Abstract
The main objective of this study was to use heating method (HM) to prepare liposome without employing any chemical solvent or detergent. Plackett-Burman design (PBD) was applied for the screening of significant process variables including the lecithin proportion, the cholesterol/lecithin ratio, the pH of solution for liposome preparation, the enzyme/lecithin ratio, the stirring time, the process temperature, the speed of stirrer, the ratio of stirrer to the tank diameter, the application of homogenization, the method of adding enzyme and centrifugation conditions on the encapsulation efficiency (EE %) of liposome and the activity of liposomal Flavourzyme (LAPU−1) (P < 0.05). Then, the response surface methodology based on the central composite design (CCD) was applied for the evaluation of the impacts of the significant mentioned variables on the EE (%) and the activity of the liposomal Flavourzyme. The results indicated that the lecithin proportion and the stirring time were the major influential variables for both responses. The most suitable formulation of the Flavourzyme-loaded liposome is 4.5 % lecithin, 45 °C temperature, 5 % Flavourzyme/lecithin ratio, 30 min stirring time and medium pH of 6. Under suitable operating conditions, the EE of liposome and the activity of the liposomal Flavourzyme were achieved as 26.5 % and 9.96 LAPU ml−1, respectively. AFM technique and size distribution clearly showed the diameter of 189 nm for the spherical shape of the Flavourzyme- loaded nanoliposome.
Keywords: Nanoliposome, Heating method, Plackett-Burman design, Response surface methodology, Flavourzyme
Introduction
In food industry, encapsulation is used to stabilize and control the release of core material (Gibbs et al. 1999). It is generally agreed that the majority of microencapsulation techniques currently used in the food industry is composed of sugar, starch, gum, protein, dextrin and alginate. Recently, liposome, spherical particles with sizes at the range of the nanometer to micrometer formed by polar lipids (Charcosset 2009; Taylor et al. 2005) has received special attention in the literature (Mozafari et al. 2008a, b). Liposome technology has generated much interest in food industry for the encapsulation of different materials such as ferrous glycinate (Ding et al. 2011), ferrous sulfate (Xia and Xu 2005), antioxidant (Mozafari et al. 2006), nisin (Taylor et al. 2007; Laridi et al. 2003; Colas et al. 2007) β-galactosidase (Rao et al. 1994) and different cheese accelerated enzymes such as lipase (Kheadr et al. 2002), Nutrease (Alkhalaf et al. 1989), chymotripsine (Laloy et al. 1998; Dufour et al. 1996), chymosine (Picon et al. 1994), neutral protease (Picon et al. 1995), cyprosine (Picon et al. 1996) bacterial and fungal protease, Flavourzyme and palatase (Kheadr et al. 2003). Different methods such as reverse-phase evaporation, dehydration-rehydration, microfludization technique and proliposome usage have been applied for liposome manufacturing with a variation of EE (%) ranging from 10 to 40.3 %. However, most of the methods (except microfluidization technique and proliposome usage) require organic solvent causing enzyme inactivation (Rao et al. 1994) which make them unsuitable for food products (Skeie 1994; Dufour et al. 1996). The disadvantages of the microfluidization technique are loss of material, contamination, relatively difficult for scaling up and the employment of very high shearing forces that can potentially damage the structure of the compounds to be encapsulated (Maa and Hsu 1999; Kasaai et al. 2003). Proliposomes are not cost-effective either and their manufacturing process is a multi-step, lengthy procedure (Song et al. 2002). Heating method (HM) is a scalable and robust method, which does not require employment of high mechanical stress, any harmful chemicals or extreme values of pH during their preparation (Mozafari and Mortazavi 2005; Mozafari et al. 2008a, b).
Flavourzyme® (EC: 3.4.11.1) is a fungal complex of exopeptidases and endoproteases used for cheese production and acceleration of cheese ripening, especially for debittering and flavor development of cheese (Fox 1993; Fox and Grufferty 1991; Kilcawley et al. 2002). Commercially, this enzyme is in a powder form obtained from Aspergillusoryzae. Kilcawley et al. (2002) showed that Flavourzyme has an endo- and amino-peptidase activity. The potential advantages of liposome encapsulation of enzyme for cheese acceleration includes usage of natural ingredients, protection of casein from early proteolysis and well partition of enzyme in the curd (El Soda 1993; Mozafari et al. 2008a, b). The mean diameter of different types of trypsine-loaded liposomes and EE (%) ranged from 110 to 1,540 nm and 10–14 % (Larivière et al. 1991). Encapsulation data of bacterial and fungal protease and lipase was investigated by Kheadr et al. (2003), in that, the EE (%) of Flavourzyme-loaded liposome was reported to be 20.2 % based on the enzyme activity and 23 % based on the protein. However, in that study the diameter of produced liposome was not reported. Dufour et al. (1996) reported that the immobilization efficiency and the mean diameter of chymotrypsin liposome were 68–96 % and 725–800 nm respectively and that the ionic strength and enzyme concentration were influenced by the immobilization efficiency and the mean diameter. All accelerated enzymes were encapsulated in liposome with traditional methods for applying in the acceleration of cheese ripening. However, enzyme-loaded liposomes show low EE (%), when using chemical solvent or detergent in liposome production (Mozafari et al. 2008a, b). While liposome size and morphology has a strong influence on liposome distribution within cheese texture, it has not been investigated yet.
The present study aimed to prepare Flavourzyme-loaded liposomes using HM as a replacer of the methods using chemical agents. Placket-Burman design (PBD) was used to assess the effects of the chemical components and process variables on the EE (%). After the screening of significant variables, the most suitable formulation of Flavourzyme-loaded liposome formulation prepared with HM was found using response surface methodology (RSM). Finally, the features of the obtained formulation about EE (%), the size and the morphology of HM liposomes were investigated.
Materials and methods
Material
Lecithin (granular form) and cholesterol (Acros Company, Geel, Belgium) were purchased and Flavourzyme (Novozymes Pvt Ltd, Tehran, Iran) was received as gift. Glycerol and triton X- 100 (Sigma Chemical Co, Sydney, Australia), L-leucine-p-nitroanilide from (Fluka, Sydney, Australia) and all other chemicals were obtained from commercial analytical grade. The water used for the solutions was purified using a Milli Q water purification unit (Millipore, MA, USA).
