Abstract
Mold association, aflatoxin B1 contamination as well as oxidative deterioration of agri-food items during storage and processing are some global task for food industries. In view of the adverse effects of some synthetic preservatives on treated food items and subsequently on consumers health, recently plant based chemicals are encouraged by food industries as better alternatives of synthetics. The present study recommends the combination (1:1:1) of Angelica archangelica essential oil: Phenyl ethyl alcohol (PEA): α- terpineol as botanical preservative against molds, aflatoxin contamination and oxidative deterioration of walnut samples. Eight mold species were procured from stored walnut samples, including some aflatoxigenic Aspergillus flavus strains. The combination inhibited growth of aflatoxigenic strain Aspergillus flavus NKDW-7 and aflatoxin B1 production at 2.25 and 2.0 μL mL−1 respectively. The IC50 value of the combination was recorded as 3.89 μL mL−1, showing strong antioxidant potential. The antifungal action of the combination showed > 90 % decrease in ergosterol content in plasma membrane of A. flavus at 2.0 μL mL−1. The LD50 of the combination, through oral administration on mice, was 9562.9 μL kg−1 body weight, indication favourable safety profile as a plant based preservative. The combination may be recommended as safe preservative against molds, aflatoxin contamination and oxidative deterioration of walnut samples.
Keywords: Aflatoxin B1, Angelica archangelica EO, Antifungal, Antioxidant, Mycoflora, Walnut
Introduction
Walnut (Juglans regia L.) is an important temperate nut fruit used as traditional food and also as ingredients of sauces, stuffing, snacks, appetizers in Mediterranean, South America, and Asian countries. Walnut fruits are the richest source of polyunsaturated fatty acids (PUFAs) (47.2 g), predominantly linoleic (38.1 g) and α-linolenic (9.1 g) acids; and protein (15.2 g); fiber (6.7 g); phosphorus (346 mg); potassium (441 mg); folate (98 μg); and vitamin E (2.9 mg) each per 100 g and low sugar (Feldman 2002). In India, Jammu and Kashmir, Uttaranchal and Himachal Pradesh are the major producers of walnut. Because of dried material from plant origin, walnut fruits are highly susceptible to contamination with storage moulds and associated mycotoxins.
In addition to microbial contamination during prolong storage, fruits and agri-commodities are also deteriorated by oxidative deterioration through free radicals which are responsible for damage of cells and adversely affect the food quality. Oxygen free radicals/reactive oxygen species (ROS) have been a source of the threat in both food systems (decreasing the shelf stability) as well as in biological systems causing chronic diseases. Adverse effects of ROS in various biomolecules such as lipids, proteins, carbohydrates, as well as nucleic acids, are well reported (Halliwell 1997).
The current strategies for the management of microbial contamination and oxidative deterioration of agri- commodities viz., low temperature, cooling, aeration, rapid drying vacuum packaging, modified atmosphere packaging (MAP) and use of synthetic preservatives/antioxidant are not sufficient to eliminate the undesirable pathogen, mycotoxins as well as to inhibit oxidative deterioration (Holley and Patel 2005; Tajkarimi et al. 2010). Although application of synthetic preservatives has greatly contributed to in control of crop pests, such measures are not appropriate for treatment of fruits and food items due to their side effects on human health (Brula and Coote 1999). Moreover, some of the synthetic preservatives used as antioxidants have been reported to enhance the mycotoxin secreting potency of associated fungi (Kumar et al. 2007; Prakash et al. 2010).
The role of different plant products as a preservative is well known since antiquity. The plant kingdom is recognized as the most efficient producer of chemical compounds, synthesizing many products that are used in defense against different pests and having antioxidant potential (Burt 2004; Holley and Patel 2005; Tajkarimi et al. 2010). Among plant products, essential oils (EOs) of higher plants and their components are gaining interest as food additives and are widely accepted by consumers because of their relatively high volatility, ephemeral and biodegradable nature (Burt 2004; Tripathi and Dubey 2004; Jaya et al. 2012; Kedia et al. 2013). Some of the EOs based formulation such as Sporan-TM (Rosemary oil), Promox-TM (Thyme), and DMC base natural (rosemary, sage, citrus oil combination) (Dayan et al. 2009; Shukla et al. 2009) are available in the market and used as antimicrobial as food preservatives.
In view of these facts, there is need of a plant based formulation which can effectively inhibit molds and aflatoxin contamination as well as oxidative deterioration of food items. In some of the earlier reports, combinations of EOs with components/ used preservative have shown better results than the individual ones because of changed chemical profile or synergistic effects between different biologically active components (Tatsadjieu et al. 2010; Prakash et al. 2012b). Therefore, in present investigation a plant based combination (1:1:1) of traditionally used plant Angelica archangelica EO, and two essential oil components viz., Phenyl ethyl alcohol, α- terpineol, widely used as flavouring, liqueur, confectionary and antibacterial agent (Hall 1960; Dorman and Deans 2000; Prajapati et al. 2003) was prepared and tested for its efficacy against storage fungi of walnut fruits, aflatoxin secretion and as free radical scavenger so as to recommend it in enhancement of shelf life of fruits. In addition, its efficacy was compared to its individual gradients so as to conclude the synergisms between its components. The safety profile of the EO based combination was assessed by determining its LD50 through oral toxicity on mice.
Materials and methods
Chemicals and equipments
All the chemicals viz. chloroform, methanol, sodium sulphate, tween-80, tween-20, toluene, isoamyl alcohol, Potato dextrose broth (PDB), SMKY (Sucrose 200 g; MgSO4 · 7H2O, 0.5 g; KNO3, 0.3 g and yeast extract, 7 g; 1 L distilled water), 2,2-diphenyl-1-picrylhydrazil (DPPH), were procured from Hi-Media Laboratories Pvt. Ltd., Mumbai, India. Phenyl ethyl alcohol (PEA) and α-terpineol were procured from Ozone International, Mumbai, India (purity of the components was >99 %). The major equipments used were hydro-distillation apparatus (Merck Specialities Pvt. Ltd., Mumbai, India), centrifuge, UV transilluminator (Zenith Engineers, Agra, India) and spectrophotometer (Systronics India Ltd., Mumbai, India).
