Abstract
Mixtures of coconut milk and gelatin solution were treated by ultrasound, mixed with maltodextrin and subsequently spray-dried to yield powder. The effects of ultrasonic power and sonication time on the microencapsulation efficiency (ME) and microencapsulation yield (MY) of coconut fat were investigated. The results indicated that increase in ultrasonic power from 0 to 5.68 W/g and in sonication time from 0 to 2.5 min augmented ME and MY of coconut fat. However, treatment with sonication power higher than 5.68 W/g led to a drop in fat ME and MY, mainly due to aggregation of fat particles and that blocked the adsorption of gelatin molecules on the particle surface.
Keywords: Coconut milk, Coconut fat, Coconut milk powder, Ultrasound, Encapsulation efficiency, Encapsulation yield
Introduction
Coconut milk is an oil-in-water emulsion extracted from grated coconut meat with or without added water. This natural product is highly susceptible to microbiological, chemical and biochemical deterioration (Waisundara et al. 2007). Therefore, over the years, many attempts have been done to extend shelf-life of coconut milk. One of the best methods for the preservation of coconut milk is spray-drying to yield powder (Seow and Gwee 1997).
According to Gharsallaoui et al. (2007), spray-drying, a process of microencapsulation, has been used for decades to microencapsulate food ingredients such as flavours, lipids, and carotenoids (core materials). Microencapsulation by spray drying consists of three basic steps: preparation of the emulsion; homogenization of the emulsion; and atomization of food material into the drying chamber. These authors reported that the first two steps significantly affected the microencapsulation of the core materials.
The first step is the formation of a fine and stable emulsion of the core material in the wall solution. The mixture is prepared by dispersing the core material into a solution of the coating agents (Gharsallaoui et al. 2007). The first coating agents used in the production of coconut milk powder were decaglycerol monostearate, sodium caseinate, and dextrin (Seow and Gwee 1997). Other coating agents such as maltodextrin, casein or skim milk, and/or corn syrup were also used for fat microencapsulation during the spray-drying (Seow and Gwee 1997). Recently, the use of gelatin as wall material for phospholipid microencapsulation by spray drying has attracted considerable interest (Bruschi et al. 2003; Gharsallaoui et al. 2007; Vinetsky and Magdassi 1997; Yoshii et al. 2001). Gelatin, a water-soluble material, has all the properties of an effective entrapping agent: high emulsifying activity, high stabilizing activity, and high tendency to form a fine dense network upon drying (Gharsallaoui et al. 2007). Furthermore, gelatin has the ability to stimulate the early formation of the surface crust, which prevents the loss of core material during spray drying (Gharsallaoui et al. 2007; Yoshii et al. 2001). However, gelatin has not been used for the spray drying of coconut milk yet.
In the second step, the emulsion is homogenized by high pressure or ultrasound to obtain the uniform and small fat droplets. The particle size distribution (PSD) of fat droplets plays an important role in the stability of emulsion (Jena and Das 2006) and in the microencapsulation efficiency by spray drying (Gharsallaoui et al. 2007). High pressure homogenization is a method of choice commonly used in many researches for enhancing the stability of coconut milk emulsion (Chiewchan et al. 2006; Tangsuphoom and Coupland 2008, 2009) and for preparing stable emulsion before spray-drying (Hogan et al. 2001; Liu et al. 2001; Yoshii et al. 2001). Recently, the use of ultrasound for homogenization of oil in water emulsion has attracted considerable attention (Kentish et al. 2008; Leong et al. 2011). In addition, ultrasound had a very good homogenization effect at high power levels compared with high pressure homogenization (Wu et al. 2000). In case of coconut milk emulsion, the modeling of particle size distribution of sonicated coconut milk emulsion was investigated by Jena and Das (2006) and the results showed that suitable sonication time reduced the fat droplet size.