Preparation of liposome
Preparation of liposome, based on the HM (Mozafari and Mortazavi 2005; Colas et al. 2007) was as follow: cholesterol was first dissolved in the glycerol (3 % w/v in final concentration) at elevated temperature (120 °C). The liposomal ingredient (lecithin and cholesterol solution) were added to preheated (40 °C, 1 min) 0.01 M tris buffer (as described in Tables 1 and 2). The temperature was controlled by a water bath in the range of ±1 °C. Enzyme addition was conducted in two ways: A) addition of enzyme, lecithin and cholesterol in buffer solution simultaneously and B) addition of cholesterol and lecithin suspension in buffer after the complete addition of the enzyme. Then the mixture was heated while stirring (approx. 500 or 900 rpm) on a hotplate stirrer (HCR2 Gerhadt Germany) with 0.5 or 0.9 ratio of stirrer to tank diameter while heating. The independent variables in PBD and CCD are shown in Tables 1 and 2 respectively. All the experiments were conducted in triplicate and samples were kept overnight in 4 °C under nitrogen atmosphere.
Table 1.
Twelve-trial PBD to study the effects of 11 factors on the encapsulation efficiency EE (%) and activity of the liposomal Flavourzyme (LAPU−1) as responses
| Run no. | pH | Cholesterol/lecithin (w/w) | Lecithin (% w/w) | Enzyme/lecithin (w/w) | Stirring time (min) | Temperature (°C) | Speed of stirrer (rpm) | Ratio of stirrer to tank diameter | Homogenization (rpm) | Way of enzyme addition | Centrifugation condition (×1,000 g) | Encapsulation efficiencya | Activity of the liposomal flavourzyme |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 0.05 | 4 | 0.2 | 5 | 50 | 1000 | 0.9 | 10000 | A | 40 | 16.47 ± 0.96 | 0.59 |
| 2 | 8 | 0.1 | 2 | 0.1 | 5 | 50 | 500 | 0.45 | 10000 | A | 45 | 5.44 ± 0.27 | 0.61 |
| 3 | 6 | 0.1 | 4 | 0.2 | 15 | 50 | 500 | 0.9 | 0 | A | 45 | 22.21 ± 2.63 | 0.67 |
| 4 | 8 | 0.05 | 4 | 0.1 | 5 | 60 | 500 | 0.9 | 0 | B | 45 | 8.73 ± 1.10 | 0.14 |
| 5 | 8 | 0.1 | 2 | 0.1 | 15 | 50 | 1000 | 0.9 | 0 | B | 40 | 9.33 ± 0.54 | 0.10 |
| 6 | 8 | 0.1 | 4 | 0.2 | 15 | 60 | 500 | 0.45 | 10000 | B | 40 | 5.83 ± 0.89 | 0.16 |
| 7 | 6 | 0.1 | 4 | 0.1 | 5 | 60 | 1000 | 0.45 | 0 | A | 40 | 8.82 ± 1.43 | 0.17 |
| 8 | 6 | 0.05 | 4 | 0.1 | 15 | 50 | 1000 | 0.45 | 10000 | B | 45 | 16.44 ± 0.97 | 0.29 |
| 9 | 6 | 0.05 | 2 | 0.1 | 15 | 60 | 500 | 0.9 | 10000 | A | 40 | 9.27 ± 2.01 | 0.1 |
| 10 | 8 | 0.05 | 2 | 0.2 | 15 | 60 | 1000 | 0.45 | 0 | A | 45 | 9.33 ± 2.1 | 0.16 |
| 11 | 6 | 0.1 | 2 | 0.2 | 5 | 60 | 1000 | 0.9 | 10000 | B | 45 | 4.99 ± 0.32 | 0.1 |
| 12 | 6 | 0.05 | 2 | 0.2 | 5 | 50 | 500 | 0.45 | 0 | B | 40 | 7.55 ± 0.54 | 0.13 |
aConfidence limit: 99 %
Table 2.
Levels of factors used in CCD
| Factors | Code | Range and levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Lecithin proportion | X1 | 2.5 | 3.5 | 4.5 |
| Stirring temperature | X2 | 40 | 50 | 60 |
| Enzyme/lecithin ratio | X3 | 0.5 | 0.1 | 0.15 |
| Stirring time | X4 | 10 | 20 | 30 |
| pH | X5 | 6 | 6.5 | 7 |
Determination of enzyme activity
Activity of Flavourzyme was measured using the procedure suggested by Kailasapathy et al. (2006) and Anjani et al. (2007), in that, L-leucine-p-nitroanilide (leu-p-Na) was used as a substrate. The activity of Flavourzyme was measured based on the reaction rate and was introduced as leucineaminopeptidaes units per milliliter (LAPU ml−1). Two replicates were made on each assay and average values were used to calculate Flavourzyme activity (micromoles of substrate release per minute per milliliter).
Determination of EE (%)
The liposomes were separated from unencapsulated enzymes by centrifugation at 4 °C according to the study conducted by Kheadr et al. (2003). One milliliter sample add to 1 ml deionized water centrifuge at 40,000 or 45,000×g (as an independent variable, X10 in PBD) for 60 or 75 min in 4 °C. Samples were washed twice with deionized water and recentrifuged. Supernatant was diluted to a final volume of 10 ml and used to determine the amount of unencapsulated enzyme (free enzyme). Pellets were suspended in deionized water to a final volume of 5 ml. Two milliliters of sample suspension was mixed with 2 ml of 2 % (w/v) Triton X-100 (Sigma) in order to disrupt the vesicles and release the enzyme.
The activity of the encapsulated and free enzyme (as LAPU ml−1) was measured to estimate the EE (%) according to the following Eq. 1:
| 1 |
Particle size characterization
The mean diameter and particle size distribution of vesicles were determined using dynamic light scattering (DLS) technique by employing a particle size analyzer 90Plus (Brookhaven Instruments Corporation, USA).
Atomic force microscopy
Atomic force microscopy (AFM) studies were performed in non-contact mode with a commercial AFM (DualScope DS 95 AFM Scanner, DME, Denmark), equipped with a D-scanner (15 mm scan size), using standard silicon tips and forces constant of 0.15–1.5 N/m. A drop (5–10 ml) of each of nano-liposome suspensions was deposited on freshly cleaved mica substrates, dried in air and visualized under AFM at room temperature.
