Abstract
Collagen from emu skins as a by-product was prepared. The skins were hardly solubilized in acetic acid, however were successfully solubilized on digestion with 10 % pepsin (w/w) for 4 days. The yield of pepsin-solubilized collagen (PSC) was about 27.3 %, on a raw weight basis. By SDS-PAGE and CM-Toyopearl 650 M column chromatography, the presence of a fourth subunit that was previously designated α4 was confirmed. The denaturation temperature of the PSC was 31.5 °C, about 6–7 °C lower than that from the porcine skins. ATR-FTIR analysis indicated that the helical arrangements of the PSC from emu skins existed and its structures of PSC were changed slightly due to the loss of N- and C-terminus domains in similar to that from the porcine skins. That is, the PSC from emu skins did not possess telopeptide chains as major portion of antigenic sites in collagen. The present study indicates that a large quantity of emu skins as by-products have potential as a good alternative source of high-quality collagen for industrial purposes in the foods, cosmetics, and pharmaceutical and biomedical fields.
Keywords: Alternative source of collagen, By-product, Collagen, Emu, Skin
Introduction
Collagen is the most primary protein of animal origin and comprised approximately 30 % of total animal protein. Collagen has been utilized to produce edible casings and heat-denatured collagen. Collagen is also used for the production of cosmetics as it has a good moisturizing property (Swatschek et al. 2002). The highest utilization of collagen is in pharmaceutical applications including production of wound dressings, vitreous implants and as carriers for drug delivery, including biodegradability and weak antigenicity (Zhang et al. 2006). The main sources of collagen and gelatin for industrial purposes are generally skins and bones of land-based animals, such as cow and pig. However, the outbreak of bovine spongiform encephalopathy, the food-and-mouth disease, transmissible spongiform encephalopathy, and avian influenza caused in anxiety among users of collagen and collagen-derived products from these land-based animals (Jongjareonrak et al. 2005) and domestic fowls. As a result, there is a strong need to develop new collagen sources. In the recent years, we reported the preparation and characterization of collagens from aquatic organisms to look at alternative collagen resources. A large amount of collagen could be obtained from these materials (Nagai 2004; Nagai and Suzuki 2000, 2002a, b; Nagai et al. 1999, 2000, 2001, 2008, 2011), although the physical and chemical properties of these collagens were different from those of land-based animals such as porcine.
The emu (Dromaius novaehollandiae) belong to the order Casuariidae and is a free-roving, large, flightless bird indigenous to Australia. Now the emu is farmed in Australia, Canada, Europe, the USA, and Japan. An adult emu (15 months old) weighing 45 kg carries up to 10 kg of body fat, from that 7 to 8 l of a thick oil is obtained by heating at 150 °C. Snowden and Whitehouse (1997) prepared oils from fat samples of emu and reported the anti-inflammatory activity of emu oils in rats. Shao et al. (1999) also reported the functional properties of restructured beef and emu steaks. Thus, emu fat and meat has been popular among the consumers. During processing, a huge amount of skin from emu is generated as a by-product. Among collagen alternatives, the emu skins provide the best source of raw material because of its high availability, no risk of disease transmission, no religious barriers, and possibility of higher yielding collagen. No information on the physicochemical properties of collagen from the emu skins, however, has been reported. The purpose of this study was to isolate collagen from the emu skins, which are the by-products from emu meat processing plants, and to investigate its potential as an important collagen source for use in the industries.
Materials and methods
Emu skin
The skins of emu (Dromaius novaehollandiae) were obtained from a local wholesale market, Hokkaido, Japan and were stored at −85 °C until used.
Pretreatment of skins
All the preparative steps were carried out at 4 °C. The skins were cut into small pieces using a scalpel. The pieces (0.5 × 0.5 cm) were homogenized for 4 days with 10 volumes of 10 % ethanol to remove the fat by changing the solution twice a day. The homogenate was squeezed using cheesecloth to remove any excessive ethanol, and then the residue was washed with distilled water for 1 day by changing the solution twice a day. After lyophilization, the dried matter was extracted for 2 days with 0.1 M NaOH to remove the noncollagenous proteins by changing the solution twice a day. The matter was squeezed using cheesecloth, and then washed with distilled water for 2 days by changing the solution twice a day. After lyophilization, the dried matter was used for the extraction of the collagen.