Until now, application of ultrasound to fat microencapsulation in the production of instant coconut milk powder has not been clearly considered. Therefore, this work was aimed to investigate the effects of sonication variables on the ME and MY of coconut fat using gelatin and maltodextrin as emulsifiers. In addition, the information obtained from this work would give a clearer understanding of phenomena that happen during the ultrasonic process of coconut milk as well as other oil-in-water emulsions.
Materials and methods
Materials
Grated coconut meat was purchased from a local market in Ben Tre, Vietnam. Wall materials used in this study included gelatin and maltodextrin. Gelatin (Bloom: 150) was originated from Gelita Australia Pty Ltd (Australia) and Maltodextrin (Dextrose equivalent: 18) was purchased from Qinhuangdao Lihua Starch Co., Ltd (China). Solvents and chemicals were obtained from Guangzhou Jinhuada Chemical Reagent Co., Ltd (China).
Experimentation
Grated coconut meat was mixed with water at a weight ratio of 1:1. Subsequently, the mixture was heated to 50 °C for 10 min, filtered through a cheesecloth and pressed to extract coconut milk. Samples of the coconut milk were collected for further analysis. In this experiment, the solid content (% w/w) and total fat content (% w/w) of coconut milk were 19.54 ± 0.19 and 14.66 ± 0.25 respectively.
Emulsifier solutions were prepared as follows:
10 % (w/w) gelatin solution was obtained by dissolving gelatin in distilled water, stirring at 750 rpm, and heating at 50 °C for 6 h.
10 % (w/w) maltodextrin solution was obtained by dissolving maltodextrin in distilled water, stirring at 750 rpm, and room temperature for 1 h.
The emulsifier solutions were then filtered through a cheesecloth to ensure that all undissolved particles were eliminated.
Effect of ultrasonic power on ME and MY of coconut fat
Mixture of coconut milk and gelatin solution was homogenized by a Model VC 750 ultrasonic probe (Sonics & Materials Inc., USA) at different power levels (2.27–6.82 W/g) for 2.5 min. Samples were taken from the obtained emulsions for further analysis. The sonicated emulsions were then mixed with maltodextrin solution and stirred by a magnetic stirrer at 750 rpm for 30 min. Our preliminary investigations (unpublished data) showed that a core (gelatin) to wall ratio (w/w) of 7.5/10 and a gelatin to maltodextrin ratio (w/w) of 4:1 were the appropriate conditions for the microencapsulation of coconut fat. These ratios were therefore chosen to carry out all experiments in this study. The solid concentration of the resultant emulsion was adjusted to 15 % (w/w) by adding distilled water. The final emulsion was spray dried by a Mobile Minor—Model E spray-drier (Niro A/S, Denmark). The spray-drier was equipped with a chamber with dimensions of 0.8 m diameter and 0.6 m height, a centrifugal atomizer, a cyclone separator and an exhaust blower. The emulsion was fed into the chamber at the rate of 22.9 mL/min by a 505S peristaltic pump (Matson-Marlow, England). The drying took place with an air inlet temperature of 160 °C, outlet temperature of 42 °C and an air pressure of 0.35 MPa at the atomizer. Control samples without ultrasonic treatment were also carried out. The powder of each run was collected for further analysis.
Effect of sonication time on ME and MY of coconut fat
In this experiment, the mixture of coconut milk and gelatin solution was homogenized by ultrasound at a suitable value of sonication power obtained from the experiment in previous section. Sonication time was varied between 0.5 and 3 min. The following steps were similar to those in section “Effect of ultrasonic power on ME and MY of coconut fat”. Control samples without ultrasonic treatments were also carried out.
The sonicated emulsion and powder of each run were sampled for further analysis.
Determination of solid content and total fat content of coconut milk emulsion
Solid content of coconut milk emulsion was determined by drying at 130 ± 3 °C until constant weight (Lakshanasomya et al. 2011).
