Abstract
The effect of postharvest treatments on storage characteristics of harvested apricots in relation to fruit quality was investigated. ‘Xiaobai’ apricots treated with 1-methylcyclopropene (1-MCP), chlorine dioxide (ClO2), calcium, and heat in sealed container and then stored at 20 °C with 90 % relative humidity (RH) for 10 days. Results showed that the treatments could reduce respiration production and MDA content, delay softening, postharvest decay, the decrease of soluble solids (SSC), and visual changes. Furthermore, the polyphenol oxidase (PPO), polygalacturonase (PG), and pectin methylesterase (PME), superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) activities were reduced by treatments. Taken together, it is suggested that ClO2 treatment might be an effective way to maintain the quality of apricot fruit except 1-MCP treatment.
Keywords: Apricots, Quality, 1-MCP, ClO2, Calcium treatment, Heat treatment
Introduction
Xiaobai apricots (Prunus armeniaca L.), an economically important variety of plant grown in China’s Xinjiang region, are famous for their crisp, juicy texture and sweet flavor. This, along with important nutrient contributions from phytochemical constituents, makes them highly favored by consumers worldwide. However, apricots mature during the hot season and have a short postharvest shelf life because they are susceptible to microbial decay, mechanical damage, and nutritional losses (Amoros et al. 1989). Apricots are climacteric and the ripening process is regulated by ethylene. Low temperatures and modified-atmosphere storage are commonly used to inhibit fruit decay and extend postharvest life. However, the apricots can develop some physiological problems, including firm and juiceless flesh and internal browning after 2–3 weeks of storage at 1–4 °C (DeMartino et al. 2002). There is still a need, therefore, for more effective apricot storage techniques.
1-methylcyclopropene (1-MCP), a synthetic cyclic olefin, blocks ethylene receptors, inhibiting ethylene action and its detrimental consequences to plant tissues (Sisler and Serek 1997). Chlorine dioxide (ClO2) is a strong oxidizing and sanitizing agent that has broad and intense biocidal efficacy. The use of ClO2 as a potential antimicrobial treatment for fruits and vegetables has risen, and the FDA now allows the ClO2 as an antimicrobial agent in water used to wash fruits and vegetables (Lee et al. 2006). Previous studies performed in our laboratory have shown that 1-MCP and ClO2 treatments exhibited significant effects on controlling ripening and senescence in many harvested fruits, such as melons, peaches, and grapes (Xiao et al. 2009; Xie et al. 2009; Zhong et al. 2006).
Postharvest calcium dips can increase calcium content without causing fruit injury, depending on the salt type and calcium concentration. Many studies have examined the effects of calcium on fruit firmness and decay after harvest (Garc et al. 1996; Picchioni et al. 1998). Heat treatment can prolong the shelf-lives of several fruit crops (Lurie 1998). It can also prevent browning in some commodities (Loaiza et al. 2003).
To the best of our knowledge, very few studies have been published regarding the effects on postharvest physiology or any optimal method for maintaining the quality of Xiaobai apricots, and what studies there are have mainly focused on qualitative characteristics or fungal resistance. The objectives of this study were to evaluate the effectiveness of four different postharvest treatments on the shelf-life of the Xiaobai apricot and to study the effects of the best treatment on the physiology and quality of apricots stored at room temperature.
Materials and methods
Plant materials
Apricots (Prunus armeniaca L.) cv. Xiaobai at commercial harvest maturity (SSC 10.5 ± 0.9 % and firmness 12.5 ± 2.2 N) were purchased from local a wholesale market in Urumqi, Xinjiang province, China. Fruits selected for uniform size and the absence of visual symptoms of any disease or blemishes were harvested. The fruits were immediately transported in bulk to the laboratory and stored at 10–15 °C overnight before processing.
Treatments
Apricots were washed with running water and dried at room temperature with forced air. Fruits were randomly divided into five treatment groups, each containing 90 fruits. Three replicates per treatment were performed and evaluations were made every day for up to 10 days at room temperature (20 ± 1 °C). Five different treatments were applied as follows:
Control: Fruits were incubated at 20 ± 1 °C for 24 h.
1-MCP: Fruits were treated with 1.0 μl L−1 1-MCP vapor in a sealed incubator at 20 ± 1 °C for 24 h.
ClO2: Fruits were treated with 1.0 μl L−1 ClO2 vapor in a sealed incubator at 20 ± 1 °C for 24 h.
Calcium: Fruits were dipped in 0.5 % CaCl2 at 20 °C for 5 min, then dried with 20 ± 1 °C forced air for 1 min.
Heat: Fruits were dipped in 34 °C hot-water for 5 min, then dried with 20 ± 1 °C forced air for 1 min.
Each treatment group was packed in three foam plates (30 fruit each), placed in perforated polyethylene bags, and then stored for 10 days at room temperature.
Application of 1-MCP and ClO2
1-MCP was obtained from a commercial powder (EthylBloc, Rohm and Haas China, Inc.) by adding to it distilled water according to the manufacturer’s instructions, with concentrations determined. ClO2 gas was released from a 0.5 g ClO2 generator and prepared as 1.0 μl L−1 concentrated stock in a 1-L sealed bottle (Guo et al. 2010).
The fruits were kept in hermetically sealed plastic drums (20 L), 1.0 μl L−1 1-MCP and 1.0 μl L−1 ClO2 gas was injected into the drums through a rubber septum. 1-MCP and ClO2 levels within the container were measured by taking 1 ml gas samples from the plastic jars and injecting them into a gas chromatograph (GC-14B, Shimadzu, Japan) with a FID detector and a fused silica capillary column. The carrier gas was N2 at 50 ml min−1, and the oven and detector were maintained at 180 °C.
Determination of respiration rate
Three groups of samples with 6 fruits per group were enclosed in a 1.9 L container and incubated for 2 h at 20 °C. A 1.0 ml gas sample was withdrawn from the headspace, and the concentration of CO2 was measured using G-3900 Gas Chromatograph (Shimadzu/Hitachi Corp. Ltd., Japan) equipped with a porapak-Q column and thermal conductivity detectors (TCD). Helium was used as the carrier gas with a head pressure of 180 kPa. The oven temperature was maintained at 50 °C. Both the injector and detector temperatures were kept at 150 °C. The respiration rates were measured as CO2 emissions and calculated and expressed in mmol kg−1 h−1 (Varoquaux et al. 2002).