Experimental design
PBD is a useful method for picking the most important factors from a long list of candidate factors and select them to realize a factorial design. Table 1 shows the selected variables, which include chemical components (the lecithin proportion (w/w %), the cholesterol/lecithin (w/w), the enzyme/lecithin (w/w) and the pHof solution for liposome preparation), and process variables (the stirring time, the process temperature, the ratio of stirrer to tank diameter, centrifugation condition, the speed of stirring, the method of adding enzyme and homogenization) at two levels. The Minitab 14 software was used to confirm the experimental matrix (with four replicates) and randomize it. The response was EE (%). The statistical analysis was done at 99 % confidence interval.
After screening by PBD, the optimization was designed based on a five-factor CCD with a total of 32 experimental runs that involved 6 axial points and 6 replicates at the center points and 1.000 alpha. In this study the independent variables were studied at three different levels, low (−1), medium (0) and high (+1), which are shown in Table 2. The dependent variables (response) are EE (%) and the activity of liposomal Flavourzyme. The model equation for analysis is given by Eq. 2:
| 2 |
Where X1, X2, …, X5 are the independent variables of the system under investigation and β1, β2, …, β5 are linear coefficients, β11, β22, … β55 are quadratic coefficients and β12, β13, …, β45 are interactive coefficient estimates with β0 having a role of a scaling constant. Analysis of variance (ANOVA) and regression analysis were done and response surface plot drawn using Minitab statistical software package 11 at 95 % confidence interval.
Results
The experimental design to find the most suitable was applied in two stages. The purpose of the first stage was to identify the significant chemical compound and process variables for the production of the Flavourzyme-loaded liposome by HM using PBD. In the second stage, the significant chemical compound and process variables resulted from PBD were optimized using a CCD in range mentioned in Table 2.
Evaluating the effects of the chemical compound and process variables by PBD
Table 1 shows the 11 selected process variables, their levels and experimental EE (%) as a response.
EE (%) and activity of the liposomal Flavourzymewere produced by HM varied from 4.99 to 22.21 % and from 0.1 to 0.67 (LAPU−1) respectively (Table 1). ANOVA of the EE (%) of Flavourzyme-loaded HM liposomes is shown in Table 3. The most significant factors affecting the EE (%) were pH (X1), the Cholesterol/lecithin (% w/w) (X2), the lecithin proportion (X3), the enzyme/lecithin ratio (X4), the stirring time (X5), the stirring temperature (X6), the method of adding enzyme (X9) and the ratio of stirrer to tank diameter (X11) (p < 0.01). The speed of stirrer (X7) and the homogenization (X8) did not show any significant impacts on the EE (%) (Table 3).
Table 3.
Statistical data for the variance analysis of the encapsulation efficiency (%) and activity of the liposomal flavourzyme (LAPU−1) encapsulated by heating method
| Variables | EE (%) | Activity of the liposomal flavourzyme (LAPU−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Coefficient | t-value | p-value Prof>F | Effect | Coefficient | t-value | p-value Prof>F | |
| X1: pH | −2.08 | −1.04 | −4.17 | 0.00 | −0.04 | −0.02 | −2.42 | 0.00 |
| X2: cholesterol/lecithin (% w/w) | −1.84 | 0.92 | 3.71 | 0.001 | −0.02 | −0.01 | −1.51 | 0.14 |
| X3: lecithin (% w/w) | 5.00 | 2.50 | 10.04 | 0.00 | 0.23 | 0.11 | 13.6 | 0.00 |
| X4: enzyme/lecithin ratio (% w/w) | −1.48 | −0.74 | −2.98 | 0.005 | −0.14 | −0.06 | −8.27 | 0.00 |
| X5: stirring time (min) | 3.34 | 1.67 | 6.70 | 0.00 | 0.05 | 0.02 | 2.94 | 0.00 |
| X6: temperature (°C) | −4.19 | −2.09 | −8.42 | 0.00 | −0.15 | −0.07 | −9.08 | 0.00 |
| X7: speed of stirrer (rpm) | 0.98 | 0.49 | 2.00 | 0.05 | 0.01 | 0.00 | 0.48 | 0.63 |
| X8: homogenization | −0.97 | −0.48 | −1.96 | 0.06 | −0.03 | −0.01 | −1.75 | 0.08 |
| X9: way of enzyme addition | 3.23 | 1.61 | 6.48 | 0.00 | 0.12 | 0.06 | 7.04 | 0.00 |
| X10: centrifugation condition | 1.18 | 0.59 | 2.39 | 0.02 | 0.04 | 0.02 | 2.71 | 0.01 |
| X11: ratio of stirrer to tank diameter | 2.52 | 1.26 | 5.07 | 0.00 | 0.11 | 0.06 | 7.06 | 0.00 |
Each value is the average of four replications
Confidence limit: 99 %
It has been approved that positive sign for each t-value indicates that high level of variable causes enhancement of response. Positive value in the model for a response represents a favorable effect and negative value indicates an inverse relationship between the response and the factor (Khosravi-Darani and Zoghi 2008). The most important variable, which considerably affected on the EE (%), was the percentage of lecithin (X3). Similar results are reported by Jahadi et al. (2012) and Xiong et al. (2009). An explanation for this observation is that lecithin is the major component in liposome formulation, while cholesterol, used as a membrane stabilizer in smaller quantity than lecithin, is the second component of the vesicles.
EE (%) increased with the elongation of stirring time (X5) and the ratio of stirrer to tank diameter (X11) suggesting the importance of these variables in increasing the liquefaction of lecithin granules in aqueous phase and the rate of liposome formation. The two modes of enzyme addition influenced EE (%). The mode B of enzyme addition increased the EE (%) more than the mode A (p < 0.01). On the contrary, increasing the pH value (X1) and the process temperature (X6) decreased the EE (%) (p < 0.01). ANOVA of the activity of the liposomal Flavourzyme (LAPU−1) produced by HM is shown in Table 3. The most significant factors affecting the activity of the liposomal Flavourzyme (LAPU−1) were the lecithin proportion (X3), the enzyme/lecithin ratio (X4), the stirring time (X5), the stirring temperature (X6), the method of adding enzyme (X9) the ratio of stirrer to tank diameter (X11) (p < 0.01). Among the all significant variables (X1, X2, X3, X4, X5, X6, X9 and X11) effects on EE (%) and (X1, X3, X4, X5, X6, X9 and X11) effects on the activity of liposomal Flavourzyme (LAPU−1), X1, X3, X4, X5, X6, X9 and X11 are influenced on both responses. The way of enzyme addition (X9) and ratio of stirrer to tank diameter (X11) were excluded from optimization process in the range of experimented level and fixed on the highest response. Between two modes for the addition of the enzyme (X9), mode B was selected, Due to the fact of the limitation of stirrer and tank diameters used for preparing HM and, the biggest ratio of stirrer to tank diameter (X11) was used to achieve as the highest response. Therefore, pH, the lecithin proportion, the enzyme/lecithin ratio, the stirring time and the stirring temperature were selected for central composite design.
