Abstract
The combined effects of enterocin A with Thymus vulgaris essential oils (EOs) against Listeria monocytogenes and Escherichia coli O157:H7 were investigated in vitro by enumeration of surviving populations of testing pathogens and minimal inhibitory concentration (MIC) determination. Enterocin A was purified to homogeneity by RP-HPLC from the culture fluid of Enterococcus strain and thyme EOs were extracted from local Thymus vulgaris plants. The major constituent of thyme EOs oils determined by GC-MS was thymol (78.4 %). Combination of enterocin A with thyme EOs showed an enhanced bactericidal effect against Listeria monocytogenes. Checkerboard assay and isobologram construction displayed a synergistic interaction between these compounds against Listeria (FIC index <0.5). Moreover, the MIC value of enterocin A has fallen fivefold (from 4.57 to 0.9 μg/ml), while the MIC of thyme EOs decreased threefold (from 3.6 to 1.2 μg/ml). Treatments with enterocin A alone did not affect the growth of the enteric pathogen E. coli O157:H7. However, the addition of thyme EOs and enterocin A yielded a synergistic antimicrobial effect against E. coli (MIC thyme EOs decrease from 2.2 to 0.71 μg/ml). This is the first report on the combined effect of enterocin A and thyme EOs against food pathogen bacteria. This combination could be useful in food bio-preservation.
Keywords: Bacteriocins, Essential oils, Food preservation, Lactic acid bacteria, Listeria, E. coli
Introduction
Undoubtedly food safety has become now the priority of many programs or research themes. However, while the producers are interested in extending the shelf life of food products, consumers demand safer natural food. The recent increases of foodborne microorganisms’ outbreaks such as Listeria (L.) monocytogenes, Escherichia (E.) coli O157:H7, Campylobacter jejuni, Yersinia enterocolitica and Vibrio parahemolyticus (Church 2004; Moore et al. 2005) encourage food industries to apply novel hurdle strategies based on “bio-preservation”.
Lactic acid bacteria (LAB) produce bacteriocins, which are natural antimicrobial peptides or proteins. These antimicrobial components have interesting potential applications in food preservation, which can meet the increasing demands of fresh and chemical additive free food products of more health conscious consumers and legal authorities. Nisin, the best-known LAB bacteriocin has been frequently shown to be safe and effective for use in food preservation over the past 30 years (Delves-Broughton 1990; Janes et al. 1999).
Enterocin A was a small, heat stable class IIa bacteriocin (Aymerich et al. 1996) and interesting as a food bio-preservative because of its strong activity against L. monocytogenes, a major biological hazard in the dairy industry (CDC 2011). However, some limitations may hinder LAB bacteriocins utilisation in many food products such as an ineffective protection against pathogenic Gram-negative bacteria, narrow pH range activity, spontaneous loss of bacteriocinogenicity, low production and emergence of bacteriocins-resistant bacteria. Therefore, it is necessary to combine bacteriocins with other food grade antimicrobial compounds such as essential oils to increase food safety and prolong shelf life.
To enhance the antimicrobial activities against various foodborne bacteria, LAB bacteriocins have been used in combination with other antibacterial agents and a range of physical treatments (Ghrairi et al. 2012). This approach has been also used to decrease the emergence of resistant strains and the dose of the antimicrobial agents. Yamazaki et al. (2004) reported that the combined effects of nisin, essential oil constituents and diglycerol fatty acid ester could reduce the viable counts of L. monocytogenes. In laboratory study, nisin and thyme EOs combination decrease the population of L. monocytogenes in minced beef during refrigerated storage (Solomakosa et al. 2008).
Despite the large flow of data, few studies, however, have been conducted to investigate the interactions between enterocin A and others antimicrobials components. Ramakrishnan et al. (2012) reported the synergistic effect of lipase with enterocin against Gram-negative bacteria. Furthermore, the enhanced antilisterial effect of enterocin AS-48 in combination with essential oils in ready-to-eat salad has been reported by Molinos et al. (2009). Even though these results were obtained from laboratory experiments, they support the idea that these combinations could be useful for a hurdle effect by adding these compounds onto the food surface or using active packaging.
The antibacterial effects of essential oils (EOs) and their components have been examined recently and their antimicrobial properties have been mostly studied in vitro (Kim et al. 1995; Ozcan et al. 2003; Bamoniri et al. 2010). The mechanism of action of EOs and their selectivity against bacteria remain poorly understood (Helander et al. 1998; Runyoro et al. 2010). According to these authors, this selectivity is due to the synergy of varied compounds and appears mainly related to disturbing the structure of the cell membrane. The main components of essential oils are terpenoids, particularly monoterpenes (C10) and sesquiterpenes (C15), as well as a variety of low molecular weight compounds (Dorman and Deans 2000). Moreover, the major EOs compounds described in diverse herbs were menthol (in mint), carvacrol (in oregano and rosemary), thymol (in thyme) and eugenol (in clove).