Fruit firmness
Fruit firmness was measured at six points on ten fruits per replicate using an Instron Harness Tester 5542 (INSTRON, Norwood, NJ, U.S.) equipped with a 12 mm-diameter cylindrical tip. Data were expressed as kg/cm2.
Determination of total phenolic content
Total phenolic content in the methanol extracts was estimated colorimetrically using Folin–Ciocalteu (FC) reagent, as described by Singleton and Rossi (1965) and expressed as mg gallic acid equivalents per 100 g of fresh mass. Briefly, 5 g of sample were extracted with 50 ml methanol. One milliliter of this extract was mixed with 9 ml of distilled H2O in a 25 ml volumetric flask; 1 ml of FC reagent was added and the mixture was shaken. After 6 min, 10 ml of Na2CO3 (7 %) were added and the volume was brought up to 25 ml with distilled H2O. After incubation at room temperature for 90 min, the absorbance of the reaction mixture was measured at 760 nm against a reagent blank using a UV–visible spectrophotometer (Shimadzu-2450, Tokyo, Japan).
Determination of PPO activity
Polyphenol oxidase (PPO) activity was determined according to Fu et al. (2007). 1.0 g of frozen tissue was homogenized in 5 ml of 0.2 mol L−1 sodium phosphate buffer (pH 6.5) containing 1 % polyvinylpyrrolidone (PVP). One unit of PPO activity was defined as the amount of enzyme that caused a 0.01 increase in absorbance at 410 nm in 1 min under the specified conditions.
Determination of MDA content
MDA content was measured according the method described by Xu et al. (2009) and expressed as μmol per gram dry weight. Apricot pulp tissue (3 g) was added to a 5.0 ml of 0.05 M phosphate buffer (pH 7.8) and 0.4 g of PVP (insoluble) at 4 °C and centrifuged at 10,000×g for 15 min. One milliliter of supernatant was added to 3.0 ml of 0.5 % (w/v) thiobarbituric acid in 10 % (w/v) trichloroacetic acid. The mixture was heated and maintained at 100 °C for 30 min and then quickly cooled on ice. After centrifugation at 10,000×g for 10 min, the levels of A532, A600, and A440 in the supernatant were recorded. The value for non-specific absorption at 600 nm was subtracted and a standard curve of sucrose (from 2.5 to 10 μmol/ml) was used to rectify the results from the interference of soluble sugars in samples, reading A532 and A440. MDA content was calculated using an absorption coefficient of 157 mmol−1 cm−1 and expressed as μmol MDA g−1 (FW)
Determination of SOD, CAT, and POD activity
Superoxide dismutase (SOD) was extracted from 2.0 g tissue with 5 ml of 50 mmol L−1 sodium phosphate buffer (pH 7.8) at 4 °C. The homogenate was centrifuged at 4,000×g for 20 min at 4 °C (Superspeed Centrifuge, TGL- 16G, Shanghai, China) and the supernatant was used to determine SOD activity by the method described by Giannopolitis and Ries (1977) in a final volume of 3 ml containing 0.1 ml crude enzyme extract. One unit of SOD activity was defined as the amount of enzyme that caused a 50 % inhibition of pyrogallol. The specific SOD activity was expressed as U mg−1 protein.
Catalase (CAT) activity was analyzed according to the methods described by Wang et al. (2005). The reaction mixture consisted of 2 ml sodium phosphate buffer (50 mmol L−1, pH 7.0), 0.5 ml H2O2 (40 mmol L−1), and 0.5 ml enzyme extract. The decomposition of H2O2 was measured by the decline in absorbance at 240 nm and 25 °C. CAT specific activity was expressed as U g−1 FW, per U = 0.1△absorbance240 nm min−1.
Peroxidase (POD) activity, using guaiacol as a substrate, was assayed by the method described by Zhang et al. (2005) in a reaction mixture (3 ml) containing 25 μl of enzyme extract, 2.78 mL of 0.05 M phosphate buffer (pH 7.0), 0.1 ml of 20 mM H2O2, and 0.1 ml of 20 mM guaiacol. An increase in POD activity at 470 nm, due to guaiacol oxidation, was recorded after 2 min. One unit of enzyme activity was defined as the amount that caused a change of 0.01 in the absorbance per minute.
Determination of PG and PME activity
Polygalacturonase (PG) activity was determined according to the method described by Zhou et al. (2000). One unit of activity was defined as 1 μg galacturonic acid released per mg protein per hour. Pectinmethylesterase (PME) activity was also determined according to the method described by Zhou et al. (2000). Five milliliters of enzyme extract was mixed with 20 ml 1 % citrus pectin and titrated with 0.01 mol L−1 NaOH to maintain pH 7.4 while the mixture was incubated at 30 °C. The reaction was measured for 30 min. One unit activity was calculated as 1 mmol L−1 NaOH consumed per mg protein per hour, and the specific activity was expressed as U mg−1 protein. The content of protein in crude enzyme extraction was assayed as mentioned above and each experiment was performed in triplicate
Quality evaluation
Fruit appearance
Three judges scored the apricots for changes in color, visible structural integrity, taste, and odor. The visual quality score was based on the following scale: 5, excellent; 4, very good; 3, good, limit of marketability; 2, fair, limit of usability; 1, poor, inedible.
Soluble solid content (SSC)
SSC was determined with a digital refractometer (Atago, Japan) and expressed as a percentage.
Weight loss
Weight loss was determined by weighing the fruits at the beginning of the experiment just after storage and every 2 days thereafter during the storage period. Weight loss was expressed as the percentage of the initial total weight lost. For each measurement, ten fruits from each treatment group were used and each experiment was performed in triplicate.
Rot index of fruits
In each treatment, 50 fruits were selected for determining the number of rotten fruits. All fruits were classified in four ranks by the extent of rot: 0, not rotten; 1, rotten surface took up less than 1/3 of the total surface; 2, rotten surface took up between 1/3 and 2/3 of the total surface; 3, the rotten surface took up more than 2/3 of the total surface. The rot index was expressed as the following equation: Rot index = ∑ (rank × quantity)/(4 × 50) × 100%.
Statistical analysis
Each experiment was repeated three times and statistical analyses were performed using Microsoft Excel software and SPSS 13.0 software. P-values were determined by t-test. Data are presented as the mean ± standard error of the mean (S.E.M.). Data were treated for multiple comparisons by analysis of variance with least significant difference (L.S.D.) between averages determined at 5 % level.
