yogurt caused a 4.54 % decrease in total cholesterol and a 7.45 % decrease in LDL-C in type 2 diabetic people (Ejtahed et al. 2011).
In many definitions on the probiotic products, there were emphases on the viability of probiotic bacteria in the product (Fuller 1989). However, several side effects such as bacteremia, endocarditis and liver abscess, have been reported in patients consuming probiotic products containing live bacteria (Kunz et al. 2004; Rautio et al. 1999; Mackay et al. 2008). Salminen et al. (1999) defined probiotics as the either microbial cell preparations or products containing non-viable cells or parts of microbial cells.
Many factors such as acidity, pH, hydrogen peroxide, temperature of storage, oxygen content, concentrations of organic acids and milk ingredients have been reported to influence the viability of probiotic bacteria and microbial metabolites during the manufacture and storage of probiotic yoghurt (Dave and Shah 1998; Cruz et al. 2013). Inulin, a prebiotic widely incorporated to probiotic dairies, has shown positive effect on the growth of probiotic bacteria in probiotic milk products (Özer et al. 2005; Akın et al. 2007). Guven et al. (2005) also investigated effect of inulin as fat replacer on titrable acidity in yogurt. However, a little information is found in the literature about the combined effect of fat contents and inulin on the proteolytic and ACE-inhibitory activities of yogurt and fermented milks. Aiming that, in the current study, the effects of different levels of fat, inulin and starter culture on the degree of proteolysis and in-vitro ACE-inhibitory activity of probiotic yogurt containing non-viable bacteria were assessed. For this purpose, yogurt samples with different fat contents were produced by inoculating different levels of starter cultures in the presence of/without inulin and then analyses were carried out.
Materials and methods
All of the chemicals used in this study were of analytical grade and purchased from Merck chemical company (Darmstadt, Germany). Angiotensin converting enzyme (ACE) and peptide substrate (N-hippuryl-histidyl-leucine hydrate) was obtained from the Sigma Aldrich Company Ltd. (United Kingdom). O-phetaldialdehyde (OPA) was also purchased from the Sigma chemical company (St Louis, MO, USA). Mixed strain culture containing L. acidophilus, Bifidobacterium animalis, Streptococcus thermophilus and L. delbrueckii subs. Bulgaricus were obtained from the Christian Hansen (Denmark). All bacterial cultures were stored at freezing conditions prior to be used in the formulation.
Preparation of probiotic yogurt
Raw milks with different fat levels (0.5 %, 3.2 % and 5.0 %) were fortified with 2 % skim milk powder, preheated to 45 °C and equally divided into two portions. Into one portion, inulin was incorporated (at the concentration of 1 % w/w) and all samples were kept at 45 °C for 4 h. After that, the samples were homogenized (using an APV homogenizer, model Gaulin 185Q, Albertslund, Denmark) at 18 Mpa and heated at 85 °C for 30 min. Then, the samples were cooled to 43 °C and inoculated with different levels (0.0, 0.5, 1.0 and 1.5 % w/w) of ABY-1 starter culture (comprised of B. animalis BB-12, L. acidophilus, L. delbrueckii subs. bulgaricus and S. thermophilus) followed by incubation at 43 °C to reach to the final pH of 4.5 after which, yogurt samples were cooled to 25 °C. The starter culture concentration of 0.0 % means that the starter culture contained only L. delbrueckii subsp. bulgaricus and S. thermophilus (without any probiotic strains). Then, in order to inactivate bacterial cells, yogurts were heated (by using tubular heat exchanger) at 85 °C for 5 min and cooled to final temperature of 25 °C. Cooled yogurts were packed into 100 mL sterile plastic cups containers under the aseptic conditions and 200 cc-tetra-pak cartons and stored at refrigerated conditions for 2 months. During this storage time, no change in the metabolites and physico-chemical properties of the samples were observed. Therefore, the results corresponding to the metabolites represent the values determined after 2 months storage.