Thymus vulgaris plants produce different essential oil chemotypes, chemically composed of differing components such as monoterpene and phenolic compounds (Thompson et al. 2003). Generally, EOs chemotypes have different biological activities due to the presence of different chemical components. Moreover, Thymus vulgaris EOs and its major antibacterial components thymol or carvacrol (Burt 2004) have been found to possess antimicrobial activity in vitro against a broad spectrum of bacteria, such as Salmonella typhimurium (Lu and Wu 2010), E. coli (Singh et al. 2002) as well as Shigella sonnei and Shigella flexneri (Bagamboula et al. 2004). According to the World Health Organization (WHO), thymol residues in food are without danger to the consumer as long as they do not exceed 50 mg/kg. Thus, thymol is also considered by many national authorities as generally recognized as safe (GRAS) (FAO/WHO 2008).
The present study was performed with the aim of better understanding the effect of enterocin A purified from E. faecium strain and thyme EOs treatment on growing cells of L. monocytogenes and E. coli O157:H7.
Materials and methods
Bacterial strain and culture condition
Cultures of E. coli O157:H7 strain were maintained on LB or brain heart infusion (BHI, Difco, Franklin Lakes, NJ, USA) at 4 °C. Listeria monocytogenes EGDe was propagated aerobically in BHI broth or agar at 37 °C.
Extraction, preparation and antibacterial activity of thyme EOs
EOs were obtained by hydrodistillation method. The plant material (about 200 g), was cut into small pieces, and placed in a flask (2 l) with double distilled water (1 l). The mixture was boiled for 2 h, collected EOs were dried with anhydrous sodium sulphate and kept at −20 °C until use.
The thyme oil was dissolved in 50 % acetonitrile first, diluted at least 5 times with BHI. The antibacterial activity was assayed against E. coli and L. monocytogenes by the agar disc diffusion method (Hernández et al. 2005). 20 μl of samples were added into sterile 6 mm filter paper discs placed on inoculated BHI agar plates. Negative control and acetonitrile solutions corresponding to the acetonitrile percentages in the test samples, were also tested. The plates were incubated at 30 or 37 °C for 24 h and the inhibition zone was measured in millimetre.
The MIC (minimal inhibitory concentration) tests were performed by the broth microdilution method (Eloff 1998; Yu et al. 2004). Serial twofold dilutions from 10–0.08 (μg/ml) of the essential oils were prepared. Ten μL of samples of each concentration were dispensed into the wells of a microplate. Each well was then inoculated with 100 μL of the bacterial suspension (105 CFU/ml) and the plate was incubated at 37 °C for 24 h. After incubation, the wells were examined for growth of microorganisms using an ELISA plate reader (Biochrom) and OD was measured at 600 nm. The MIC is defined as the lowest concentration of the essential oil at which the bacterium does not demonstrate visible growth.
Gas chromatography conditions
The identification of volatile constituents was conducted by gas-chromatography in Hewlett-Packard 6890 Series (Palo Alto, CA, USA) equipment, with selective mass detector HP 5973, injector split/splitless, capillary column VF-5MS (30 m × 0.25 mm × 0.25 μm). Temperature: injector = 250 °C, column = 50 °C, 5 °C.min−1, 250 °C (1 min). Carrier gas (He) = 1.0 mL.min−1. Retention indices (RI) have been obtained according to the method of Van den and Kratz (1963). The standards used were from Sigma (St. Louis, MO, USA).
Preparation of enterocin A from cell-free supernatant
Enterocin A was purified to homogeneity from the culture fluid of E. faecium MMT21 by 60 % ammonium sulphate precipitation, C18 solid phase chromatography on Sep-pak C18 cartridge (Waters, Millipore, USA), and RP-HPLC as previously reported (Ghrairi et al. 2008). The solvent system used was a gradient of acetonitrile/trichloroacetric acid 0.01 % starting at 15 % acetonitrile. Chromatograms were recorded at 280 nm. Bacteriocin solution was filtered sterilized by 0.2 μm pore filter (Millipore Corp., Belford, MA, USA) and tested for bacteriocin activity against L. monocytogenes EGDe by the agar well diffusion method (Tagg et al. 1976). Protein concentration was determined by the bicinchoninic acid procedure as described by the supplier with bovine serum albumin as a standard (Sigma). The critical dilution assay was used for bacteriocin titration (Ghrairi et al. 2004).
The lowest concentration of enterocin A (MIC) that inhibits the growth of the microorganism being tested was detected by well-agar diffusion method. Bacteriocin concentrations used in this experiment ranged from 0.53 μg/ml to 8.5 μg/ml.