Isolation of plant essential oil
The root samples of A. archangelica were collected from the Botanical garden, Banaras Hindu University, Varanasi, India, during August 2011. The plant was identified with the help of relevant taxonomic literature/flora and their voucher specimen (Api./Ang-07/2011) was deposited in the herbarium of the Laboratory of Herbal Pesticides, Department of Botany, BHU, Varanasi. The root samples were thoroughly washed thrice with distilled water and then subjected to hydrodistillation (4 h) in Clevenger’s apparatus (Prakash et al. 2010). The EO of root samples of A. archangelica was collected separately in sterilized glass vial. The water traces from the essential oil was removed by adding anhydrous sodium sulphate thereafter the EO was kept at 4 °C in dark for further experiments.
Collection of Walnut samples
Walnuts (Juglans regia L.) samples were procured from the local market of Jammu and Varanasi, India in the month of May to June 2011. The collected samples were stored in sterilized polythene bags to prevent further contamination and were stored at 10 °C until analysis.
Moisture content and pH of Walnut
Fifty grams of walnut sample was dried at 100 °C in hot air oven for 24 h and moisture content was calculated based on difference with the fresh weight (Mandeel 2005). For pH, 1 g of walnut sample was finely ground using mortar-pestle and 1:10 (sample: distilled water) suspension was prepared and stirred for 24 h. The pH of the suspension was recorded using electronic pH meter (Prakash et al. 2010).
Mycological analysis of Walnut
Mycological analysis of walnut samples was carried out by serial dilution method Aziz et al. (1998). Prior to mycological screening seed samples were surface sterilized by 1 % NaOCl for 5 minutes thereafter rinsed with distilled water thrice. Thereafter, 10 g of powdered samples of two selected region was homogenized in 90 mL sterile distilled water in an Erlenmeyer flask (250 ml). Five fold serial dilutions were prepared and 1 mL of aliquot (10−4) of each sample was inoculated on a Petri dish containing 10 mL freshly prepared PDA medium. Ten replicates of each sample were prepared and incubated (27 ± 2 °C) for 7 days. Different fungal colonies were counted and species were identified following Gilman 1998. The percent occurrence frequency of fungal species were calculated following formula;
Detection of aflatoxigenic isolates of Aspergillus flavus
A total of 24 isolated (12 from each region) of A. flavus procured from walnut samples were screened for their ability for production of aflatoxin B1 (AFB1) following Kumar et al. (2007). A 5 mm fungal disc (7 days old culture) of each selected A. flavus isolate grown on PDA medium was cut with the help of sterilized cork borer aseptically and inoculated on 25 mL of the SMKY medium in 100 mL flask containing streptomycin (300 mg L−1) for controlling bacterial growth and was kept for 10 days incubation period at 27 ± 2 °C in B.O.D (Biochemical oxygen demand) incubator. Thereafter, the content of each flask was filtered (Whatman No. 1) and filtrate was extracted with 20 mL chloroform. The extract was evaporated to dryness on water bath and re-dissolved in 1 mL chloroform. Fifty microliters of chloroform extract was spotted on TLC plates along with the standard of AFB1 and developed in toluene:isoamylalcohol:methanol (90:32:2 v/v/v). The plate was air dried and AFB1 was observed in UV-transilluminator (360 nm). The appearance of blue fluorescent spot in the UV transilluminator by different A. flavus isolates was recorded as their toxin producing ability.
The amount of aflatoxin secreted by the A. flavus isolates in medium was quantified by Thin Layer Chromatography (TLC) followed by spectrophotometry. The blue color fluorescent spot in TLC plate were scratched and dissolved in 5 mL cold methanol and centrifuged at 5,000 rpm (5 min). Absorbance of the supernatant was recorded at 360 nm and AFB1 was calculated following Kumar et al. (2007).
- D
absorbance
- M
molecular weight (312)
- E
molar extinction coefficient AFB1 (21800)
- L
path length (1 cm)
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of Angelica archangelica EO, Phenyl ethyl alcohol and α- terpineol alone and in combination (1:1:1) against Aspergillus flavus (NKDW-7)
The minimum inhibitory concentration (MIC) of A. archangelica EO, PEA and α- terpineol alone and in EO-based combination (1:1:1 v: v: v) against most toxigenic isolates of A. flavus (NKDW-7) was determined by broth dilution method reported earlier by and Shukla et al. 2009. A 5 mm diameter disk of A. flavus (NKDW-7) was cut from the periphery of 7 day old colony was inoculated aseptically on PDB medium amended with A. archangelica EO, PEA and α- terpineol alone and as combination (1:1:1 v: v: v) at varying concentrations ranged between (0.25 μL mL−1 and 5.0 μL mL−1). The concentrations were prepared separately by dissolving their requisite amount in 0.5 ml 4 % tween-20 and were then added to 9.5 ml of PDB medium in culture tube. The tube containing only PDB medium and A. flavus (NKDW-7) without any treatment served as controls. Both the treatment and control sets were incubated for 10 days at 27 ± 2 °C in B.O.D incubator. The tube showing no visible growth of test mold after 10 days of incubation periods were recorded as MIC value of test EO, component, and their EO-based combination. For MFC, 5 mm disk of A. flavus (NKDW-7) of medium from the test tube showing no visible growth was subcultured on freshly prepared treatment-free PDA plates. MFC is the lowest concentration test compound at which there was no revival of growth of the inhibited fungal inoculums on treatment-free PDA plates.