Determination of degree of proteolysis
The proteolytic activities of microbial strains in the probiotic yogurt were evaluated according to the method described by Donkor et al. (2007) using OPA. 2.5 ml of yogurt was added to 5 ml of 0.75 % TCA in a test tube. The mixture was vigorously vortexed and filtered through a wathman filter paper. Then, 200 μL of filtrate was added to 3 ml of OPA reagent and incubated at room temperature for 2 min. Finally, the absorbance of the mixture was read at 340 nm using a UV-visible spectrophotometer (model CE 2502, 3000 series, CECIL instruments, Cambridge, England). OPA reagent was freshly prepared according to the procedure previously described by Church et al. (1983). Briefly, 2.5 ml sodium dodecyl sulfate (SDS) (20 % w/w) was transferred into a glass flask containing 25 ml 100 mM sodium tetra borat. Then, 40 mg OPA reagent (previously dissolved in 1 ml pure methanol) was added to the flask. Finally, 150 μl of β-mercaptoethanol was added to the flask and the mixture was reached to the final volume of 50 ml with distilled water.
Preparation of ACE-inhibitory extracts
Extracts containing water soluble ACE-inhibitory components were prepared similar to the method previously described by Donkor et al. (2007). 100 ml of yogurt samples were centrifuged at 15000 × g (Sigma 3-30 K) for 10 min. The supernatant was separated and filtered through a 0.45 μm membrane filter. Then, the filtrate was freeze-dried (Alpha freeze dryer, model 1-2 L-D, Christ, Netherland) and stored at −80 °C until used for further analyses. Just prior to analyze, 1 g freeze-dried powder was dissolved in 1 ml Hepes buffer (pH = 8). To prepare the buffer solution, 1.192 g Hepes powder and 1.752 g NaCl were dissolved in 90 ml deionized water and diluted to 100 ml. Total protein contents of the supernatants were also determined by the Bradford method using bovine serum albumin as the standard.
ACE inhibitory activity
The inhibitory activities of the extracts toward ACE were evaluated similar to the methods of Donkor et al. (2005) and Tsai et al. (2008). Briefly, 25 μl substrate (3 mM hippuryl-histidyl-leucine in Hepes buffer), 25 μl ACE solution and 25 μl extract were mixed and incubated at 37 for 30 min in a Thermomixer apparatus (Comfort MTP). Then, 50 μl HCl (1 M) was added to the mixture to stop the reaction. The ACE-inhibitory activity was determined by measuring the contents of hippuryc acid released from the reaction between the enzyme and substrate using high pressure liquid chromatography (HPLC) (Tsai et al. 2008). For this purpose, after filtering through 45 μm filter, 20 μl of incubated solution was manually injected to a HPLC (Shimadzu, Japan) equipped with a LC-10ADVP pump and Bondapak® C18μ analytical column (250 × 46 mm, 10 μm). The chromatograms corresponding to the injected samples were monitored at 228 nm (SPD-10AV detector). The mixture of 10 mM KH2PO4 and methanol (1:1 V/V, pH = 3) was used as the mobile phase. To prepare control solution, distilled water was used instead of sample extract. The extent of inhibition was calculated as follows:
Where, Ac is the absorbance of the control, As is the absorbance of reaction mixture (sample) and Ab is the absorbance of blank (when the stop solution was added before the reaction occurred). The potential of supernatants in reducing the activity of ACE was also evaluated by calculating IC50 of the samples. For this purpose, the ACE-inhibitory activities of different volumes (3, 5, 7 and 25 μl) of supernatant solutions (incubated with enzyme and substrate) were separately determined.
Statistical analysis
All of the experiments and determinations were performed in duplicate. Data were statistically analyzed using the full factorial experiments by Statistical Analysis System (SAS) release 9.1 (SAS Institute, Inc., Cary, NC). The main factors were % fat (at three levels), inulin (at two levels) and % starter cultures (at four levels). The mean values were compared with each other using the Least Significant Difference (LSD) at 95 % confidence level.
Results and discussion
Degree of proteolysis
Proteolysis is one of the most important biochemical processes carried out in fermented milk products during fermentation (Donkor et al. 2007). Table 1 presents the relative degrees of proteolysis obtained from differences between the amounts of free amino groups (determined by measuring the absorbance of filtrates at 340 nm in the presence of OPA reagent) from probiotic yogurts and those in unfermented UHT milk. In comparison to the UHT milk as the control, degrees of proteolysis in all yogurt samples were higher, indicating the proteolytic activities of microorganisms inoculated to the milk.