Inactivation experiments using suspended cells
Cultures of L. monocytogenes EGDe or E. coli O157:H7 grown overnight in BHI broth at 37 °C were inoculated to a final cell density of 106 CFU/ml in Erlenmeyer flasks (50 mL) containing BHI medium with enterocin A (2.33 μg/ml), thyme EOs (1.8 μg/ml) or combination of enterocin A/thyme EOs. Incubation was carried at 37 °C in a rotary shaker. At appropriate intervals, samples were removed for measurement of biomass by 10-fold serial dilution method in BHI, appropriate dilutions were plated on BHI agar (1.2 % w/v agar) and then incubated at 37 °C for 24 h. Controls were carried out in the same conditions but in the absence of antimicrobial compounds. The results were expressed as CFU/ml and presented as the median of two independent measures.
Determination of synergy between enterocin A and thyme EOs
Combinations of the concentrations below each MIC level that caused complete inhibition of microbial growth of L. monocytogenes were evaluated across serial dilutions of enterocin A and thyme EOs by checkerboard technique using a sterile 96-well microplates and BHI broth (Orhan et al. 2005; NCCLS 2003). All aliquots were prepared using the same solvents (acetonitrile + BHI) as in the MIC assay and blank solutions containing the solvent at concentrations equivalent to those in the test solutions were also prepared. The fractional inhibitory concentration index (FICI) combination was calculated. FICI is determined by dividing the MIC of each antimicrobial compound when used in combination by the MIC of each compound when used alone.
Where MICEntA and MICEOs are the MICs of enterocin A and thyme EOs, respectively. FICI > 4 defines antagonism FICI 0.5-4 expresses no interaction (indifference), FICI < 0.5 defines synergism.
An isobologram analysis was constructed to establish graphically the synergic effect by blotting MICs using Microsoft Excel 2011 (Microsoft corporation, USA). Based on these isobolograms, the points below the trendline are synergistic; those above the line indicates antagonistic and those just next to the lines are additive.
Results
Essential oils composition and activity
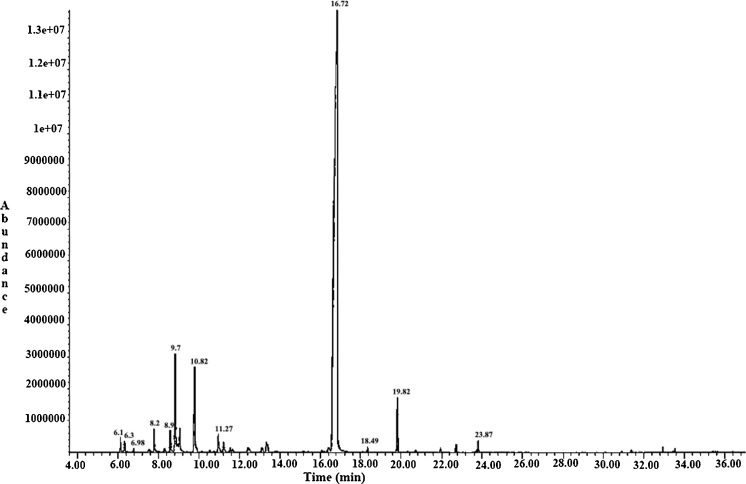
The retention data and chemical composition of T. vulgaris EOs are shown in Fig. 1 and Table 1. The EO yield from thyme, expressed in relation to dry weight of plant material (%, w/w), was about 0.3 %. Twelve constituents were identified in the thyme EOs samples, representing the 93.8 % of the total oils. Thymol (78.4 %), eucalyptol (4.45 %), linalool (4.1 %) and the monoterpene hydrocarbons α-pinen (2.23 %), were the predominant components of thyme EOs in the present study.
Fig. 1.
Chromatogram of T. vulgaris essential oil
Table 1.
Chemical composition of essential oils of Thymus vulgarus
| Constituents | Composition (%) | Retention time |
|---|---|---|
| α-pinène | 0.1 | 6.1 |
| β-pinène | 0.2 | 6.3 |
| β-myrcène | 0.01 | 6.983 |
| Alpha-phellandrène | 0.8 | 8.2 |
| Alpha terpinene | 0.6 | 8.9 |
| 1,3 benzène-1methyl-2-(1methylethyl) | 1.03 | 10.82 |
| Eucalyptol | 4.45 | 9.8 |
| Terpinen4ol | 4.1 | 11.27 |
| Thymol | 78.4 | 16.7 |
| Carvacrol | 0.67 | 18.5 |
| Linalol | 2.23 | 19.82 |
| Bicyclo(5,2,0)nonane | 1.3 | 23.8 |
The results of the disk-diffusion method showed very high activity against L. monocytogenes and E. coli O157: H7 and similar inhibition zones were measured (18–20 mm).