Effect of Angelica archangelica EO, Phenyl ethyl alcohol and α- terpineol alone and in combination (1:1:1) on aflatoxin B1 synthesis
For aflatoxin inhibitory efficacy different amounts of A. archangelica EO, PEA and α- terpineol alone and in EO-based combination (1:1:1 v: v: v) were added to SMKY broth medium to achieve final concentration ranged between (0.25 and 5.0 μL mL−1). The concentrations were prepared separately by dissolving their requisite amount in 0.5 ml 4 % tween-20 and were then added to 24.5 ml of SMKY broth medium in 100 mL Erlenmeyer flasks. Thereafter, A 5 mm diameter disk of A. flavus (NKDW-7) was cut from the periphery of 7 day old colony was inoculated aseptically on SMKY medium. The isolation and quantification of aflatoxin B1 in given treatments were calculated by the method described by Kumar et al. (2007).
DPPH free radical scavenging activity of Angelica archangelica EO, Phenyl ethyl alcohol and α-terpineol alone and in combination (1:1:1)
Free radical scavenging activity of Angelica archangelica EO, PEA and α- terpineol alone and as combination (1:1:1) were determined through spectrophotometeric assay by recording the extent of bleaching of the purple-colored methanolic solution of DPPH to yellow Prakash et al. (2010). Different concentrations (1.0 to 10.0 μL mL−1 within the intervals of 0.5 μL mL−1) were prepared for the Angelica archangelica EO and EO-based combination; (25 to 150 μL mL−1 within the intervals of 25.0 μL mL−1) for PEA and α- terpineol and (1.0 to 10.0 μg mL−1 within the intervals of 1.0 μg mL−1) for BHT (as a control) were added to 0.004 % methanolic solution of DPPH and kept in dark at room temperature (25 ± 2 °C) for 30 min. Thereafter, the absorbance was taken against a blank at 517 nm using spectrophotometer. Reduction in absorbance of the sample as compared to blank was measured as potential of DPPH free radical scavenging of test samples. IC50 (the concentration responsible for the 50 % neutralization of DPPH radicals) was calculated from the graph plotted on percentage inhibition and concentration. Percent inhibition (I%) of free radical was calculated as
where, Ablank is the absorbance of the blank (without any test samples), and Asample is the absorbance of the test samples.
Fungitoxic spectrum of EO-based combination against the isolated molds species
Based on the preliminary results the EO-based combination was further tested for its MIC value against all the food borne molds species isolated from walnut by the method reported earlier by Shukla et al. 2009.
Effect of EO-based combination on ergosterol content in the plasma membrane of test fungus Aspergillus flavus (NKDW-7)
The ergosterol content in the plasma membrane of test fungus A. flavus (NKDW-7) was determined by method described earlier by Tian et al. 2012 with slight modifications. 50 μL spore suspension of test fungus A. flavus containing 106spores mL−1 was inoculated in 100 mL flask amended with SMKY medium containing different concentrations viz., 0, 0.50, 1.0, 1.50, 2.0, 2.25 μL mL−1 of (1:1:1) combination of A. archangelica:PEA:α-terpineol. Thereafter, flasks were incubated for 4 days at 28 ± 2 °C in B.O.D incubator. After incubation period mycelia was harvested and washed twice with distilled water. The net wet weight of the cell pellet was determined. Thereafter, 5 mL of 25 % alcoholic potassium hydroxide solution (25 g KOH and 35 ml sterile distilled water, brought to 100 ml with absolute ethanol) was added to each sample and vortex mixed for 2 min followed by incubation at 85 ± 2 °C for 4 h in water bath. Thereafter, sterols were extracted from each sample by adding a mixture of 2 ml sterile distilled water and 5 mL n-heptane. Then, the mixture was sufficiently mixed by vortex for 2 min allowing the layers to separate for 1 h at room temperature. Thereafter, n-heptane layer was analyzed by scanned spectrophotometry between 230 and 300 nm. The presence of ergosterol (at 281.5 nm) and the late sterol intermediate 24(28) dehydroergosterol (at 230 and 281.5 nm) in the n-heptane layer led to a characteristic curve. The ergosterol amount was calculated as a percentage of the weight of the fungal mycelia and was based on the absorbance and wet weight of the fungal mycelia (initial pellet weight). The ergosterol amount was calculated by the given formula by Tian et al. 2012.
% ergosterol + %24(28) dehydroergosterol = (A281.5/290)/pellet weight,
%24(28) dehydroergosterol = (A230/518)/pellet weight,
% ergosterol = (%ergosterol + %24(28)dehydroergosterol)—%24(28)dehydroergosterol
Where; 290 and 518 are the E values (in percentages per cm) determined for crystalline ergosterol and 24(28) dehydroergosterol, respectively, and pellet weight is the net wet weight (g).
Safety assessment of EO-based combination by determination of LD50 on mice
The safety limits of EO-based combination was determined by recording LD50 values (lethal dose of oil per unit body weight for killing 50 % population ) on mice (Mus musculus L., average weight 30.0 g, age 3 months) of the same sex (Prakash et al. 2011). Mice were procured from the Institute of Medical Sciences, Banaras Hindu University, Varanasi, and were kept in a laboratory under controlled environmental conditions (25 ± 2 °C) for 20 days to acclimatize prior to LD50 experiments. A stock solution of tween-80 and distilled water (1:2) was prepared. Different doses of EO-based combination (0.025 to 0.5 mL) were mixed separately with 0.5 ml stock solution and were orally administered to each group of animal (10 mice) separately with the help of fine syringe and catherator. In control set equal dose of tween-80 and distilled water solution and administered orally to the mice. After 4 h, the mortality of the test animals was recorded and LD50 of EO-based combination calculated by probit analysis (Finney 1971). After experiments the alive animals were killed by chloroform treatment and were buried in the soil of botanical garden BHU, Varanasi following the ethical precautions.