Table 1.
Degree of proteolysis, Angiotensin 1 converting enzyme (ACE)-inhibitory activities and corresponding IC50 of probiotic yogurt investigated in this study
| Treatments (different levels, % w/w) | ACE-inhibitory activitya | IC50 a | Proteolysis | ||
|---|---|---|---|---|---|
| Fat | Inulin | Starter | |||
| 0.5 | 0.0 | 0.0 | 78.5gh | 1.52de | 0.83bcde |
| 0.5 | 92 b | 0.955gh | 0.63fghijk | ||
| 1.0 | 92 b | 1.125fg | 0.75cdef | ||
| 1.5 | 92.5b | 0.93gh | 0.84abcde | ||
| 1.0 | 0.0 | 82.5efg | 1.155fg | 0.52kl | |
| 0.5 | 82.5efg | 0.995g | 0.62fghijk | ||
| 1.0 | 84.5def | 1.76cd | 0.73defgh | ||
| 1.5 | 85def | 3.455a | 0.75cdefg | ||
| 3.2 | 0.0 | 0.0 | 81.5efgh | 1.09fg | 0.71defghij |
| 0.5 | 100.5a | 0.66hi | 0.61ghijkl | ||
| 1.0 | 79gh | 1.1fg | 0.72defghi | ||
| 1.5 | 85.5cde | 0.95gh | 0.98a | ||
| 1.0 | 0.0 | 72.5j | 1.69cde | 0.89abc | |
| 0.5 | 77.5hi | 2.36b | 0.59ijkl | ||
| 1.0 | 88.5bcd | 1.56de | 0.66fghij | ||
| 1.5 | 90 bc | 1.415ef | 0.71defghij | ||
| 5.0 | 0.0 | 0.0 | 77.5hi | 0.975gh | 0.59hijkl |
| 0.5 | 91b | 0.42i | 0.82bcde | ||
| 1.0 | 92.5b | 1.07g | 0.85abcd | ||
| 1.5 | 91b | 0.94gh | 0.90ab | ||
| 1.0 | 0.0 | 73.5ij | 1.56de | 0.58jkl | |
| 0.5 | 100 a | 0.355i | 0.70efghij | ||
| 1.0 | 80.5fgh | 1.92c | 0.48l | ||
| 1.5 | 86cde | 2.28b | 0.67fghij | ||
aIC50: The sample concentration (mg/ml) required to inhibit 50 % of the ACE activity
abcdefghijIn each column, means with different letters are significantly different (p < 0.05) for a particular treatment
0.0 % starter means that the mix starter culture contained only Lactobacillus delbrueckiisubs. bulgaricus and Streptoccusthermophilus (without probiotic bacteria)
The effects of different parameters (%fat, inulin and %starter culture) on the protolysis were varied (Fig. 1). Results of relative proteolysis in probiotic-free samples (samples with 0 % starter culture) and those containing probiotic bacteria showed that the presence of probiotic bacteria L. acidophilus and B. animalis did not increase the degree of proteolysis, except for the samples inoculated with 1.5 % mix starter culture (Fig. 1a). Our result is in consistent with some previous scientific findings reporting that probiotic bacteria such as bifidobacterium spp. and L. acidophilus have no proteolytic activity and grow slowly in milk (Klaver et al. 1993). Dave and Shah (1998) also in a study on the effect of ingredient supplementation on viability of probiotic bacteria in yogurt concluded that bifidobacteria lacked proteolytic activity. They suggested that it could be grown in dairies by adding casein hydrolysates or by co-culturing bifidobacteria with proteolytic species (Dave and Shah 1998). However, some published literatures have addressed positive effect of probiotic bacteria on the proteolysis. Donkor et al. (2007) reported that as a consequence of growth of probiotic bacteria in yogurt the levels of released peptides increased. Increase in the proteolysis may lead to improve survival of some probiotic microorganism in yogurt (Donkor et al. 2007). On the other hand, although increase in the concentrations of starter culture containing both yogurt commercial bacteria and probiotic bacteria (up to 1 %) did not increase degree of proteolysis, our results showed that degree of proteolysis was significantly higher in the samples inoculated with the highest level of starter culture (1.5 % w/w). This may be due to the increase in the levels of L. delbrueckii subsp. bulgaricus which is a highly proteolytic organism (Cruz et al. 2013).
