Abstract
There is currently much interest in phytochemicals as bioactive molecules of food. Functional foods are an emerging field in food science due to their increasing popularity among health conscious consumers. Flaxseed is cultivated in many parts of world for fiber, oil as well as for medicinal purposes and also as nutritional product. In this review, nutrients, anti-nutrients, functional properties, processing, metabolism and health benefits of bioactive molecules viz., essential fatty acids, lignans and dietary fiber of flaxseed are discussed.
Keywords: Flaxseed, Functional properties, Nutritional quality, Processing, Alpha-linolenic acid, Dietary fiber, Lignans, Health benefits
Introduction
Flax (Linum usitassimum) belonging to family Lineaceae, is a blue flowering annual herb that produces small flat seeds varying from golden yellow to reddish brown color. Flaxseed possesses crispy texture and nutty taste (Morris 2007; Rubilar et al. 2010). Flaxseed is also known as linseed and these terms are used interchangeably. Flaxseed is often used to describe flax when consumed by humans while linseed denotes when it is used specifically for industrial applications (Morris 2007). Almost all parts of linseed plant are utilized for various purposes. Seed contains oil which after refining is used for edible purpose (Singh et al. 2011a, b). The stem yields fiber of good quality possessing high strength and durability. Humans have been consuming flaxseed since ancient times. It has been cultivated for fiber as well as for medicinal purposes and as nutritional product (Tolkachev and Zhuchenko 2000). Currently, it is cultivated in more than 50 countries, predominantly in the Northern hemisphere. Canada is the world’s largest producer and exporter of flaxseeds (Oomah 2001). The important flaxseed growing countries include India, China, United States, and Ethiopia (Oomah and Mazza 1998; Singh et al. 2011a, b). India ranks first among the leading flaxseed producing countries in terms of acreage accounting 23.8 % of the total and third in production contributing to 10.2 % of the world’s production (Singh et al. 2011a, b). In India flaxseed is mainly cultivated in Madhya Pradesh, Maharashtra, Chattisgarh and Bihar. It is interesting to know that flaxseed was native of India and was a staple food crop. In India, flaxseed is still being consumed as food and as well as for medicinal purposes (Shakir and Madhusudan 2007). It enjoys a good status among oilseeds because of its versatile uses. It has emerged as an attractive nutritional food because of its exceptionally high content of alpha-linolenic acid (ALA), dietary fiber, high quality protein and phytoestrogens. Flaxseeds contain about 55 % ALA, 28–30 % protein and 35 % fiber (Carter 1993; Rubilar et al. 2010; Rabetafika et al. 2011). Flaxseed has been the focus of growing interest for the nutritionists and medical researchers due to its potential health benefits associated with its biologically active components—ALA, lignan-Secoisolariciresinol diglycoside (SDG) and dietary fiber (Toure and Xueming 2010).
Flaxseed is establishing importance in the world’s food chain as a functional food. Functional food can be defined as the food or food ingredients that may provide physiological benefits and helps in preventing and/or curing of diseases (Al-Okbi 2005). Presently, flaxseed has new prospects as functional food because of consumer’s growing interest for food with superb health benefits. Owing to its excellent nutritional profile and potential health benefits, it has become an attractive ingredient in the diets specially designed for specific health benefits (Oomah 2001). ALA is one of the essential polyunsaturated fatty acid and reported to exhibit anti-inflammatory, anti-thrombotic and anti-arrhythmic properties (Simopoulos 1999). Nutritionists all over the world suggest incorporation of omega 3 fatty acid sources in the diet. Flaxseed serves as the best omega 3 fatty acid source to the non-fish eaters. Edible flaxseed products include the whole flaxseed, ground meal and extracted oil or mucilage. These products have been proposed as nutritional additives in the preparation of a number of dietary items such as baked cereal products, ready to eat cereals, fiber bars, salad toppings, meat extenders, bread, muffins and spaghetti (Singh et al. 2011a, b). Inspite of the multiple clinical evidences of flaxseeds, people are still unaware about its nutritional as well as therapeutic benefits.
Nutritional composition
Among the functional foods, flaxseed has emerged as a potential functional food being good source of alpha-linolenic acid, lignans, high quality protein, soluble fiber and phenolic compounds (Oomah 2001). The composition of flaxseed is presented in Table 1 (Morris 2007; Gopalan et al. 2004; Payne 2000). Chemical composition of flaxseed depends upon growing environment, genetics and processing conditions (Morris 2007). The lipid content of flaxseed varies from 37 to 45 g/100 g of the seed as reported by various scientists (Carter 1993; Payne 2000; Morris 2007). Cotyledons are the major oil storage tissues, containing 75 % of the seed oil (Rubilar et al. 2010; Singh et al. 2011a, b). Flaxseed oil constitutes 98 % triacylglycerol, phospholipids and 0.1 % free fatty acids (Mueller et al. 2010). On an average it contains 21 % protein. Majority of the protein is concentrated in the cotyledons (Rabetafika et al. 2011). Major protein fractions are globulin (26–58 %) and albumin (20–42 %). Nutritional value and amino acid profile of flaxseeds are comparable to that of soya proteins (Madhusudan and Singh 1985; Oomah and Mazza 1993). Flaxseed protein is rich in arginine, aspartic acid and glutamic acid, while lysine is limiting (Singh et al. 2011a, b; Chung et al. 2005). High cysteine and methionine contents improve the antioxidant levels, thus helps in reducing risk of cancer (Oomah 2001). The processing conditions, dehusking and defatting affect the protein content. The defatted and dehusked meals have high protein content (Oomah and Mazza 1997, 1998) Flaxseed proteins exhibit antifungal properties against Alternaria solani, Candida albicans and Aspergillus flavus (Xu et al. 2008a, b).
Table 1.
Nutritional composition of flaxseed
| Nutrients | Amount per 100 g of edible flaxseed |
|---|---|
| Moisture (g) | 6.5 |
| Protein (N × 6.25) (g) | 20.3 |
| Fat (g) | 37.1 |
| Minerals (g) | 2.4 |
| Crude fiber (g) | 4.8 |
| Total dietary fiber (g) | 24.5 |
| Carbohydrates (g) | 28.9 |
| Energy (kcal) | 530.0 |
| Potassium | 750.0 |
| Calcium (mg) | 170.0 |
| Phosphorous (mg) | 370.0 |
| Iron (mg) | 2.7 |
| Vitamin A (μg) | 30.0 |
| Vitamin E (mg) | 0.6 |
| Thiamine (B1) (mg) | 0.23 |
| Riboflavin (B2) (mg) | 0.07 |
| Niacin (mg) | 1.0 |
| Pyridoxine (mg) | 0.61 |
| Pantothenic acid | 0.57 |
| Biotin (μg) | 0.6 |
| Folic acid (μg) | 112 |
Flaxseed is the richest source of phytoestrogens (lignans). The amount of secoisolariciresinol diglycoside (SDG) varies from 77 to 209 mg SDG/tbsp. of whole flaxseed (Morris 2007; Toure and Xueming 2010). Flaxseed contains very low level of carbohydrates (1 g/100 g) and thus contributing very little to total carbohydrates intake (Morris 2007).
Flaxseeds contain a good amount of phenolic compounds. These phenolic compounds are well known for anticancer and anti-oxidative properties. Basically, flaxseeds have three different types of phenolic compounds–phenolic acids, flavonoids and lignans. Major phenolic acids present in defatted flaxseed are ferulic acid (10.9 mg/g), chlorogenic acid (7.5 mg/g), gallic acid (2.8 mg/g). Other phenolic acids include p-coumaric acid glucosides, hydroxycinnamic acid glucosides and 4-hydroxybenzoic acid that are present in low quantities (Beejmohun et al. 2007; Mazza 2008). Flavone C- and Flavone O-glycosides are the major flavonoids found in flaxseeds (Mazza 2008).
It serves as a good source of minerals especially, phosphorous (650 mg/100 g), magnesium (350–431 mg/100 g), calcium (236–250 mg/100 g) and has very low amount of sodium (27 mg/100 g) (Morris 2007). It contains highest amount of potassium 5600–9200 mg/kg among various foods and high potassium intake is inversely related to blood platelet aggregation, free radicals in blood and stroke incidence (Carter 1993). Flaxseed contains small amounts of water-soluble and fat-soluble vitamins. Vitamin E is present as γ-tocopherol, amounting to 39.5 mg/100 g. γ-tocopherol is an antioxidant providing protection to cell proteins and fat from oxidation; promotes sodium excretion in urine, which may help in lowering of blood pressure and heart disease risks and Alzheimer disease (Morris et al. 2005; Morris 2007).
Anti-nutrients
Flaxseeds contain anti-nutrients that may have adverse influence on the health and well-being of human population. Cyanogenic glycosides are the major anti-nutrients and are fractionated into linustatin (213–352 mg/100 g), neolinustatin (91–203 mg/100 g), linmarin (32 mg/100 g). The content of these three glycosides depend upon cultivar, location etc. (Oomah et al. 1992). Fiber type linseed has a higher percentage of glycosides than the seed type, and ripe seed contains less glycoside than the immature seed. Whole flaxseed contains 250–550 mg/100 g cyanogenic glycoside (Singh et al. 2011a, b). In the intestine, cyanogenic glycosides release hydrogen cyanide, a potent respiratory inhibitor, by intestinal β-glycosidase that produces thiocyanates. Thiocyanates interfere with iodine uptake by thyroid gland and long term exposure aggravates iodine-deficiency disorders, goiter and cretinism. Cyanogenic glycosides are heat labile and easily destroyed by processing methods namely autoclaving, microwave roasting, pelleting and by certain detoxifying enzymes such as β-glycosidases, releasing hydrogen cyanide which can be evaporated by using steam (Cunnane et al. 1993; Feng et al. 2003; Yamashita et al. 2007).
Phytic acid, another anti-nutrient present in flaxseed, ranges from 23 to 33 g/kg of the flaxseed meal (Oomah et al. 1996a, b). Phytic acid interferes with the absorption of calcium, zinc, magnesium, copper and iron. It is a strong chelator, forming protein and mineral-phytic acid complexes and thus reducing their bioavailability (Erdman 1979; Akande et al. 2010). Clinical studies reveal that flaxseed fed rats had no effect on their Zn status (Ratnayake et al. 1992). Ganorkar and Jain (2013) have also reviewed that flaxseed antinutrients have lesser impact on human health as compared to that of soyabean and canola. Linatine (antipyrodoxidine factor) has been identified as a vitamin B6 antagonist in case of chicks. While in humans, flaxseeds are not found to be associated with vitamin B6 deficiency (Dieken 1992; Ratnayake et al. 1992). Trypsin inhibitors are also reported in flaxseed, though activity is insignificant as compared to soybean and canola seeds (Bhatty 1993).
