Abstract
It has been proposed that the decline in protein synthesis observed in aging organisms may result from a decrease in elongation factor EF-1 alpha. Transgenic Drosophila melanogaster flies carrying an additional copy of the EF-1 alpha gene under control of a heat-inducible promoter have an extended lifespan, further indicating that the EF-1 alpha gene may play an important role in determining longevity. To test this hypothesis, we have quantitated EF-1 alpha mRNA, EF-1 alpha protein, and the EF-1 alpha complex-formation activity in these transgenic flies. Furthermore, we have tested whether the transgene construct is functional--i.e., whether transgenic mRNA is induced when flies are grown at higher temperature. The results show that although there is a clear difference in mean lifespan between the EF-1 alpha transgenic (E) flies and the control transgenic (C) flies, E flies do not express more EF-1 alpha protein or mRNA than C flies kept at the same experimental conditions. Although the transgene can be induced when E flies are heat-shocked at 37 degrees C, transgenic mRNA is not detectable in E flies aged at 29 degrees C. In both lines, the loss in catalytic activity with age is the same. We conclude that the E flies examined here do not live longer because of overexpressing the EF-1 alpha gene.
Full text
PDF
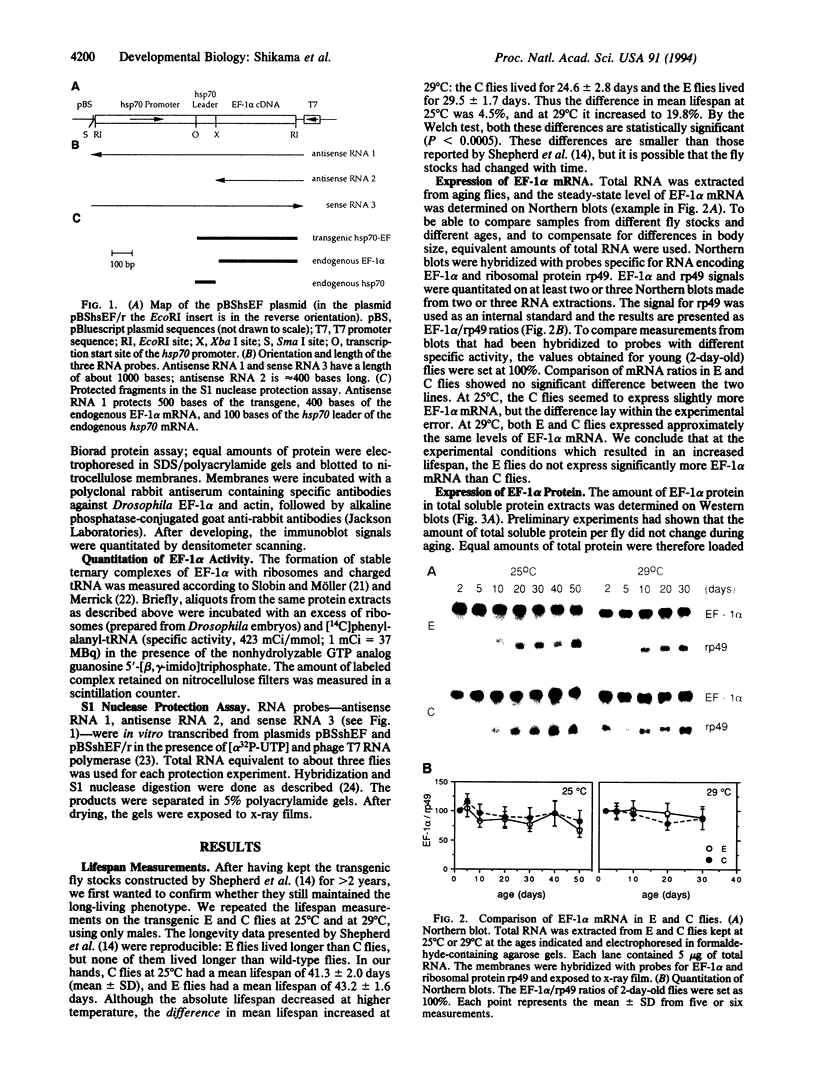
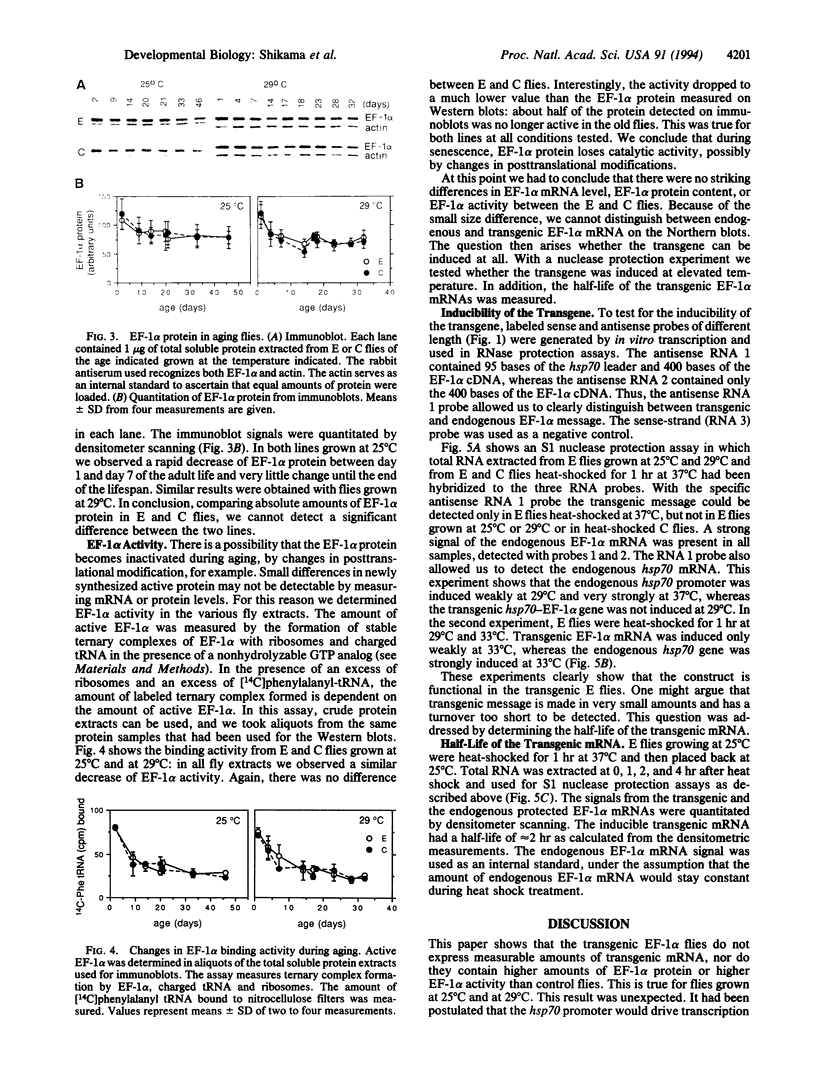
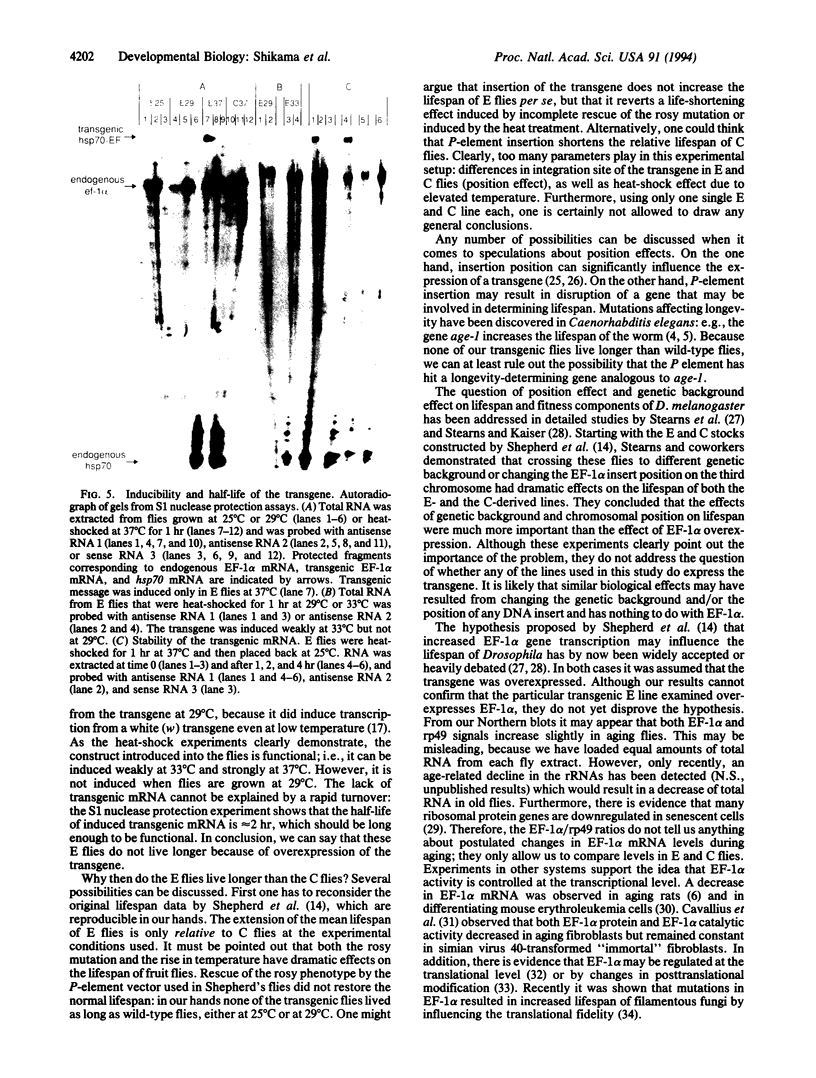

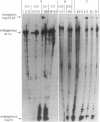
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavallius J., Rattan S. I., Clark B. F. Changes in activity and amount of active elongation factor 1 alpha in aging and immortal human fibroblast cultures. Exp Gerontol. 