Fig. 1.
The main effects of starter cultures (a), fat contents (b) and inulin (c) on the relative degree of proteolysis in the probiotic yogurts investigate in this study. 0.0 % starter means that the mix starter culture contained only Lactobacillus delbrueckiisubs. bulgaricus and Streptoccusthermophilus (without probiotic bacteria)
In general, fat contents showed no effects on the proteolytic activities of bacteria (Fig. 1b). In contrast, proteolysis was negatively affected by the presence of inulin in the yogurt (Fig. 1c). Similar results can be found in the study performed by Ramchandran and Shah (2008). No significant difference was reported between degree of proteolysis in yogurt containing 2 % Raftiline HP (inulin) and that in control yogurt. However, they observed that total proteolysis was higher in the samples supplemented with 3 % Raftiline HP (inulin) in comparison to the samples without or with 2 % Raftiline. Nevertheless, our observation was in contrast with some findings indicating effects of polysaccharides and inulin on proteolysis in yogurt. Degree of proteolysis was higher in the low-fat yogurt inoculated with ropy exo-polysaccharide (EPS) producing S. thermophiles in comparison to the yogurt containing non EPS producing S. thermophiles (Ramchandran and Shah 2009a). Additionally, supplementation of probiotic yogurt containing B. animalis Bb-12 with 5 % inulin resulted in increased proteolysis (Vasiljevic et al. 2007).
ACE-inhibitory activities
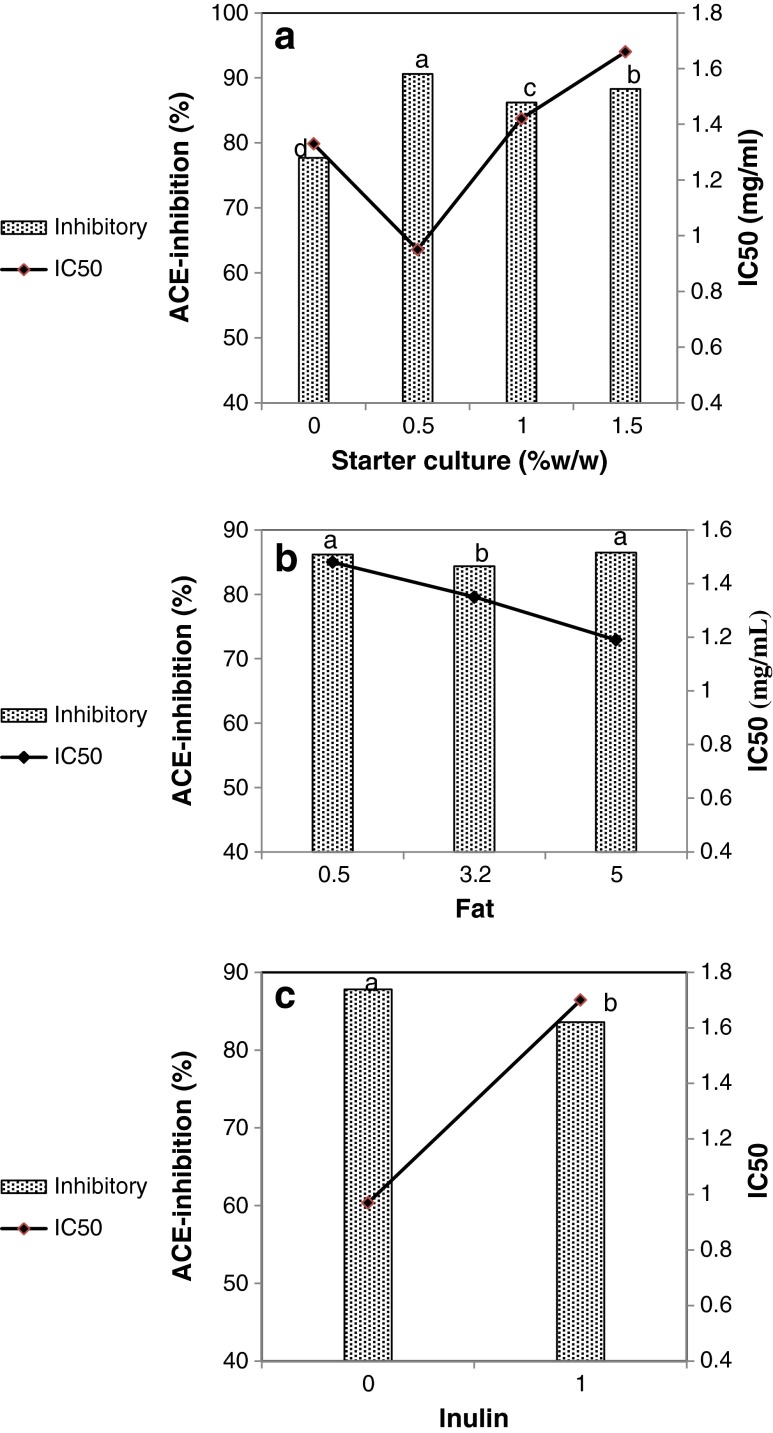
Results of relative in-vitro ACE-inhibitory activities and IC50 of yogurt samples are presented in Table 1. Results revealed that both probiotic and regular yogurt samples inhibited the activity of ACE enzyme with relative inhibition ranging from 72.5 % to 100 %. IC50 ranged from 0.95 to 3.46 mg/ml. As shown in Fig. 2a, ACE-inhibitory activities in probiotic samples (containing 0.5, 1.0 and 1.5 % starter culture) were statistically higher than those in regular samples (0 % starter cultures) containing only yogurt commercial bacteria. However, the relation between ACE-inhibitory activity and different concentrations of probiotic bacteria was not in a linear trend. Inoculation of 0.5 % starter culture showed the most potent effect on ACE-inhibitory activity. IC50 were also found to be at the least levels in the samples inoculated with 0.5 % starter culture. Decrease in ACE-inhibitory activity and increase in IC50 in the samples propagating with higher levels of starter culture (samples with 1 % and 1.5 % starter culture compared to samples with 0.5 %) may be the result of further degradation of active peptides by the bacteria.