Results and discussion
Respiration rate
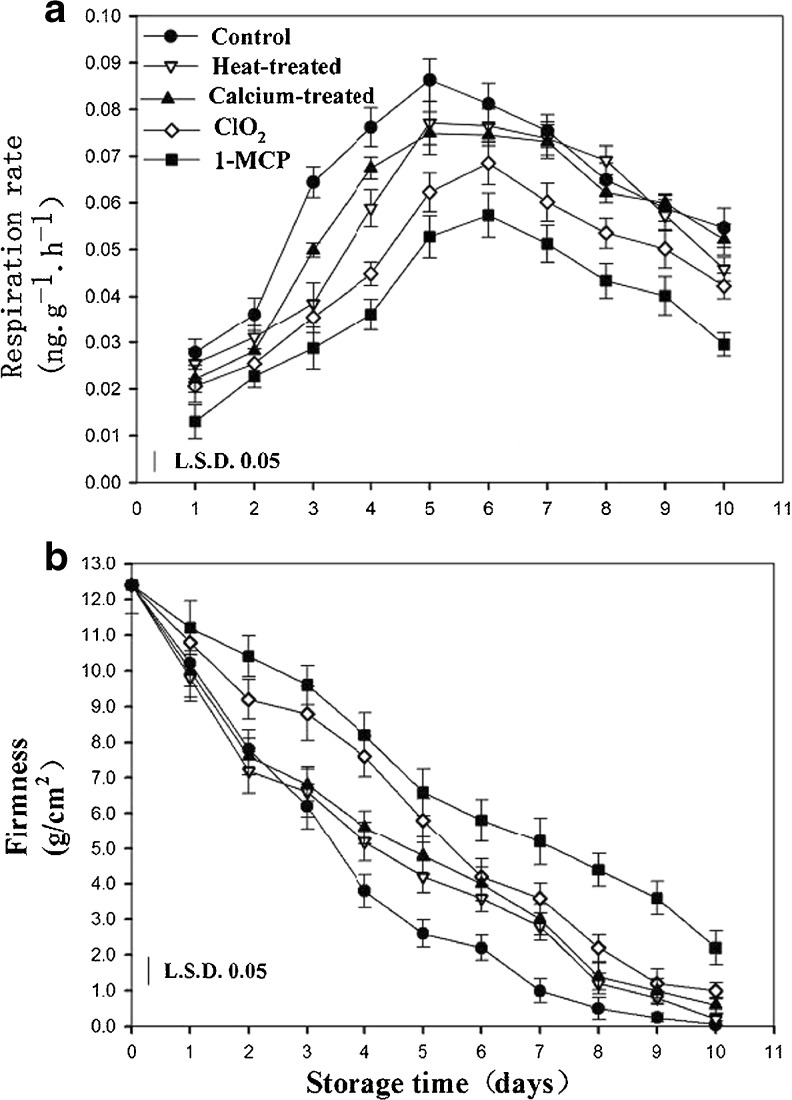
Respiration is a major factor contributing to postharvest loss which provides the energy for plant biochemical processes, and accelerates plant ripening and senescence. In the present study, the respiration rates in control fruit increased rapidly, reaching a maximum at day 5, and then gradually decreased during storage (Fig. 1a). The respiration rates in 1-MCP, ClO2, calcium and heat treated fruit exhibited a similar trend to the control while the levels of CO2 production were lower than that in the control fruit for the initial 9 days. After that day, the CO2 production in fruit treated with calcium and heat treatments had no significant differences compared to the control fruit, but the levels of CO2 production in 1-MCP and ClO2 treated fruit were significantly lower during the entire storage period. The value of peaks in 1-MCP, ClO2, calcium and heat treated fruit were reduced about A% B%, C%, and D% respectively, and the peaks were retarded for 1 day in 1-MCP and ClO2 treated fruit compared to the control fruit. These results indicate that 1-MCP and ClO2 are more effective than the other two treatments in inhibition of respiration rates during 10 days of storage at 20 °C, which are in agreement with the previous reports suggesting the beneficial effects of 1-MCP and ClO2 in reducing respiration rates in apricots (De Martino et al. 2006; Zhong et al. 2006; Guo et al. 2013).
Fig. 1.
Effects of different postharvest treatments on respiration rate (a) and firmness (b) of apricot fruits during storage at room temperature. Vertical bars in the chart represent the standard error (n = 3)
Fruit firmness and weight loss
The softening in apricots is one of the most important features during ripening, directly influencing postharvest storage and commercial value of the fruit. In this work, firmness in control fruit declined rapidly during storage indicating that the fruits softened quickly (Fig. 1b). There was almost no obvious change of firmness among the control, calcium and heat treated fruit during the first 3 days of storage. 1-MCP and ClO2 treatments resulted in significantly higher firmness compared to the control fruit, which were 5.8 and 5.2-fold higher than that of in control fruit. The results demonstrated that these treatments, especially 1-MCP treatment, delayed the decrease of firmness, which was consistent with a previous study by De Martino et al. (2006) who showing less tissue deformability treated by 1-MCP. We observed that the firmness in apricots dropped dramatically with the increase in respiration rates before the fifth day, while after the 8th day there was no marked change in both respiration rates and firmness. These results indicate that accelerated respiration rates are susceptible to leading to fruit softening.
Fruit weight loss is mainly associated with respiration and moisture evaporation through the skin. The thin skin of apricot fruits makes them susceptible to rapid loss of water resulting in shriveling and deterioration. In our study, the loss of water in control fruit increased gradually during storage (Table 1). Weight loss in 1-MCP, ClO2, calcium, and heat treated fruit remained at a significantly low level compared to the control fruit at all-time points examined. At the end of the storage period, control fruit showed a 2.81 % loss in weight, while the weight losses in fruit treated with 1-MCP, ClO2, calcium, and heat were 1.65 %, 2.02 %, 2.21 %, and 2.29 %, respectively. It has been reported that the use of vapors of main components of several essential oils reduced weight loss in grapes and cherries (Serrano et al. 2005; Valverde et al. 2005), the mechanism of the protective effect still being unknown.
Table 1.