Flaxseed as functional food
Flaxseed is considered as functional food owing to the presence of three main bioactive components—alpha-linolenic acid, lignans and dietary fiber.
Alpha-linolenic acid
Alpha-linolenic acid is the main functional component of flaxseed. It serves as an exclusive source of omega-3 fatty acid in the vegetarian diets (Riediger et al. 2009). Flaxseed oil is rich in polyunsaturated fatty acid (73 % of total fatty acid), moderate in monounsaturated fat (18 %) and low amount of saturated fat (9 %) (Cunnane et al. 1993; Dubois et al. 2007). It is rich in both the essential fatty acids—alpha-linolenic acid (ALA), and linolenic acid (LA). Fatty acids are termed as essential because both they are required by the body but body cannot synthesize them, therefore need to be supplied in the diet. Human body lacks the enzymes which are required for the synthesis of these essential fatty acids (de Lorgeril et al. 2001).
Metabolism
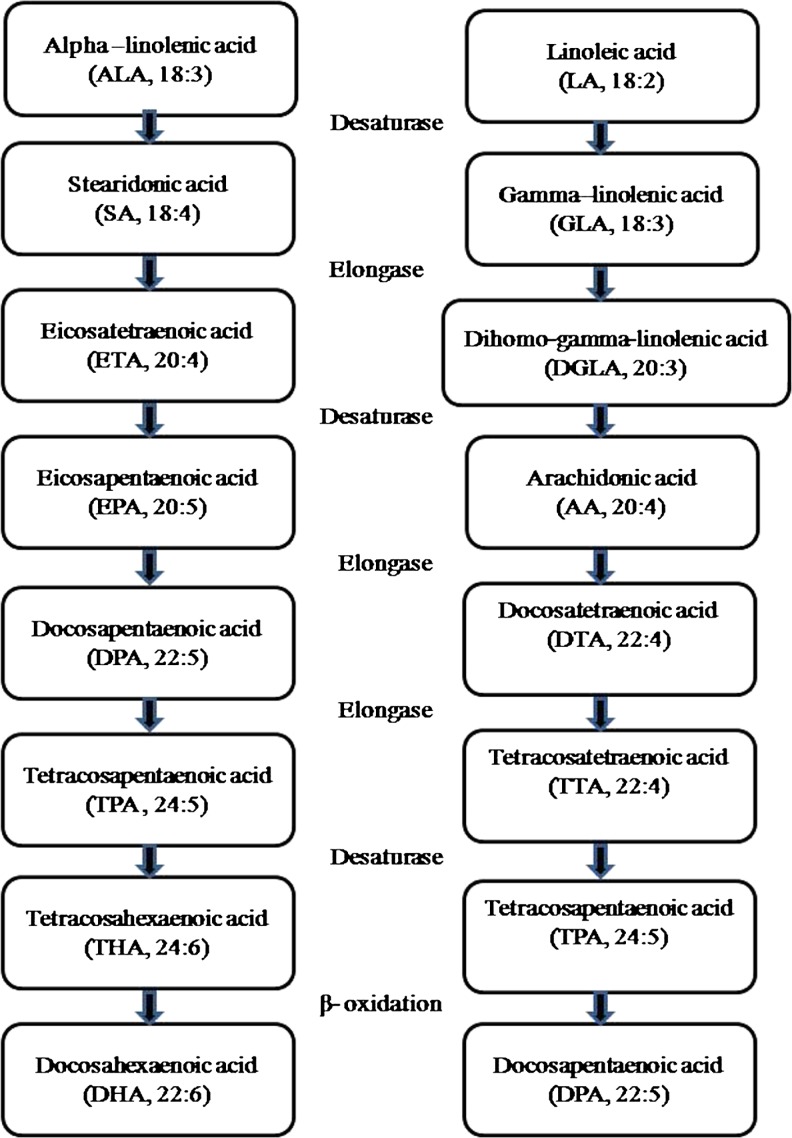
Omega-3 fatty acid is known as essential fatty acid because humans cannot introduce a double bond beyond the ninth carbon from carboxyl end of fatty acid. The metabolism of essential fatty acids is depicted in Fig. 1. ALA serves as the precursor for the synthesis of polyunsaturated fatty acids—EPA (Eicosapentaenoic acid) and DHA (Docosahexanoic acid). During the transformation of ALA into EPA and DHA, a series of fatty acids belonging to n-3 PUFA family are also synthesized via desaturation and elongation reactions in the presence of specific desaturases and elongases. Similarly, linolenic acid is also synthesized using similar enzymatic reactions. It has been reported that the conversion of ALA to EPA and DHA is not very efficient in humans and animals and there exist competition between both the fatty acids for the same enzymes. Lower order animals are known to have such enzymatic activities which are capable of converting n-6 fatty acid to n-3 fatty acids, while mammals lack such activities. But recent research findings indicated that mice are the only mammals possessing the enzymes capable of converting n-6 fatty acid to n-3 fatty acid (Spychalla et al. 1997; Lunn and Theobald 2006; Kang 2007).
Fig. 1.
Flowchart for metabolism of essential fatty acids
Long chain PUFAs, EPA and DHA are further metabolized by the enzymes cyclooxygenase and lipoxygenase to eicosanoids, prostaglandins, leukotrienes. Among these eicosanoids, E2 series prostaglandins, leukotrienes B4 derived from linoleic acid are the key metabolites which are responsible for many inflammatory diseases like cardiovascular diseases and arthritis, while eicosanoids and E3 series prostaglandins derived from linolenic acid have anti-inflammatory responses (James et al. 2000; Funk 2001; Barcelo-Coblijn and Murphy 2009; Kaur et al. 2012). Therefore, it is advised that human beings should consume a diet that contains a balanced ratio of omega-3 and omega-6 essential fatty acids. The two groups of essential fatty acids compete with each other for placement within cell membranes. If the intercellular environment has a higher proportion of one type of fatty acid as compared to the other, it is likely that the predominant fatty acid will be incorporated into cell membrane, resulting in adverse effects in the fluidity of the cell membrane affecting cellular functions and overall health of the cell. If there is an equal proportion of both the essential fatty acid in the intercellular environment, there is selective preference for omega-3 fatty acid. Both these fatty acids have opposing, yet necessary, influences over physiological functions (Lunn and Theobald 2006; Kaur et al. 2012).
EPA and DHA can be converted endogenously into different metabolites known as resolvins, neuroprotectins and protectins. The resolvins act as potent anti-inflammatory mediator. In particular, they function to limit the extent of inflammation by blocking the actions of prostanoids, and also by helping to clear site of inflammation from breakdown products of inflammatory process. Resolvins and protectins promote resolution in oral, lung, kidney, skin, gastrointestinal and various other inflammations to maintain homeostasis by activating specific mechanisms. DHA is converted into neuroprotectins which exhibit neuroprotective effects (Simpolous 2011; Macmohan and Godson 2004). In order to maintain good health, it is therefore important that both fatty acids should be present in a balanced ratio.
Ratio of omega-6 to omega-3 fatty acid
Fatty acid profiles of various oilseeds are reported in Table 2. It is evident from the data that flaxseed contains highest amount of linolenic acid followed by soybeans and mustard oil, while sunflower and safflower oils contain large amount of linoleic acid which may leads to various diseases. Over the past 100 to 150 years, the consumption of vegetable oils from corn, sunflower seeds, safflower seeds, cottonseeds and soybeans has greatly increased, which resulted in drastic imbalance of the essential fatty acids. Today, the ratio of omega-6 to omega-3 fatty acid is shifted to 20–30:1 in western diets and the situation is even worse in case of Indian diets where this ratio attains a high value of 38–50:1 which reveals that more of omega-6 fatty acids are incorporated into the cell membrane (Simpolous 2004; Pella et al. 2003). Therefore, the cellular functions support more of the pro-inflammatory processes than anti-inflammatory processes. Simple dietary choices, which favour foods containing omega-3 fatty acids, can ameliorate this imbalance. The recommended ratio of omega-6 to omega-3 fatty acids may be in the range of 4:1 to 10:1, and omega-6 and omega-3 fatty acid intakes should account for at least 3 and 0.5 % of total energy intake, respectively (de Lorgeril et al. 2001; Tolkachev and Zhuchenko 2000; WHO 2003). These variations in the ratio of fatty acids may be considered as a deciding factor for the development of therapeutic doses for prevention of colorectal cancer, asthma and rheumatoid arthritis (Arend and Dayer 1995).
Table 2.
Fatty acid profile of various oilseeds
| Fatty acid | Flaxseed | Mustard | Soyabean | Rice bran | Corn | Sesame | Safflower | Olive | Sunflower |
|---|---|---|---|---|---|---|---|---|---|
| Saturated | 10 | 8 | 15.7 | 21.3 | 14.8 | 15.7 | 9.1 | 15.3 | 12.8 |
| Monounsaturated | 18.5 | 62.4 | 24.2 | 42.4 | 28.1 | 40.1 | 13.9 | 73.8 | 22.4 |
| Polyunsaturated | 71.8 | 31.5 | 59.8 | 35.9 | 57.1 | 45.7 | 77.3 | 10 | 66 |
| Linoleic acid (n6) | 16.8 | 21.6 | 52.1 | 34.6 | 56.1 | 45.3 | 76.5 | 9.4 | 65.6 |
| Linolenic acid (n3) | 55 | 9.9 | 7.8 | 1.2 | 1 | 0.4 | 0.8 | 0.6 | 0.5 |
| n6/n3 | 0.3 | 2.2 | 6.7 | 2 | 56 | 113 | 7.4 | 16 | 131 |
Dubois et al. 2007
Health benefits
A large number of clinical studies have recognized the tremendous potential of n-3 polyunsaturated fatty acids against inflammatory mediators like prostaglandins E2, leukotriene B4, TNF-α, interleukin, and cytokines. These clinical studies revealed that n-3 polyunsaturated fatty acids are helpful in prevention of coronary heart diseases, atherosclerosis, rheumatoid arthritis and asthma (Arend and Dayer 1995; Kremer 2000). Daily intake of 3 g EPA and DHA for more than 12 weeks was found to be effective in reducing the inflammation of rheumatoid arthritis (Kremer 2000). It has also been reported that the consumption of omega-3 dietary supplements lead to significant reduction of nonsteroidal anti-inflammatory drugs (Arend and Dayer 1995). Flaxseed oil supplementation for about 4 weeks resulted in protecting the mice against Streptococcus pneumonia infection (Saini et al. 2010). Flaxseed and its oil reduces the growth of tumors at the later stage of carcinogenesis; whereas, mammalian lignan precursor exert the greatest inhibitory effect on the growth of new tumors (Thompson et al. 1996). The role of flaxseed oil in tumors prevention is attributed to its high alpha-linolenic acid. The fatty acid composition of the tumors revealed higher incorporation of alpha-linolenic acid which in turn resulted in suppression of the growth of the tumor cells (Gonzalez et al. 1991; Thompson 1996; Gabor and Abraham 1986).