Fig. 2.
The main effects of starter cultures (a), fat contents (b) and inulin (c) on the Angiotensin 1converting enzyme (ACE)-inhibitory and corresponding IC50 of the probiotic yogurts investigate in this study. 0.0 % starter means that the mix starter culture contained only Lactobacillus delbrueckii subs. bulgaricus and Streptoccus thermophilus (without probiotic bacteria)
Effects of fat contents on the ACE-inhibitory activity were varied (Fig. 2b). Yogurt samples with 0.5 % and 5.0 % fat showed higher ACE inhibition than did yogurt samples with 3.2 % fat. On the other hand, as fat contents increased from 0.5 % to 5.0 %, IC50 of the samples increased. Difference in IC50 may be attributed to difference in the types and quality of peptides released in the yogurt (Donkor et al. 2007) and the contents of soluble proteins (Ramchandran and Shah 2009a). No reports were found in literature on the effects of fat content on ACE-inhibitory activity of probiotic yogurt for comparison.
The main trend corresponding to the effects of the presence of inulin on ACE-inhibitory activities and IC50 is shown in Fig. 2c. Supplementation of yogurt with 1 % (w/w) inulin resulted in decrease in the ACE-inhibitory activity. This may be due to lower levels of peptides produced in inulin-containing yogurt as the result of proteolysis. This hypothesis is confirmed by the results of proteolysis test as mentioned above; yogurts with 1 % inulin showed lower proteolytic activities. Our finding is well in agreement with previously published results. Fuglsang et al. (2003) observed that ACE-inhibitory activity was greater in the samples with higher degree of proteolysis. Ramchandran and Shah (2008) also reported a good correlation between proteolysis and ACE-inhibition in the yogurt samples. However, inconsistent reports to our finding on the effect of inulin and polysaccharides on ACE-inhibitory activity and IC50 are found in literature. Ramchandran and Shah (2009b, 2010) showed that low-fat yogurt and low-fat probiotic yogurt both containing inulin had higher ACE-inhibitory activities than the samples without inulin. In another study, the presence of EPS from S. thermophilus in low-fat yogurt showed no effect on ACE inhibitory (Ramchandran and Shah 2009a). Prebiotics such as inulin at high levels (50–200 g/kg) have shown hypocholestrolemic effect (Ramchandran and Shah 2011).