Effects of different postharvest treatments on quality parameters of Xiaobai apricots during storage at room temperature
| Parameters | Storage time (d) | Treatments | ||||
|---|---|---|---|---|---|---|
| Control | 1-MCP | ClO2 | Calcium | Heat | ||
| Fruit appearance | 3 | 4.0d | 4.7a | 4.6ab | 4.4b | 4.2c |
| 6 | 3.0d | 4.1a | 3.9ab | 3.5b | 3.3c | |
| 9 | 2.0 c | 3.6 a | 3.2 ab | 2.7 b | 2.5 b | |
| SSC (%) | 3 | 10.5 ± 0.3a | 10.8 ± 0.2a | 10.5 ± 0.3a | 10.7 ± 0.3a | 10.3 ± 0.2a |
| 6 | 15.7 ± 0.4b | 16.0 ± 0.2a | 16.2 ± 0.2a | 15.5 ± 0.5b | 15.5 ± 0.4b | |
| 9 | 15.8 ± 0.5 a | 16.5 ± 0. 5 a | 16.5 ± 1.2 a | 15.3 ± 0.6 a | 14.5 ± 0.8 a | |
| Weight loss (%) | 3 | 0.54 ± 0.12a | 0.25 ± 0.08d | 0.37 ± 0.07c | 0.49 ± 0.11b | 0.37 ± 0.08c |
| 6 | 1.74 ± 0.38 | 0.98 ± 0.11c | 1.08 ± 0.62bc | 1.59 ± 0.78b | 1.53 ± 0.18b | |
| 9 | 2.81 ± 0.25 a | 1.65 ± 0.22 c | 2.02 ± 0.33 bc | 2.21 ± 0.48 b | 2.29 ± 0.52 b | |
| Rot index (%) | 3 | 3.86a | 0b | 0b | 0b | 0b |
| 6 | 46.2 ± 2.2a | 3.2 ± 0.2 d | 7.5 ± 0. 5c | 12.8 ± 3.3bc | 16.5 ± 1.8b | |
| 9 | 87.6 ± 4.5 a | 34.3 ± 2.4 d | 47.5 ± 3.2 c | 53.8 ± 4.1 bc | 60.3 ± 3.5 b | |
Values = Means f standard deviations (n = 3)
Values in the same row with different small letters are significantly different (P < 0.05)
Fruit appearance, soluble solid content (SSC) and rot index of fruit
Appearance is a major criterion for determining the acceptability of products. As shown in Table 1, visual quality scores in control fruit gradually decreased to 3 at day 6, which indicated that fruit is good for market (limit of marketability). However, in treated fruits, visual quality scores showed the same trends as in the control and remained at significantly high levels compared to the control fruit during storage (Fig. 1f). At day 3, visual quality scores in fruit treated by 1-MCP, ClO2, calcium, and heat were about 1.4-, 1.3-, 1.2-, 1.1-folds than that of control fruit.
The SSC in control fruit exhibited a similar trend to the treated fruit, increased continuously during storage. However, there was almost no obvious change of SSC between the control and treated fruit during apricots storage. It was found that the rot index in control fruit increased rapidly during 9 days storage (Table 1). The rot indexes in treated fruit shared the same trends as in the control fruit, but significantly lower than that in the control fruit during storage, and no rot was observed in treated fruit at day 3. At day 9, the rot index in 1-MCP, ClO2, calcium, and heat treated fruit were reduced by 60.8 %, 45.8 %, 38.6 % and 31.2 %, respectively.
The dates above suggest that 1-MCP and ClO2 are effective in preserving quality of apricot fruit, indicating that ClO2 is not only an antimicrobial agent, but it also could maintain quality of fruit during ripening process. Numerous reports have demonstrated the beneficial effects of 1-MCP treatment on fruits and vegetables. However, Mahmoud et al. (2007) found that strawberries treated with ClO2 did not affect quality parameters including external colour and visual appearance. Other authors have detected negative effect of ClO2 treatment on sensory quality of fruits and vegetables, such as white blushing (Sy et al. 2005). The difference could be attributed to the fruit, the time of harvest and the ClO2 concentration used.
Total phenolic content and PPO activity
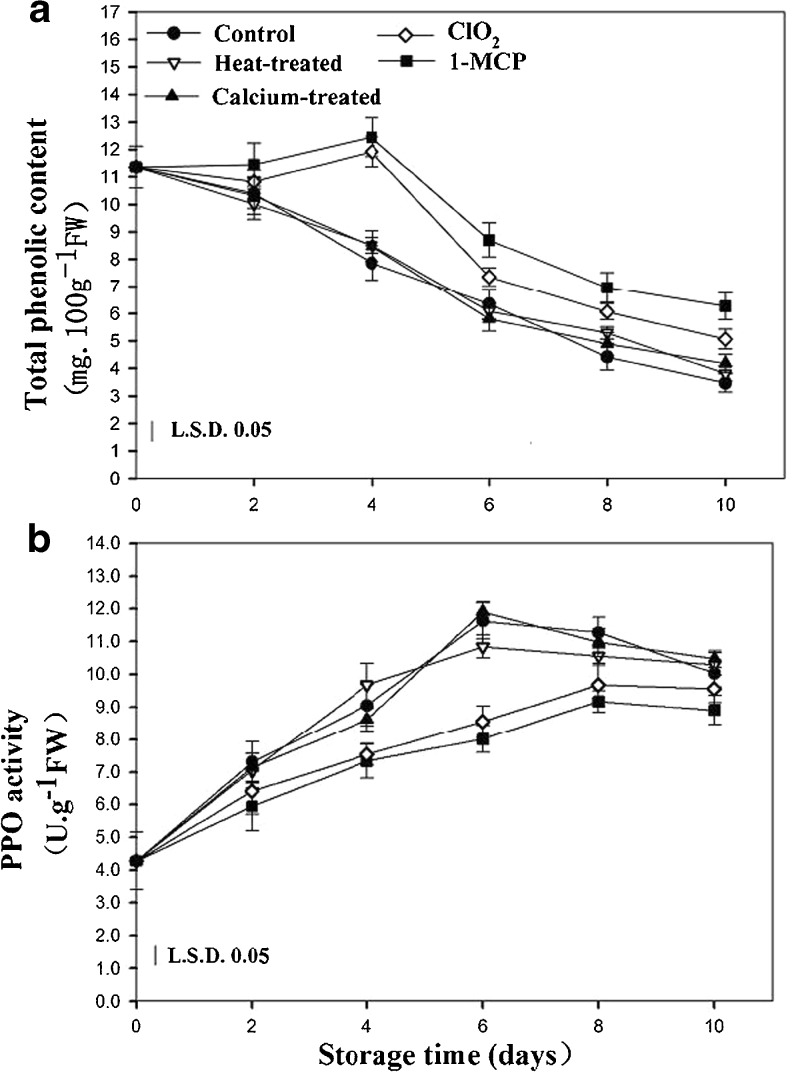
It is generally accepted that PPO-mediated oxidation of phenols results in the formation of brown substances in fruits and vegetables (López-Serrano and Ros Barceló 1999). For this reason, both phenolic content and PPO activity have been found to be closely related to the degree of browning (Lee et al. 1990). In the present study, the total phenolic content in control fruit decreased during storage (Fig. 2a). The total phenolic content in calcium and heat treated fruits exhibited similar trends to the control, but no significant difference was notated during the entire storage period. It is found that the total phenolic content in 1-MCP and ClO2 treated fruit gradually decreased with a notable increase at day 4, while the contents of the total phenolic are significantly higher than the other treatments.
