Abstract
Aspartame was used in the manufacture of kalakand instead of sucrose. Sensory evaluation revealed that aspartame when used in the preparation of kalakand at a level of 0.065 % scored the highest in terms of sweetness perception and resembled control. Aspartame sweetened kalakand possessed the same desirable sweetness, colour, body and texture/consistency and mouthfeel even after 7 days of storage at 6–8 °C. Significant increase in titratable acidity of control as well as aspartame sweetened kalakand was observed during storage. However, only a slight drop in pH was observed in all samples on storage. The titratable acidity was higher in aspartame sweetened products than the corresponding control samples. Lightness (L*) was less in control samples with sucrose than the aspartame sweetened kalakand during storage. Total plate counts were higher in aspartame sweetened kalakand than its corresponding control throughout the storage period. Total plate counts increased linearly for both aspartame sweetened kalakand and control. A solid phase extraction method was standardized for the isolation of aspartame in kalakand. HPLC analytical conditions were standardized for separation of aspartame and its degradation products diketopiperazine and L-phenylalanine. HPLC analysis revealed that aspartame did not degrade in kalakand during storage establishing its stability in these products.
Keywords: Aspartame, Safety, Kalakand, Degradation
Introduction
Aspartame (N-L-α-aspartyl-L-phenylalanine methyl ester), discovered in 1965, is a low-calorie sweetener with a sugar-like taste but is approximately 200 times sweeter than sucrose. It is a white dipeptide, crystalline in nature. It is unique among low calorie sweeteners; in that it is completely broken down by the body to its components—the amino acids, aspartic acid and phenylalanine, and a small amount of methanol (Mazur 1974). Aspartame is the most widely used artificial sweetener and has captured 50 % of the world market since its introduction in 1981. The risks of aspartame ingestion would be in the toxicity of its metabolism products. The safety issues that have been raised in the past about aspartame have included: (1) the possibility of toxicity from methanol, one of the breakdown products of aspartame; (2) elevations in plasma concentrations of phenylalanine and aspartic acid, which could result in increased transport of these amino acids into the brain, altering the brain’s neurochemical composition; (3) the possibility of neuroendocrine changes, particularly increased concentrations in the brain, synaptic ganglia and adrenal medulla of catecholamines derived from phenylalanine and its hydroxylation product, tyrosine; and (4) a postulated link with epilepsy and brain tumours. All these areas have been addressed in the pre-1988 literature and in recent reviews (Meldrum 1993; Lajtha et al. 1994; Tschanz et al. 1996). Therefore, it is important to study the thermal behavior of foodstuffs because the kinetic factors, temperature and time, can provoke decomposition of components into new compounds. Chemical interactions can occur during industrial processing, which may necessitate the development of better methodologies for quality control applications.
Consumption of sweets is an integral part of Indian dietary system and diabetic-friendly traditional sweets are a new category for such products; the production of which is being contemplated by many enterprising manufacturers. With increased consumer interest in reducing sugar intake, food products made with sweeteners rather than sugar have become more popular. Recent change in legislation in India (PFA 2004) now permits the use of aspartame in sweets like halwa, khoa, burfi, rasogolla, gulabjamun and other milk products upto 200 ppm as per the limits prescribed under proper label declaration. This can lead to partial or whole replacement of sucrose in sweetened dairy products like kalakand.
Kalakand is one of the most popular khoa-based sweets having granular fused mass, held together in a loosely compact body with off-white to light caramel colour. Kalakand is a milk product where milk is coagulated with small quantities of citric acid followed by desiccation and addition of sugar to obtain a product with a granular texture, caramel flavour, and paste-like consistency. Replacement of sugar with aspartame in kalakand may result changes in its physicochemical attributes. Since application of aspartame in kalakand is new, qualitative and quantitative information on sweeteners degradation in dairy systems is required. No work has been done on the estimation and stability of aspartame in indigenous dairy products. Therefore, study was carried out to optimize the rate of addition of aspartame for obtain an organoleptically acceptable product and determine the stability of aspartame during storage.
Material and methods
Preparation of kalakand
Pooled buffalo milk was collected from the Experimental Dairy, NDRI, Karnal. The artificially sweetened kalakand was prepared according to the method of Suresh and Jha (1994) with slight modification (Fig. 1) for aspartame containing product. A control sample of kalakand was also prepared using sugar and citric acid procured from the local market.