Flaxseed possesses antioxidant and hepatoprotective properties. Several studies advocated the cholesterol lowering benefits of flaxseed meal (Cunnane et al. 1993; Ridges et al. 2001; Bhathena et al. 2003). A study on hypercholesterolemic rats fed on flaxseed chutney supplemented diet (15 %) revealed significant reduction in LDL cholesterol and total serum cholesterol and no change in HDL cholesterol. In CCl4 intoxicated rats, lipid peroxidation products were neutralized by flaxseed lignans (Shakir and Madhusudan 2007). Several clinical studies showed that EPA and DHA play a major role in reducing depression symptoms. During depression or stress proinflammatory cytokines such as TNF-α, interferon gamma etc. are produced. Increased of n-6 fatty acid to n-3 fatty acid ratio may lead to the production of proinflammatory cytokines which causes depression and mood swings in elderly persons (Maes et al. 1996; Tiemeier et al. 2003; Locke and Stoll 2001).
Lignans
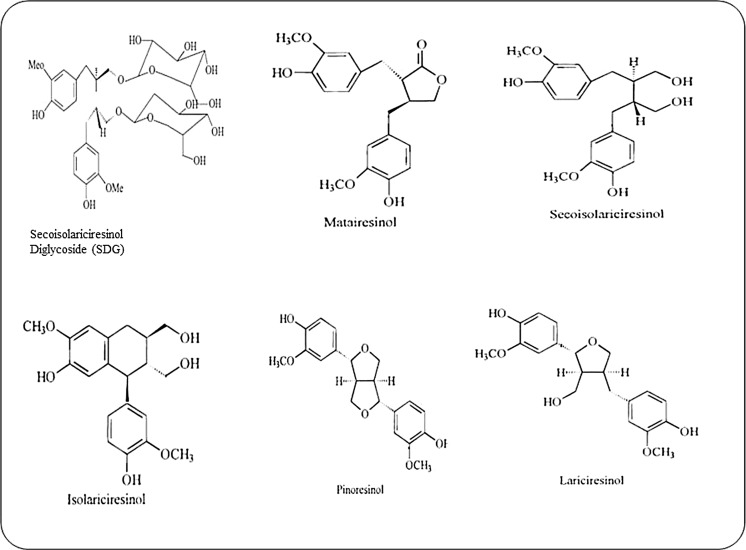
Lignans are phytoestrogens, which are abundantly available in fiber rich plants, cereals (wheat, barley, and oats), legumes (bean, lentil, soybean), vegetables (broccoli, garlic, asparagus, carrots) fruits, berries, tea and alcoholic beverages. Flaxseed contains about 75–800 times more lignans than cereal grains, legumes, fruits and vegetables (Mazur et al. 2000; Meagher and Beecher 2000; Murphy and Hendrich 2002; Hosseinian and Beta 2009). Secoisolariciresinol diglycoside (SDG) is the major lignan of flaxseed, alongwith minor contents of matairesinol, pinoresinol, lariciresinol and isolariciresinol (Meagher et al. 1999; Sicilia et al. 2003; Krajcova et al. 2009). SDG ranges from 11.7 to 24.1 mg/g in defatted flour and 6.1 to 13.3 mg/g in whole flaxseed flour (Johnsson et al. 2000).
Lignans are the diphenolic compounds synthesised by the coupling of two coniferyl alcohol residues existing in cell wall of higher plants (Toure and Xueming 2010; Westcott and Muir 2003). Secoisolariciresinol (SECO) is produced by acid hydrolysis of secoisolariciresinol diglycoside. Secoisolariciresinol diglycoside existing bound form as a complex of five secoisolariciresinol diglycoside residues held together by four HMGA (3-hydroxy-3-methylglutaric acid) residues in the outer layers of the seed (Kamal-Eldin et al. 2001; Raffaelli et al. 2002; Muir 2006). Structures of the flaxseed lignans complied from different sources (Toure and Xueming 2010; Meagher et al. 1999) are presented in Fig. 2.
Fig. 2.
Structure of various lignans present in flaxseed
Metabolism
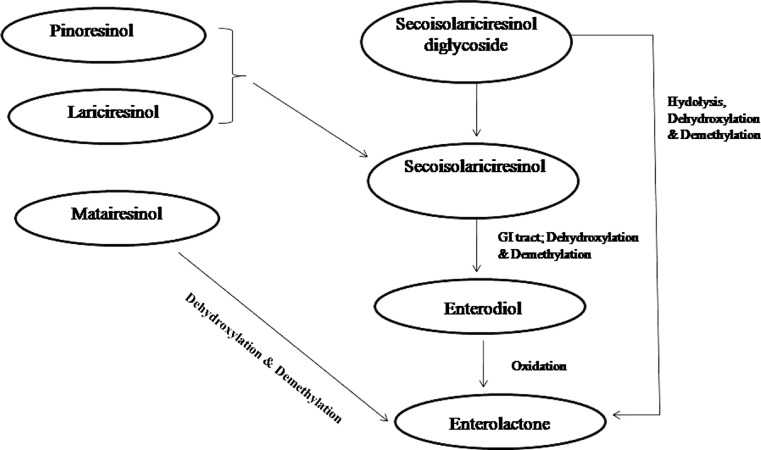
SDG is metabolized by bacteria in the colon of humans to synthesize mammalian lignans known as enterodiol (END) and enterolactone (ENL) (Chen et al. 2007). In human body, the lignans are acted upon by the gastrointestinal microflora to release SECO, non-sugar moiety of SDG. Further hydroxylation and demethylation by the microflora, lead to the production of mammalian lignan-enterodiol (END), which is then oxidized to give enterolactone (ENL) (Morris 2007; Hu et al. 2007; Toure and Xueming 2010). The metabolism of flaxseed lignans is presented in Fig. 3. Bacteroides as well as Clostridia have been identified to release the glucosyl moieties from SDG to yield SECO (Clavel et al. 2006; Struijs et al. 2009). Peptostreptococcus productus, Eubacterium callanderi, Eubacterium limosum and Bacteroides methylotrophicum are found to be responsible for carrying out demethylation reactions, while dehydroxylation reaction are carried out by Eubacterium lentum (Wang et al. 2000; Clavel et al. 2007). The dehydrogenation of END into ENL has been carried out by several Clostridia and Ruminococcus sp. (Clavel et al. 2007; Jin et al. 2007). The END and ENL, so formed can be excreted in faeces or are absorbed by the human colon and enter the circulation.
Fig. 3.
Flowchart depicting the metabolism of flaxseed lignans
Health benefits
Epidemiological studies indicate that phytoestrogens rich diets reduce the risk of various hormone dependent cancers, heart diseases and osteoporosis (Krajcova et al. 2009; Toure and Xueming 2010). Research studies also demonstrate the ability of SDG to scavenge hydroxyl free radicals and shown that it is a potent antioxidant Human body produces free radicals during the oxidation of fats, proteins and carbohydrates. Free radicals damages tissues, membrane lipids, nucleic acids, proteins which may cause cancer, lung diseases, neurological diseases, premature aging and diabetes (Prasad 1997; Toure and Xueming 2010; Singh et al. 2011a, b). Anticancer activity of lignans is attributed to its ability to scavenge hydroxyl free radicals (Prasad 1997; Hu et al. 2007; Sok et al. 2009). SECO, SDG also play an important role in reduction of hypercholesterolemia, atherosclerosis, hypertension and diabetes (Prasad 2000, 2004). Daily administration of 100 mg SDG was found to be effective in reducing blood cholesterol and hepatic diseases risk in moderately hypercholesterolemic men (Fukumitsu et al. 2010). Flaxseed lignans behaviour depends on biological levels of estrogen hormone. At normal levels of estrogen, it exhibit antagonistic activity, but in postmenopausal phase when estrogen level is low, flaxseed lignans acts as weak estrogen (Sok et al. 2009; Toure and Xueming 2010; Saini et al. 2010). The mammalian lignans stimulate the synthesis of sex hormone binding globulin, which binds sex hormones and reduce their circulation in blood stream, and decrease their biological activity and thus reducing the risk of developing cancer (Thompson et al. 1996). These mammalian lignans are believed to act by binding to estrogen receptors on cell membranes in the same manner as body’s own steroids do but not as powerful as endogenous estrogens (Sok et al. 2009; Morris 2007; Toure and Xueming 2010). Lignans, enterodiol and enterolactone are believed to be partly responsible for growth inhibition of human prostate cancer (Westcott and Muir 2003).
Flaxseed lignans play an important role in preventing various types of cancer specially the hormone sensitive ones. Flax lignans are reported to have antioxidant property which presumably is the main reason of the anticancer activity (Schweigerer et al. 1992; Prasad 1997). The lower incidences of prostate and breast cancers in Asian men and women compared to European men and women has been speculated to be due to the higher consumption of diets rich in fruits and vegetables. (Adlercreutz 1990; Morton et al. 1997). Various clinical studies imply that lignans prevent breast cancer by balancing the hormonal mechanisms. The lignans inhibit the aromatase activity in adipose tissue resulting in the circulation of estrogen (Sturgeon et al. 2008; Adlercreutz et al. 1993).
In postmenopausal women, lignans act as weak estrogens, while at normal estrogen levels, lignans act as estrogen antagonists (Wang et al. 1994; Hutchins and Slavin 2003). Dietary flaxseed moderately lowers the serum levels of steroid sex hormones which are implicated in development of breast cancer in obese postmenopausal women (Sturgeon et al. 2008).