Statistical analysis
All experiments except mycoflora analysis and ergosterol content were repeated thrice and data are the mean ± standard error. Data of aflatoxin B1 subjected to one way ANOVA. Means were separated by Tukey’s multiple range tests when ANOVA was significant (p < 0.05). Probit analysis was performed to estimate lethal dose (LD50) with its 95 % fiducial limits. The analysis of data was performed with the SPSS program version 16.0.
Results
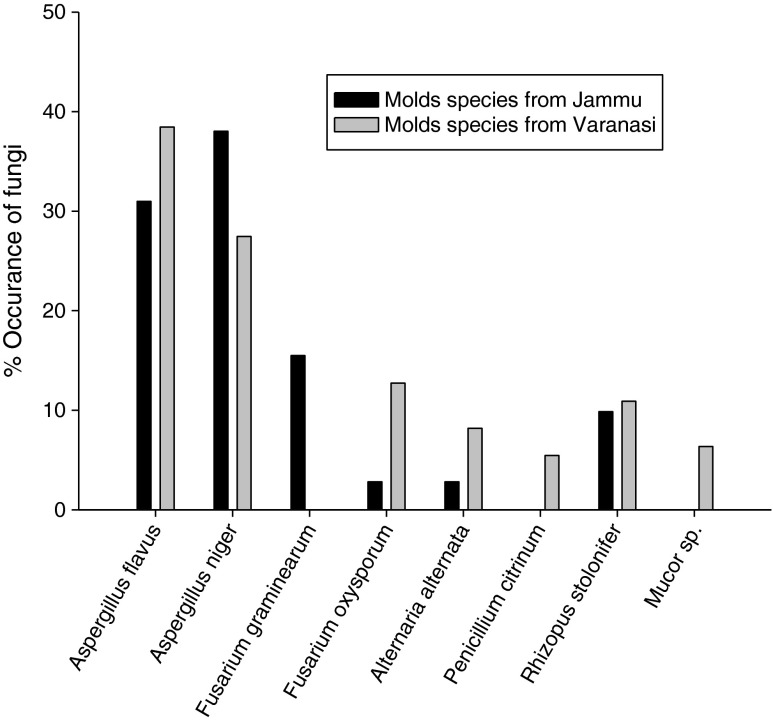
During hydrodistillation the yield of A. archangelica L. root oil ranged between (0.1 % and 0.12 %). The moisture content and pH of the walnut samples was recorded to be 10.5 ± 0.45 and (4.9 to 5.2) respectively for those collected from Jammu region while 14.43 ± 1.02 and (5.2 to 5.8) respectively for Varanasi region. Result of mycoflora analysis of walnut samples of two different climatic zones, Jammu and Varanasi depicted that fruit samples of both regions were contaminated with the storage molds (Fig. 1). A total of 08 fungal species were isolated by serial dilution methods. The results revealed A. flavus (30.98 and 38.45), and A. niger (38.02 and 27.45) as dominated fungal species over other species in both Jammu and Varanasi region respectively. The percent occurrence frequency of different fungal species associated with the walnut is summarized in (Fig. 1).
Fig. 1.
Per cent occurrence of different mold species associated with Walnut seed samples collected from Jammu and Varanasi regions
A total of 24 (twelve from each region) A. flavus isolates were randomly selected for screening of aflatoxin producing ability where 07 isolates from Varanasi region and only two from Jammu region were found positive for aflatoxigenic potential based on the prominent blue color fluorescent spot on TLC plate . During qualitative estimation based on aflatoxin B1 content (μg L−1) through spectrophotometer, the isolate NKDW-7 of A. flavus isolated from fruits of Varanasi region was found strongest toxigenic (509.45 μg L−1); while the strain NKDW-8 of A. flavus isolated from those of Jammu region as lowest (74.49 μg L−1). Hence, toxigenic strain A. flavus (NKDW-7) was selected for the detailed investigation for entire antimicrobial assay and is maintained in the laboratory for future reference.
During determination of MIC value of A. archangelica oil, α-terpineol, PEA and their combination (1:1:1 v:v:v) against the test mold A. flavus (NKDW-7), A. archangelica EO, could not inhibited growth of test mold species A. flavus (NKDW-7) up to 5.0 μL mL−1 while, α-terpineol, PEA and EO-based combination inhibited growth at 1.75, 2.0 and 2.25 μL mL−1 respectively. However, none of them showed MFC up to 5.0 μL mL−1, as the revival growth of inhibited mold disk was observed when it was inoculated in fresh PDA medium suggesting fungi static nature of test samples (Table 1).
Table 1.
Minimum inhibitory concentration (MIC) of A. archangelica oil, α-terpineol, phenyl ethyl alcohol and their EO-based combination against toxigenic strain of A. flavus NKDW-7
| Test components | MIC (μL mL−1) | MFC (μL mL−1) |
|---|---|---|
| A. archangelica oil | >5.00 | >5.00 |
| α-terpineol | 1.75 | >5.00 |
| Phenyl ethyl alcohol | 2.00 | >5.00 |
| EO-based combination | 2.25 | >5.00 |
The results of aflatoxin inhibitory activity revealed that α-terpineol, PEA and the combination exhibited complete inhibition of aflatoxin production by test fungus A. flavus (NKDW-7) at 1.5 μL mL−1, 1.75 μL mL−1, 2.00 μL mL−1 respectively in the SMKY medium (Table 2). However, A. archangelica oil as such could not inhibited aflatoxin production up to 5.0 μL mL−1.
Table 2.