Conclusion
Supplementation of probiotic yogurt with inulin negatively affected degree of proteolysis and also ACE-inhibition activity of produced yogurt. Proteolysis also was not influenced by variable levels of fat, but was affected by high concentration (1.5 % w/w) of probiotic bacteria and commercial yogurt bacteria. Effects of fat contents and concentrations of starter culture on ACE-inhibitory activity were significant and varied. As ACE-inhibition is attributed to the presence of bioactive peptides released from milk casein during fermentation, more studies are needed to characterize such peptides and to investigate relations between peptide size and biological activities.
Acknowledgments
This work was financially supported by the University of Tehran and Iranian center of excellence for application of modern technologies for producing functional foods and drinks.
References
- Akın M, Akın M, Kırmacı Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. Food Chem. 2007;104:93–99. doi: 10.1016/j.foodchem.2006.11.030. [DOI] [Google Scholar]
- Church FC, Swaisgood HE, Porter GH, Catignani GL. Spectrophotometric Assay using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- Cruz A, Castro W, Faria J, Bolini H, Celeghini R, Raices R, Oliveira C, Freitas M, Conte Júnior C, Mársico E. Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Res Int. 2013;51:723–728. doi: 10.1016/j.foodres.2013.01.028. [DOI] [Google Scholar]
- Dave R, Shah N. Ingredient supplementation effects on viability of probiotic bacteria in yogurt. J Dairy Sci. 1998;81:2804–2816. doi: 10.3168/jds.S0022-0302(98)75839-4. [DOI] [PubMed] [Google Scholar]
- Donkor O, Henriksson A, Vasiljevic T, Shah NP. Probiotic strains as starter cultures improve angiotensin-converting enzyme inhibitory activity in soy yogurt. J Food Sci. 2005;70:M375–M381. doi: 10.1111/j.1365-2621.2005.tb11522.x. [DOI] [Google Scholar]
- Donkor ON, Henriksson A, Singh T, Vasiljevic T, Shah NP. ACE-inhibitory activity of probiotic yoghurt. Int Dairy J. 2007;17:1321–1331. doi: 10.1016/j.idairyj.2007.02.009. [DOI] [Google Scholar]
- Ejtahed H, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, Akbarian-Moghari A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94:3288–3294. doi: 10.3168/jds.2010-4128. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RJ, Murray BA. Bioactive peptides and lactic fermentations. Int J Dairy Technol. 2006;59:118–125. doi: 10.1111/j.1471-0307.2006.00250.x. [DOI] [Google Scholar]
- Fuglsang A, Rattray FP, Nilsson D, Nyborg NCB. Lactic acid bacteria: inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie Van Leeuwenhoek. 2003;83:27–34. doi: 10.1023/A:1022993905778. [DOI] [PubMed] [Google Scholar]
- Fuller R. Probiotics in man and animals. J Applied Bact. 1989;66:365. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- Guven M, Yasar K, Karaca O, Hayaloglu A. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. Int J Dairy Technol. 2005;58:180–184. doi: 10.1111/j.1471-0307.2005.00210.x. [DOI] [Google Scholar]
- Klaver FAM, Kingma F, Weerkamp A. Growth and survival of bifidobacteria in milk. Nederlands Melk en Zuiveltijdschrif. 1993;47:151–164. [Google Scholar]
- Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J pediatr Gastr Nutr. 2004;38:457–458. doi: 10.1097/00005176-200404000-00017. [DOI] [PubMed] [Google Scholar]
- Laragh JH. L’hypertension. La Recherche. 1979;10:1068–1076. [Google Scholar]
- Leclerc PL, Gauthier SF, Bachelard H, Santure M, Roy D. Antihypertensive activity of casein-enriched milk fermented by Lactobacillus helveticus. Int Dairy J. 2002;12:995–1004. doi: 10.1016/S0958-6946(02)00125-5. [DOI] [Google Scholar]