MIC values are presented in Table 2. T. vulgaris essential oils showed very high effectiveness against the two bacterial strains tested, as MIC values obtained were very low. The MIC value was obtained lowest 2.2 μg/ml against E. coli O157:H7, while it was obtained 3.6 μg/ml against L. monocytogenes EGDe.
Table 2.
Minimal inhibitory concentration (MIC) of enterocin A, thyme EOs or enterocin A/thyme EOs combination against L. monocytogenes and E. coli O157:H7
| Organism | MICa | ||
|---|---|---|---|
| Enterocin A (μg/ml) | Thyme EOs (μg/ml) | EntA/thyme EOs | |
| L. monocytogenes EGDe | 4,57 | 3.6 | 0.9/1.2 |
| E. coli O157:H7 | no effect | 2.2 | 3,4/0.71 |
aThe minimum concentration at which microbial growth was not observed within 48 h at 37 °C
Purification, antimicrobial activity and MIC of enterocin A
Enterocin A used in our experiments was obtained from cultured broths of the producer strain E. faecium MMT21 in MRS medium by ammonium sulphate precipitation, followed by C18 solid phase chromatography and RP-HPLC. After the last step, the bacteriocin was eluted as a single peak of activity corresponding to a chromatographic retention time of 22.5 min (Fig. 2). The proteins in the active peak were apparently homogeneous, as judged by electrophoresis (data not shown), and were used as purified bacteriocin in further examinations. Bacteriocin recovery was about 0.8 %.
Fig. 2.
Final RP-HPLC of purified enterocin A from E. faecium MMT21 strain. Dark arrow indicates the active fraction against L. monocytogenes. The purified peptides were applied to C18 column and eluted using a linear gradient of acetonitrile (15–80 %) containing 0.1 % TFA at a flow rate 1 ml/min
The MIC of enterocin A against L. monocytogenes EGDe was about 4.57 μg/ml based on the concentration evaluated in this study (Table 2). As expected, enterocin A was not active against the target strain E. coli O157: H7.
Effect of antimicrobial agents on bacterial growth
In vitro antimicrobial effect of enterocin A in combination with T. vulgaris EOs was investigated against L. monocytogenes and E. coli O157:H7 planktonic cells in BHI broth at 37 °C for up to 18 h.
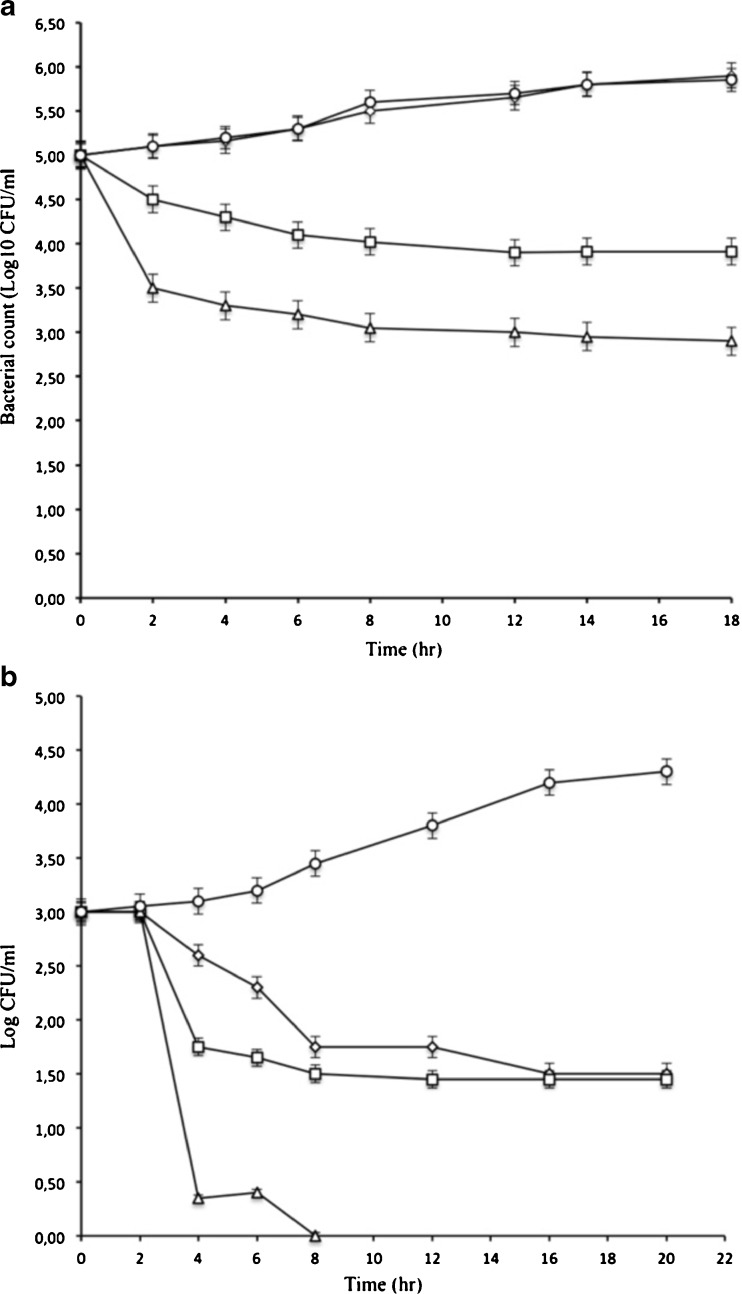
Six hours of contact with enterocin A (2.3 μg/ml) or T. vulgaris EOs (1.8 μg/ml) alone reduced L. monocytogens population by 1.3 and 2 log cfu/mL, respectively (Fig. 3, panel a). Control experiments with no additions showed a stable viable count over the 18 h of incubation in BHI broth. However, L. monocytogenes EGDe was undetectable after 8 h in the presence of combined enterocin A/thyme EOs. This result show very clearly the synergistic effect of this combination and the antimicrobial effect was augmented by enrichment with combined antimicrobial compounds.