Fig. 2.
Effects of different postharvest treatments on total phenolic content (a) and PPO activity (b) of apricot fruits during storage at room temperature. Vertical bars in the chart represent the standard error (n = 3)
The PPO activity in control fruit increased gradually and reached a maximum at day 6, and then decreased steadily (Fig. 3c). In calcium and heat treated fruit, PPO activity showed the same trend as in the control but no obvious changes were observed compared to the control fruit during apricots storage. The activities of PPO in 1-MCP and ClO2 treated fruit were significantly reduced and the peaks were retard for 2 days.
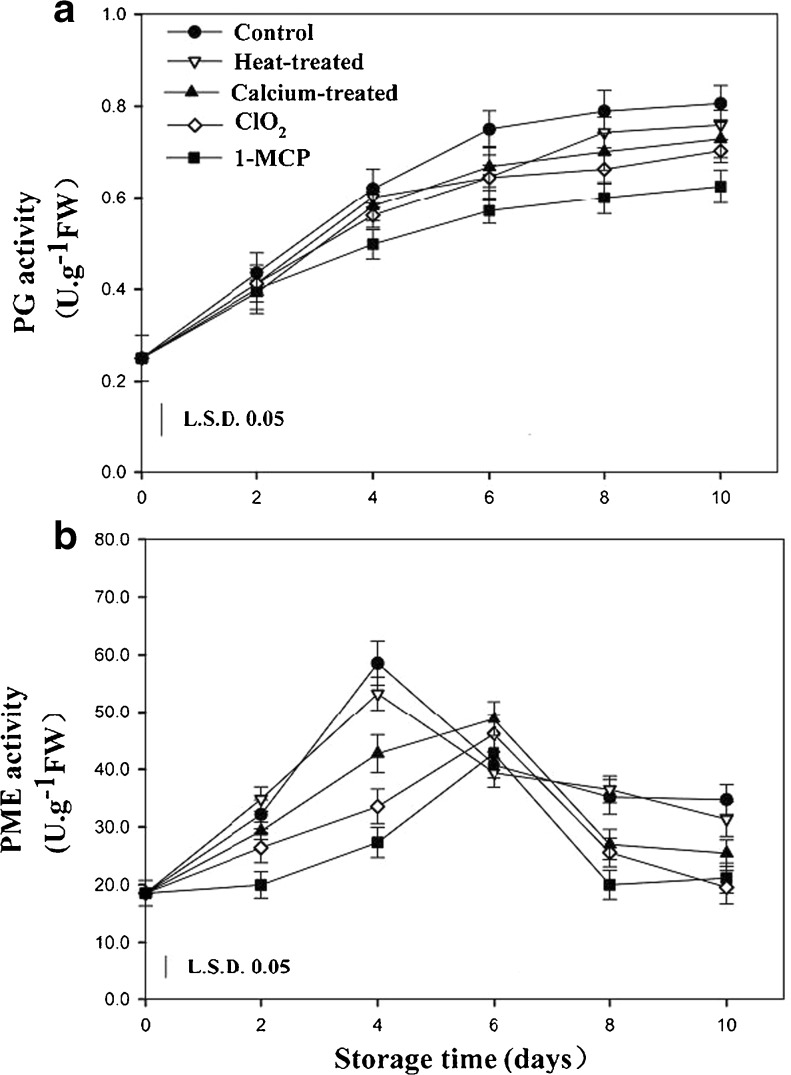
Fig. 3.
Effects of different postharvest treatments on PG (a) and PME (b) activity of apricot fruits during storage at room temperature. Vertical bars in the chart represent the standard error (n = 3)
These results showed that total phenolic content was continuously reduced, while the tendency of PPO activity first increased and then decreased. Similar results were reported by Xu et al. (2009) who reported that these phenomena might be explained by an increase in the consumption of phenols alongside increased PPO activity during fruit senescence and, to a certain degree, the gradual decrease in PPO activity could be due to a lack of substrates. Our study indicated that 1-MCP and ClO2 treated fruits showed dramatically higher total phenolic content and lower PPO activity than other fruits.
PG and PME activity
PG is considered to be one of the most important enzymes in soft ripening of fruits as PG breaks down the pectin polysaccharides (Hadfield and Bennett 1998). Partial inactivation of PG can result in reduction of pectin solubilisation and slower softening (Fernández-Trujillo et al. 2000). In the present study, the activity of PG in control fruit increased gradually during storage at 20 °C, while the PG activity in treated fruit had no significantly differences compared to the control fruit in the initial 2 days (Fig. 3a). After that day, 1-MCP treatment significantly reduced PG activity, but ClO2, calcium, and heat treatments showed no obvious change compared to the control fruit. PG needs de-esterified homogalacturonic acid as a substrate, and PE can catalyze the de-methylation of the C6 carboxylic acid group in galacturonosyl residues, so PE could be necessary for optimal PG activity (Lurie et al. 2003).