Application of CCD for production Flavourzyme-loaded liposome
The cholesterol was first dissolved in the glycerol (3 % w/v in final concentration) at elevated temperature (120 °C) in cholesterol/lecithin ratio (% w/w) 0.05 %. After complete addition of Flavourzyme in 0.01 M tris buffer, cholesterol suspension and lecithin were added in buffer. Then the mixture was heated while stirring (900 rpm) on a hotplate stirrer (HCR2 Gerhadt Germany) with 0.9 ratio of stirrer to tank diameter while heating. All samples were kept overnight in 4 °C under nitrogen atmosphere. Centrifugation was used at 45,000×g for 75 min duo to the separation of the free enzyme from encapsulated. The lecithin proportion (X1), the process temperature on hot plate (X2), the enzyme/lecithin ratio (X3), the stirring time on hot plate (X4) and pH of solution (X5) were selected for next step using CCD.
The EE (%) and the activity of liposomal Flavourzyme of liposome (LAPU−1) were optimized using RSM. By taking the EE (%) and the activity of liposomal Flavourzyme(LAPU−1) as the responses value (Y), 32 experiments were designed, in which 1–26 were the factorial experiments, 27–32 were the zero-point tests. The Zero-point tests were performed in six replicates to estimate the impact of the random errors. The experimental design used for the study and the activity of liposomal Flavourzyme as well as experimental and predicted EE (%) are shown in Table 4.
Table 4.
Central composite design for evaluation of process variables on the activity of the liposomal flavourzyme (LAPU−1) and encapsulation efficiency EE (%)
| No. | Variables | EE (%) | Activity of the liposomal flavourzyme | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lecithin (%) | Enzyme/lecithin | Temperature (°C) | Time (min) | pH | Experimental | Predicted | Experimental | Predicted | |
| 1 | 2.5 | 5 | 40 | 10 | 7 | 4.50 ± 0.12 | 3.06 | 0.82 ± 0.08 | 0.77 |
| 2 | 4.5 | 5 | 40 | 10 | 6 | 16.85 ± 0.96 | 17.38 | 4.16 ± 0.14 | 4.16 |
| 3 | 2.5 | 5 | 65 | 10 | 6 | 0.37 ± 0.05 | 0.10 | 0.40 ± 0.02 | 0.29 |
| 4 | 4.5 | 5 | 65 | 10 | 7 | 4.28 ± 0.84 | 4.25 | 2.80 ± 0.08 | 2.91 |
| 5 | 4.5 | 15 | 40 | 10 | 6 | 4.33 ± 0.25 | 3.57 | 2.06 ± 0.09 | 1.98 |
| 6 | 4.5 | 15 | 40 | 10 | 7 | 13.89 ± 1.05 | 14.27 | 11.99 ± 1.82 | 12.14 |
| 7 | 2.5 | 15 | 65 | 10 | 7 | 3.22 ± 0.5 | 3.63 | 2.33 ± 0.07 | 2.37 |
| 8 | 4.5 | 15 | 65 | 10 | 6 | 11.30 ± 1.96 | 11.84 | 11.21 ± 2.01 | 11.30 |
| 9 | 2.5 | 5 | 40 | 30 | 6 | 8.13 ± 0.87 | 9.26 | 1.15 ± 0.02 | 1.02 |
| 10 | 4.5 | 5 | 40 | 30 | 7 | 17.35 ± 2.15 | 15.85 | 4.33 ± 0.05 | 4.43 |
| 11 | 2.5 | 5 | 65 | 30 | 7 | 7.84 ± 1.02 | 8.41 | 1.09 ± 0.02 | 1.08 |
| 12 | 4.5 | 5 | 65 | 30 | 6 | 21.51 ± 2.23 | 20.72 | 3.90 ± 0.04 | 3.94 |
| 13 | 2.5 | 15 | 40 | 30 | 7 | 5.20 ± 0.45 | 5.12 | 2.72 ± 0.03 | 2.74 |
| 14 | 4.5 | 15 | 40 | 30 | 6 | 23.29 ± 2.14 | 23.53 | 24.38 ± 2.13 | 24.26 |
| 15 | 2.5 | 15 | 65 | 30 | 6 | 8.95 ± 0.96 | 9.02 | 2.38 ± 0.01 | 2.35 |
| 16 | 4.5 | 15 | 65 | 30 | 7 | 8.10 ± 1.02 | 9.50 | 6.97 ± 0.07 | 7.16 |
| 17 | 2.5 | 10 | 52.5 | 20 | 6.5 | 7.34 ± 1.12 | 8.13 | 3.03 ± 0.02 | 3.40 |
| 18 | 4.5 | 10 | 52.5 | 20 | 6.5 | 18.38 ± 1.45 | 17.59 | 11.40 ± 1.01 | 10.61 |
| 19 | 3.5 | 10 | 40 | 20 | 6.5 | 6.63 ± 0.6 | 8.13 | 4.53 ± 0.03 | 4.43 |
| 20 | 3.5 | 10 | 65 | 20 | 6.5 | 6.50 ± 0.92 | 5.01 | 2.24 ± 0.01 | 1.92 |
| 21 | 3.5 | 5 | 52.5 | 20 | 6.5 | 7.51 ± 0.62 | 9.38 | 1.90 ± 0.01 | 2.14 |
| 22 | 3.5 | 15 | 52.5 | 20 | 6.5 | 12.20 ± 1.00 | 9.61 | 8.12 ± 0.07 | 7.86 |
| 23 | 3.5 | 10 | 52.5 | 10 | 6.5 | 5.65 ± 0.24 | 6.76 | 4.07 ± 0.05 | 4.31 |
| 24 | 3.5 | 10 | 52.5 | 30 | 6.5 | 13.25 ± 2.24 | 12.23 | 5.59 ± 0.04 | 5.69 |
| 25 | 3.5 | 10 | 52.5 | 20 | 6 | 12.09 ± 1.07 | 11.43 | 5.45 ± 0.07 | 5.98 |
| 26 | 3.5 | 10 | 52.5 | 20 | 7 | 7.71 ± 0.45 | 7.57 | 4.22 ± 0.02 | 4.02 |
| 27 | 3.5 | 10 | 52.5 | 20 | 6.5 | 7.85 ± 0.54 | 9.50 | 4.89 ± 0.06 | 5.00 |
| 28 | 3.5 | 10 | 52.5 | 20 | 6.5 | 9.02 ± 0.68 | 9.50 | 4.87 ± 0.03 | 5.00 |
| 29 | 3.5 | 10 | 52.5 | 20 | 6.5 | 10.92 ± 1.00 | 9.50 | 5.32 ± 0.04 | 5.00 |
| 30 | 3.5 | 10 | 52.5 | 20 | 6.5 | 8.90 ± 0.91 | 9.50 | 5.15 ± 0.02 | 5.00 |
| 31 | 3.5 | 10 | 52.5 | 20 | 6.5 | 10.68 ± 1.07 | 9.50 | 5.22 ± 0.02 | 5.00 |
| 32 | 3.5 | 10 | 52.5 | 20 | 6.5 | 8.19 ± 0.71 | 9.50 | 4.76 ± 0.05 | 5.00 |
Confidence limit: 95 %
Multiple regressions were used to analyze the data and thus polynomial equation was derived from regression analysis as follow (Eq. 3):
| 3 |
Stepwise elimination of insignificant terms was done. X3, X4 and X5 show insignificant quadratic effect on EE (%) by low p-value (p < 0.05) are showed in fitted model Eq. 3. So the model was modified to reduced fitted model of Eq. 4:
| 4 |