Fig. 3.
Growth of L. monocytogenes EGDe (a) and E. coli O157:H7 (b) in BHI broth in the presence of enterocin A, thyme EOs and both. The concentration of each agent is 1/2 MIC except for E. coli test. Results were obtained from duplicate. Enterocin A (diamond), thyme EOs (white square), EntA + thyme EOs (triangle), blank test (white circle)
The activities of enterocin A and thyme EOs in combination were also investigated in vitro using the checkerboard microtiter plate assay. The MIC value of enterocin A falls fivefold (from 4.57 to 0.9 μg/ml), while the MIC of thyme EOs decreased threefold (Table 2). The fractional inhibitory concentration index (FICI) recorded synergy (FICI <0.5) in conjunction mixture between the enterocin A and thyme EOs against L. monocytogenes EGDe. This result was also demonstrated through isobologram analyses (Fig. 4). In fact, this observation indicated the more efficacy of combined treatment than mono-treatment for Listeria eradication.
Fig. 4.
Isobologram of enterocin A and thyme oils against L. monocytogenes EGDe
At a concentration of 1.1 μg/ml, thyme EOs reduced the count of E. coli O157:H7 from 105 to less than 104 CFU/ml after 8 h of incubation (Fig. 3, panel b). Combination of 1.1 μg/ml thyme EOs with 2.6 μg/mL of enterocin A reduced substantially the number of E. coli O157:H7 compared with the control.
The MIC of T. vulgaris EOs against E. coli O157:H7 was 2.2 μg/ml. In the checkerboard technique, the combinations of thyme EOs with enterocin A (3.4 μg/ml) resulted in a threefold decrease in its MIC (Table 2). Thus, effective concentrations of EOs have become more effective in combination with enterocin A against E. coli O157:H7.
Discussion
L. monocytogenes and E. coli O157:H7 are important pathogenic bacteria frequently responsible for food infections (CDC 2011). These bacteria contaminate a wide range of foodstuffs such as dairy products, egg products, meat and vegetable, and are often resistant to many antimicrobial compounds used in food industries. Improving the effectiveness and decreasing the amount of chemical compounds used for preservation are the two basic objectives in the development of strategies based on “bio-preservation” (Ghrairi et al. 2012).
Enterocin A is class IIa bacteriocin known to be more effective at inhibiting Gram-positive bacteria compared with Gram-negative bacteria (Boziaris and Adams 1999). The resistance of Gram-negative bacteria is attributed to the protective outer membrane that forms the outermost layer of the cell envelope (Helander et al. 1998). Natural hydrophobic organic compounds, such as essential oils, are known to have prominent outer membrane disintegrating properties (Sikkema et al. 1994). Therefore, it could be expected that combining potent membrane-active EOs with enterocin A may enhance the inhibitory effect against Gram-negative bacteria.
Our results reveal the importance of enterocin A as it has strong antibacterial activity against bacteria like L. monocytogenes EGDe attested. However, it remains inactive against Gram-negative bacteria. This bacteriocin was easily purified from commercial broth media by RP-HPLC and has interesting traits (e.g., wide pH range activity, solubility, and thermo-stability), which makes it an amenable antimicrobial for application in foods. In addition, this bacteriocin has been expressed in Lc. lactis (Martín et al. 2007) and in yeasts (Borrero et al. 2012) to improve food preservation. In this study, the MIC of enterocin A is lower than other known bacteriocins like nisin Z (Ghrairi et al. 2008) or mesentericin Y130 (Morisset et al. 2004).