Dietary fiber
Flaxseeds serve as a good source of both soluble and insoluble dietary fiber. Flaxseed holds a unique place among the oilseeds due to presence of mucilage located in outer layers of the seed (Singh et al. 2011a, b). Flaxseed mucilage has gained momentum due to its superb health benefits and potential functional properties (Susheelamma 1987; Mazza and Biliaderis 1989). It contains 35–45 % of fibre and two-third is insoluble and one third is soluble fiber. Insoluble fiber consists of cellulose, hemicellulose and lignin (Morris 2007; Oomah and Mazza 1993). Most of the soluble fiber of flaxseed appears to be the mucilage of seed coat. It makes up 7–10 % of seed weight (Mazza and Biliaderis 1989). Soluble fiber in the form of mucilaginous material consists mainly of water soluble polysaccharides; its recovery and purity vary with the extraction conditions. The water binding capacity of flaxseed mucilage is reported to be about 1600–3000 g of water/ 100 g of solids. High water binding capacity of flaxseed is attributed due to the presence of polysaccharides in the seed coat (Fedenuik and Biliaderis 1994; Wanasundara and Shahidi 1997).
Mucilage of flaxseed consists of acidic and neutral polysaccharides. The neutral fraction constitutes L-arabinose, D-xylose and D-galactose and arabinoxylan and acidic fraction contains L-rhamnose, L-fucose, L-galactose and D-galactouronic acid (Wanasundara and Shahidi 1997). Functionally, these polysaccharides possess similar properties to guar gum (Wanasundara and Shahidi 1994; Tarpila et al. 2005). The mucilage can be extracted by water and exhibit good foam-stability properties (Susheelamma 1987).
Metabolism
Dietary fiber of flaxseed reaches the large intestine and is fermented by colonic microflora with production of short chain fatty acids (SCFA), hydrogen, carbon dioxide, methane and biomass and exhibit laxative effects (Kritchevsky 1979). In the large intestine, both soluble and insoluble fibers have their bulking effect resulting in increasing both dry and wet weight of the colon contents and faeces. Soluble fiber increases water binding, initially by the binding capacity of its macromolecules, later by increasing the mass of microbial cells. The contribution of soluble fiber to faecal weight was insignificant compared to insoluble fiber. Recent studies, however, have shown that it is of the same magnitude (Malkki 2004).
Health benefits
Water-binding capacity of flaxseed insoluble fiber increases the intestinal bulk which is useful in the treatment of constipation, irritable bowel syndrome and diverticular disease. Soluble fiber from flaxseed mucilage increases the viscosity of intestinal contents and delays gastric emptying and nutrient absorption Inclusion of flaxseed mucilage in the diet of broiler chicks resulted in decreased faecal digestibility of fat and fatty acids while protein digestion was unaffected. Intestinal viscosity of the broiler chicks increased on addition of flaxseed mucilage in the diet (Rebole et al. 2002). Traditionally, dietary fiber is used for the treatment of constipation, irritable bowel syndrome (Cann et al. 1984; Tarpila et al. 2005). Dietary fiber delays gastric emptying, regulate post prandial blood glucose levels and helpful in prevention of constipation (Spiller 1994). Flaxseed fiber plays an important role in lowering the blood glucose levels. Studies demonstrated that insoluble fiber slows down the release of sugar in the blood and thus help in reducing blood glucose levels to great extent (Thakur et al. 2009; Kapoor et al. 2011). Soluble gum of the flaxseed may be helpful in the prevention of cardiovascular diseases by exhibiting hypocholesterolemic effect (Jenkins et al. 1987; Cunnane et al. 1994). Kristensen et al. 2012 studied the effect of differently processed flax fibers on the fat excretion and energy balance. It was observed that flax fiber enriched drink lowered the cholesterol to a large extent as compared to fiber enriched bread. However, the consumption of fiber bread increased the fecal fat excretion and maintained proper energy balance. Studies have shown that the high intake of dietary fibers is beneficial for the prevention of obesity in both men and women (Du et al. 2010).
Processing
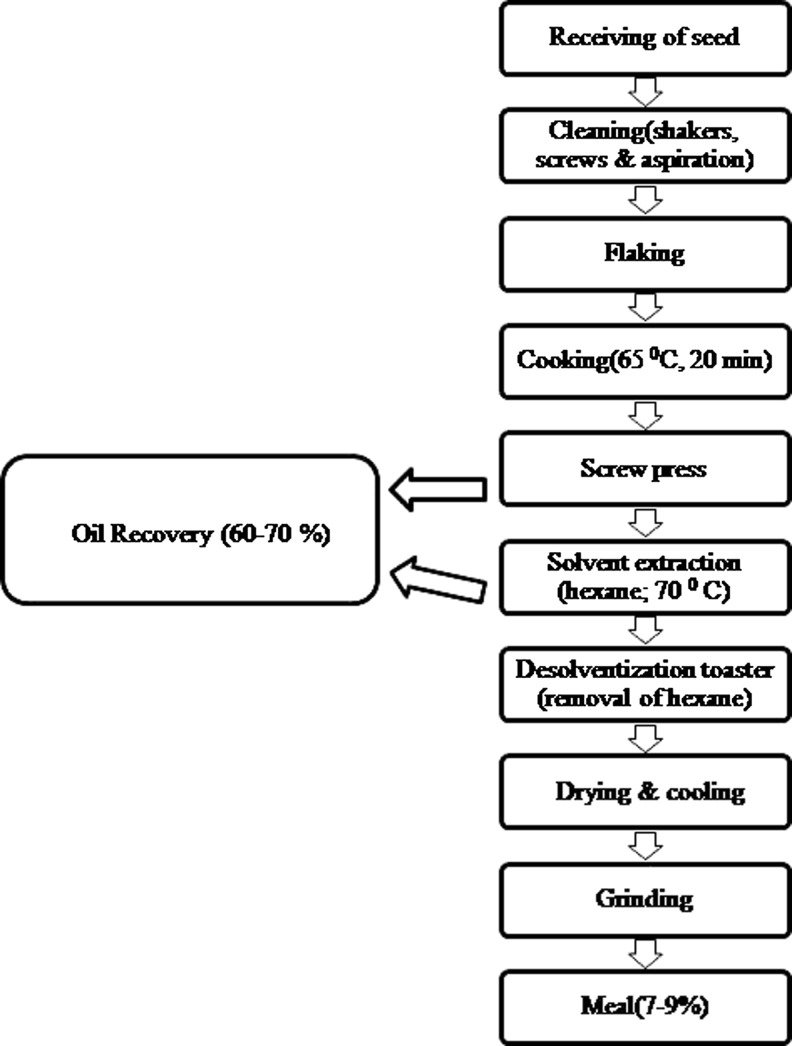
Commercial processing of flaxseeds is carried out to obtain oil and various by-products. Compositional changes during processing are of prime importance to food, feed and nutraceuticals industry. Processing of flaxseeds at commercial level involves multiple steps as shown in Fig. 4 (Oomah and Mazza 1998). The amount of heat as well as extraction time influence the quality of oilseed meal as excessive heating reduce the nitrogen digestibility and net protein utilization (Young 1982). During the processing of flaxseed to meal, protein, carbohydrates and mineral levels increased significantly which contribute to decrease in lipid content. Protein solubility of seeds declined while processing to meal but substantial increase in solubility of protein were observed in flaxseed flakes which may be credited to the increased surface exposure of protein during conditioning treatment. Heat treatment affects the quality of protein and therefore decreases the protein solubility. (Madhusudan and Singh 1984; Oomah and Mazza 1998). However, the total cyanogenic contents of the meal remained unaltered by processing. Several attempts are being made from time to time to reduce the cyanogenic compounds of the flaxseeds. One of the simple and convenient methods to eliminate the cyanogenic glycosides by use of exogenous enzymes to produce hydrogen cyanide from the flaxseed meal and then subjected to steam for evaporation of hydrogen cyanide (Yamashita et al. 2007). Similarly, autoclaving, microwave roasting, pelleting of flaxseeds resulted in significant reduction in cyanogenic glycoside content of the meal without lowering nutritional quality of the seed (Feng et al. 2003).
Fig. 4.
Processing of flaxseed
Extraction of oil
Commercially majority of the flaxseed is processed for extraction of oil which is then used for paints, coatings, linoleum, inks, floor coverings, etc. (Tolkachev and Zhuchenko 2000). Industrial oil is not suitable for food or feed, but the residual meal can be used as feed for cattle. The very high content of alpha-linolenic acid content of flaxseed make it susceptible to autoxidation, leading to deterioration of quality, therefore flaxseed oil extraction has been done by cold pressing and solvent extraction methods. Even after cold extraction of flax oils, it is strongly recommended that oil should be stored in dark glass bottles, supplemented with antioxidants to avoid quality deterioration (Lukaszewicz et al. 2004) In India, various techniques are used for the extraction of flaxseed oil, namely bullock driven ghanis (Kohlu), power driven rotary ghanis hydraulic press and screw-press oil expellers (Singh et al. 2011a, b). Flaxseed oil is generally screw pressed without heat treatment as well no refining is done except for sedimentation and filtration (Wiesenborn et al. 2005). Fresh unrefined oil has a pleasant nutty flavor and attractive golden color. The oil recovery using double stage compression screw press ranges from 86 to 92 %. However, various pretreatments viz., the adjustment of moisture content, (Singh and Bargale 2000) use of enzymes (Shankar et al. 1997) steam treatment, cooking (Singh et al. 2011a, b) prior to pressing results in significant improvement in oil recovery. Decreasing the moisture content of the flaxseeds from 13.8 and 6.5 % resulted in significant increase in oil recovery varying from 44.4 and 81 % (Singh et al. 2011a, b).
Solvent extraction of oilseeds using hexane is usually carried out for recovery of high quality oil and retention of polar lipids (Nash and Frankel 1986). Cold pressing results in only partial recovery of the oil; therefore, pressing of the seeds is followed by solvent extraction at high temperatures to achieve maximum oil recovery. But the alpha-linolenic acid is degraded by exposure to high temperature; therefore, supercritical fluid extraction technique can be a boon to such oils. Supercritical carbon dioxide (SC-CO2) is the most often used supercritical fluid for purpose of oil extraction as the low critical temperature of CO2 (31 °C) allows extraction of heat sensitive compounds without quality deterioration. Lipid composition of the flaxseed oils obtained by both SC-CO2 and petroleum ether extraction were studied and it was found that the alpha-linolenic acid content was higher in case of the oil extracted using SC-CO2 as compared to oil extracted using petroleum ether (Bozan and Temelli 2002). Ultrasonic power is also employed for the extraction of flaxseed oil. A study revealed that ultrasonic assisted extraction of oil resulted in enhanced recovery of oil with increased ultrasonic power. Ultrasonic assisted extraction saves time and lesser solvent consumption (Zhang et al. 2008).