The Regression analysis of the experiments showed that the lecithin proportion (X1), the enzyme/lecithin ratio (X3) and the stirring time (X4) had positive linear effects and the stirring temperature (X2) and the pH of solution (X5) had negative effects on the EE (%) (p < 0.05). Among the five variables, the lecithin proportion had dramatically highest linear coefficient effect (4.725) on EE (%) followed by the stirring time on the hot plate (2.734), the pH of solution (−1.931), the stirring temperature on the hot plate (−1.561) and the enzyme/lecithin ratio (0.119). The Positive and negative signs in front of the terms indicate synergistic and antagonistic effects, respectively (Bas and Boyaci 2007). The Lecithin proportion and the stirring temperature on hot plate show significant positive and negative quadratic effects on EE (%) respectively. The findings revealed that suitable EE (%) is obtained at higher stirring temperature and lecithin concentration. On the other hand, decreased EE (%) occurs as a result of more increased level of stirring temperature. Interactions among these parameters were also significant. The interaction between the stirring temperature on the hot plate- enzyme/lecithin ratio, the enzyme/lecithin ratio-stirring time and the stirring time- pH of solution were significant (p < 0.05). By contrast, the interactions between the lecithin proportion- stirring temperature on the hot plate, the lecithin proportion- enzyme/lecithin ratio, the lecithin proportion- stirring time on the hot plate, the lecithin proportion- pH of solution, the stirring temperature on the hot plate- stirring time on the hot plate, the stirring temperature on hot plate- pH of solution and the enzyme/lecithin ratio- pH of the solution were found to be insignificant (p < 0.05). Consequently, these were excluded from the regression Eq. (4) used for this model.
A polynomial model describing the correlation between the activity of liposomal Flavourzyme and significant five variables (after stepwise elimination of insignificant terms) was obtained in Eq. (5):
| 5 |
The regression analysis of the experiments showed that the lecithin proportion (X1), the stirring temperature (X2), the enzyme/lecithin ratio (X3), and the stirring time (X4) and the pH of solution (X5) show significant linear effects on liposomal Flavourzyme activity (p < 0.05). But only the lecithin proportion and the stirring temperature on the hot plate show significant positive and negative quadratic effects on the activity of liposomal Flavourzyme respectively (p < 0.05). In addition, interactions among some of these parameters were also significant (p < 0.05). Furthermore, these terms were existed in the regression Eq. (5) used for this model.
The analysis of variance for the EE (%) and the activity of liposomal Flavourzyme (LAPU−1) are given in Table 5. ANOVA gives the value of the model and may explain whether this model adequately fits the variation observed in EE (%) and the activity of liposomal Flavourzyme (LAPU−1) produced using HM with process variables. If the F-test for the model is significant at 5 % (p < 0.05), then the model is fit and can adequately explain the observed variation. If the F-test for lack of fit is significant (p < 0.05), then a more complicated model is required to fit the data. The R2 value for Eqs. (4) and (5) were 0.956 and 0.977 respectively, indicating that there were good agreements between the experimental and predicted values from the models. When the value of R (multiple correlation coefficients) is closer to 1, it shows a better correlation between the observed and the predicted values. P value for the lack of fit of (0.410) and (0.102) indicated that the experimental data obtained fitted well with the model and explained the effects of the lecithin proportion, the stirring temperature on hotplate (°C), the Flavourzyme/lecithin ratio, the stirring time (min) and the pH on the EE (%) and the activity of liposomal Flavourzyme (LAPU−1) produce by HM.
Table 5.
Analysis of variance for encapsulation efficiency EE (%) and activity of liposomal flvourzyme (LAPU−1) which prepared by heating method using central composite design criterion
| Source | EE (%) | Activity of liposomal flavourzyme (LAPU−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| DF | MS | F-value | P-value | DF | MS | F-value | P-value | |
| Regression | 12 | 68.27 | 34.56a | 0.00 | 17 | 37.36 | 291.19a | 0.00 |
| Linear | 5 | 129.45 | 65.58a | 0.00 | 5 | 8.55 | 66.65a | 0.00 |
| Square | 2 | 20.50 | 10.38a | 0.00 | 2 | 7.44 | 58.00a | 0.00 |
| Interaction | 5 | 26.11 | 13.22a | 0.00 | 10 | 18.47 | 144.02a | 0.00 |
| Residual error | 19 | 1.97 | 14 | 0.12 | ||||
| Lack of fit | 14 | 2.10 | 1.31 | 0.41 | 9 | 0.17 | 3.29 | 0.102 |
| Pure error | 5 | 1.61 | 5 | 0.05 | ||||
| Total | 31 | 31 | ||||||
aSignificant at p < 0.05
The response surface plot is the theoretical three-dimensional plot showing the relationship between the response and the independent variables (Bas and Boyaci 2007). The response surface plots were shown in Fig. 1.