Our results showed that PME activity in control fruit increased gradually, reaching a maximum at day 4, and then a decline occurred (Fig. 3b). No significant differences between the control and heat treated fruits during the 10 days of storage. The activities of PME in 1-MCP, ClO2 and calcium treated fruits were significantly lower than that in the control fruit and the value of peaks were reduced by A %, B %, and C % respectively compared to the control fruit. So the apricots treated with 1-MCP,ClO2 and calcium can maintain significantly higher firmness than the other fruits after 2 days of storage, in agree with our results (Fig 1b). There may be different mechanism of promoting the firmness of peaches between 1-MCP and ClO2 treatments. 1-MCP treatment could influence membrane lipid composition of peach (Zhu et al. 2006) in the storage life and preserve its cellular integrity (Zhu et al. 2009). Therefore, protection of the biomembrane and the cellular integrity may be the reason that 1-MCP treatment can maintain firmness of apricots. It is presumed that ClO2 treatment could maintain fruit firmness attributing to preservation of tissue integrity by killing bacteria to further reduce the rate of rot and water loss (Table 1).
MDA content
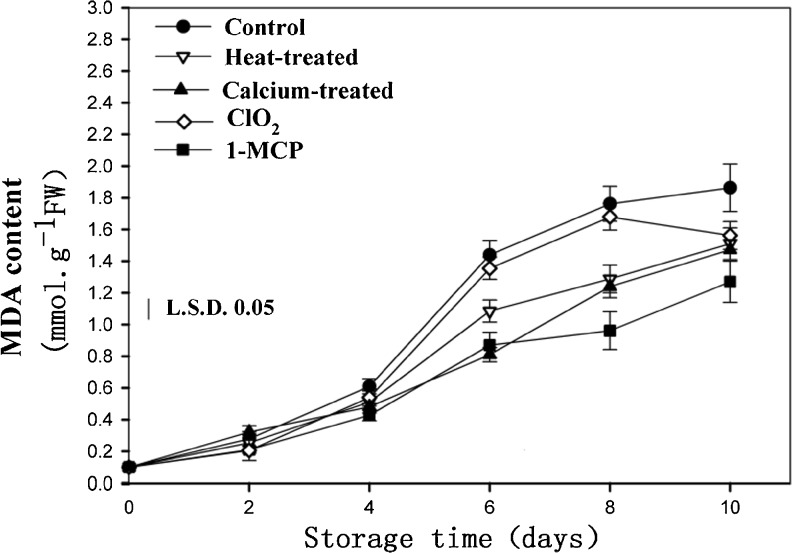
MDA is one of the most important products of membrane lipid peroxidation during vegetable senescence. The level of MDA can be used as an index to evaluate vegetable senescence (Yan et al. 2004). As can be seen, there was an increment in the MDA difference during storage as a consequence of the ripening of the fruit, but there was no obvious difference between the control and treated fruit during the first 4 days storage (Fig. 4). However, the MDA content in fruit treated with 1-MCP, calcium, and heat were significantly lower than that in the control fruit as reported for fruit. The result indicated that 1-MCP could protect cell membrane integrity and reduce loss of membrane semi-permeability and membrane lipid peroxidation
Fig. 4.
Effects of different postharvest treatments on MDA content of apricot fruits during storage at room temperature. Vertical bars in the chart represent the standard error (n = 3)
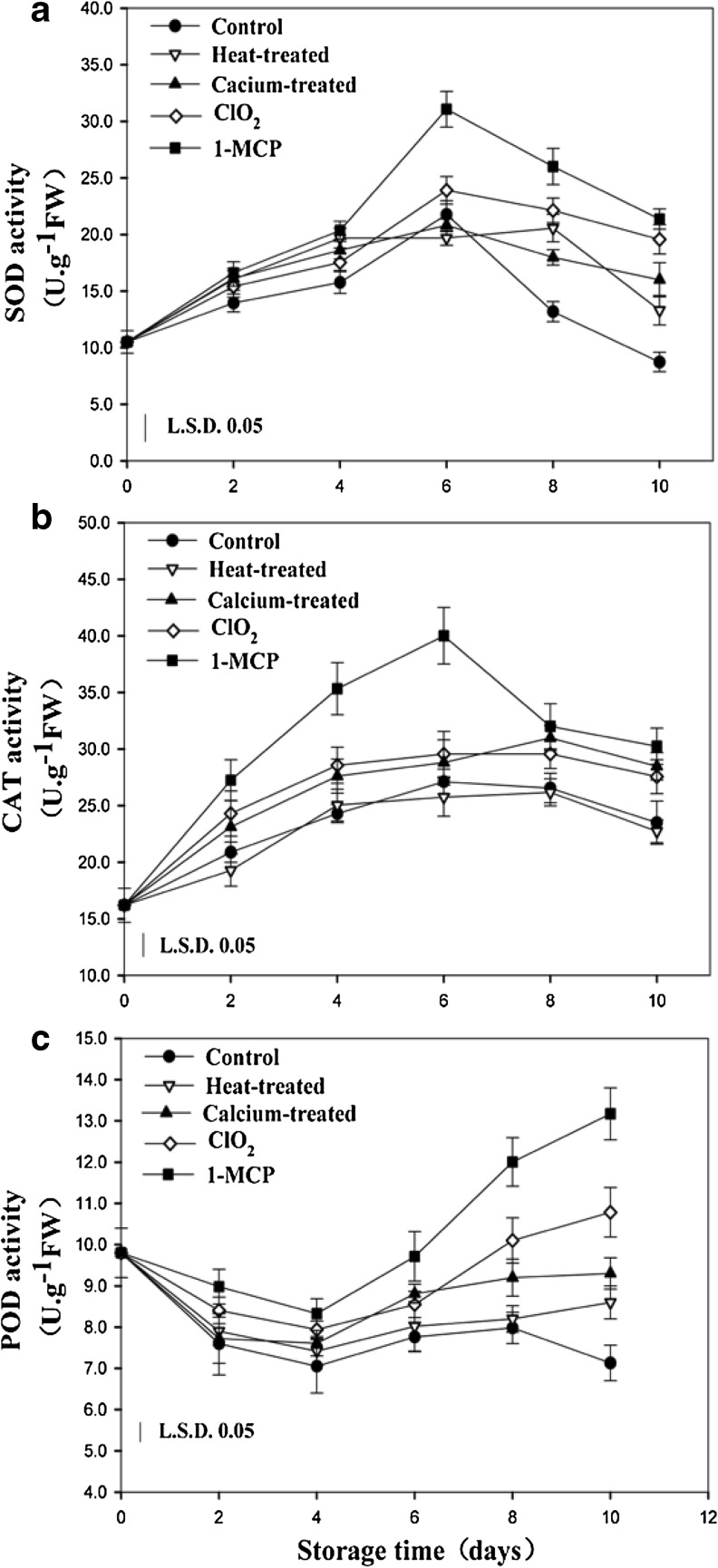
SOD, CAT, and POD activity
Through MDA content are widely used by researchers to indirectly evaluate cell membrane integrity (Aghdam and Bodbodak 2013), oxidative stress from excess oxygen species (ROS) has been associated with the appearance of membrane in fruits (Hodges et al. 2004). The toxicity of ROS is due to their reactions with numerous cell components causing a cascade of oxidative reactions and the consequent inactivation of enzymes including SOD, CAT and POD (Scandalios 1993). As shown in Fig. 5, the activity of SOD in control fruit increased gradually, reached a peak at day 6, and then decreased during storage. SOD activity in fruit treated with ClO2, calcium, and heat had no significant differences compared to the control fruit while the activity of SOD in fruit treated with 1-MCP remained at significantly higher level than the control fruit.