The total fat content of coconut milk emulsion was determined by using a method proposed by Lakshanasomya et al. (2011). Ten milliliters of coconut milk emulsion was taken into the fat extraction flask for analysis. Firstly, 1.5 ml of ammonium hydroxide was added and mixed followed by 10 ml of alcohol (95 %) and the contents were again well mixed. Secondly, 25 ml diethyl ether was added to the flask, then it was shook vigorously for 1 min. Finally, 25 ml of light petroleum ether (b.p. 40–60 °C) was added and the flask was shook vigorously for 1 min. After separation was complete, the fat solution was transferred into a petri dish and the petri dish was dried at 102 ± 2 °C for 1 h and weighed. The total fat content was calculated as the difference between weight of petri dish with fat and weight of initial petri dish.
Determination of surface fat content, encapsulated fat content, and total fat content of coconut milk powder
The fat on the surface of coconut milk powder particles was determined by a method described by Jafari et al. (2008). One gram of coconut milk powder was accurately weighed into the extraction flask. Subsequently, 25 ml of petroleum ether (b.p. 40–60 °C) was added and the mixture was shook vigorously for 10 min. The mixture was then filtered through a cloth. The filtrate was transferred into the petri dish, dried at 102 ± 2 °C for 1 h and weighed. The surface fat content was calculated as the difference between weight of petri dish with fat and weight of initial petri dish.
The total fat content of coconut milk powder was determined by using a method described by Lakshanasomya et al. (2011). One gram of coconut milk powder was accurately weighed into the fat extraction flask. Water was added to complete the volume to 10 ml and mixed. The total fat content in the emulsion was then determined similarly to the process in section “Determination of solid content and total fat content of coconut milk emulsion”.
The encapsulated fat content was calculated as a difference of the total fat content and the surface fat content of the powder obtained.
Microencapsulation efficiency (ME) and microencapsulation yield (MY) of coconut fat
ME and MY were calculated by formulas reported by Shu et al. (2006).
ME was defined as a ratio between the mass of the encapsulated fat and the mass of the total fat in the coconut milk powder.
MY was defined as a ratio between the mass of the total fat of the coconut milk powder and the mass of the total fat of the emulsion before spray drying.
Scanning electron microscopy (SEM) of coconut milk powder
The powder sample was placed on one surface of a double-faced adhesive tape and coated with gold by using an ion-coater E-102 (Hitachi- Japan). The S-4800 model scanning electron microscope (Hitachi, Japan) was used to study the outer surface of the coconut milk powder. The examination was operated at an accelerating voltage of 2 kV. The S-4800 software (Hitachi, Japan) was used to present the micrographs of the powder microstructure.
PSD of coconut milk emulsion
Particle size distributions of raw and homogenized coconut milk emulsion were assessed by using a Model LA 920 laser diffraction particle analyzer (Horiba, Japan). Samples were diluted to approximately 0.005 wt% in an effort to avoid multiple scattering effect. The average particle size was calculated as a volume mean diameter, or d43 (d43 = ∑ ni. D4i/∑ni. D4i) (Tangsuphoom and Coupland 2008) where ni is the number of the droplets of diameter Di.
Statistical analysis
All experiments were performed in triplicate. Mean values were considered significantly different when P < 0.05. One-Way analysis of variance was performed using the software Statgraphics Centurion XV.