Thyme EOs can be considered suitable antimicrobial since it has the GRAS status and are available in large quantity in Tunisia with a potential of production of 2000 T every year. We chose to submit the entire extracts of thyme EOs to antimicrobial activity studies. Indeed, extracts may be more beneficial than isolated constituents since a bioactive individual component can change its properties in the presence of other compounds present in the extracts. Although, major components of thyme EOs such as thymol, eucalyptol and carvacrol have the main roles in the anti-microbial activity (Kim et al. 1995).
This study showed that EOs from Tunisian T. vulgaris has potent activity against E. coli 0157:H7 and the psychotrophic pathogen L. monocytogenes. T. vulgaris EOs showed a strong antimicrobial activity with MIC < 4 μg/ml against the bacteria tested. It was also observed that the thyme EOs used belongs to the thymol chemotype. Thymol is generally recognized as a safe food and many publications have reported thymol had better activity than carvacrol (Bouddine et al. 2012). Solomakosa et al. (2008) showed that the combined addition of thyme EOs at 0.6 % and nisin at 500 or 1000 IU/g showed a synergistic activity against L. monocytogenes. The authors suggested that such combination could represent a promising approach to control Listeria growth.
The present work aimed to study the synergistic interaction between enterocin A and thyme EO. Enterocin A and thyme EOs combination reduced viable counts of L. monocytogenes in BHI broth below detection limits after 18 h of treatment. Checkerboard testing was also employed for testing enterocin A and thyme EOs combination. The reduction in Listeria growth was analysed for each compound in the presence of the other. The result is expressed as the fractional inhibitory concentration (FIC) index. An FIC index less than or equal to 0.5 indicates synergy and an index greater than four indicates antagonism. When the FIC index is greater than 0.5 and less than four, indicates indifference (Johnson et al. 2004). Our results clearly indicate a synergistic antilisterial effect between enterocin A and thyme EOs (FIC < 0.5). Similarly, Molinos et al. (2009) demonstrated that antilisterial activity of AS-48 (30 μg/g), a cyclic bacteriocin produced by E. faecalis, in the Russian type salad which was strongly enhanced by essential oils (thyme verbena, thyme red, Spanish oregano, ajowan, tea tree, clove, and sage oils tested at 1 %). The authors speculated that the simultaneous use of two or more antibacterials could be useful not only to decrease the bacteriocin doses, but also to avoid regrowth of survivor’s cells. Another study along the same line examined essential oil constituents such as carvacrol and thymol, in combination with nisin (Yamazaki et al. 2004). The authors showed high-level of growth reduction of L. monocytogenes and suggested that the use of several EOs compounds would improve food quality, especially the sensory quality, due to the low dosage of essential oils. Thus, the use of such antimicrobial compounds that work synergistically with other stresses (osmotic, pH, temperature) as hurdle technology can extend the shelf life of many food products and prevent the survival and regrowth of pathogens.
Synergism among antilisterial agents have been investigated to achieve higher levels of food safety standards (Kim et al. 2008; Friedly et al. 2009); but no report has been found or published in the literature on the synergistic effect of enterocin A and thyme EOs against L. monocytogenes. The exact mechanism of action of the essential oil and bacteriocins is not known, but some authors hypothesized that these components cause disruption of the cell membrane (Hyldgaard et al. 2012). Since the bactericidal effects of these compounds were thought to be due to the action at the cytoplasmic membrane, two mechanisms by which antimicrobial synergy can arise: enhancing the uptake of other antimicrobial agents present including the uptake of the antimicrobial peptide itself or enhancing the permeabilization of the bacterial membrane which permits the leakage or the passage of a variety of molecules and ions. Although, its may not be the only factor contributing to increase of the antilisterial activity. Further elucidation of the mechanisms of antimicrobial actions of EOs would provide insights that may prove useful for technological applications.
Previous reports have stated that the outer membrane of E. coli was rigid and that enterocin A could kill the E. coli only when used in conjunction with membrane disturbing compounds such as EDTA, sodium tripolyphosphate or at low and high pH (Ananou et al. 2005). In our study, we found that thyme EOs and enterocin A synergistically and significantly inhibited the growth of E. coli in medium broth at very low dosage. When in combination with sub MIC concentration of T. vulgaris EOs (1.1 μg/ml), enterocin A (2.3 μg/ml) effectively increased the anti-Escherichia coli activity of the EOs. Furthermore, the presence of antimicrobial effect against E. coli O157:H7 was also seen in EOs concentration as low as 3 fold (0.71 μg/ml) of its MIC when combined with enterocin A. In fact, this combination showed a greater inhibition ratio compared with the single treatment.
The biocidal mode of action of EOs and their components on bacteria has been reviewed in the past (Burt 2004). EOs are known to interact with the outer membrane layer of Gram-negative bacteria, making this outer protective shield more permeable. It is possible that thyme EOs may cause an increase in this permeability that could make enterocin A easily accessible to the cytoplasmic membrane. After these, the mode of bacterial killing is not clear.