Extraction of collagen
The extraction of collagen was performed as described previously (Nagai et al. 2008). The matter was treated for 2 days with 10 volumes of 0.5 M acetic acid along with gentle stirring. The extract was centrifuged at 50,000 × g for 1 h. The supernatants were pooled and salted to isolate and purify the type I collagen by selective salt precipitation. The collagen was isolated by the addition of NaCl to a final concentration of 0.9 M NaCl in 0.5 M acetic acid, followed by precipitation with 2.3 M NaCl in 0.05 M Tris–HCl buffer (pH 7.5). The resultant precipitate was obtained by centrifugation at 50,000 × g for 1 h, and was dissolved in a minimum volume of 0.5 M acetic acid, dialyzed against 0.1 M acetic acid for 2 days by changing the solution once a day, distilled water for 3 days by changing the solution twice a day, and then lyophilized (acid-soluble collagen: ASC). The residue from acetic acid extraction was washed with distilled water, suspended in 0.5 M acetic acid, and digested with 10 % (w/w) pepsin (EC 3.4.23.1; 2 x crystallized; 3,085 U/mg protein, Sigma-Aldrich Co., St. Louis, MO) by continuous stirring for 4 days. The viscous solution was centrifuged at 50,000 × g for 1 h, and the supernatants were pooled and dialyzed to inactivate the pepsin against 0.02 M Na2HPO4 (pH 7.2) for 3 days by changing the solution twice a day. After centrifugation at 50,000 × g for 1 h, the precipitate was dissolved in 0.5 M acetic acid and salted out by the addition of solid NaCl to a final concentration of 0.9 M NaCl in 0.5 M acetic acid, followed by precipitation with 2.3 M NaCl in 0.05 M Tris–HCl buffer (pH 7.5). The resultant precipitate was obtained by centrifugation at 50,000 × g for 1 h, and the precipitate was dissolved in a minimum volume of 0.5 M acetic acid, dialyzed against 0.1 M acetic acid for 2 days by changing the solution twice a day, distilled water for 3 days by changing the solution twice a day, and then lyophilized (PSC).
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed as described previously (Nagai et al. 2008). Molecular weight marker proteins [myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase B (97 kDa), and bovine serum albumin (66 kDa)] were purchased from the Sigma-Aldrich Co. (USA).
Amino acid composition
An amino acid analysis was performed as described previously (Nagai et al. 2008).
Ultraviolet spectra
The ultraviolet absorption spectra of the PSC from the skins of emu was recorded by a PerkinElmer model Lambda 11 (PerkinElmer, Tokyo, Japan) UV/VIS spectrophotometer. The PSC was dissolved in 0.5 M acetic acid and then the viscous solution was centrifuged at 50,000 × g for 30 min to remove the undissolved collagen fibre. The supernatants were used in this analysis.
Peptide mapping
The peptide mapping was performed as described previously (Nagai et al. 2008). The molecular weight markers [myosin (205 kDa), β-galactosidase (116 kDa), phosphorylase (97.4 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), bovine pancreas trypsinogen (24 kDa), and soybean trypsin inhibitor (20.1 kDa)] were obtained from Sigma-Aldrich Co., (USA).
CM-Toyopearl 650 M column chromatography
CM-Toyopearl 650 M column chromatography was performed using 10 mg of the PSC as described previously (Nagai et al. 2008). The subunit components were detected at 230 nm and the fractions indicated by the numbers were examined by SDS-PAGE using 7.5 % gel.
Denaturation temperature
Denaturation temperature was measured by the method of Nagai et al. (2008).
Attenuated total reflectance-fourier transform infrared (ATR-FTIR) spectroscopy
The ATR-FTIR spectra were collected at 20 °C and 40 % relative humidity by coupling the ATR accessory (ATR PRO410-S: JASCO Co., Tokyo, Japan) to a JASCO (Tokyo, Japan) FT/IR-4100 type A instrument. The IR spectrometer bench was equipped with a globar source, a KBr beam splitter, and a triglycine sulfate detector. The ATR sampling device utilized a diamond internal reflection element embedded into a ZnSe support/focusing element in a single reflection configuration. The spectra were obtained over the range of 4,000–650 cm−1 at 4 cm−1 resolution. The resultant spectra were analyzed using an IR Protein Secondary Structure Analysis Program (JASCO Co., Tokyo, Japan).
Results and discussion
Preparation of collagen
The emu skins were treated with ethanol to remove the fat. A large amount of fat were extracted. The yield of the lyophilized matter was about 35.6 % on a raw weight basis. The matter was treated with NaOH to remove the noncollagenous proteins. As a result, the yield of the lyophilized matter was about 31.3 % on a raw weight basis. Next, the matter was treated to solubilize the ASC with acetic acid, but was hardly solubilized. The PSC was readily solubilized from the residue from the acetic acid extraction. Finally, the PSC was successfully solubilized by gentle stirring for 4 days with limited pepsin digestion. Then the PSC was purified by differential salt precipitation. The yield of the PSC was very high, about 27.3 % on a raw weight basis. The result was explained by the fact that collagen molecules in emu skin were most likely cross-linked by covalent bonds through the condensation of aldehyde groups at the telopeptide region as well as the inter-molecular cross-linking, leading to a decrease in the solubility of collagen (Zhang et al. 2007). However, with limited pepsin digestion, the cross-linked molecules at the telopeptide region were cleaved, resulting in further extraction: pepsin could cleave effectively at the telopeptide region of collagen from the emu skins.