Effect of α-terpineol, phenyl ethyl alcohol (PEA) and their EO-based combination on mycelia biomass and aflatoxin B1 production by A. flavus (NKDW-7) in SMKY medium
| Conc. (μL mL-1) | α-terpineol | % inhibition | % inhibition | PEA | % inhibition | % inhibition | EO-based combination | % inhibition | % inhibition | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDW | AFB1 | MDW | AFB1 | MDW | AFB1 | |||||||
| CNT | 449.5 ± 32.5a | 0.0 | 509.5 ± 40.1a | 0.0 | 449.5 ± 32.5a | 0.0 | 509.5 ± 40.1a | 0.0 | 449.5 ± 32.5a | 0.0 | 509.5 ± 40.1a | 0.0 |
| 0.25 | 253.5 ± 11.5b | 43.6 | 377.8 ± 22.9b | 25.8 | 198.5 ± 13.5b | 55.8 | 323.6 ± 11.4bc | 36.5 | 205.5 ± 4.5b | 54.3 | 332.1 ± 22.9b | 34.8 |
| 0.50 | 153.5 ± 09.5c | 65.8 | 320.6 ± 11.4b | 37.1 | 150.0 ± 2.0bc | 66.6 | 326.3 ± 5.72bc | 35.9 | 104.0 ± 3.0c | 76.8 | 223.3 ± 17.2c | 56.2 |
| 0.75 | 89.0 ± 02.0d | 80.2 | 188.9 ± 28.6c | 62.9 | 134.5 ± 8.5c | 70.1 | 257.6 ± 51.5c | 49.4 | 86.5 ± 5.5cd | 80.7 | 183.2 ± 11.5cd | 64.1 |
| 1.00 | 30.6 ± 01.5e | 93.2 | 211.7 ± 40.1c | 58.4 | 106.0 ± 10.0c | 76.4 | 114.5 ± 11.4d | 77.5 | 77.0 ± 3.0cd | 82.8 | 131.6 ± 17.2de | 74.2 |
| 1.25 | 20.2 ± 0.8e | 95.5 | 28.6 ± 5.7d | 94.4 | 36.5 ± 1.5d | 91.9 | 62.9 ± 17.2d | 87.7 | 39.1 ± 2.0de | 91.3 | 108.8 ± 5.7de | 78.6 |
| 1.50 | 9.5 ± 1.5e | 97.9 | 00.00d | 100 | 19.0 ± 2.1d | 95.8 | 40.1 ± 11.23d | 92.1 | 22.5 ± 5.5e | 94.9 | 74.4 ±7.13ef | 85.4 |
| 1.75 | 00.00e | 100 | 00.00d | 100 | 12.1 ± 1.2d | 97.3 | 00.00d | 100 | 20.8 ± 2.1e | 95.4 | 69.89 ± 3.78ef | 86.5 |
| 2.00 | 00.00e | 100 | 00.00d | 100 | 00.00d | 100 | 00.00d | 100 | 10.0 ± 1.0e | 97.8 | 00.00f | 100 |
| 2.25 | 00.00e | 100 | 00.00d | 100 | 00.00d | 100 | 00.00d | 100 | 00.00e | 100 | 00.00f | 100 |
Value are mean (n = 3) ± SE. The means followed by same letter in the same column are not significantly different according to ANOVA and Tukey’s multiple-comparison tests
Conc. Concentration; MDW Mycelial dry weight (mg); AFB1 Aflatoxin B1 content (μg L-1)
The antioxidant activity of A. archangelica oil, α-terpineol, PEA and their combination were determined in terms of their IC50 values by DPPH free radical scavenging assay are presented in Table 3. The IC50 of A. archangelica EO (1.04 μL mL−1), α-terpineol (66.6 μL mL−1), and their EO-based combination (3.89 μL mL−1) while, PEA could inhibited only 4.33 % up to 150 μL mL−1. The IC50 value of synthetic antioxidant BHT was found to be 7.4 μg mL−1 (Table 3).
Table 3.
DPPH free radical scavenging activity of A. archangelica oil, α-terpineol, phenyl ethyl alcohol and its EO-based combination and Butylated hydroxyl toluene (BHT)
| S.N. | Sample | DPPH (IC50) |
|---|---|---|
| 1 | Angelica L. oila | 1.04 ± 0.18 |
| 2 | α-terpineola | 66.6 ± 1.24 |
| 3 | Phenyl ethyl alcoholc | nf |
| 4 | EO based combinationa | 3.89 ± 0.14 |
| 5 | BHTb | 7.4 ± 0.21 |
nf not found. Value are mean (n = 3) ± SE
aμL mL-1; bμg mL-1; cIC50 value not found up to 150 μL mL-1
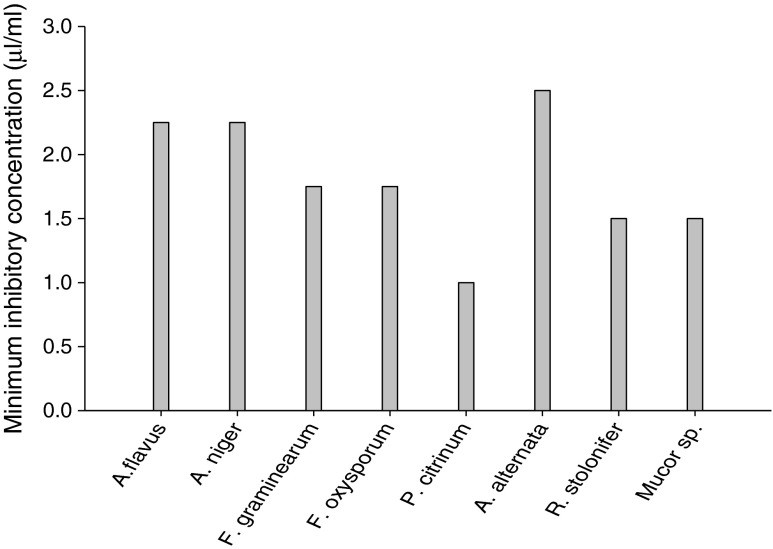
The fungitoxic spectrum of EO-based combination was found in following order Penicillium citrinum (1.0 μL mL−1) > Rhizopus stolonifer, Mucor sp. (1.5 μL mL−1) > Fusarium graminearum, Fusarium oxysporum (1.75 μL mL−1) > Aspergillus flavus, Aspergillus niger (2.25 μL mL−1) > Alternaria alternata (2.5 μL mL−1). Hence, the EO-based combination inhibited the growth of all molds species at its respective MICs showing broad fungitoxic spectrum (Fig. 2). Penicillium citrinum was recorded as the most susceptible as it was inhibited completely by the EO-based combination even at 1.0 μL mL−1.