Dehulling
Dehulling leads to removal of outer layer of the seed coat and hence increasing the availability of nutrients. As it is well known that flaxseeds passes through the gut as such and useful nutrients are not absorbed by the body, therefore efforts are being made to remove the hard outer layers of the seeds by using different methods viz., crushing, dehulling, grinding etc. Dehulling removes the hull which results in the elimination most of the mucilage and crude fiber (Dev and Quensel 1988). Dehulled seed contain high level of protein and low carbohydrate content, which makes the flaxseed as potential ingredient for food and feed products (Oomah and Mazza 1997). The hull of flaxseed contain an outer layer which is tough and fibrous containing negligible amounts of oil and protein and inner layer of the hull is soft and has small amount of oil as well as protein. Both these layers of hull are intact; therefore they are considered as single component (Mandokhot and Singh 1979). Several attempts have been made to remove the outer mucilaginous layers of the flaxseed. Traditionally, mucilage has been removed by aqueous extraction methods. The aqueous extraction method involves soaking of seeds in water at temperature preferably between 50 and 80 °C with occasional stirring followed by filtration. The extract is treated with acetone, washed, dialyzed against water and then lyophilized. Hot water extraction result in high yields of mucilage as compared to cold water extraction (Fedenuik and Biliaderis 1994; Cui and Mazza 1996). The mucilage obtained using aqueous extraction is known to be free of specially cotyledon fractions, but this extraction is not economical as it involves various steps including filtration, centrifugation, drying etc. Dry dehulling is also practiced using mechanical methods. Simple process of dry dehulling involves fractionation of ground flaxseeds using graded sieves and air separation method (Dev and Quensel 1988; Smith et al. 1946). Method adopted by Smith et al. 1946 revealed that adjusting the moisture content of the seed before grinding results in significant improved separation of hull with less contamination of cotyledon fractions. The dehulled meal is rich in crude fiber and crude protein. Dehulling also leads to quality deterioration of the dehulled meal because abrasive force used for dehulling may results in removal of some growth promoting factors as well as some protein fraction (Mandokhot and Singh 1979). Most common method used for dry dehulling of flaxseed is TADD (Tangential Abrasive Dehulling Device). This is a simple method for assessing dehulling quality; it requires small sample size and is useful for routine testing in breeding programmes. Dehulling done by TADD method revealed that reduction in moisture content of the seed resulted in exponential increase in hull recovery. However, the amount of hull recovered was independent of speed of rotating disk and dehulling time (Oomah and Mazza 1997). Dehulling of flaxseeds by TADD resulted in significant increase in oil and protein contents and decrease in carbohydrates. Water hydration capacity and viscosity are reduced upon dehulling as the hull contains good percentage of fiber and mucilage which accounts for these properties. The hull fraction contains highest palmitic acid and low amounts of stearic and oleic acid as compared to whole and dehulled flaxseeds. The very high content of dehulled meal makes it an attractive unusual source of protein, whereas, high amount of carbohydrates in hull fraction makes it a good source of dietary fiber to human nutrition (Oomah and Mazza 1997).
Flaxseed as food
As ingredient
In functional foods arena, flaxseed has resurged as a new potential functional ingredient with a vast array of medical benefits. Flaxseed supplemented food products are gaining popularity because of its high content of polyunsaturated fatty acids, protein, soluble fiber and phytochemicals. It is utilized as a versatile ingredient in various types of food products (Table 3). It is convenient to use flax seeds as whole or milled in batters, dough and various baked products. Flaxseed-water mixture serve as egg substitute in the diet of vegetarians especially in baked products pancakes, muffins and cookies. These baked goods are slightly gummier and chewier, and have low loaf volume than normal. One tablespoon of milled flaxseed (approx. 15 g) along with three tablespoon of water (approx. 45 mL) substitute for one egg. Flaxseed gum (0.45 % w/w) can be utilized for stabilization of emulsion in case of salad dressings (Stewart and Mazza 2000). Functional properties of flaxseed constituents are presented in Table 4. Flaxseed products are quiet stable for a longer period at ambient temperature despite of generous amount of alpha-linolenic acid. Several studies justify the above statement that storing the milled flaxseed for about 4 months did not result in deterioration of quality (Singh et al. 2011a, b). Similarly, bread prepared with the flaxseed oil cake at the rate of 10 and 15 % had peroxide levels well below the threshold limits after the 6 months of storage (Ogunronbi et al. 2011). Flaxseed is also being incorporated in the feed of animals to improve the nutritional quality of the meat and fat obtained from them. Omega-3 enriched eggs, pork products are now-a-days available commercially (Kassis et al. 2011).
Table 3.
Flaxseed as functional ingredient in various food products
| Flaxseed as ingredient | Processing methods | Products | Characteristics | References |
|---|---|---|---|---|
| Flaxseed flour + wheat flour | Baking | Bread | Acceptable in terms of sensory and nutritional characteristics, specific volume of bread decreased with increase in flaxseed concentration | Lipilina and Ganji 2009 |
| Flaxseed flour | Baking | Cookies | 15 % supplementation improved alpha-linolenic acid content, acceptable sensory and rheological characteristics | Rajiv et al. 2011 |
| 20 % supplementation acceptable, however, with increase in supplementation level, spread factor decreased | Hussain et al. 2006 | |||
| 12 % supplementation acceptable without affecting sensory and physical characteristics | Khouryieh and Aramouni 2012 | |||
| Flaxseed (raw & roasted) flour (along with wheat flour) | Baking | Muffins | Softer products with better nutritional quality and overall acceptability at 20 % level of supplementation | Chetana et al. 2010 |
| Flaxseed flour (full fat and partial defatted) | Baking | Chapatti | Total, soluble and insoluble dietary fibers, essential amino acid contents and alpha-linolenic acid content of chapattis and breads increased | Hussain 2009 |
| Ground flaxseed | Baking | Beef patties | Cooking losses improved, good nutritional characteristics at 3–6 % supplementation | Bliek and Turhan 2009 |
| Ground whole flaxseed and ground flaxseed hull | Extrusion | Macaroni | Lipids remained stable during processing, free fatty levels reduced | Lee et al. 2004 |
| ALA and SDG remained stable over 32 week storage period | Hall et al. 2005 | |||
| Flaxseed flour | Extrusion | Pasta | Retardation of microbial activity | Manthey et al. 2008 |
| Flaxseed with corn meal | Extrusion | Puffs | 15 % incorporation produced good puffed product, however, lignan content decreased by 25–52 % | Wu et al. 2007 |
| Flaxseed with corn & soy flour | Extrusion | Puffs | Good puffing characteristics at 10 % supplementation, low color scores and poor textural characteristics | Alpaslan and Hayta 2006 |
| SDG (lignan content) isolated from flaxseed | Pasteurization, fermentation, milk renneting | Dairy products | SDG remained stable during processing as well as storage | Hyvarinen et al. 2006a |
| Baking | Baked products | Hyvarinen et al. 2006b | ||
| Linseed polysaccharide | Steaming | Idli | Good soft texture and overall acceptability | Susheelamma 1989 |
| Defatted crushed flaxseed | Fermentation | Spoonable/drinkable snack | Acceptable oat and buckwheat supplemented flaxseed product with probiotic property | Salimnen et al. 2010 |
| Ground flaxseed | Roasting | Energy rich bar | 15 % flaxseed + 45 % sweeteners along with other ingredients had acceptable quality, nutritionally as well as organoleptically | Mridula et al. 2011 |
| Flaxseed oil | Homogenization | Ice cream | Oil act as foam stabilizer, improved meltdown time and soft creamy texture | Goh et al. 2006 |
Table 4.
Functional properties of flaxseed constituents
| Functional ingredient | Applications | References |
|---|---|---|
| Mucilage | Emulsifier & stabilizer in sauces, sausages, meat emulsions, salad dressings | Stewart and Mazza 2000 |
| Anti-staling agent in baked products | Lipilina and Ganji 2009 | |
| Improves cooking quality of noodles | Kishk et al. 2011 | |
| Functional food ingredient (interaction of mucilage and protein regulate blood glucose level) | Singer et al. 2011 | |
| Protein | Stabilizer & emulsifier in ice cream, sauces and meat emulsions | Martinez-Flores et al. 2006 |
| Antifungal property | Xu et al. 2008a, b | |
| Viscoelastic texture to extruded pastes for breakfast cereals and snacks | Wu et al. 2010 | |
| Enhances nutrition in gluten free meal | Gambus et al. 2009 | |
| Egg and gelatin substitute in baked goods and ice cream | Shearer and Davies 2005 | |
| Functional food ingredient | Moller et al. 2008 |
Edible oil
As already discussed, flaxseed oil is rich in polyunsaturated fatty acids specifically the alpha-linolenic acid. Decades back, flaxseed was used mainly in the manufacturing of drying oil, paints, coating, and printing inks etc. But recently there has been resurgence in the use of flaxseed oil for edible purposes because of its nutraceuticals values. Fresh flaxseed is golden yellow in colour, has neutral taste and is very sensitive to heat, light, oxygen; therefore it is usually extracted by cold pressing when it is meant for edible purposes (Choo et al. 2007a, b). Since, flaxseed oil has very high amount of alpha-linolenic acid, it is highly susceptible to oxidation, rancidity and poor sensory quality (Hosseinian et al. 2004; Wiesenborn et al. 2005). The oxidation products serve as a causative agent for chronic diseases.
Flaxseed oil is not recommended for high temperature processing. Studies reported that the high temperature treatment of the oil result in the alteration of its nutritional composition. It has been reported that elevated temperature deteriorate the alpha-linolenic acid (heat labile) which is not desirable in terms of its associated health benefits (Choo et al. 2007a, b). In China, stir frying of flaxseed oil at 150 °C is very common for cooking purposes (Pan 1990). Some studies state that stir frying of flaxseed oil upto177°C did not cause any loss to the quality of the oil (Hadley 1996). Tocopherols, plastochromanol-8, phenolic acids, flavonoids, lignans and chlorophyll pigments act as natural antioxidants in various vegetable oils and these are also present in fair amounts in flaxseed oil (Choo et al. 2007a, b). Storage as well as heat treatment resulted in destruction of these natural antioxidants, thus deterioration of the oil quality and shelf-stability (Tautorus and McCurdy 1990; Li et al. 1996). Attempts are being made in terms of encapsulation of the oil with gelatin-arabic gum capsules, which provide protection against oxidative products formed during processing or storage (Liu et al. 2010).
Due to its high alpha-linolenic acid content, it has multiple industrial applications. Based on the scientific research on the stupendous health benefits of linolenic acid it has attracted the intellectuals from various fields are doing sincere efforts to widen its food applications. Plant breeders, food technologists and nutritionists are using conventional and molecular approaches for altering the fatty acid profile of flaxseed and create its competitive food market. In this respect, initiative was taken by Green 1995 reduced the alpha-linolenic acid of flaxseed oil, to less than 5 %. Flax council has given the name solin for such cultivars containing less than 5 % alpha-linolenic acid. The high levels of palmitic acid, oleic acid, linoleic acid in the solin cultivars make them suitable for manufacturing of margarines and shortenings (Hosseinian et al. 2004).