Fig. 1.
Flowchart for manufacture of kalakand
Optimization of aspartame levels in kalakand
Aspartame levels were optimized by sensory evaluation of kalakand using the basis of its acceptance by a panel of eight judges on a 9-point hedonic scale score card. The levels of aspartame tried for kalakand were in the range of 0.045–0.075 % on 6 % sucrose equivalence milk basis.
Aspartame standards
Nutrasweet powder (Nutrasweet Company, Augusta, GA, USA) was used as aspartame standard and its degradation products standards were 2, 5-diketopiperazine, L-Phenylalanine (Sigma-Aldrich, Lovfs, MO, USA). The chemicals used in this study were of analytical grade reagent. All aqueous solutions were prepared using reverse-osmosis quality water produced by a Milli-RO 15 plus Milli-Q purification system (Millipore, Bedford, MA, USA).
Equipment
The vacuum filtration assembly was supplied by the Millipore corporation Bedford, MA, USA; the solid phase extraction C18 cartridge by Supelco (Bellfonte, PA, USA); the solid phase extraction vacuum manifold by VisiprepTM DL 9 Supelco; Bellfont, PA, USA; and HPLC system by Shimadzu (LC10A, detector UV, SPD-M10AV, Japan).
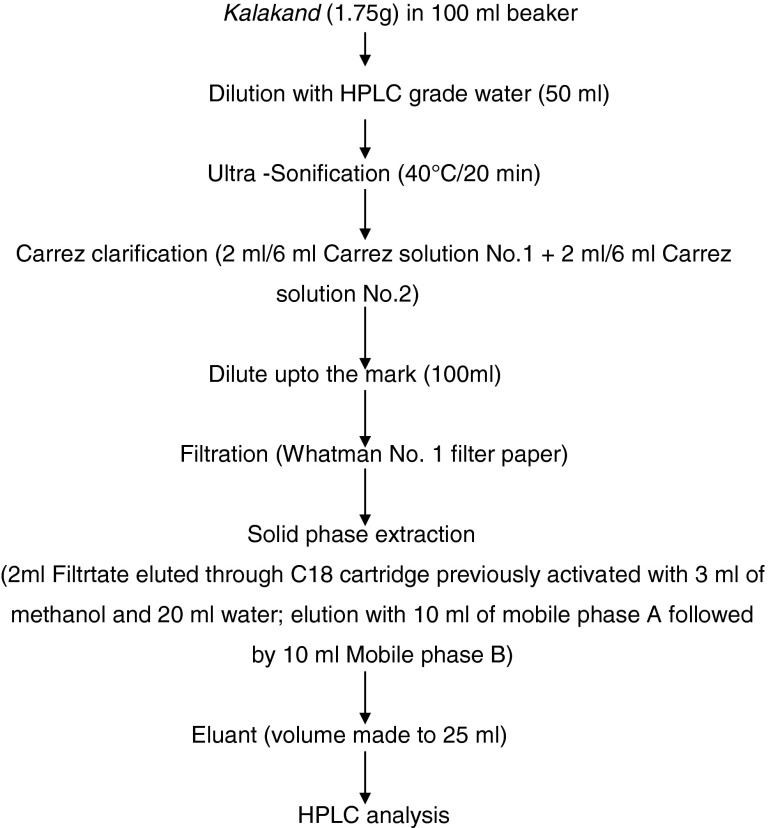
Solid Phase Extraction (SPE) of aspartame from Kalakand
The sample preparation procedure (Fig. 2) used for isolation of aspartame from kalakand was essentially based upon the method of BS EN:12856 (1999) used for semisolid and solid products. A blank experiment was also performed simultaneously.
Fig. 2.