Fig. 5.
Effects of different postharvest treatments on SOD (a), CAT (b), and POD (c) activity of apricot fruits during storage at room temperature. Vertical bars in the chart represent the standard error (n = 3)
Changes in CAT activity were consistent with the changes of SOD activity, increased slightly and then decreased during storage. The activity of CAT in heat treated fruit had no obvious change compared to the control fruit, while CAT activity in ClO2 and calcium treated fruit was higher than that of control fruit. 1-MCP treatment significantly induced the activity of CAT activity during storage. POD activity in control fruit showed slightly decrease until day 4, and then increase sharply in the rest of storage days. In the treated fruit, the activity of POD showed the same trends as in the control and exhibited a higher level than that of control fruit.
The coordinated action of antioxidant enzymes such as SOD, CAT and POD are very important for scavenging ROS to protect cell membranes, which is thought to be a major mechanism of resistance to senescence (Imahori et al. 2008; Sevillano et al. 2009). Our results suggest that the activities of these enzymes, to some extent, were induced by treatments. The results obtained here confirm ClO2 made by our lab may be an effective method to induce the activities of SOD, CAT and POD to enhance the oxidation resistance of apricot fruit.
Conclusions
The results of the above experiments indicate that, relative to controls, all of the four treatments contributed to maintaining the quality of apricots by reducing respiration rate, MDA content, and the activity levels of both PPO and pectase (PG and PME), delaying softening, decay development, and changes in visual quality as well as increasing the antioxidant capacity and activity of antioxidant enzymes. Specifically, 1-MCP treatment exhibited certain psychological and biochemical roles for postharvest quality of apricots during storage at room temperature. The inhibition of ripening and the subsequent delay in softening caused by the 1-MCP treatment involved the maintenance of membrane integrity, alleviation of lipid peroxidation, and enhancement of antioxidant ability and free radical scavenging capacity.
These results demonstrated that 1-MCP treatment might be a useful way of maintaining the quality and extending the shelf-life of apricots during whole storage at room temperature.
Acknowledgments
Our research was supported by China Postdoctoral Science Foundation funded project (2012M521826) '12th Five-Year Plan' National Key Technology R & D program of China (No. 2011BAD27B01-01-02).
Contributor Information
Bin Wu, Email: xjuwubin0320@sina.com.
Feng-bin Che, Email: chefbxaas@sina.com.
References
- Aghdam MS, Bodbodak S. Physiological and biochemical mechanisms regulating chilling tolerance in fruits and vegetables under postharvest salicylates and jasmonates treatments. Sci Hortic. 2013;156:73–85. doi: 10.1016/j.scienta.2013.03.028. [DOI] [Google Scholar]
- Amoros A, Serrano M, Riquelme F, Romojaro F. Levels of ACC and physical and chemical parameters in peach development. J Hortic Sci. 1989;64(6):171–175. [Google Scholar]
- De Martino G, Vizovitis K, Botondi R, Bellincontro A, Mencarelli F. 1-MCP controls ripening induced by impact injury on apricots by affecting SOD and POX activities. Postharvest Biol Technol. 2006;39(1):38–47. doi: 10.1016/j.postharvbio.2005.09.002. [DOI] [Google Scholar]
- Demartino G, Massantini R, Botondi R. Temperature affects impact injury on apricot fruit. Postharvest Biol Technol. 2002;25:145–149. doi: 10.1016/S0925-5214(01)00165-X. [DOI] [Google Scholar]
- Fernández-Trujillo JP, Cano A, Artés F. Interactions among cooling, fungicide and postharvest ripening temperature on peaches. Int J Refrig. 2000;23(6):457–465. doi: 10.1016/S0140-7007(99)00067-5. [DOI] [Google Scholar]
- Fu Y, Zhang K, Wang N, Du J. Effects of aqueous chlorine dioxide treatment on polyphenol oxidases from Golden Delicious apple. LWT Food Sci Technol. 2007;40:1362–1368. doi: 10.1016/j.lwt.2006.11.001. [DOI] [Google Scholar]
- Garc AJM, Herrera S, Morilla A. Effects of postharvest dips in calcium chloride on strawberry. J Agric Food Chem. 1996;44:30–33. doi: 10.1021/jf950334l. [DOI] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. J Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Wu B, Xiao W-L,Wang, J-D (2010) Preparation and application of slow-released solid chlorine dioxide preservatives. Food Sci 31(18):441–444.