Conclusion
Various clinical trials revealed that the flaxseed constituents provide disease preventive and therapeutic benefits. This encourages development of new branded healthy and functional foods using flaxseeds, oil and cakes. More in vivo studies are required to ascertain the health benefits of flaxseed constituents and to know the minimum amount of flaxseed required to explore its therapeutic potential for all population groups including pregnant and lactating women and to know possible problems posed by its overdose. There is need for the development of rapid, reproducible and economic techniques for the analysis of nutraceuticals from flaxseed.
References
- Adlercreutz H. Western diet and western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Investig Suppl. 1990;201:3–23. [PubMed] [Google Scholar]
- Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;42:147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- Akande KE, Doma UD, Agu HO, Adamu HM. Major anti nutrients found in plant protein sources: their effect on nutrition. Pak J Nutr. 2010;9:827–832. [Google Scholar]
- Al-Okbi SY. Highlights on functional foods, with special reference to flaxseed. J Nat Fibers. 2005;2(3):63–68. [Google Scholar]
- Alpaslan M, Hayta M. The effects of flaxseed, soy and corn flours on the textural and sensory properties of a bakery product. J Food Qual. 2006;29:617–627. [Google Scholar]
- Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n3 fatty acids: benefits for human health and a role in maintaining tissue n3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. doi: 10.1016/j.plipres.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Beejmohun V, et al. Microwave-assisted extraction of the main phenolic compounds in flaxseed. Phytochem Anal. 2007;18:275–282. doi: 10.1002/pca.973. [DOI] [PubMed] [Google Scholar]
- Bhathena SJ, Ali AA, Haudenschild C, Latham P, Ranich T, Mohamed AI, Hansen CT, Velasquez MT. Dietary flaxseed meal is more protective than soy protein concentrate against hypertriglycerdemia and steatosis of the liver in an animal model of obesity. J Am Coll Nutr. 2003;22:157–164. doi: 10.1080/07315724.2003.10719289. [DOI] [PubMed] [Google Scholar]
- Bhatty RS. Further compositional analyses of flax: mucilage, trypsin inhibitors and hydrocyanic acid. J Am Oil Chem Soc. 1993;70:899–904. [Google Scholar]
- Bliek AE, Turhan S. Enhancement of the nutritional status of beef patties by adding flaxseed flour. Meat Sci. 2009;82:472–477. doi: 10.1016/j.meatsci.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Bozan B, Temelli F. Supercritical CO2 extraction of flaxseed. J Am Oil Chem Soc. 2002;79:231–235. [Google Scholar]
- Cann PA, Read NW, Holdsworth CD. What is the benefit of coarse wheat bran in patients with irritable bowel syndrome? Gut. 1984;24:168–173. doi: 10.1136/gut.25.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JF. Potential of flaxseeds and flaxseed oil in baked goods and other products in human nutrition. Cereal Foods World. 1993;38:754–759. [Google Scholar]
- Chen J, Liu X, Shi Y, Ma C. Determination of the lignan secoisolariciresinol diglucoside from flaxseed (Linum usitatissimum) by HPLC. J Liq Chromatogr Relat Technol. 2007;30:533–544. [Google Scholar]
- Chetana, Sudha ML, Begum K, Ramasarma PR. Nutritional characteristics of linseed/flaxseed (Linum usitatissimum) and its application in muffin making. J Texture Stud. 2010;41:563–578. [Google Scholar]
- Choo W, Birch J, Dufour JP. Physicochemical and stability characteristics of flaxseed oils during pan-heating. J Am Oil Chem Soc. 2007;84:735–740. [Google Scholar]
- Choo W, Birch J, Dufour JP. Physiochemical and quality chartacteristicsof cold-pressed flaxseed oils. J Food Comp Anal. 2007;20:201–211. [Google Scholar]
- Chung M, Lei B, Li-Chan E. Isolation and structural characterization of the major protein fraction from Nor Man flaxseed (Linum usitatissimum L.) Food Chem. 2005;90:271–279. [Google Scholar]
- Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12:140–147. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Clavel T, Lippman R, Gavini F, Dore J, Blaut M. Clostridium saccharogumia spnov., and Lactonifactorlongoviformisgen. nov., spnov., two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside. Syst Appl Microbiol. 2007;30:16–26. doi: 10.1016/j.syapm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Cui W, Mazza G. Physiochemical characteristics of flaxseed gum. Food Res Int. 1996;29:397–402. [Google Scholar]
- Cunnane SC, et al. High linolenic acid flaxseed (Linum usitatissimum): some nutritional properties in humans. Br J Nutr. 1993;69:443–453. doi: 10.1079/bjn19930046. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TMS, Jenkins DJA. Nutritional attributes of flaxseed in healthy young adults. Am J Clin Nutr. 1994;61:62–68. doi: 10.1093/ajcn/61.1.62. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M, Salen P, Laporte F, de Leiris J. Alpha-linolenic acid in the prevention and treatment of coronary heart disease. Eur Heart J Suppl D. 2001;3:D26–D32. [Google Scholar]
- Dev DK, Quensel E (1988) Preparation and functional properties of linseed protein products containing differing levels of mucilage. J Food Sci 53:1834–1837, 1857
- Dieken H. Use of flaxseed as a source of omega-3 fatty acids in human nutrition. Proc Flax Inst. 1992;54:1–4. [Google Scholar]
- Du H, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91:329–336. doi: 10.3945/ajcn.2009.28191. [DOI] [PubMed] [Google Scholar]
- Dubois V, Breton S, Linder M, Fanni J, Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol. 2007;109:710–732. [Google Scholar]
- Erdman JW. Oilseed phytates: nutritional implications. J Am Oil Chem Soc. 1979;56:736–741. [Google Scholar]
- Fedenuik RW, Biliaderis CG. Composition and physiochemical properties of linseed (Linum usitatissimum) mucilage. J Agric Food Chem. 1994;42:240–247. [Google Scholar]
- Feng D, Shen Y, Chavez ER. Effectiveness of different processing methods in reducing hydrogen cyanide content of flaxseed. J Sci Food Agric. 2003;83:836–841. [Google Scholar]
- Fukumitsu S, Aida K, Shimizu H, Toyoda K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr Res. 2010;30:441–446. doi: 10.1016/j.nutres.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandlins and leukotrienes: advances in eicasanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gabor H, Abraham S. Effect of dietary menhaden oil on tumor cell loss and the accumulation of mass of a transplantable mammary adenocarcinoma in BALB/c mice. J Natl Cancer Inst. 1986;76:1223–1231. [PubMed] [Google Scholar]
- Gambus H, Gambus F, Pastuszka D. Quality of gluten-free supplemented cakes and biscuits. Int J Food Sci Nutr. 2009;60:31–50. doi: 10.1080/09637480802375523. [DOI] [PubMed] [Google Scholar]
- Ganorkar PM, Jain RK. Flaxseed—a nutritional punch. Int Food Res J. 2013;20:519–525. [Google Scholar]
- Goh KKT, Ye A, Dale N. Characterisation of ice cream containing flaxseed oil. Intl J Food Sci Technol. 2006;41:946–953. [Google Scholar]
- Gonzalez MJ, Schemmel RA, Gray J, Dugan L, Sheffield LG, Welsch CW. Effect of dietary fat on growth of MCF-7 and MDAMB231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis. 1991;12:1231–1235. doi: 10.1093/carcin/12.7.1231. [DOI] [PubMed] [Google Scholar]
- Gopalan C, Sastri R, Balasubramanian SC. Nutritive value of Indian foods. Hyderabad: National Institute of Nutrition, ICMR; 2004. [Google Scholar]
- Green A (1995) Linola-new flaxseed breed low in alpha-linolenic acid. Australian New Crops Newsletter
- Hadley M (1996) Stability of flaxseed oil used in cooking/stir frying. In: Proceedings of the 56th Flax Institute of the United States of America, North Dakota, pp 55–59
- Hall CA, III, Manthey FA, Lee RE, Niehaus M. Stability of α-linolenic acid and secoisolariciresinol diglucoside in flaxseed-fortified macaroni. J Food Sci. 2005;70:C483–C489. [Google Scholar]
- Hosseinian FS, Beta T. Patented techniques for the extraction and isolation of secoisolariciresinol diglucoside from flaxseed. Recent Patents Food Nutr Agric. 2009;25:25–31. doi: 10.2174/2212798410901010025. [DOI] [PubMed] [Google Scholar]
- Hosseinian FS, Rowland GG, Bhirud PR, Dyck JH, Tyler RT. Chemical composition and physicochemical and hydrogenation characteristics of high-palmitic acid solin (low-linolenic acid flaxseed) oil. J Am Oil Chem Soc. 2004;81:185–188. [Google Scholar]
- Hu C, Yuan YV, Kitts DD. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem Toxicol. 2007;45:2219–2227. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Hussain S (2009) Utilization of flaxseed as a functional food. PhD Thesis submitted at National Institute of Food Science and Technology University of Agriculture, Faisalabad, Pakistan
- Hussain S, Anjum FM, Butt MS, Khan MI, Asghar A. Physical and sensoric attributes of flaxseed flour supplemented cookies. Turk J Biol. 2006;30:87–92. [Google Scholar]
- Hutchins AM, Slavin JL. Effects of flaxseed on sex hormone metabolism. In: Thompson LU, Cunnane SC, editors. Flaxseed in human nutrition. 2. Champaign: AOCS Press; 2003. pp. 126–149. [Google Scholar]
- Hyvarinen HK, Pihlava J, Hiidenhovi JA, Hietaniemi V, Korhonen HJT, Ryhanen E. Effect of processing and storage on the stability of flaxseed lignan added to dairy products. J Agric Food Chem. 2006;54:8788–8792. doi: 10.1021/jf061285n. [DOI] [PubMed] [Google Scholar]
- Hyvarinen HK, Pihlava J, Hiidenhovi JA, Hietaniemi V, Korhonen HJT, Ryhanen E. Effect of processing and storage on the stability of flaxseed lignan added to bakery products. J Agric Food Chem. 2006;54:48–53. doi: 10.1021/jf0507590. [DOI] [PubMed] [Google Scholar]