Previously we have tried to prepare collagens from aquatic organisms, and its yields were very high as follows: ASCs from Japanese sea bass, chub mackerel, and bullhead shark skins: 44.7–51.4 % (Nagai and Suzuki 2000); PSCs from diamondback squid outer skins: 35.6 % (Nagai 2004), paper nautilus outer skins: 50.0 % (Nagai and Suzuki 2002a), Sepia lycidas outer skins: 35.0 % (Nagai et al. 2001), and ocellate puffer fish skins: 44.7 %, (Nagai and Suzuki 2002b), respectively. Furthermore, we tried to prepare and characterize the collagen from common minke whale unesu. The collagen was readily solubilized from the unesu by pepsin digestion, about 28.4 % yield on a raw weight basis (Nagai et al. 2008). Other researchers have also reported the yields of skin collagen as follows: channel catfish ASC (25.8 %) and PSC (38.4 %) (Liu et al. 2007), deep-sea redfish ASC (47.5) and PSC (92.2 %) (Wang et al. 2007), and grass carp PSC (46.6 %) (Zhang et al. 2007). Jaroenviriyapap and Vittayanont (2009) tried to prepare collagens from tracheas of chicken, duck, and ostrich as by-products. Cliché et al. (2003) reported that the collagen was prepared from chicken skins by limited pepsin digestion and the yield of PSC was 38.9 % on a dry weight basis. It suggests the possibility for the PSC from the emu skins to have potential as a good alternative source of high-quality collagen for industrial purposes.
SDS-PAGE
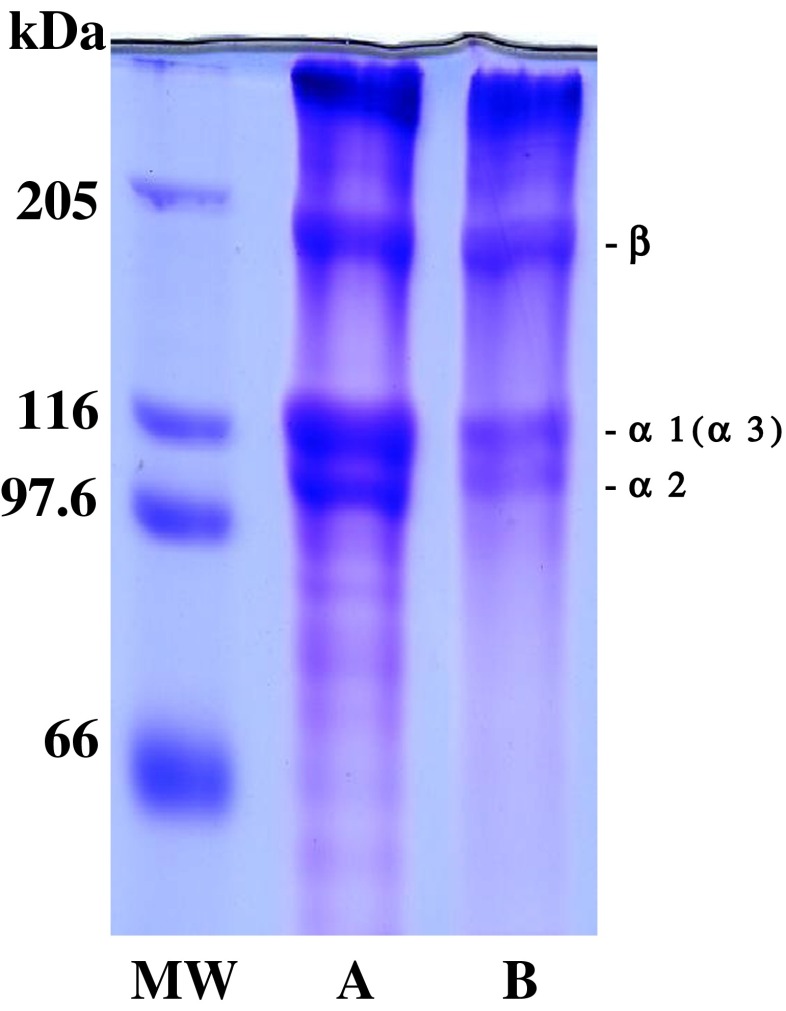
The PSC from the emu skins was estimated by SDS-PAGE using 7.5 % gel. As a result, the two distinct bands were confirmed in the stained gel in mobility in the α region (Fig. 1): this collagen existed as trimers consisting of two distinct α chains, such as α1 and α2. The existence of an α3 chain was not identified under this electrophoretic conditions. A large amount of high molecular weight component, β chain was detected in it. By limited pepsin digestion, β-component might be cleaved into α components, as evidenced by the increased band intensity of the α chains. The positions of the chains of PSC from the emu skins were similar to those from the porcine skins (Fig. 1). These results indicate that the PSC from the emu skins may have a chain composition of (α1)2α2 heterotrimer are the type I collagen as a major component in mammalian collagen, such as porcine and in poultry collagens, such as chicken (Cliché et al. 2003) and duck and ostrich (Jaroenviriyapap and Vittayanont 2009).