Flowchart for isolation of aspartame from Kalakand
HPLC analysis
High-performance liquid chromatography analysis of reference standards of aspartame, degradation products and sample isolates from kalakand were performed under the following set of standardized conditions:
| Column stationary phase | : Shimpak C18, S-5 μm, 120A, 250 × 4.6 mm ID | |
| Phase | : Reverse phase | |
| UV detector wavelength | : 200 nm | |
| Mobile Phase (A) | : 0.02 M phosphate buffer, pH 5.0: acetonitrile | (97:3) |
| Mobile Phase (B) | : 0.02 M phosphate buffer, pH 3.5: acetonitrile | (80:20) |
| Flow rate | : 1 ml/min | |
| Run time | : 30 min. | |
| Maximum pressure | : 400 kgmf |
Binary gradient programming
| Time (min) | B concentration (%) | Actual pressure observed (Kgmf) |
|---|---|---|
| 0.1 | 0 | 80 |
| 8 | 0 | 80 |
| 13 | 100 | 160 |
| 25 | 100 | 160 |
| 27 | 0 | 80 |
| 30 | STOP | 80 |
Standard solutions of aspartame and degradation products
Ten milligram each of aspartame and its degradation products were dissolved in a 10 mL mixture of mobile phases A and B (1:1) to give stock standard solutions of concentration 1 mg/mL were pipetted out into separate 10 mL volumetric flasks and the volume made up to the mark with mixture of mobile phases A and B (1:1), to give standard solution of concentration 10 ng/μL. Also, 100 μL from each of the standard solutions (1 mg/mL) were pipetted out into a 10 mL volumetric flask and volume made up to the mark with mixture of mobile phases A and B (1:1), to give a mixture of aspartame and degradation products in standard solutions of concentration 10 ng/μL. A 5-point calibration curves were plotted for aspartame and its degradation products. Curves were prepared representing 50, 100, 200 and 250 ng concentration and their corresponding peak areas. The correlation coefficient of 0.99 for aspartame as well as its degradation products showed the linearity of the system. Recovery procedures of aspartame and its degradation products were performed at 800 and 625 ppm, respectively in kalakand.
Sample preparation and HPLC analysis
The sample preparation was standardized to 1.75 g of kalakand to ensure completely fat free extraction through C18 cartridges. The amounts of Carrez solutions 1 and 2 required for the precipitation of proteins in kalakand was standardized as 6 mL each. Elution was done with 10 mL of mobile phase B for efficient recovery of aspartame and its degradation products.
Storage and analysis of kalakand
Control and artificially sweetened samples of kalakand (with best selected sweetener level) were stored at refrigerated temperature (6–8 °C) and samples were analysed at 0, 3 and 7 days of storage for titratable acidity and pH IS: SP-18 Part XI (1981), microbiological examination IS: SP-18 Part I (1980) using Plate Count Agar; incubation of the plates was carried out at 37 °C for 48 h.
Sensory evaluation
The samples of kalakand were evaluated for the sweetness, colour and appearance, body and texture and overall acceptability by a panel of 8 judges from faculty and students of Dairy Chemistry Division, National Dairy Research Institute, Karnal using a 9-point hedonic scale score card (Amerine et al. 1965).
Colour measurement
The colour of kalakand was measured by using a Colourflex (Hunter lab, Reston, VA, USA) with universal software 9 version 4.10. The light source was xenon flash lamp. Sample was filled upto 2/3rd of the capacity in a cylindrical glass bowl (2.5 cm height and 5 cm diameter) and readings were noted down. Data was received through the software in terms of L* [lightness, range from zero (black) to 100 (white)], a* [redness, range from +60 (red) to −60(green)] and b* [yellowness, range from +60 (yellow) to −60(blue)] values of the international colour system.
Statistical analysis
In all experiments, one-way/two-way analysis of variance (ANOVA) with a subsequent least significant difference (LSD) test was applied for multiple sample comparison. This was done to test for any significant differences (P < 0.05) in the mean values of all the groups as described by Snedcor and Cochran (1989). Three replications of each experiment were analyzed for statistical analysis.
Results and discussion
Optimization of aspartame levels in kalakand
Sensory evaluation revealed that aspartame when used at the levels of 0.055, 0.060 and 0.070 % in kalakand (Table 1) resulted in lower sensory scores than control. The level of 0.065 % in kalakand scored highest in terms of sweetness perception and resembled control. Colour and appearance and body and texture scores were significantly lower in aspartame sweetened kalakand as compared to control. However, overall acceptability of aspartame sweetened kalakand was lower (P < 0.05) than the corresponding controls. This is because sugar (sucrose) not only contributes towards the sweetness but also helps in development of desirable flavor, body and texture and characteristic colour through maillard browning and caramelisation. Hence, on the basis of sensory evaluation, out of the above three levels, the level of 0.065 % in kalakand was finally selected for the manufacture.