Fig. 2.
Minimum inhibitory concentrations of EO-based combination against isolated mold species from walnut seed samples
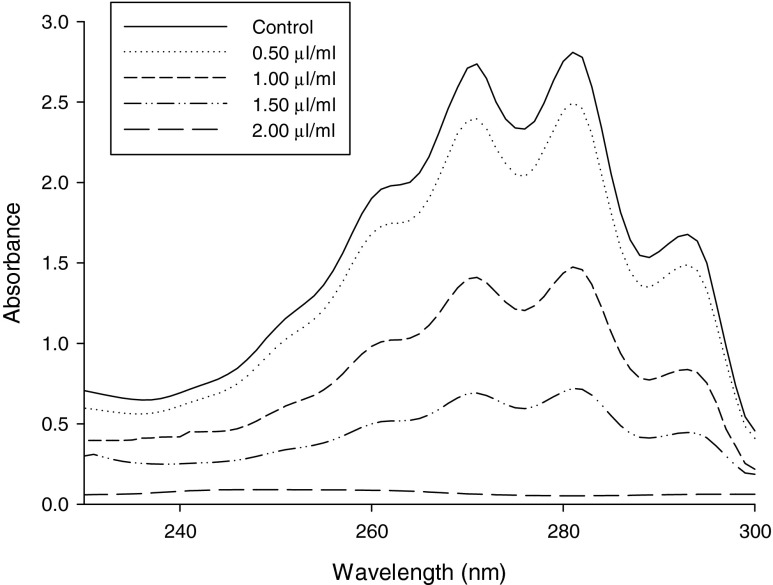
The effects of EO-based combination of A. archangelica EO, α-terpineol, PEA on ergosterol content in the plasma membrane of A. flavus (NKDW-7) was recorded to assess the antifungal mechanism of action. A dose dependent decrease in ergosterol content was observed on increasing concentration of the EO-based combination. A reduction percentage of the ergosterol content as compared with the control was found to be 10.58, 41.56, 65.29, 94.35 and 100 % respectively for 0.5, 1.0, 1.5, 2.0, and 2.5 μL mL−1concentrations (Fig. 3).
Fig. 3.
Inhibition of ergosterol biosynthesis on different concentrations of EO-based combination in plasma membrane of A. flavus (NKDW-7)
During safety profile trials on mice, the LD50 values of the EO-based combination determined through oral administration was calculated to be 9562.9 μL kg−1 body weight.
Discussion
The results of present investigation revealed that walnut seed samples were contaminated with various mold species along with the toxigenic strain of A. flavus. The findings are supported by the results of earlier observation where Aspergillus spp. and its associated toxins were one of the most predominant molds species in some of dry fruit samples (Abdel-Hafez and Saber 1993; Kumar et al. 2011; Zubair et al. 2011). Moisture content of walnut samples of Varanasi region was found to be more than the prescribed storage condition (13.5 %) as reported by Prakash et al. 2012c and supported the growth of molds and the toxigenic strains. Although, the moisture content of walnut seed samples of Jammu region was safe for storage but during mycological screening the samples were found to be contaminated with the molds species. Hence it may be concluded that chemical profile of substrate along with storage conditions also influence the growth of molds as earlier emphasized by Prakash et al. (2010). However, percent occurrence of different molds species was found to be different in Varanasi and Jammu regions. This may be due to the significant difference in storage practices, moisture, climatic conditions, Varanasi region being warmer and humid having favorable conditions for molds growth and proliferation with respect to Jammu region.
During screening of aflatoxigenic potential of A. flavus strains, only two strains from Jammu region and seven from Varanasi exhibited the potential to produce aflatoxin in liquid medium. The presence of toxigenic strain of A. flavus in walnut samples would be responsible for both qualitative and quantitative biodeteriorations. Amongst the toxigenic strains of A. flavus, the strain NKDW-7 isolated from walnut samples of Varanasi region was found to be highly toxigenic, and was therefore, selected for detailed investigation.
In the present investigation, the MIC value of α-terpineol (1.75 μL mL−1), Phenyl ethyl alcohol (2.0 μL mL−1) and their combination with A. archangelica oil (2.25 μL mL−1) was found to be lower than the some of the earlier reported essential oils viz. Cicuta virosa (5.0 μL mL−1), Cinnamomum jensenianum (8.0 μL mL−1), Curcuma longa L. (>10.0 μL mL−1), Piper nigrum L. (>10.0 μL mL−1), Pogostemon cablin Benth. (>10.0 μL mL−1) and frequently used food preservative viz. sodium benzoate (>10.0 μL mL−1) (Tian et al. 2011, 2012; Prakash et al. 2012c). Low MIC value of EO-based combination supports its recommendation at a lower dose which would be economical for the stakeholders. Since α-terpineol, PEA and the EO-based combination showed fungistatic properties the findings strengthen their application as an ideal antifungal agent in integrated pest management program which prefers static nature of preservatives rather than their cidal activity.