- Mackay AD, Taylor MB, Kibbler CC, Hamilton-miller JMT. Lactobacillus endocarditis caused by a probiotic organism. Clin Microb Infect. 2008;5:290–292. doi: 10.1111/j.1469-0691.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Moslehishad M, Ehsani MR, Salami M, Mirdamadi S, Ezzatpanah H, Niasari-Naslaji A, Moosavi-Movahedi AA. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int Dairy J. 2013;29:82–87. doi: 10.1016/j.idairyj.2012.10.015. [DOI] [Google Scholar]
- Özer D, Akin S, Özer B. Effect of inulin and lactulose on survival of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-02 in acidophilus-bifidus yoghurt. Food Sci Technol Int. 2005;11:19–24. doi: 10.1177/1082013205051275. [DOI] [Google Scholar]
- Pihlanto-Leppala A. Bioactive peptides from bovine whey proteins: opioid and ACE-inhibitory peptides. Trends Food Sci Tech. 2001;11:347–356. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Ramchandran L, Shah NP. Growth, proteolytic and ACE-I activities of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus and rheological properties of low-fat yogurt as influenced by the addition of Raftiline HP. J Food Sci. 2008;73:368–374. doi: 10.1111/j.1750-3841.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Ramchandran L, Shah NP. Effect of exopolysaccharides on the proteolytic and angiotensin-I converting enzyme-inhibitory activities and textural and rheological properties of low-fat yogurt during refrigerated storage. J Dairy Sci. 2009;92:895–906. doi: 10.3168/jds.2008-1796. [DOI] [PubMed] [Google Scholar]
- Ramchandran L, Shah NP. Effect of exopolysaccharides and inulin on the proteolytic, angiotensin-I-converting enzyme- and alpha-glucosidase-inhibitory activities as well as on textural and rheological properties of low-fat yogurt during refrigerated storage. Dairy Sci Technol. 2009;89:583–60. doi: 10.1051/dst/2009039. [DOI] [PubMed] [Google Scholar]
- Ramchandran L, Shah NP. Characterization of functional, biochemical and textural properties of synbiotic low-fat yogurts during refrigerated storage. LWT Food Sci Technol. 2010;43:819–827. doi: 10.1016/j.lwt.2010.01.012. [DOI] [Google Scholar]
- Ramchandran L, Shah NP. Yogurt can beneficially affect blood contributors of cardiovascular health status in hypertensive rats. J Food Sci. 2011;76:131–136. doi: 10.1111/j.1750-3841.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- Rautio M, Jousimies-somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, Koskela M. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin infect diseases. 1999;28:1159–1160. doi: 10.1086/514766. [DOI] [PubMed] [Google Scholar]
- Saarela M, Mogensen G, Fonden R, Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotechnol. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- Salminen S, Ouwehand A, Benno Y, Lee Y. Probiotics: how should they be defined? Trends Food Sci Tech. 1999;10:107–110. doi: 10.1016/S0924-2244(99)00027-8. [DOI] [Google Scholar]
- Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure–lowering effect in hypertensive subjects. Am J Clin Sci. 2003;77:326–330. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- Sipola M, Finckenberg P, Santisteban J, Korpela R, Vapaatalo H, Nurminen ML (2001) Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J Phys Pharm 52:745–54 [PubMed]
- Tsai JS, Chen TJ, Sun Pan B, Gong SD, Chung MY. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008;106:552–558. doi: 10.1016/j.foodchem.2007.06.039. [DOI] [Google Scholar]
- Vasiljevic T, Kealy T, Mishra VK. Effects of β-Glucan addition to a probiotic containing Yogurt. J Food Sci. 2007;72:405–411. doi: 10.1111/j.1750-3841.2007.00454.x. [DOI] [PubMed] [Google Scholar]