Table 1.
Sensory quality of kalakand containing different levels of aspartame
| Characteristics | Control | Aspartame levels | |||
|---|---|---|---|---|---|
| 0.055 % | 0.060 % | 0.065 % | 0.070 % | ||
| 550 ppm | 600 ppm | 650 ppm | 700 ppm | ||
| Sweetness | 8. 0 ± 0.00a | 6.7 ± 0.34b | 6.7 ± 0.25b | 7.7 ± 0.16a | 6.2 ± 0.37b |
| Body &texture | 7.6 ± 0.17a | 6.3 ± 0.25b | 6.4 ± 0.22b | 6.4 ± 0.31b | 6.5 ± 0.18b |
| Colour & appearance | 7.6 ± 0.18a | 6.9 ± 0.14b | 7.0 ± 0.18b | 7.0 ± 0.25b | 7.0 ± 0.18b |
| Overall acceptability | 7.7 ± 0.16a | 6.1 ± 0.22b | 6.2 ±0.25b | 7.1 ± 0.25c | 6.8 ± 0.18c |
Means in each row with different superscripts (a, b, c) were significantly different (LSD test, P < 0.05) from each other. Data are presented as means ± SD (n = 8)
Analysis of kalakand during storage
Sensory profile
Sensory scores during storage (Table 2) revealed that aspartame-sweetened kalakand resembled the control in retaining the sensory profile (sweetness, colour and appearance, body and texture, and overall acceptability) at all periods of storage. However, aspartame sweetened kalakand ranked lower (P < 0.05) than control. The scores in control kalakand could be attributed to sucrose. On other hand, addition of aspartame resulted in insufficient water-binding capacity due to lower quantity of addition leading to product with a slightly lower score.
Table 2.
Changes in quality of aspartame (650 ppm) sweetened kalakand during storage at 6–8 °C
| Particulars of kalakand | Storage (Days) | ||
|---|---|---|---|
| 0 | 3 | 7 | |
| Sensory quality | |||
| Sweetness | |||
| Control | 8.1 ± 0.12Aa | 8.1 ± 0.12Aa | 8.0 ± 0.12Aa |
| Aspartame | 7.6 ± 0.16Ba | 7.6 ± 0.161Ba | 7.5 ± 0.17Ba |
| Color and appearance | |||
| Control | 8.0 ± 0.18Aa | 7.8 ± 0.12Aa | 7.8 ± 0.20Aa |
| Aspartame | 7.6 ± 0.18Ba | 7.5 ± 0.18Ba | 7.2 ± 0.31Ba |
| Body and texture | |||
| Control | 7.8 ± 0.12Aa | 7.8 ± 0.12Aa | 8.1 ± 0.12Aa |
| Aspartame | 6.7 ± 0.36Ba | 6.8 ± 0.22Ba | 6.5 ± 0.17Ba |
| Overall acceptability | |||
| Control | 8.0 ± 0.26Aa | 8.0 ± 0.26Aa | 8.0 ± 0.17Aa |
| Aspartame | 7.1 ± 0.27Ba | 7.0 ± 0.27Ba | 6.6 ± 0.48Ba |
| Physicochemical | |||
| Titratable acidity (%LA) | |||
| Control | 0.35 ± 0.01Aa | 0.37 ± 0.00Ab | 0.40 ± 0.00Ac |
| Aspartame | 0.34 ± 0.00Aa | 0.41 ± 0.00Bb | 0.43 ± 0.00Bc |
| pH | |||
| Control | 6.37 ± 0.00Aa | 6.35 ± 0.01Aa | 6.29 ± 0.00Ab |
| Aspartame | 6.35 ± 0.00Aa | 6.32 ± 0.03Ab | 6.25 ± 0.00Bc |
| L (light ness) | |||
| Control | 65.7 ± 0.30Aa | 64.7 ± 0.30Aa | 63.2 ± 0.41Ab |
| Aspartame | 66.5 ± 0.16Aa | 65.6 ± 0.05Aa | 65.3 ± 0.12Ba |
| A (red ness) | |||
| Control | 6.2 ± 0.09Aa | 7.2 ± 0.18Ab | 7.2 ± 0.05Ab |
| Aspartame | 5.9 ± 0.22Aa | 6.3 ± 0.10Aa | 6.9 ± 0.11Ab |
| B (yellow ness) | |||
| Control | 31.8 ± 0.10Aa | 30.9 ± 0.15Aa | 30.7 ± 0.23Aa |
| Aspartame | 30.5 ± 0.11Aa | 31.2 ± 0.13Aa | 30.5 ± 0.10Aa |
| Microbiological quality | |||
| Total plate count (log CFU/ml) | |||
| Control | 1.6 × 103 ± 0.32Aa | 3.6 × 103 ± 0. 92Ab | 5.6 × 103 ± 0.26Ac |
| Aspartame | 2.0 × 103 ± 0. 28Aa | 5.0 × 103 ± 0.69Bb | 10.3 × 103 ± 1.04Bc |
Means in each row with different superscripts (a, b, c) were significantly different (LSD test, P < 0.05) from each other. Means in a column with different superscripts (A, B) were significantly different (LSD test, P < 0.05) from each other. Data are presented as means ± SD (n = 8)