Results and discussion
Effect of ultrasonic power on ME and MY of coconut fat
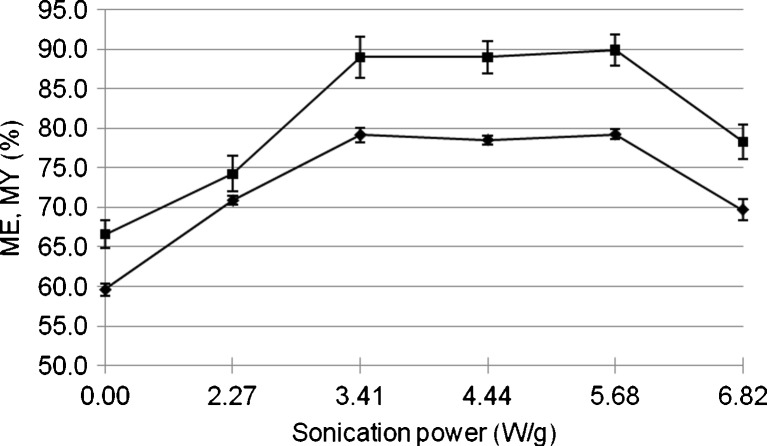
The results in Fig. 1 demonstrate that the ME and MY reached the highest level when the sonication power was between 3.41 and 5.68 W/g, then decreased when the ultrasonic power was higher than 5.68 W/g. The reasons for these changes can be explained through the effects of ultrasonic power on PSD of coconut milk emulsion. PSD of the sonicated coconut milk (treated at 3.41 W/g and 6.82 W/g) and non-sonicated coconut milk was evaluated. Emulsion without sonication exhibited a trimodal distribution as shown in Fig. 2. By contrast, emulsion sonicated at 3.41 W/g had a monomodal distribution. An interesting result from Fig. 2 was that emulsion homogenized at 6.82 W/g (the highest applied power) had a bimodal distribution.
Fig. 1.
Effect of sonication power on ME (black diamond) and MY (black square)
Fig. 2.
Effect of sonication power on fat particle size distribution of sonicated coconut milk emulsions: 3.41 W/g (black square) and 6.82 W/g (black triangle) and control (0 W/g, black diamond)
The application of low frequency ultrasound is known to cause acoustic cavitation which plays an important role in the reduction of primary droplets of oil-in-water emulsion (Kentish et al. 2008). Therefore, it would be expected that increase in ultrasonic power would improve the amount of shear force that breaks up primary droplets into the smaller ones. However, the high mean particle size (d43 ≈ 9.7 μm) was obtained in the emulsion homogenized at the highest power level (6.82 W/g). By contrast, emulsion homogenized at 3.41 W/g had the smallest particle size (d43 ≈ 6 μm). Decrease in particle size lead to increase in surface area of fat particle where gelatin molecules adsorb on. As a consequence, ME and MY of coconut fat increased when fat droplet size decreased.
Based on the results obtained, we proposed a hypothesis about the effects of sonication power on ME and MY of coconut fat. Small droplet size was reported to prevent flocculation of the droplets (Tadros 2005). Consequently, decrease in particle size will facilitate the adsorption of gelatin on the droplet surface. As a result, ME and MY increased. However, when the sonication power reached the “over-processing” levels, the coalescence of droplets enhanced the formation of bigger droplets or “aggregates” with higher diameter. Consequently, the adsorption of gelatin on the newly formed droplet interface was obstructed. In this case, we supposed that gelatin molecules just partially adsorbed on the droplet surface. As a consequence, microencapsulation efficiency of gelatin declined. In all experiments, sonicated coconut milk with gelatin was added with maltodextrin before spray-dried to yield powder. Suitable concentration of maltodextrin was believed to decrease the number of cracks on the surface of powder particles (Sheu and Rosenberg 1998). ME and MY of coconut fat were therefore enhanced. A convincing result that strongly supported our hypothesis was that powder particles in SEM pictures from 6.82 W/g sonicated and non-sonicated emulsions appeared agglomerated (Fig. 3). This phenomenon was probably due to the formation of “aggregates”. The aggregates of fat droplets in the emulsion could result in the formation of aggregates of particles in the powder obtained. By contrast, few agglomerated particles were observed in SEM picture of the powder from 3.41 W/g ultrasonic emulsion. According to Hogan et al. (2001) the powder particles that appeared highly agglomerated had the high level of surface fat content. Therefore, coconut milk powder that exhibited aggregated particles had low ME and MY of coconut fat.
Fig. 3.