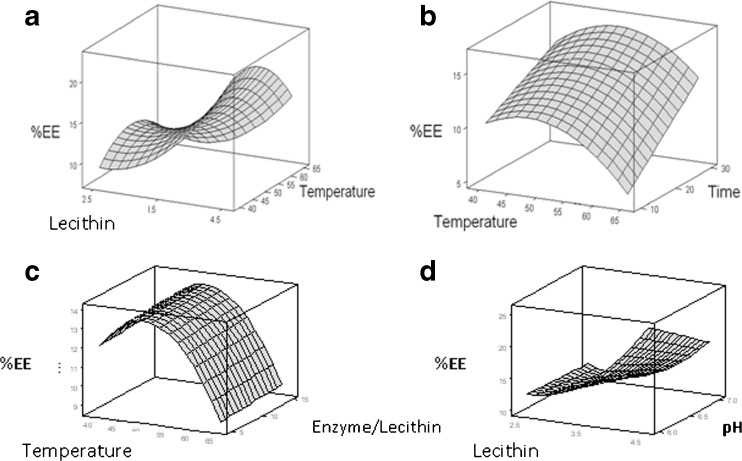
Fig. 1.
Response surface showing the effect of experimental factor on the encapsulasion efficiency (EE %) of the flavourzyme-loaded liposome which prepared by heating method. a Effect of the lecithin proportion and the process temperature, b Effect of the process temperature and the stirring time, c Effect of the process temperature and the enzyme/lecithin ratio, d Effect of the lecithin proportion and pH
Figure 1a shows the response surface plot for the impact of the lecithin proportion and the stirring temperature to the acquired EE (%) at fixed enzyme/lecithin ratio (10 %), the stirring time on the hot plate (20 min) and the pH of solution (6.5). Figure 1a presents a saddle point as the critical point. The saddle point is an inflexion point between a relative maximum and a relative minimum (Bezerra et al. 2008), while lecithin proportion and stirring temperature on the hot plate were varied (2.5–4.5 %) and (40–65 °C), respectively. The response surface plot shows that EE (%) increased with the increase of the lecithin proportion. Therefore, higher lecithin proportion could be favorable to entrap the Flavourzyme into the HM liposome. The results were in line with those suggested by Xiong et al. (2009), in that, they reported that an increase in phosphatidyl choline (PC) proportion caused an increase in EE (%) when a loaded liposomal formulation was studied using RSM.
At the fixed lecithin proportion, the stirring temperature on hotplate could increase the EE (%). However, when the stirring temperature on hotplate exceeded 44 °C, the EE (%) began to decrease. it was not advisable to use higher stirring temperature on hotplate in order to enhance EE (%). Increased temperature leads to enhance solubility of lecithin but above 44 °C, the inactivated enzyme causes decreased efficiency. As shown in the Eq. (4) EE (%) was found to be a function of the linear and quadratic effects of the stirring temperature on hotplate. The linear effect was positive and the quadratic effect was negative.
Figure 1b shows the response surface plate for the stirring temperature and the time to the acquired EE (%) at fixed lecithin proportion (3.5 %), the enzyme/lecithin ratio (10 %) and pH of solution (6.5). Figure 1b shows that the maximum point is outside the experimental design (Bezerra et al. 2008). The response surface indicated that EE (%) would be enhanced by the increment of stirring time on hotplate. The maximum acquired EE (%) could be obtained at time of 30 min.
Figure 1c and d show the response surface for the impacts of temperature and enzyme/lecithin ratio and lecithin proportion and pH to EE (%) respectively. Figure 1d showed that EE (%) would be decrement by increment of pH to 7.
The regression models in the Eqs. (4) and (5) are fitted to the experimental data and the most suitable formulation of the Flavourzyme-loaded liposome which produced using HM is 4.5 % lecithin, 44 °C temperature, 5 % Flavourzyme/lecithin ratio, 29.9 stirring time and medium pH 6.
Characterization of the Flavourzyme loaded liposome
In order to evaluate the optimized formulation of Flavourzyme loaded liposome, which is produced by modified HM without solvent and chemical detergent, the EE (%), size and shape of liposome were studied. The EE (%) of the most suitable Flavourzyme-loaded liposome was 26.5 %. The Dynamic light scattering measurement revealed an average diameter of the produced enzyme loaded liposome was 173.9 ± 0.47 nm. Consequently, they were claimed in a nanometer scale and named Flavourzyme-loaded nanoliposome. Its polydispersity index was 0.258. This index ranged from 0.0 to 1.0. This result showed particle size distribution of Flavourzyme-loaded nanoliposome was narrow. These results are comparable to those of Colas et al. (2007) who prepared nisin-loaded nanoliposome. EE (%), particle size and polydispersity index of nisin-loaded nanoliposome were varied from 11.7 to 54.2 (%), 190 to 284 nm and 0.184 to 0.281, respectively. Flavourzyme has been encapsulated by the proliposome technique with EE (%) of 20.2 ± 1.9 (%) based on the enzyme activity and 23 ± 2.9 (%) based on protein (Kheadr et al. 2003).