Titratable acidity and pH
There was no apparent difference in initial titratable acidity of the control and aspartame-sweetened kalakand (Table 2). It is evident that there was a significant (P < 0.05) increase in acidity of control as well as aspartame sweetened kalakand during storage. There was a significant difference (P < 0.05) in titratable acidity between control and Kalakand sweetened with aspartame. This difference in titratable acidity may be due to the slight preservative effect of sucrose, which led to the retarded microbial growth. The pH of aspartame-sweetened kalakand decreased (P > 0.05) during storage. The pH of control and aspartame sweetened kalakand were similar initially as well as at different periods of storage.
Colour measurement
Changes in lightness L* and redness a* values was not significant (P > 0.05) during storage for both control and artificially sweetened kalakand (Table 2). The slight change in these colour parameters was due to the Maillard type of browning reaction in both control and aspartame containing samples. It was also noted that the lightness was less in control samples than aspartame sweetened kalakand during the entire period of storage, which might be due to slight caramelization in presence of sucrose. Results obtained were in accordance with the results of Gothwal and Bhavdasan (1991), who reported an increase in browning as a result of storage in khoa.
Microbiological examination
Total plate counts were higher (P < 0.05) in aspartame sweetened product than their corresponding control throughout the storage period (Table 2) as there was no preservation/protection effect of these sweeteners unlike sugar, which ultimately led to higher microbial counts. There was an increase (P < 0.05) in the total plate counts in both the control kalakand and the aspartame sweetened products during the storage period.
Stability of aspartame
Mazur (1974) has discussed the degradation of aspartame into its degradation products like diketopiperazine, L-phenylalanine, Aspartic acid, Methanol etc. with structure. Aspartame, diketopiperazine and L-phenylalanine gave λmax at 200 nm. Figure 3 represents the HPLC chromatogram of these three components at 200 nm under the standardized analytical conditions. The degradation products are eluted by mobile phase A and the main sweetener aspartame by mobile phase B.
Fig. 3.
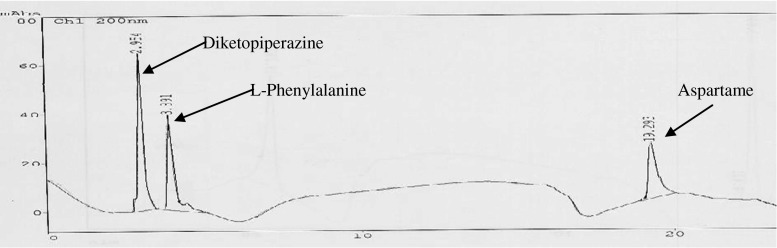
HPLC chromatogram of aspartame and its degradation products standards
The regression equations and correlations coefficient obtained are depicted in the Table 3. The correlation coefficient of 0.99 for aspartame as well as degradation products showed linearity of the system. The detection limits were in the range of 10–30 ng. Lawrence and Charbonneau (1988) also reported similar observations for aspartame. However, no information is available in the literature regarding the detection limits of these degradation products of the sweetener. Samples of kalakand sweetened with optimized aspartame levels were isolated and analyzed over HPLC under the standardized set of conditions. The recovery of the method was 95–98 %.
Table 3.