During the aflatoxin inhibitory assay of A. archangelica oil, α-terpineol, PEA and their combination, a positive correlation between the subsequent decrease in mycelium growth and aflatoxin B1 production with increasing concentrations was found in all treatment sets except A. archangelica oil. α -terpineol, PEA and the combination caused complete inhibition of aflatoxin B1 production at concentration below their MIC. This may be due to their different mode of action on fungal growth inhibition and aflatoxin suppression as has been reported in the oils of Lippia alba, Thyme oils (Shukla et al. 2009; Rasooli and Abyaneh 2004). The findings of present investigation are supported by earlier observation of that the inhibition of AFB1 synthesis below the MIC value is attributed by reduced fungal growth as well as inhibition of carbohydrate catabolism in A. flavus by acting on some key enzymes along with the lack of sporulation in fungal mycelia (Tian et al. 2011; Prakash et al. 2012b).
Apart from microbial infestation and aflatoxin contamination, the shelf life of food items is also reduced by toxic reactive oxygen species (ROS) molecules causing oxidative stresses and biodegradation. In addition, the metabolic products of aflatoxin B1 (AFB1-8,9-exoepoxides) have also been reported responsible for the stimulation of the lipid peroxidation by enhancement of highly reactive molecules (ROS) (Choy 1993). Therefore, the antioxidant activity of A. archangelica oil, α-terpineol, PEA and their combination along with the commonly used synthetic antioxidant butylated hydroxytoluene (BHT), was tested through DPPH free radical scavenging assay. DPPH method is polarity independent, frequently employed method for determination of antioxidant activity (Prakash et al. 2011; Kedare and Singh 2011). Highest radical scavenging activity was shown by BHT > A. archangelica oil > EO-based combination > α-terpineol while, PEA was recorded as non radical scavenging agent as it could inhibit only 4.33 at 150 μL mL−1. Based on the carcinogenic reports, the large scale application of BHT is restricted by food industries (Wichi 1986). Therefore, in order to prolong the shelf life of food items against oxidative deterioration, there is strict need to developed plant based safe antioxidant agent.
In the present investigation, the EO-based combination of A. archangelica oil, α-terpineol, PEA showed better efficacy in inhibition of molds growth, aflatoxin production, as well as antioxidant agent with respect to intact oil and the individual α-terpineol, phenyl ethyl alcohol. The combination also exhibited broad fungitoxic spectrum against all the isolated mold species from walnut. The findings thus strengthen its application for complete protection of food items from different molds species and also in qualitative control of aflatoxin contamination and lipid per oxidation.
The antifungal action of plant EO and EO based combination has been supposed to be because of their lipophilic nature, responsible for disruption of plasma membrane and thereby negatively affect the chief cellular components particularly mitochondria as has been supported by the transmission electron microscopy studies on EO treated A. flavus culture by Tian et al. 2011, 2012.
Ergosterol, a sterol, is a component of yeast and fungal cell membranes, Because of this ergosterol is a obvious target for antifungal drugs. Keeping this point in view the antifungal activity of EO-based combination was also assessed in terms of decrease in ergosterol content with the different concentrations. Our results reveal that EO-based combination can induce a considerable impairment of the ergosterol biosynthesis by A. flavus. Thus, the plasma membrane is an important site for the antifungal mechanism of EO based combination. The finding are supported by the earlier findings of some workers with azole antifungal drugs and some essential oils, inhibit fungal cell growth, because of the disruption of normal sterol biosynthetic pathways resulting in a decrease of ergosterol biosynthesis (Kelly et al. 1995).
To assess the safety limit of EO-based combination regarding its application as food preservative, its LD50 values was calculated through oral administration on mice (acute oral toxicity LD50) which is widely used and accepted method for determination of safety profile of plant products. The LD50 values of EO-based combination were found to be 9562.9 μl kg−1 as per body weight of mice. The high LD50 values was found to be higher than the some well known botanicals viz., azadirachtin (>5,000 mg kg−1), pyrethrum (350–500 mg kg−1) and carvone (1,640 mg kg−1), food preservatives sorbic acid (LD50 3,200 mg kg−1), propionic acid (LD50 3,500–4,300 mg kg−1), formic acid (LD50 700 mg kg−1), acetic acid (LD50 3,530 mg kg−1) and benzoic acid (LD50 2,000–2,500 mg kg−1) and some commercial fungicides including Bavistin (1,500 mg kg−1) and Wettable Sulfur (5,000 mg kg−1) (Prakash et al. 2012a). Thus the EO-based combination may be recommended as safe biorational plant based preservatives.
In conclusion, the EO-based combination of A. archangelica oil, α-terpineol, PEA has industrial potential as safe plant based preservative in view its efficacy against storage molds, aflatoxin production, as well as antioxidant agent. In addition high LD50 value of EO-based combination strengthens its non mammalian toxicity during its application as a food preservative. However, further investigations during storage conditions are required to evaluate its cost-efficacy, mode of action and efficacy in food system before possible large-scale application.
Acknowledgment
Bhanu Prakash is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance as Senior Research Fellow (SRF) and Dr. Priyanka Singh is thankful to Department of Science and Technology (DST) New Delhi for financial assistance as DST- Fast Track Young Scientist.