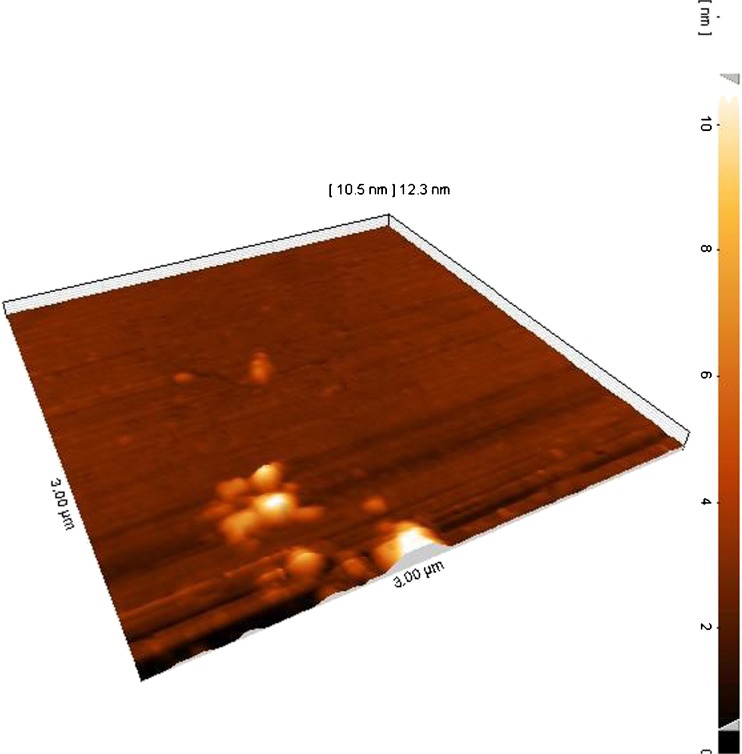
Flavourzyme-loaded nanoliposomes were visualized by AFM in Fig. 2. Atomic force microscopy (AFM), one of the techniques belonging to the family of scanning probe microscopes with dimensional resolution approaching 1 A°, has revolutionized imaging of the nanosamples (Binning et al. 1986; Khosravi-Darani et al. 2007). AFM ability to explore samples under variety of environmental conditions, without the need to perform any sample preparation such as staining, labeling, fixation, etc. including biological specimens in an aqueous/physiological environment, or in air at different temperatures, or under controlled humidity, make it a very versatile characterization technique, with lateral and vertical resolution (Sahoo and Labhasetwar 2003; Prater et al. 1991; Khosravi-Darani et al. 2007; Colas et al. 2007). AFM is being widely used to study the size and adsorption behavior of vesicles on solid substrates (Mozafari 2005; Colas et al. 2007). Figure 2 shows a representative 3D image of Flavourzyme-loaded nanoliposome and successful formation of liposome with closed and spherical structure. However, a little aggregation between liposomes was observed in Fig. 2. This figure was in line with Colas et al. (2007) which showed a representative 3D AFM image of partially dehydrated nisin-loaded nanoliposome.
Fig. 2.
A three-dimensional AFM image of partially dehydrated optimized the flavourzyme-loaded nanoliposome formulation prepared by modified heating method
Conclusion
This study aimed to prepare Flavourzyme-loaded liposomes using HM. The PBD was used to assess the relative importance of the chemical components and the process variables on the EE (%) and activity of liposomal Flavourzyme (LAPU−1). The results showed the significant impact of the pH of solution, the lecithin proportion (%w/w), the Enzyme/Lecithin ratio, the temperature of process (°C), the stirring time (min), the ratio of stirrer to the tank diameter and the way of enzyme addition for liposome preparation on the both responses; EE (%) and activity of liposomal Flavourzyme (LAPU−1).
After screening of the variables, the preparation of the Flavourzyme-loaded liposome prepared by HM was done using RSM. All the above mentioned variables showed profound impacts on the EE (%) and the activity of liposomal Flavourzyme (LAPU−1). The highest EE (%) and the activity of liposomal enzyme were obtained under the following condition: lecithin concentration, the Flavourzyme/lecithin ratio, temperature, stirring time and pH were 4.5 %, 5 %, 44 °C, 29.9 min and 6 respectively. The liposome formulation reported here possesses nanoscale in size, narrow size distribution and high EE (%). The results of particle size and polydispersity index are comparable to nisin-loaded nanoliposome also, EE (%) of encapsulated Flavourzyme was compared to those entrapped by technique of proliposome (Kheadr et al. 2003; Colas et al. 2007). The Application of the atomic force microscopy confirmed successful formation of the Flavourzyme-loaded nanoliposome.
Acknowledgments
We would like to thank the National Nutrition and Food Technology Research Institute (NNFTRI) Tehran, Iran, for financial support of this research project and Novozymes Pvt Ltd (Tehran, Iran) for sending Flavourzyme as gift.
References
- Alkhalaf W, Soda ME, Gripon JC, Vassal L. Acceleration of cheese ripening with liposomes-entrapped proteinase: influence of liposomes net charge. J Dairy Sci. 1989;72:2233–2238. doi: 10.3168/jds.S0022-0302(89)79352-8. [DOI] [Google Scholar]
- Anjani K, Kailasapathy K, Phillips M (2007) Impact of alginate-chitosan encapsulated flavourzyme on peptide and amino acid profiles in Cheddar cheese. Int Dairy J 17:79
- Bas D, BoyacI IH. Modeling and optimization I: usability of response surface methodology. J Food Eng. 2007;78:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Binning G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Charcosset C. Preparation of emulsions and particles by membrane emulsification for the food processing industry. J Food Eng. 2009;92:241–249. doi: 10.1016/j.jfoodeng.2008.11.017. [DOI] [Google Scholar]
- Colas JC, Shi W, Rao VSNM, Omri A, Mozafari MR, Singh H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron. 2007;38:841–847. doi: 10.1016/j.micron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Ding B, Zhang X, Hayat K, Xia S, Jia C, Xie M, Liu C. Preparation, characterization and the stability of ferrous glycinate nanoliposomes. J Food Eng. 2011;102:202–208. doi: 10.1016/j.jfoodeng.2010.08.022. [DOI] [Google Scholar]
- Dufour P, Vuillemard JC, Laloy E, Simard RE. Characterization of enzyme immobilization in liposomes prepared from proliposomes. J Microencapsul. 1996;13:185–194. doi: 10.3109/02652049609052906. [DOI] [PubMed] [Google Scholar]
- El Soda M. Accelerated maturation of cheese. Int Dairy J. 1993;3:531–544. doi: 10.1016/0958-6946(93)90030-4. [DOI] [Google Scholar]
- Fox PF. Cheese: chemistry, physics and microbiology. Victoria, Canada: Chapman & Hall; 1993. [Google Scholar]
- Fox PF, Grufferty MB (1991) Exogenous enzyme in dairy technology. In: Fox PF (ed) Food enzymology, vol. 1. Elsevier Applied Science, London, UK, pp 219–69
- Gibbs BF, Kermasha S, Alli I, Mulligan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50:213–224. doi: 10.1080/096374899101256. [DOI] [PubMed] [Google Scholar]
- Jahadi M, Khosravi-Darani K, Ehsani MR, Mozafari MR, Saboury AA, Seydahmadian F, Vafabakhsh Z. Evaluating the effects of process variables on protease-loaded nano-liposome production by Plackett-Burman design for utilizing in cheese ripening acceleration. Asian J Chem. 2012;24(9):3891–3894. [Google Scholar]