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr Suppl. 2000;71:343S–348S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Kalmusky J. Low glycemic index diet in hyperlipidemia: use of traditional starchy foods. Am J Clin Nutr. 1987;46:66–71. doi: 10.1093/ajcn/46.1.66. [DOI] [PubMed] [Google Scholar]
- Jin JS, Kakiuchi J, Hattori M. Enantioselective oxidation of enterodiol to enterolactone by human intestinal bacteria. Biol Pharm Bull. 2007;30:2204–2206. doi: 10.1248/bpb.30.2204. [DOI] [PubMed] [Google Scholar]
- Johnsson P, Kamal-Eldin A, Lundgren LN, Aaman P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J Agric Food Chem. 2000;48:5216–5219. doi: 10.1021/jf0005871. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Peerlkamp N, Johnsson P, Andersson R, Andersson RE, Lundgren LN, Aman P. An oligomer from flaxseed composed of secoisolariciresinol diglucoside and 3-hydroxy-3-methyl glutaric acid residues. Photochemistry. 2001;58:587–590. doi: 10.1016/s0031-9422(01)00279-5. [DOI] [PubMed] [Google Scholar]
- Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Essent Fat Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor S, Sachdeva R, Kochhar A. Flaxseed: a potential treatment of lowering blood glucose and lipid profile among diabetic females. Ind J Nutr Diet. 2011;48:529–536. [Google Scholar]
- Kassis NM, Gigliotti JC, Beamer SK, Tou JC, Jaczynski J. Characterization of lipids and antioxidant capacity of novel nutraceutical egg products developed with omega-3-rich oils. J Sci Food Agric. 2011 doi: 10.1002/jsfa.4542. [DOI] [PubMed] [Google Scholar]
- Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods—a review. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouryieh H, Aramouni F. Physical and sensory characteristics of cookies prepared with flaxseed flour. J Sci Food Agric. 2012 doi: 10.1002/jsfa.5642. [DOI] [PubMed] [Google Scholar]
- Kishk YMK, Elsheshetawy HE, Mahmoud EAM. Influence of isolated flaxseed mucilage as a non-starch polysaccharide on noodle quality. Int J Food Sci. 2011;46:661–668. [Google Scholar]
- Krajcova A, Schulzova V, Hajslova J, Bjelkova M. Lignans in flaxseed. Czech J Food Sci. 2009;27:252–255. [Google Scholar]
- Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:349S–351S. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Jensen MG, Aarestrup J, Petersen KEN, Sondergaard L, Mikkelsen MS, Astrup A. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depends on food type. Nutr Metab. 2012;9:8. doi: 10.1186/1743-7075-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D. Metabolic effects of dietary fiber (clinical nutrition symposium) West J Med. 1979;130:123–127. [PMC free article] [PubMed] [Google Scholar]
- Lee RE, Manthey FA, Hall CA., III Content and stability of hexane extractable lipid at various steps of producing macaroni containing ground flaxseed. J Food Process Preserv. 2004;28:133–144. [Google Scholar]
- Li SX, Cherain G, Hardin RT, Sim JS. Storage, heating and tocopherols affect cholesterol oxide formation in food oils. J Agric Food Chem. 1996;44:3830–3834. [Google Scholar]
- Lipilina E, Ganji V. Incorporation of ground flaxseed into bakery products and its effect on sensory and nutritional characteristics—a pilot study. J Foodserv. 2009;20:52–59. [Google Scholar]
- Liu S, Low NH, Nickerson MT. Entrapment of flaxseed oil within gelatin-gum arabic capsules. J Am Oil Chem Soc. 2010;87:809–815. [Google Scholar]
- Locke CA, Stoll AL. Omega-3 fatty acid in major depression. World Rev Nutr Diet. 2001;89:173–185. doi: 10.1159/000059784. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz M, Szopa J, Krasowska A. Susceptibility of lipids from different flax cultivars to per oxidation and its lowering by added antioxidants. Food Chem. 2004;88:225–231. [Google Scholar]
- Lunn J, Theobald HE. The health effects of dietary unsaturated fatty acids. British Nutrition Foundation. Nutr Bull. 2006;31:178–224. [Google Scholar]
- Macmohan B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol Ren Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- Madhusudan KT, Singh N. Effect of heat treatment on the functional properties of linseed meal. J Sci Food Agric. 1984;35:29–35. [Google Scholar]
- Madhusudan KT, Singh N. Isolation and characterization of major protein fraction (12 S) of flaxseed proteins. J Agric Food Chem. 1985;33:673–677. [Google Scholar]
- Maes M, Smith R, Christophe A, Cosyns P, Desnydes R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteroyl esters and increased C20:4 omega-6/ C20:5 omega-3 ratio in cholesteroyl esters and phospholipids. J Affect Disord. 1996;38:35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- Malkki Y. Trends in dietary fiber research and development: a review. Acta Aliment. 2004;33:39–62. [Google Scholar]
- Mandokhot VM, Singh N. Studies on linseed (Linum usitatissimum) as a protein source for poultry. I. Process of demucilaging and dehulling of linseed and evaluation of processed materials by chemical analysis and with rats and chicks. J Food Sci Technol. 1979;16:25–31. [Google Scholar]
- Manthey FA, Sinha S, Wolf-Hall CE, Hall CA., III Effect of flaxseed flour on shelf life of refrigerated pasta. J Food Process Preserv. 2008;32:75–87. [Google Scholar]
- Martinez-Flores H, Barrera E, Garnica-Romo M, Penagos C, Saavedra J, Macazaga-Alvarez R. Functional characteristics of protein flaxseed concentrated obtained applying a response surface methodology. J Food Sci. 2006;71:495–498. [Google Scholar]
- Mazur W, Uehara M, Wahala K, Adlercreutz H. Phytoestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br J Nutr. 2000;83:381–387. [PubMed] [Google Scholar]
- Mazza G (2008) Production, Processing and Uses of Canadian Flax. First CGNA International Workshop, Temuco, Chile, August 3–6
- Mazza G, Biliaderis CG. Functional properties of flaxseed mucilage. J Food Sci. 1989;54:1302–1307. [Google Scholar]
- Meagher LP, Beecher GR. Assessment of data on the lignin content of foods. J Food Comp Anal. 2000;13:935–947. [Google Scholar]
- Meagher LP, Beecher GR, Flanagan VP, Li BW. Isolation and characterization of the lignans, isolariciresinol and pinoresinol, in flaxseed meal. J Agric Food Chem. 1999;47:3173–3180. doi: 10.1021/jf981359y. [DOI] [PubMed] [Google Scholar]
- Moller NP, Scholz-Ahrens KE, Ross N, Schrezenmeir J. Bioactive peptides and proteins from foods: indication for health effects. Eur J Nutr. 2008;47:171–182. doi: 10.1007/s00394-008-0710-2. [DOI] [PubMed] [Google Scholar]
- Morris DH (2007) Flax—a health and nutrition primer, 4th edn. Available from: www.flaxcouncil.ca
- Morris MC, Evans DA, Tangney CC. Relation of the tocopherol forms to incident alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- Morton MS, Chan PSF, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Montero L, Correia R, Lloyd S, GriYths K. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mridula D, Singh KK, Barnwal P. Development of omega-3 rich energy bar with flaxseed. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Eisner P, Yoshie-Stark Y, Nakada R, Kirchoff E. Functional properties and chemical composition of fractionated brown and yellow linseed meal (Linum usitatissimum L.) J Food Eng. 2010;98:453–460. [Google Scholar]
- Muir AD. Flax lignans—analytical methods and how they influence our understanding of biological activity. J AOAC Int. 2006;89:1147–1157. [PubMed] [Google Scholar]
- Murphy PA, Hendrich S. Phytoestrogens in foods. Adv Food Nutr Res. 2002;44:195–246. doi: 10.1016/s1043-4526(02)44005-3. [DOI] [PubMed] [Google Scholar]
- Nash AM, Frankel EM. Limited extraction of soybeans with hexane. J Am Oil Chem Soc. 1986;63:244–246. [Google Scholar]
- Ogunronbi O, Jooste PJ, Abu J, Merwe B. Chemical composition, storage stability and effect of cold-pressed flaxseed oil cake inclusion on bread quality. J Food Process Preserv. 2011;35:64–79. [Google Scholar]
- Oomah BD. Flaxseed as a functional food source. J Sci Food Agric. 2001;81:889–894. [Google Scholar]
- Oomah BD, Mazza G. Flaxseed proteins—a review. Food Chem. 1993;48:109–114. [Google Scholar]
- Oomah BD, Mazza G. Effect of dehulling on chemical composition and physical properties of flaxseed. Lebensm Wiss Technol. 1997;30:135–140. [Google Scholar]
- Oomah BD, Mazza G. Compositional changes during commercial processing of flaxseed. Ind Crop Prod. 1998;9:29–37. [Google Scholar]
- Oomah BD, Mazza G, Kenaschuk EO. Cyanogenic compounds in flaxseed. J Agric Food Chem. 1992;40:346–348. [Google Scholar]
- Oomah BD, Kenaschuk EO, Mazza G. Phytic acid content of flaxseed as influenced by cultivar, growing season and location. J Agric Food Chem. 1996;44:2663–2666. [Google Scholar]
- Oomah BD, Mazza G, Kenaschuk EO. Dehulling characteristics of flaxseed. Lebensm Wiss Technol. 1996;29:245–250. [Google Scholar]
- Pan Q (1990) Flax production, utilization and research in China. In: Proceedings of the 53rd Flax Institute of the United States of America, North Dakota, pp 59–63
- Payne TJ. Promoting better health with flaxseed in bread. Cereal Foods World. 2000;45(3):102–104. [Google Scholar]
- Pella D, Dubnov G, Singh RB, Sharma R, Berry EM (2003) Effects of an Indo-Mediterranean diet on the omega-6/ omega-3 ratio in patients at high risk of coronary artery disease. The Indian Paradox. Vol.92. Basel, Karger: World Rev Nutr Diet 74–80 [DOI] [PubMed]
- Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol Cell Biochem. 1997;168:117–123. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- Prasad K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol. 2000;9:220–225. doi: 10.1007/BF01623898. [DOI] [PubMed] [Google Scholar]
- Prasad K. Antihypertensive activity of secoisolariciresinol diglucoside (SDG) isolated from flaxseed: role of guanylatecyclase. Int J Angiol. 2004;13:7–14. [Google Scholar]