Regression equation and correlation coefficient of aspartame and its degradation products
| Product | Regression equation | R2 correlation coefficient |
|---|---|---|
| Aspartame | Y = 1903.2X − 2026.6 | 0.9992 |
| Diketopiperazine | Y = 3978.7X − 2951.8 | 0.9998 |
| L-Phenylalanine | Y = 2740.6X − 1130.5 | 0.9987 |
Stability of aspartame in kalakand during storage
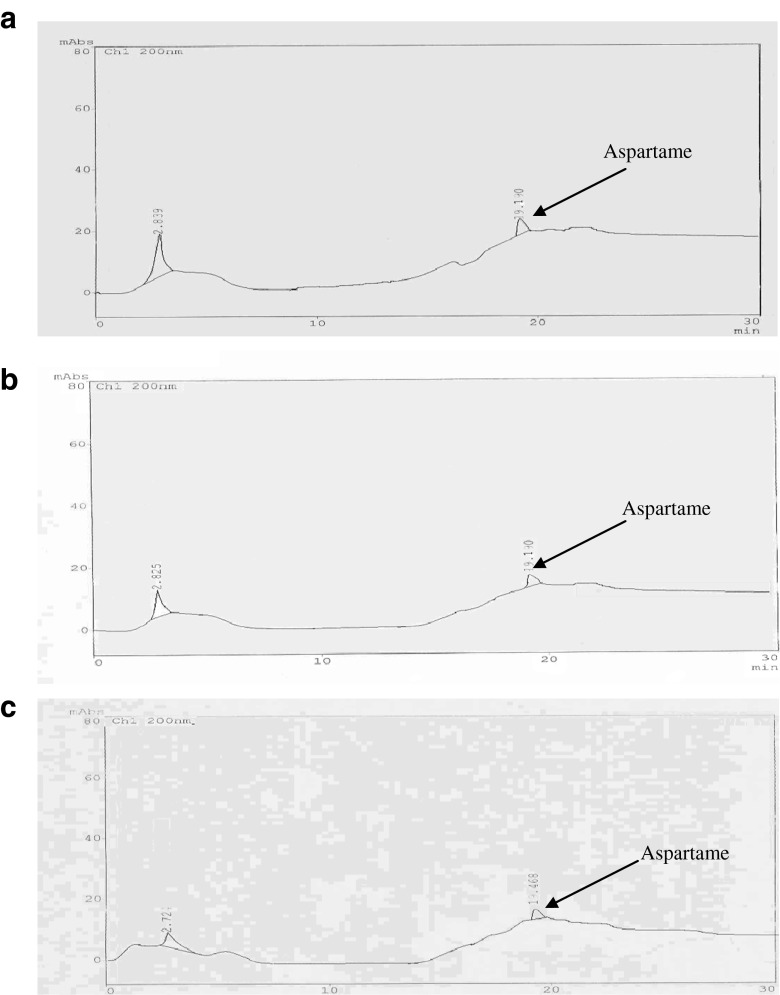
Aspartame in kalakand maintained level of 638.30 ppm, 633.94 ppm and 625.77 ppm on day 0,3 and 7 of storage with recovery of 98.20,97.53 and 96.27 % respectively. It is evident from the data that the aspartame levels remained unchanged even upto 7 days of storage in kalakand, establishing thereby the stability of aspartame during storage.
HPLC chromatograms (Fig. 4) obtained on 0, 3rd and 7th days of storage for kalakand also supported this observation as there was no peak other than aspartame in these chromatograms. This established that aspartame was not degraded during storage in kalakand samples under investigation. This might be due to pH of kalakand (6.2) and its low moisture content (aw = 0.764). Bell and Labuza (1994) observed that decreasing the pH from 6.7 to 6.4 doubled the stability of aspartame in the dairy beverages sweetened with aspartame. Arman and Temiz (1988) evaluated aspartame degradation in reduced moisture toothpaste at pH 6.05. They found a half life of 114 days at 40 °C for a system with water activity (aw) determined experimentally to be 0.76. Aspartame is unstable if subjected to prolonged heating and therefore cannot be used in baking or cooking unless added at the end of the cooking process (Kroger et al. 2006), as has been done in the preparation of sweetened kalakand in the present study.
Fig. 4.