- Guo Q, Lv X, Xu F, et al. Chlorine dioxide treatment decreases respiration and ethylene synthesis in fresh-cut ‘Hami’ melon fruit. Int J Food Sci Technol. 2013;48(9):1775–1782. doi: 10.1111/ijfs.12149. [DOI] [Google Scholar]
- Hadfield KA, Bennett AB. Polygalacturonases: many genes in search of a function. J Plant Physiol. 1998;117(2):337–343. doi: 10.1104/pp.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DM, Lester GE, Munro KD, Toivonen PMD. Oxidative stress: importance for postharvest quality: oxidative stress: postharvest fruits and vegetables. J Am Soc Hortic Sci. 2004;39(5):924–929. [Google Scholar]
- Imahori Y, Takemura M, Bai J. Chilling-induced oxidative stress and antioxidant responses in mume (Prunus mume) fruit during low temperature storage. Postharvest Biol Technol. 2008;49(1):54–60. doi: 10.1016/j.postharvbio.2007.10.017. [DOI] [Google Scholar]
- Lee CY, Kagan V, Jaworski AW, Brown SK. Enzymic browning in relation to phenolic compounds and polyphenoloxidase activity among various peach cultivars. J Agric Food Chem. 1990;38:99–101. doi: 10.1021/jf00091a019. [DOI] [Google Scholar]
- Lee SY, Dancer GI, Chang SS, Rhee MS, Kang DH. Efficacy of chlorine dioxide gas against alicyclobacillus acidoterrestris spores on apple surfaces. Int J Food Microbiol. 2006;108:364–368. doi: 10.1016/j.ijfoodmicro.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Loaiza VJG, Mangrich ME, Campos VR, Saltveit ME. Heat shock reduces browning of fresh-cut celery petioles. Postharvest Biol Technol. 2003;27:305–311. doi: 10.1016/S0925-5214(02)00118-7. [DOI] [Google Scholar]
- López-Serrano M, Ros Barceló A (1999) H2O2-mediated pigment decay in strawberry as a model system for studying color alterations in processed plant foods. J Agric Food Chem 47:824–827 [DOI] [PubMed]
- Lurie S. Postharvest heat treatments. Postharvest Biol Technol. 1998;14(3):257–269. doi: 10.1016/S0925-5214(98)00045-3. [DOI] [Google Scholar]
- Lurie S, Zhou H-W, Lers A, Sonego L, Alexandrov S, Shomer I. Study of pectin esterase and changes in pectin methylation during normal and abnormal peach ripening. Physiol Plant. 2003;119(2):287–294. doi: 10.1034/j.1399-3054.2003.00178.x. [DOI] [Google Scholar]
- Mahmoud BSM, Bhagat AR, Linton RH. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and salmonella enterica on strawberries by chlorine dioxide gas. J Food Microbiol. 2007;24(7–8):736–744. doi: 10.1016/j.fm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Picchioni GA, Watada AE, Conway WS, Whitaker BD, Sams CE. Postharvest calcium infiltration delays membrane lipid catabolism in apple fruit. J Agric Food Chem. 1998;46:2452–2457. doi: 10.1021/jf971083e. [DOI] [Google Scholar]
- Scandalios JG. Oxygen stress and superoxide dismutases. J Plant Physiol. 1993;101(1):7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Martínez-Romero D, Castillo S, Guillén F, Valero D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov Food Sci Emerg. 2005;6(1):115–123. doi: 10.1016/j.ifset.2004.09.001. [DOI] [Google Scholar]
- Sevillano L, Sanchez-Ballesta MT, Romojaro F, Flores FB. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J Sci Food Agric. 2009;89(4):555–573. doi: 10.1002/jsfa.3468. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sisler EC, Serek M. IInhibitors of ethylene responses in plants at the receptor level: recent developments. Anglais. 1997;100:577–582. [Google Scholar]
- Sy KV, Murray MB, Harrison MD, Beuchat LR. Evaluation of gaseous chlorine dioxide as a sanitizer for killing salmonella, escherichia coli O157:H7, Listeria monocytogenes, and yeasts and molds on fresh and fresh-cut produce. J Food Prot. 2005;68(6):1176–1187. doi: 10.4315/0362-028x-68.6.1176. [DOI] [PubMed] [Google Scholar]
- Valverde JM, Guillen F, Martinez-Romero D, Castillo S, Serrano M, Valero D. Improvement of table grapes quality and safety by the combination of modified atmosphere packaging (MAP) and eugenol, menthol, or thymol. J Agric Food Chem. 2005;53(19):7458–7464. doi: 10.1021/jf050913i. [DOI] [PubMed] [Google Scholar]
- Varoquaux P, Gouble B, Ducamp MN, Self G. Procedure to optimize modified atmosphere packaging for fruit. Fruits. 2002;57(5–6):313–322. doi: 10.1051/fruits:2002028. [DOI] [Google Scholar]
- Wang YS, Tian SP, Xu Y. Effects of high oxygen concentration on pro- and anti-oxidant enzymes in peach fruits during postharvest periods. Food Chem. 2005;91:99–104. doi: 10.1016/j.foodchem.2004.05.053. [DOI] [Google Scholar]
- Xiao LM, Zhong M, Wu B, Wang JD. Effects of 1-methylcyclopropene and chlorine dioxide on preservation of Xinjiang oblate-peaches. Chin J Food Sci. 2009;12:276–280. [Google Scholar]
- Xie SZ, Wu B, Zhong M, Wang JD. Effect of 1-methylcyclopropene on preservation of fresh-cut Hami melon. Chin J Food Sci. 2009;10:278–281. [Google Scholar]
- Xu WT, Peng XL, Luo YB, Wang JA, Guo X, Huang KL. Physiological and biochemical responses of grapefruit seed extract dip on ‘Redglobe’ grape. LWT Food Sci Technol. 2009;42:471–476. doi: 10.1016/j.lwt.2008.09.002. [DOI] [Google Scholar]
- Yan ZM, Lin J, Sheng BL, Li XG, Yang QS. Effects of 1-MCP treatment on pear storage at room temperature. Chin J Agric Sci. 2004;20:189–193. [Google Scholar]
- Zhang Z, Pang X, Xuewu D, Ji Z, Jiang Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005;90:47–52. doi: 10.1016/j.foodchem.2004.03.023. [DOI] [Google Scholar]
- Zhong M, Wu B, Wang JD, Wu JM, Wei LH. Effect of chlorine dioxide on ripening of ‘Xiaobai’ apricots. Eur Food Res Technol. 2006;223:791–795. doi: 10.1007/s00217-006-0271-7. [DOI] [Google Scholar]
- Zhou HW, Ben AR, Lurie S. Pectin esterase, polygalacturonase and gel formation in peach pectin fractions. Phytochemistry. 2000;55:191–195. doi: 10.1016/S0031-9422(00)00271-5. [DOI] [PubMed] [Google Scholar]
- Zhu S, Liu M, Zhou J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol Technol. 2006;42(1):41–48. doi: 10.1016/j.postharvbio.2006.05.004. [DOI] [Google Scholar]
- Zhu L-Q, Zhou J, Shu-Hua Z, Lai-Hui G. Inhibition of browning on the surface of peach slices by short-term exposure to nitric oxide and ascorbic acid. Food Chem. 2009;114(1):174–179. doi: 10.1016/j.foodchem.2008.09.036. [DOI] [Google Scholar]