- Rabetafika HN, Remoortel VV, Danthine S, Paquot M, Blecker C. Flaxseed proteins: food uses and health benefits. Int J Food Sci Technol. 2011;46:221–228. [Google Scholar]
- Raffaelli B, Hoikkala A, Leppala E, Wahala K. Enterolignans. J Chromatogr. 2002;777:29–43. doi: 10.1016/s1570-0232(02)00092-2. [DOI] [PubMed] [Google Scholar]
- Rajiv J, Indrani D, Prabhasankar P, Rao GV. Rheology, fatty acid profile and storage characteristics of cookies as influenced by flax seed (Linum usitatissimum) J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake WMN, Behrens WA, Fischer PWF, L’Abbe MR, Mongeau R, Beare-Rogers JL. Flaxseed: chemical stability and nutritional properties. Proc Flax Inst. 1992;54:37. [Google Scholar]
- Rebole A, Rodriguez ML, Ortiz LT, Alzueta C, Centeno C, Trevino J. Mucilage in linseed: effects on the intestinal viscosity and nutrient digestion in broiler chicks. J Sci Food Agric. 2002;82:1171–1176. [Google Scholar]
- Ridges L, Sunderland R, Moerman K, Meyer B, Astheimer L, Howe P. Cholesterol lowering benefits of soy and linseed enriched foods. Asia Pac J Clin Nutr. 2001;10:204–211. doi: 10.1046/j.1440-6047.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n6:n3 fatty acid ratio, with fish or flaxseed oil, in high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr. 2009;47:153–160. doi: 10.1007/s00394-008-0709-8. [DOI] [PubMed] [Google Scholar]
- Rubilar M, Gutiérrez C, Verdugo M, Shene C, Sineiro J. Flaxseed as a source of functional ingredients. J Soil Sci Plant Nutr. 2010;10:373–377. [Google Scholar]
- Saini A, Harjai K, Mohan H, Punia RPS, Chhibber S. Long-term flaxseed oil supplementation diet protects BALB/c mice against Streptococcus pneumonia infection. Med Microbiol Immunol. 2010;199:27–34. doi: 10.1007/s00430-009-0132-7. [DOI] [PubMed] [Google Scholar]
- Salimnen HK, Kauppinen TV, Virtanen HT, Rananiemi TS, Ryhanen HE-L (2010) Fermented Food Product. United States Patent Application Publication. Patent No.: US 2010/0203194 A1
- Schweigerer L, Christeleit K, Fleischmann G, Adlercreutz H, Wahala K, Hase T, Schwab R, Ludwig R, Fotsis T. Identification in human urine of a natural growth inhibitor of cells derived from solid pediatric tumors. Eur J Clin Investig. 1992;22:260–264. doi: 10.1111/j.1365-2362.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Shakir KAF, Madhusudan B. Hypocholesterolemic and hepatoprotective effects of flaxseed chutney: evidence from animal studies. Int J Clin Biochem. 2007;22:117–121. doi: 10.1007/BF02912893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar D, Agarwal YC, Sarker BC, Singh BPN. Enzymatic hydrolysis in conjuction with conventional pre-treatments to soyabean for enhanced oil availability and recovery. J Am Oil Chem Soc. 1997;74:1543–1547. [Google Scholar]
- Shearer AEH, Davies CGA. Physicochemical properties of freshly baked and stored whole-wheat muffins with and without flaxseed meal. J Food Qual. 2005;28:137–153. [Google Scholar]
- Sicilia T, Niemeyer HB, Honig DM, Metzler M. Identification and stereochemical characterization of lignans in flaxseed and pumpkin seeds. J Agric Food Chem. 2003;51:1181–1188. doi: 10.1021/jf0207979. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Essential fatty acids in health and chronic diseases. Am J Clin Nutr. 1999;70:560–569. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- Simpolous AP. Omega-6/omega-3 essential fatty acid ratio and chronic diseases. Food Rev Int. 2004;20:77–90. [Google Scholar]
- Simpolous AP (2011) Evolutionary aspects of diet: the omege-6/ omega-3 ratio and the brain. MolNeurbiol. Published online: 29 January, 2011. Humana Press
- Singer FAW, Taha FS, Mohammad SS, Gibriel A, El- Nawaway M. Preparation of mucilage/protein products from flaxseed. Am J Food Technol. 2011;6:260–278. [Google Scholar]
- Singh J, Bargale PC. Development of a small capacity double stage compression screw press oil expression. J Food Eng. 2000;43:75–82. [Google Scholar]
- Singh KK, Jhamb SA, Kumar R. Effect of pretreatments on performance of screw pressing for flaxseed. J Food Pocess Eng. 2011 [Google Scholar]
- Singh KK, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Criti Rev Food Sci Nutr. 2011;51:210–222. doi: 10.1080/10408390903537241. [DOI] [PubMed] [Google Scholar]
- Smith AK, Johnsen VL, Beckel AC. Linseed proteins—alkali dispersion and acid precipitation. Ind Eng Chem. 1946;38:353–356. [Google Scholar]
- Sok D, Cui HS, Kim MR. Isolation and bioactivities of furfuran type lignan compounds from edible plants. Recent Patents Food Nutr Agric. 2009;1:87–95. doi: 10.2174/2212798410901010087. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Pharmacology of dietary fiber. Pharmacol Ther. 1994;62:407–427. doi: 10.1016/0163-7258(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Spychalla JP, Kinney AJ, Browse J. Identification of an animal omega-3 fatty acid desaturase by heterologous expression in Arabidopsis. Proc Natl Acad Sci U S A. 1997;94:1142–1147. doi: 10.1073/pnas.94.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Mazza G. Effect of flaxseed gum on quality and stability of a model salad dressing. J Food Qual. 2000;23:373–390. [Google Scholar]
- Struijs K, Vincken JP, Gruppen H. Bacterial conversion of secoisolariciresinol and anhydrosecoisolariciresinol. J Appl Microbiol. 2009;107:308–317. doi: 10.1111/j.1365-2672.2009.04209.x. [DOI] [PubMed] [Google Scholar]
- Sturgeon SR, Heersinka JL, Volpeb SL, Bertone-Johnsona ER, Puleoa E, Stanczykc FZ, Sabelawskid S, Wahalae K, Kurzerf MS, Bigelowa C. Effect of dietary flaxseed on serum levels of estrogens and androgens in postmenopausal women. Nutr Cancer. 2008;60:612–618. doi: 10.1080/01635580801971864. [DOI] [PubMed] [Google Scholar]
- Susheelamma NS. Isolation and properties of linseed mucilage. J Food Sci Technol. 1987;24:103–106. [Google Scholar]
- Susheelamma NS. Functional role of linseed (Linum usitatissimum L.) polysaccharide in steamed pudding (idli) J Food Sci Technol. 1989;26:16–20. [Google Scholar]
- Tarpila A, Wennberg T, Tarpila S. Flaxseed as a functional food. Curr Top Nutraceutical Res. 2005;3:167–188. [Google Scholar]
- Tautorus CL, McCurdy AR. Effect of randomization on oxidative stability of vegetable oils at two different temperatures. J Am Oil Chem Soc. 1990;67:525–530. [Google Scholar]
- Thakur G, Mitra A, Pal K, Rousseau D. Effect of flaxseed gum on reduction of blood glucose & cholesterol in type 2 diabetic patients. Int J Food Sci Technol. 2009;60:126–136. doi: 10.1080/09637480903022735. [DOI] [PubMed] [Google Scholar]
- Thompson LU, Rickard SE, Orcheson LJ, Seidl MM. Flaxseed and its lignan and its oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis. 1996;17:1373–1376. doi: 10.1093/carcin/17.6.1373. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, van Tuijl HR, Hofman A, Kiliaan A, Breteler MMB. Plasma fatty acid composition and depression are associated in the elderly: the Rotterdam Study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- Tolkachev ON, Zhuchenko AA. Biologically active substances of flax: medicinal and nutritional properties (a review) Pharm Chem J. 2000;34:360–367. [Google Scholar]
- Toure A, Xueming X. Flaxseed lignans: source, biosynthesis, metabolism, antioxidant activity, bio-active components and health benefits. Compr Rev Food Sci Food Saf. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- Wanasundara JP, Shahidi F. Functional properties and amino acid composition of solvent extracted flaxseeds meals. Food Chem. 1994;49:45–51. [Google Scholar]
- Wanasundara PKJPD, Shahidi F. Removal of flaxseed mucilage by chemical and enzymatic treatments. Food Chem. 1997;59:47–55. [Google Scholar]
- Wang C, Makela T, Hase T, Adlercreutz H, Kurzer MS. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol. 1994;50:205–212. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Meselhy MR, Li Y, Qin GW, Hattori M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem Pharm Bull. 2000;48:1606–1610. doi: 10.1248/cpb.48.1606. [DOI] [PubMed] [Google Scholar]
- Westcott ND, Muir AD. Chemical studies on the constituents of Linum spp. In: Muir AD, Westcott ND, editors. Flax: the genus Linum. London: Taylor & Francis; 2003. pp. 55–73. [Google Scholar]
- WHO (2003) Diet, nutrition and the prevention of chronic diseases. WHO Technical Report, Series 916 [PubMed]
- Wiesenborn D, Kangas N, Tostenson K, Hall C, III, Chang K. Sensory and oxidative quality of screw-pressed flaxseed oil. J Am Oil Chem Soc. 2005;82:887–892. [Google Scholar]
- Wu W, Huff HE, Hsieh F. Processing and properties of extruded flaxseed-corn puff. J Food Process Preserv. 2007;31:211–226. [Google Scholar]
- Wu M, Li D, Wang LJ, Ozkan N, Mao ZH. Rheological properties of extruded dispersions of flaxseed-maize blend. J Food Eng. 2010;98:480–491. [Google Scholar]
- Xu Y, Hall C, III, Wolf-Hall C. Antifungal activity stability of flaxseed protein extracts using response surface methodology. J Food Sci. 2008;73:M9–M14. doi: 10.1111/j.1750-3841.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Hall C, III, Wolf-Hall C. Antifungal activity stability of flaxseed protein extracts using response surface methodology. Food Microbiol Saf. 2008;73:M9–M14. doi: 10.1111/j.1750-3841.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Sano T, Hashimoto T, Kanazawa K. Development of a method to remove cyanogen glycosides from flaxseed meal. Int J Food Sci Technol. 2007;42:70–75. [Google Scholar]
- Young LG (1982) Effects of processing on nutritive value of feeds: oilseeds and oilseed meals. In: Miloslav Jr R (ed) Handbook of nutritive value of processed food. CRC series in Nutrition, vol. 2, animal feedstuffs. CRC Press, Boca Raton pp 213–221
- Zhang ZS, Wang Li J, Li D, Jiao SS, Chen DX, Mao ZH. Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol. 2008;62:192–198. [Google Scholar]