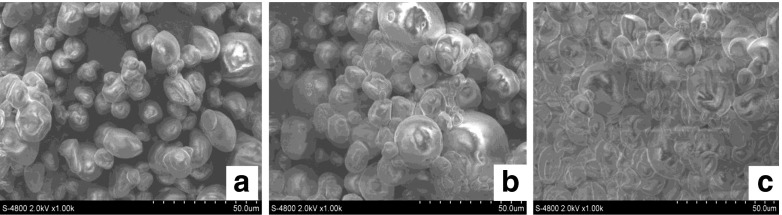
SEM pictures of coconut milk powder produced from sonicated coconut milk at different ultrasonic powers: 3.41 W/g (a), 6.82 W/g (b) and 0 W/g (c, control sample)
A similar trend between droplet size and applied sonication power has been observed by Jafari et al. (2008) and Kentish et al. (2008) who homogenized the internal phase of fish oil and flax seed oil with the emulsifiers of proteins and Tween 40, respectively. These studies showed that when the ultrasonic power reached an “over-processing” level, droplet aggregation was enhanced. Generally, ultrasound increases ME and MY of fat when the ultrasonic power lower than the “over-processing” level. Very high ultrasonic power decreases ME and MY of fat.
Effect of sonication time on ME and MY of coconut fat
Figure 4 shows that prolongation of sonication time resulted in the increase in ME and MY of coconut fat. The sonication time of 2.5 min was suitable for coconut fat microencapsulation, in which ME and MY of the treated samples increased 22.6 % and 24.0 % respectively as compared to ME and MY of the non-treated samples. Treatment with longer time did not significantly increase ME and MY of coconut fat.
Fig. 4.
Effect of sonication time on ME (black diamond) and MY (black square) of coconut fat (The ultrasonic power was 3.41 W/g)
In order to clearly understand the effects of sonication time on ME and MY of coconut fat, we determined the PSD of coconut milk emulsion treated at 3.41 W/g with different sonication times: 0 min (control sample), 1 min, 2 min, 2.5 min and 3 min. As shown in Fig. 5, coconut milk treated at 2, 2.5 and 3 min had a monomodal distribution. By contrast, non-treated and 1 min treated coconut milk with gelatin emulsion had a trimodal and bimodal distribution, respectively. The average particle size (D43) of the non-treated, 1 min, 2 min, 2.5 min, 3 min treated coconut milk were 10.3, 7.6, 7.4, 6.2 and 6.0 μm, respectively. Similar trend between sonication time and droplet size reduction was observed by Jena and Das (2006) for coconut fat microencapsulated by gum acacia and maltodextrin. As a result of droplet size reduction, ME and MY increased when sonication time increased.
Fig. 5.
Effect of sonication time on fat particle size distribution of coconut milk with gelatin emulsions: 0 min (control sample) (black diamond), 1 min (black square), 2 min (black triangle), 2.5 min (x) and 3 min (+) (The ultrasonic power was 3.41 W/g)
Conclusion
Ultrasonic treatment increased ME and MY of coconut fat due to its ability to break fat droplets into smaller size droplets. ME and MY reached the highest levels when the sonication power increased from 3.41 to 5.68 W/g. However, when the sonication power increased from 5.68 to 6.82 w/g, the formation of “aggregates” with high diameter blocked the adsorption of gelatin on particle surface and that led to a drop in MY and MY of coconut milk fat. Suitable sonication time for coconut fat microencapsulation was 2.5 min.