1986;21(3):149–157. doi: 10.1016/0531-5565(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Friedman D. B., Johnson T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988 Jan;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B., Johnson T. E. Three mutants that extend both mean and maximum life span of the nematode, Caenorhabditis elegans, define the age-1 gene. J Gerontol. 1988 Jul;43(4):B102–B109. doi: 10.1093/geronj/43.4.b102. [DOI] [PubMed] [Google Scholar]
- Giller T., Brunner L., Pick L., Brack C. A homologous in vitro system to analyze transcription of a mouse immunoglobulin mu heavy-chain gene. Eur J Biochem. 1988 Mar 15;172(3):679–685. doi: 10.1111/j.1432-1033.1988.tb13942.x. [DOI] [PubMed] [Google Scholar]
- Kurasawa Y., Numata O., Katoh M., Hirano H., Chiba J., Watanabe Y. Identification of Tetrahymena 14-nm filament-associated protein as elongation factor 1 alpha. Exp Cell Res. 1992 Nov;203(1):251–258. doi: 10.1016/0014-4827(92)90062-d. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Savereide P., Lefebvre P., Dasgupta S. The predicted amino acid sequence of a centrosphere protein in dividing sea urchin eggs is similar to elongation factor (EF-1 alpha). J Cell Sci. 1990 Feb;95(Pt 2):231–236. doi: 10.1242/jcs.95.2.231. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Ngo N. In vitro assembly of multiprotein complexes containing alpha, beta, and gamma tubulin, heat shock protein HSP70, and elongation factor 1 alpha. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3028–3032. doi: 10.1073/pnas.90.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Merrick W. C., Dever T. E., Kinzy T. G., Conroy S. C., Cavallius J., Owens C. L. Characterization of protein synthesis factors from rabbit reticulocytes. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):235–240. doi: 10.1016/0167-4781(90)90173-y. [DOI] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K., Toriyama M., Miyazaki M., Murofushi H., Hosoda S., Endo S., Sakai H. The mitotic apparatus-associated 51-kDa protein from sea urchin eggs is a GTP-binding protein and is immunologically related to yeast polypeptide elongation factor 1 alpha. J Biol Chem. 1990 Feb 25;265(6):3240–3247. [PubMed] [Google Scholar]
- Riis B., Rattan S. I., Clark B. F., Merrick W. C. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990 Nov;15(11):420–424. doi: 10.1016/0968-0004(90)90279-k. [DOI] [PubMed] [Google Scholar]
- Roth W. W., Bragg P. W., Corrias M. V., Reddy N. S., Dholakia J. N., Wahba A. J. Expression of a gene for mouse eucaryotic elongation factor Tu during murine erythroleukemic cell differentiation. Mol Cell Biol. 1987 Nov;7(11):3929–3936. doi: 10.1128/mcb.7.11.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Shepherd J. C., Walldorf U., Hug P., Gehring W. J. Fruit flies with additional expression of the elongation factor EF-1 alpha live longer. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7520–7521. doi: 10.1073/pnas.86.19.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silar P., Picard M. Increased longevity of EF-1 alpha high-fidelity mutants in Podospora anserina. J Mol Biol. 1994 Jan 7;235(1):231–236. doi: 10.1016/s0022-2836(05)80029-4. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Möller W. Purification and properties of an elongation factor functionally analogous to bacterial elongation factor Ts from embryos of Artemia salina. Eur J Biochem. 1978 Mar;84(1):69–77. doi: 10.1111/j.1432-1033.1978.tb12142.x. [DOI] [PubMed] [Google Scholar]