Conclusions
Synergic interaction is defined as a combined effect greater than expected from the additive effect of the individual antimicrobial compounds. In this study, the combination of enterocin A with thyme essential oil exhibited synergistic activities against L. monocytogenes and E. coli O157:H7 even at low concentration. Our data suggest that such combinations could be exploited so as suitable control strategies for these pathogens, considering both economical aspects and the flavor of food. In addition, the synergistic effect of enterocin A and EOs may minimize the development of microbial resistance.
Acknowledgments
This work was partially supported by a grant from the Ministry of High Education, Tunisia.
References
- Ananou S, Galvez A, Martinez-Bueno M, Maqueda M, Valdivia E. Synergistic effect of enterocin AS-48 in combination with outer membrane permeabilizing treatments against Escherichia coli O157:H7. J Appl Microbiol. 2005;99:1364–1372. doi: 10.1111/j.1365-2672.2005.02733.x. [DOI] [PubMed] [Google Scholar]
- Aymerich T, Holo H, Havarstein LS, Hugas M, Garriga M, Nes IF. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagamboula CF, Uyttendaele M, Debevere J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004;21:33–42. doi: 10.1016/S0740-0020(03)00046-7. [DOI] [Google Scholar]
- Bamoniri A, Ebrahimabadi AH, Mazoochi A, Behpour M, Kashi FJ, Batooli H. Antioxidant and antimicrobial activity evaluation and essential oil analysis of Semenovia tragioides Boiss from Iran. Food Chem. 2010;122:553–558. doi: 10.1016/j.foodchem.2010.03.009. [DOI] [Google Scholar]
- Borrero J, Kunze G, Jiménez JJ, Böer E, Gútiez L, Herranz C, Cintas LM, Hernández PE. Cloning, production and functional expression of the bacteriocin enterocin A, produced by Enterococcus faecium T136, by the Yeasts Pichia pastoris, Kluyveromyces lactis, Hansenula polymorpha and Arxula adeninivorans. Appl Environ Microbiol. 2012;78:5956–5961. doi: 10.1128/AEM.00530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouddine L, Louaste L, Achahbar S, Chami N, Chami F, Remmal A. Comparative study of the antifungal activity of some essential oils and their major phenolic components against Aspergillus niger using three different methods. Afr J Biotechnol. 2012;11:14083–14087. doi: 10.5897/AJB11.3293. [DOI] [Google Scholar]
- Boziaris IS, Adams MR. Effect of chelators and nisin produced in situ on inhibition and inactivation of Gram negatives. Int J Food Microbiol. 1999;53:105–113. doi: 10.1016/S0168-1605(99)00139-7. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- CDC (2011) Estimates of foodborne illness in the United States. http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html
- Church D. Major factors affecting the emergence and re-emergence of infectious diseases. Clin Lab Med. 2004;24:559–586. doi: 10.1016/j.cll.2004.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves-Broughton J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–117. [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Eloff JNP. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2008) Food and Agriculture Organization of the United Nations/World Health Organization. Microbiological hazards in fresh fruits and vegetables. Microbiological Risk Assesment Series. Rome (Italy)
- Friedly EC, Crandall PG, Ricke SC, Roman M, O’Bryan C, Chalova VI. In vitro antilisterial effects of citrus oil fractions in combination with organic acids. J Food Sci. 2009;74:67–72. doi: 10.1111/j.1750-3841.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- Ghrairi T, Manai M, Berjaud JM, Frère J. Occurrence of anti-Listeria activity in lactic acid bacteria strains isolated from rigouta, a traditional Tunisian cheese. J Appl Microbiol. 2004;97:621–628. doi: 10.1111/j.1365-2672.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Ghrairi T, Frère J, Berjaud JM, Manai M. Purification and characterisation of bacteriocins produced by Enterococcus faecium from Tunisian rigouta cheese. Food Control. 2008;19:162–169. doi: 10.1016/j.foodcont.2007.03.003. [DOI] [Google Scholar]
- Ghrairi T, Chaftar N, Hani K. Bacteriocins: recent advances and opportunities. Progress in food preservation. Chap. 23. USA: Wiley-Blackwell Edition; 2012. pp. 485–512. [Google Scholar]
- Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, von Wright A. Characterization of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- Hernández T, Canales M, Avila JG, García AM, Martínez A, Caballero J, De Vivar AR, Lira R. Composition and antibacterial activity of essential oil of Lantana achyranthifolia Desf. (Verbenaceae) J Ethnopharmacol. 2005;96:551–554. doi: 10.1016/j.jep.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes ME, Nannapneni R, Johnson MG. Identification and characterization of two bacteriocin-producing bacteria isolated from garlic and ginger root. J Food Prot. 1999;62:899–904. doi: 10.4315/0362-028x-62.8.899. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Macdougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. Combination antifungal therapy. Antimicrob Agents Chemother. 2004;48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Marshall MR, Wei CI. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 1995;43:2839–2845. doi: 10.1021/jf00059a013. [DOI] [Google Scholar]
- Kim EL, Choi NH, Bajpai VK, Kang SC. Synergistic effect of nisin and garlic shoot juice against Listeria monocytogenes in milk. Food Chem. 2008;110:375–382. doi: 10.1016/j.foodchem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wu C. Reduction of Salmonella enterica contamination on grape tomatoes by washing with thyme oil, thymol, and carvacrol as compared with chlorine treatment. J Food Protect. 2010;73:2270–2275. doi: 10.4315/0362-028x-73.12.2270. [DOI] [PubMed] [Google Scholar]
- Martín MM, Gutiérrez J, Criado R, Herranz C, Cintas LM, Hernández PE. Cloning, production and expression of the bacteriocin enterocin A produced by Enterococcus faecium PLBC21 in Lactococcus lactis. Appl Microbiol Biotechnol. 2007;76:667–675. doi: 10.1007/s00253-007-1044-3. [DOI] [PubMed] [Google Scholar]
- Molinos AC, Abriouel H, López RL, Ben Omar N, Valdivia E, Gálvez A. Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food Chem Toxicol. 2009;46:2216–2223. doi: 10.1016/j.fct.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Moore JE, Corcoran D, Dooley JSG, Fanning S, Lucey B, Matsuda M, McDowell DA, Mégraud F, O’Mahony R, Millar BC, O’Riordan L, O’Rourke M, Rao JR, Rooney PJ, Sails A, Whyte P. Campylobacter. Vet Res. 2005;36:351–382. doi: 10.1051/vetres:2005012. [DOI] [PubMed] [Google Scholar]
- Morisset D, Berjeaud JM, Marion D, Lacombe C, Frère J. Mutational analysis of mesentericin Y105, an anti-Listeria bacteriocin, for determination of impact on bactericidal activity, in vitro secondary structure, and membrane interaction. Appl Environ Microbiol. 2004;70:4672–4680. doi: 10.1128/AEM.70.8.4672-4680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCLS . Methods for dilution antimicrobial susceptibility tests for bacteria. 6. USA: NCCLS; 2003. [Google Scholar]
- Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;1:140–143. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan G, Sagdic O, Ozcan M. Note: Inhibition of pathogenic bacteria by essential oils at different concentrations. Food Sci Technol Int. 2003;9:85–88. doi: 10.1177/1082013203009002003. [DOI] [Google Scholar]
- Ramakrishnan V, Narayan B, Halami PM. Combined effect of enterocin and lipase from Enterococcus faecium NCIM5363 against food borne pathogens: mode of action studies. Curr Microbiol. 2012;65:162–169. doi: 10.1007/s00284-012-0138-z. [DOI] [PubMed] [Google Scholar]
- Runyoro D, Ngassapa O, Vagiona K, Aligiannis N, Graikou K, Chinou I. Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chem. 2010;119:311–316. doi: 10.1016/j.foodchem.2009.06.028. [DOI] [Google Scholar]
- Sikkema J, De Bont JAM, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- Singh N, Singh RK, Bhunia AK, Stroshine L. Efficacy of chlorine dioxide, ozone, and thyme essential oils or a sequential washing in killing Escherichia coli O157:H7 on lettuce and baby carrots. Lebensm Wiss Technol. 2002;35:720–729. doi: 10.1006/fstl.2002.0933. [DOI] [Google Scholar]
- Solomakosa N, Govarisa A, Koidisb P, Botsoglouc N. The antimicrobial effect of thyme essential oil, nisin, and their combination against Listeria monocytogenes in minced beef during refrigerated storage. Food Microbiol. 2008;25:120–127. doi: 10.1016/j.fm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Tagg JR, Dajani AS, Wannamaker LW. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Chalchat JC, Michet A, Linhart YB, Ehlers B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J Chem Ecol. 2003;29:859–880. doi: 10.1023/A:1022927615442. [DOI] [PubMed] [Google Scholar]
- Van den D, Kratz H. Generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chrom. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Yamamoto T, Kawai Y, Inoue N. Enhancement of antilisterial activity of essential oil constituents by nisin and diglycerol fatty acid ester. Food Microbiol. 2004;21:283–289. doi: 10.1016/j.fm.2003.08.009. [DOI] [Google Scholar]
- Yu JQ, Lei JC, Yu HD, Cai X, Zou GL. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochem. 2004;65:881–884. doi: 10.1016/j.phytochem.2004.02.005. [DOI] [PubMed] [Google Scholar]