References
- Bruschi ML, Cardoso MLC, Lucchesi MB, Gremião MPD. Gelatin microparticles containing propolis obtained by spray-drying technique: preparation and characterization. Int J Pharm. 2003;264:45–55. doi: 10.1016/S0378-5173(03)00386-7. [DOI] [PubMed] [Google Scholar]
- Chiewchan N, Phungamngoen C, Siriwattanayothin S. Effect of homogenizing pressure and sterilizing condition on quality of canned high fat coconut milk. J Food Eng. 2006;73:38–44. doi: 10.1016/j.jfoodeng.2005.01.003. [DOI] [Google Scholar]
- Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Hogan SA, McNamee BF, O’Riordan ED, O’Sullivan M. Emulsification and microencapsulation properties of sodium caseinate/carbohydrate blends. Int Dairy J. 2001;11:137–144. doi: 10.1016/S0958-6946(01)00091-7. [DOI] [Google Scholar]
- Jafari SM, Assadpoor E, Bhandari B, He Y. Nano-particle encapsulation of fish oil by spray drying. Food Res Int. 2008;41:172–183. doi: 10.1016/j.foodres.2007.11.002. [DOI] [Google Scholar]
- Jena S, Das H. Modeling of particle size distribution of sonicated coconut milk emulsion: effect of emulsifiers and sonication time. Food Res Int. 2006;39:606–611. doi: 10.1016/j.foodres.2005.12.005. [DOI] [Google Scholar]
- Kentish S, Wooster TJ, Ashokkumar M, Balachandran S, Mawson R, Simons L. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol. 2008;9:170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- Lakshanasomya N, Danudol A, Ningnoi T. Method performance study for total solids and total fat in coconut milk and products. J Food Compos Anal. 2011;24:650–655. doi: 10.1016/j.jfca.2010.10.002. [DOI] [Google Scholar]
- Leong TSH, Wooster TJ, Kentish SE, Ashokkumar M. Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem. 2011;16:721–727. doi: 10.1016/j.ultsonch.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Liu X-D, Atarashi T, Furuta T, Yoshii H, Aishima S, Ohkawara M, Linko P. Microencapsulation of emulsified hydrophobic flavors by spray drying. Dry Technol. 2001;19:1361–1374. doi: 10.1081/DRT-100105293. [DOI] [Google Scholar]
- Seow CC, Gwee CN. Coconut milk: chemistry and technology. Int J Food Sci Technol. 1997;32:189–201. doi: 10.1046/j.1365-2621.1997.00400.x. [DOI] [Google Scholar]
- Sheu T-Y, Rosenberg M. Microstructure of microcapsules consisting of whey proteins and carbohydrates. J Food Sci. 1998;63:491–494. doi: 10.1111/j.1365-2621.1998.tb15770.x. [DOI] [Google Scholar]
- Shu B, Yu W, Zhao Y, Liu X. Study on microencapsulation of lycopene by spray-drying. J Food Eng. 2006;76:664–669. doi: 10.1016/j.jfoodeng.2005.05.062. [DOI] [Google Scholar]
- Tadros T (2005) Applied surfactants: principles and applications. Wiley - VCH
- Tangsuphoom N, Coupland JN. Effect of surface-active stabilizers on the microstructure and stability of coconut milk emulsions. Food Hydrocoll. 2008;22:1233–1242. doi: 10.1016/j.foodhyd.2007.08.002. [DOI] [Google Scholar]
- Tangsuphoom N, Coupland JN. Effect of surface-active stabilizers on the surface properties of coconut milk emulsions. Food Hydrocoll. 2009;23:1801–1809. doi: 10.1016/j.foodhyd.2008.12.002. [DOI] [Google Scholar]
- Vinetsky V, Magdassi S. Microencapsulation by Surfactant–Gelatin insoluble complex: effect of pH and surfactant concentration. J Colloid Interface Sci. 1997;189:83–91. doi: 10.1006/jcis.1997.4783. [DOI] [Google Scholar]
- Waisundara VY, Perera CO, Barlow PJ. Effect of different pre-treatments of fresh coconut kernels on some of the quality attributes of the coconut milk extracted. Food Chem. 2007;101:771–777. doi: 10.1016/j.foodchem.2006.02.032. [DOI] [Google Scholar]
- Wu H, Hulbert GJ, Mount JR. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov Food Sci Emerg Technol. 2000;1:211–218. doi: 10.1016/S1466-8564(00)00020-5. [DOI] [Google Scholar]
- Yoshii H, Soottitantawat A, Liu X-D, Atarashi T, Furuta T, Aishima S, Ohgawara M, Linko P. Flavor release from spray-dried maltodex-trin/gum Arabic or soy matrices as a function of storage relative humidity. Innov Food Sci Emerg Technol. 2001;2:55–61. doi: 10.1016/S1466-8564(01)00019-4. [DOI] [Google Scholar]