References
- Abdel-Hafez AII, Saber SM. Mycoflora and mycotoxin of hazelnut (Corylus avellana L.) and walnut (Juglans regia L.) seeds in Egypt. Zentralbl Mikrobiol. 1993;148:137–147. doi: 10.1016/S0232-4393(11)80117-4. [DOI] [PubMed] [Google Scholar]
- Aziz NH, Youssef YA, El-Fouly MZ, Moussa LA. Contamination of somecommon medicinal plant samples and spices by fungi and their mycotoxins. Bot Bull Acad Sinica. 1998;39:279–285. [Google Scholar]
- Brula S, Coote P. Preservative agents in foods mode of action and microbial resistance mechanisms. Int J Food Microbiol. 1999;50:1–17. doi: 10.1016/S0168-1605(99)00072-0. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods– a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Choy WN. A review of the dose-response induction of DNA adducts by aflatoxin B1 and its implication in quantitative cancer risk assessment. Mutat Res. 1993;296:181–198. doi: 10.1016/0165-1110(93)90010-K. [DOI] [PubMed] [Google Scholar]
- Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorg Med Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr. 2002;132:1062S–1101S. doi: 10.1093/jn/132.5.1062S. [DOI] [PubMed] [Google Scholar]
- Finney JD. Probit analysis. London: Cambridge University Press; 1971. p. 333. [Google Scholar]
- Gilman JC. A manual of soil fungi. Delhi: Biotech Book; 1998. [Google Scholar]
- Hall RL. Recent progress in the consideration of flavoring ingredients under the food additives amendment. Food Technol. 1960;14:488–495. [Google Scholar]
- Halliwell B. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;55:44–49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- Holley RA, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. doi: 10.1016/j.fm.2004.08.006. [DOI] [Google Scholar]
- Jaya, Singh P, Prakash B, Dubey NK (2012) Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J Food Sci Technol. DOI 10.1007/s13197-012-0698-8 [DOI] [PMC free article] [PubMed]
- Kedare SB, Singh R. P (2011) Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia A, Prakash B, Mishra PK, Singh P, Dubey NK (2013) Botanicals as eco friendly iorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—a review. J Food Sci Technol DOI 10.1007/s13197-013-1167-8 [DOI] [PMC free article] [PubMed]
- Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. Mode of action and resistance to azole antifungals associated with the formation of 14amethylergosta- 8, 24(28)-dien-3b, 6a-diol. Biochem Biophys Res Commun. 1995;207:910–915. doi: 10.1006/bbrc.1995.1272. [DOI] [PubMed] [Google Scholar]
- Kumar R, Mishra AK, Dubey NK, Tripathi YB. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol. 2007;115:159–164. doi: 10.1016/j.ijfoodmicro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Kumar A, Shukla R, Singh P, Prakash B, Dubey NK. Chemical composition of Ocimum basilicum L. essential oil and its efficacy as a preservative against fungal and aflatoxin contamination of dry fruits. Int J Food Sci Technol. 2011;46:1840–1846. doi: 10.1111/j.1365-2621.2011.02690.x. [DOI] [Google Scholar]
- Mandeel QA. Fungal contamination of some imported species. Mycopatholgia. 2005;159:291–298. doi: 10.1007/s11046-004-5496-z. [DOI] [PubMed] [Google Scholar]
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. A handbook of medicinal plants: a complete source. Jodhpur: Agrobios Publisher; 2003. [Google Scholar]
- Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int J Food Microbiol. 2010;142:114–119. doi: 10.1016/j.ijfoodmicro.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Prakash B, Shukla R, Singh P, Mishra PK, Dubey NK, Kharwar RN. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res Int. 2011;44:385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- Prakash B, Singh P, Kedia A, Dwivedy AK, Singh A, Dubey NK. Mycoflora and aflatoxin analysis of Arachis hypogaea L. and assessment of Anethum graveolens L. seed and leaf essential oils against isolated fungi, aflatoxin production and their antioxidant activity. J Food Saf. 2012;32:481–492. doi: 10.1111/jfs.12011. [DOI] [Google Scholar]
- Prakash B, Singh P, Kedia A, Singh A, Dubey NK. Efficacy of essential oil combination of Curcuma longa L. and Zingiber officinale Rosc. as a postharvest fungitoxicant, aflatoxin inhibitor and antioxidant agent. J Food Saf. 2012;32:279–288. doi: 10.1111/j.1745-4565.2012.00378.x. [DOI] [Google Scholar]
- Prakash B, Singh P, Mishra PK, Dubey NK. Safety assessment of Zanthoxylum alatum Roxb. essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of Piper nigrum L. fruits. Int J Food Microbiol. 2012;153:183–191. doi: 10.1016/j.ijfoodmicro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Rasooli I, Abyaneh MR. Inhibitory effects of Thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control. 2004;15:479–483. doi: 10.1016/j.foodcont.2003.07.002. [DOI] [Google Scholar]
- Shukla R, Kumar A, Singh P, Dubey NK. Efficacy of Lippia alba (Mill.) N.E. Brown essential oil and its monoterpene aldehyde constituents against fungi isolated from some edible legume seeds and aflatoxin B1 production. Int J Food Microbiol. 2009;135:165–170. doi: 10.1016/j.ijfoodmicro.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Tatsadjieu NL, Yaouba A, Nukenine EN, Ngassoum MB, Mbofung CMF. Comparative study of the simultaneous action of three essential oils on Aspergillus flavus and Sitophilus zeamaisMotsch. Food Control. 2010;21:186–190. doi: 10.1016/j.foodcont.2009.05.004. [DOI] [Google Scholar]
- Tian J, Ban X, Zeng H, He J, Huang B, Youwei W. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int J Food Microbiol. 2011;145:464–470. doi: 10.1016/j.ijfoodmicro.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012;130:520–527. doi: 10.1016/j.foodchem.2011.07.061. [DOI] [Google Scholar]
- Tripathi P, Dubey NK. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol. 2004;32:235–245. doi: 10.1016/j.postharvbio.2003.11.005. [DOI] [Google Scholar]
- Wichi HC. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol. 1986;24:1127–1130. doi: 10.1016/0278-6915(86)90298-X. [DOI] [PubMed] [Google Scholar]
- Zubair A, Ud-Din Z, Saleemullah, Khan SA, Shah HU, Khan BA, Ali E. Fatty acid profile and aflatoxin contamination of walnuts (Juglans regia) ARPN J Agric Biol Sci. 2011;6:1–8. doi: 10.3844/ajabssp.2011.1.6. [DOI] [Google Scholar]