- Stearns S. C., Kaiser M. The effects of enhanced expression of elongation factor EF-1 alpha on lifespan in Drosophila melanogaster. IV. A summary of three experiments. Genetica. 1993;91(1-3):167–182. doi: 10.1007/BF01435996. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. Expression of the Drosophila white gene under the control of the hsp70 heat shock promoter. EMBO J. 1985 Dec 30;4(13B):3765–3772. doi: 10.1002/j.1460-2075.1985.tb04146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D., Cottrelle P., Iborra F., Buhler J. M., Sentenac A., Fromageot P. Elongation factor 1 alpha from Saccharomyces cerevisiae. Rapid large-scale purification and molecular characterization. J Biol Chem. 1985 Mar 10;260(5):3084–3089. [PubMed] [Google Scholar]
- Thomas G., Thomas G. Translational control of mRNA expression during the early mitogenic response in Swiss mouse 3T3 cells: identification of specific proteins. J Cell Biol. 1986 Dec;103(6 Pt 1):2137–2144. doi: 10.1083/jcb.103.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walldorf U., Hovemann B., Bautz E. K. F1 and F2: Two similar genes regulated differently during development of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5795–5799. doi: 10.1073/pnas.82.17.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster G. C., Webster S. L. Specific disappearance of translatable messenger RNA for elongation factor one in aging Drosophila melanogaster. Mech Ageing Dev. 1984 Mar;24(3):335–342. doi: 10.1016/0047-6374(84)90118-0. [DOI] [PubMed] [Google Scholar]
- Wilson C., Bellen H. J., Gehring W. J. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- Yang F., Demma M., Warren V., Dharmawardhane S., Condeelis J. Identification of an actin-binding protein from Dictyostelium as elongation factor 1a. Nature. 1990 Oct 4;347(6292):494–496. doi: 10.1038/347494a0. [DOI] [PubMed] [Google Scholar]