- Kailasapathy K, Perera C, Phillips M (2006) Evaluation of alginate–starch polymers for preparation of enzyme microcapsules. Int J Food Eng 2(2):1556–3758
- Kasaai MR, Charlet G, Paquin P, Arul J. Fragmentation of chitosan by microfluidization process. Innov Food Sci Emerg Technol. 2003;4:403–413. doi: 10.1016/S1466-8564(03)00047-X. [DOI] [Google Scholar]
- Kheadr EE, Vuillemard LC, El-Deeb SA. Acceleration of cheddar cheese lipolysis by using liposome-entrapped lipases. J Food Sci. 2002;67:485–492. doi: 10.1111/j.1365-2621.2002.tb10624.x. [DOI] [Google Scholar]
- Kheadr EE, Vuillemard JC, El-Deeb SA. Impact of liposome-encapsulated enzyme cocktails on cheddar cheese ripening. Food Res Int. 2003;36:241–252. doi: 10.1016/S0963-9969(02)00166-7. [DOI] [Google Scholar]
- Khosravi-Darani K, Zoghi A. Comparison of pretreatment strategies of sugarcane baggase: experimental design for citric acid production. Bioresour Technol. 2008;99:6986–6993. doi: 10.1016/j.biortech.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Khosravi-Darani K, Pardakhty A, Honarpisheh H, Rao VSNM, Mozafari MR. The role of high-resolution imaging in the evaluation of nanosystems for bioactive encapsulation and targeted nanotherapy. Micron. 2007;38:804–818. doi: 10.1016/j.micron.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilcawley KN, Wilkinson MG, Fox PF. Determination of key enzyme activities in commercial peptidase and lipase preparations from microbial or animal sources. Enzym Microb Technol. 2002;31:310–320. doi: 10.1016/S0141-0229(02)00136-9. [DOI] [Google Scholar]
- Laloy E, Vuillemard JC, Dufour P, Simard R. Release of enzymes from liposomes during cheese ripening. J Control Release. 1998;54:213–222. doi: 10.1016/S0168-3659(97)00265-4. [DOI] [PubMed] [Google Scholar]
- Laridi R, Kheadr EE, Benech RO, Vuillemard JC, Lacroix C, Fliss I. Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. Int Dairy J. 2003;13:325–336. doi: 10.1016/S0958-6946(02)00194-2. [DOI] [Google Scholar]
- Larivière B, El Soda M, Soucy Y, Trépanier G, Paquin P, Vuillemard JC. Microfluidized liposomes for the acceleration of cheese ripening. Int Dairy J. 1991;1:111–124. doi: 10.1016/0958-6946(91)90003-Q. [DOI] [Google Scholar]
- Maa YF, Hsu CC. Performance of sonication and microfluidization for liquid-liquid Emulsification. Pharm Dev Technol. 1999;4:233–240. doi: 10.1081/PDT-100101357. [DOI] [PubMed] [Google Scholar]
- Mozafari MR. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005;10:711–719. [PubMed] [Google Scholar]
- Mozafari MR, Mortazavi SM. Nanoliposomes from fundamentals to recent developments. Victoria, Canada: Trafford Publishing; 2005. [Google Scholar]
- Mozafari MR, Flanagan J, Matia-Merino L, Awati A, Omri A, Suntres ZE, Singh H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J Sci Food Agric. 2006;86:2038–2045. doi: 10.1002/jsfa.2576. [DOI] [Google Scholar]
- Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18(4):309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Mozafari MR, Khosravi-Darani K, Borazan GG, Cui J, Pardakhty A, Yurdugul S. Encapsulation of food ingredients using nanoliposome technology. Int J Food Prop. 2008;11:833–844. doi: 10.1080/10942910701648115. [DOI] [Google Scholar]
- Picon A, Gaya P, Medina M, Nuaez M. The effect of liposome encapsulation of chymosin derived by fermentation on Manchego cheese ripening. J Dairy Sci. 1994;77:16–23. doi: 10.3168/jds.S0022-0302(94)76923-X. [DOI] [Google Scholar]
- Picon A, Gaya P, Medina M, Nunez M. The effect of liposome-encapsulated Bacillus subtilis neutral proteinase on Manchego cheese rippening. J Dairy Sci. 1995;78:1238–1247. doi: 10.3168/jds.S0022-0302(95)76743-1. [DOI] [Google Scholar]
- Picon A, Serrano C, Gaya P, Medina M, Nunez M. The effect of liposome-encapsulated cyprosins on Manchego cheese ripening. J Dairy Sci. 1996;79:1699–1705. doi: 10.3168/jds.S0022-0302(96)76535-9. [DOI] [Google Scholar]
- Prater CB, Wilson MR, Garnaes J, Massie J, Elings VB, Hansma PK. Atomic force microscopy of biological samples at low temperature. J Vac Sci Technol B. 1991;9:989–991. doi: 10.1116/1.585442. [DOI] [Google Scholar]
- Rao DR, Chawan CB, Veeramachaneni R. Liposomal encapsulation of β-Galactosidase: comparision of two methods of encapsulations and in vitro lactose digesibility. J Food Biochem. 1994;18:239–251. doi: 10.1111/j.1745-4514.1994.tb00500.x. [DOI] [Google Scholar]
- Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–1120. doi: 10.1016/S1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- Skeie S. Developments in microencapsulation science applicable to cheese research and development. A review. Int Dairy J. 1994;4:573–595. doi: 10.1016/0958-6946(94)90035-3. [DOI] [Google Scholar]
- Song KH, Chung SJ, Shim CK. Preparation and evaluation of proliposomes containing salmon calcitonin. J Control Release. 2002;84:27–37. doi: 10.1016/S0168-3659(02)00238-9. [DOI] [PubMed] [Google Scholar]
- Taylor TM, Davidson PM, Bruce BD, Weiss J. Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr. 2005;45:587–605. doi: 10.1080/10408390591001135. [DOI] [PubMed] [Google Scholar]
- Taylor TM, Gaysinsky S, Davidson PM, Bruce BD, Weiss J. Characterization of antimicrobial-bearing liposomes by zeta-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007;2:1–9. doi: 10.1007/s11483-007-9023-x. [DOI] [Google Scholar]
- Xia S, Xu S. Ferrous sulfate liposomes: preparation, stability and application in fluid milk. Food Res Int. 2005;38:289–296. doi: 10.1016/j.foodres.2004.04.010. [DOI] [Google Scholar]
- Xiong Y, Guo D, Wang L, Zheng X, Zhang Y, Chen J. Development of nobiliside A loaded liposomal formulation using response surface methodology. Int J Pharm. 2009;371:197–203. doi: 10.1016/j.ijpharm.2008.12.031. [DOI] [PubMed] [Google Scholar]