HPLC chromatograms of sample isolates of aspartame sweetened kalakand during storage
Conclusion
Sensory profile during storage studies at 6–8 °C revealed that aspartame-sweetened kalakand resembled the control kalakand in retaining sweetness. The optimum level of aspartame was found to be 0.065 % in kalakand on the basis of sensory evaluation. However, aspartame-sweetened kalakand ranked lower than the control in sensory, acidity and microbial load. A solid phase extraction (SPE) method was standardized for the isolation of aspartame from kalakand followed by HPLC. High-performance liquid chromatography analytical conditions were standardized for the separation of aspartame and its degradation product diketopiperazine and L-phenylalanine over a C18 column at UV 200 nm. Recovery of the method was 90–97 %. The results of the present investigation have established the successful use of aspartame in the preparation of the indigenous dairy product kalakand.
Abbreviations
- UV
Ultraviolet
- Kg
Kilogram
- mL
Mililitre
- ng
Nanogram
- μL
Microlitre
- ppm
Parts per million
- cm
Centimeter
- λmax
Absorption maxima
- aw
Water activity
- nm
Nanometer
- SD
Standard deviation
- LSD
Least significant difference
References
- Amerine MA, Pangborn RM, Roessler EB. Principles of sensory valuation of food. New York: Academic; 1965. [Google Scholar]
- Arman, Temiz Stability of aspartame in tooth pastes. Acta PharmTurc. 1988;30:28–32. [Google Scholar]
- Bell LN, Labuza TP. Aspartame stability in commercially sterilized flavoured dairy beverages. J Dairy Sci. 1994;77(1):34–38. doi: 10.3168/jds.S0022-0302(94)76925-3. [DOI] [PubMed] [Google Scholar]
- BSEN:12856 (1999) Foodstuffs—Determination of acesulfame-K, aspartame and saccharin—High performance liquid chromatographic method. Cited by Wood R, Foster L, Key P (2004). In: Analytical methods for food additive, Woodhead Publshing Ltd., CRC Press, pp 231–252
- Gothwal PP, Bhavdasan MK. Studies on the browning characteristics in dairy products. Indian J Dairy Sci. 1991;45(3):146–151. [Google Scholar]
- IS:SP-18 (Part I) ISI Handbook for food analysis. New Delhi: Bureau of Indian Standards, Manak Bhavan; 1980. [Google Scholar]
- IS:SP-18 (Part XI) ISI handbook for food analysis-dairy products. New Delhi: Bureau of Indian Standards, Manak Bhavan; 1981. [Google Scholar]
- Kroger M, Meister K, Kava R. Low-calorie sweeteners and other sugar substitutes: a review of the safety issues. Compr Rev Food Sci Food Saf. 2006;5:35–47. doi: 10.1111/j.1541-4337.2006.tb00081.x. [DOI] [Google Scholar]
- Lajtha A, Reilly MA, Danlop DS. Aspartame consumption: lack of effects on neural function. J Nutr Biochem. 1994;5:226–283. doi: 10.1016/0955-2863(94)90032-9. [DOI] [Google Scholar]
- Lawrence JF, Charbonneau CF. Determination of seven artificial sweeteners in diet food preparations by reverse-phase liquid chromatography with absorbance detection. J Assoc Off Anal Chem. 1988;71(5):934–937. [PubMed] [Google Scholar]
- Mazur RH. Aspartic acid-based sweeteners. In: Inglett GE, editor. ACS sweetener symposium. Westport: AVI Publishing; 1974. [Google Scholar]
- Meldrum BS. Amino acids as dietary excitotoxins: a contribution to understanding neurodegenerative disorders. Brain Res. 1993;18:293–314. doi: 10.1016/0165-0173(93)90014-Q. [DOI] [PubMed] [Google Scholar]
- Prevention of food adulteration (1st amendment) rules Notification GSR No. 388(E) Prev Food Adulteration Cases. 2004;64(2):1–25. [Google Scholar]
- Snedcor GW, Cochran WG. Statistical methods. 8. Amsterdam: Lowa University Press; 1989. [Google Scholar]
- Suresh I, Jha YK. Optimization of the process for kalakand manufacture and extension of its shelf life. J Food Sci Technol. 1994;31(5):389–394. [Google Scholar]
- Tschanz C, Butchko HH, Stargel WW, Kotsonis FN, editors. The clinical evaluation of a food additive: Assessment of aspartame. Boca Raton: CRC Press; 1996. p. 308. [Google Scholar]