Fig. 1.
SDS-polyacrylamide gel electrophoresis pattern of the collagens from the skins of porcine and emu on 7.5 % gels containing 3.5 M urea. (MW): high molecular marker: (A): porcine skin collagen; (B): emu skin PSC
Amino acid composition
The amino acid composition of PSC from the emu skins was investigated and expressed as residues per 1,000 total residues. Glycine (331 residues) was the most abundant amino acid and accounted for about one third among the total amino acids in the PSC (Table 1). There were relatively high contents of proline (121 residues), alanine (110 residues), hydroxyproline (97 residues), and glutamic acid (75 residues), decreasing in that order. Tryptophan and cysteine were not detected. In general, type I collagen has low contents of cysteine (~0.2 %) and methionine (~1.24–1.33 %) (Owusu-Apenten 2002). It is well known about the existence of imino acids, such as proline and hydroxyproline in collagen. Higher the imino acids content, greater is the stability of the helices of collagen. The molecular structure of collagen is maintained mainly by restrictions on changes in the secondary structure of the polypeptide chain, imposed by the pyrrolidine rings of proline and hydroxyproline, and also maintained partially by the hydrogen bonding ability of the hydroxyl group of hydroxyproline. The total contents of the imino acids in this collagen from the emu skins were very high (218 residues), 21.8 %, similar to that in collagen from the largefin longbarbel catfish skins (21.3 %; Zhang et al. 2009). Moreover, this was fairly higher than those in collagens from as follows: ocellate puffer skin (17.0 %; Nagai and Suzuki 2002b) and cuttlefish outer skin (18.6–18.8 %; Nagai 2004; Nagai et al. 2001). Moreover, other researchers also reported total contents of imino acid in collagen from the skins: bigeye snapper (19.3 %; Kittiphattanabawon et al. 2005), bullfrog (16.7 %; Li et al. 2004), channel catfish [17.1 (ASC) and 17.7 % (PSC); Liu et al. 2007], brown backed toadfish (17.0 %; Senaratne et al. 2006), deep-sea redfish (16.0 %; Wang et al. 2007), walleye pollack (18.4 %; Yan et al. 2008), and grass carp (18.6 %; Zhang et al. 2007), respectively.
Table 1.
Amino acid composition of PSC from emu skins
| Amino acid | Residues/1000 |
|---|---|
| Hydroxyproline | 97 |
| Hydroxylysine | 4 |
| Aspartic acid | 48 |
| Threonine | 19 |
| Serine | 30 |
| Glutamic acid | 75 |
| Proline | 121 |
| Glycine | 331 |
| Alanine | 110 |
| Valine | 17 |
| Methionine | 7 |
| Isoleucine | 13 |
| Leucine | 25 |
| Tyrosine | 3 |
| Phenylalanine | 14 |
| Lysine | 30 |
| Histidine | 5 |
| Arginine | 51 |
| Total | 1000 |
Hydroxyproline is derived from proline by post-translational hydroxylation, mediated by prolylhydroxylase. It is well known that the degree of hydroxylation of proline influences the thermal stability of the collagen: a higher degree of hydroxylation is associated with a higher denaturation temperature of the collagen. The degree of hydroxylation of proline residues in the PSC from the emu skins was calculated to be about 44.5 % (Table 1). On the other hand, we reported the degrees of hydroxylation of proline residues in the collagens as follows: cuttlefish outer skin (47.8–47.9 %; Nagai 2004; Nagai et al. 2001), ocellate puffer skin (39.4 %; Nagai and Suzuki 2002b), common minke whale unesu (39.2 %; Nagai et al. 2008), respectively. In this respect results reported by other researchers are as follows: bigeye snapper (39.9 %; Kittiphattanabawon et al. 2005), bullfrog (32.3 %; Li et al. 2004), channel catfish (42.9 %; Liu et al. 2007), brown backed toadfish (45.3 %; Senaratne et al. 2006), deep-sea redfish (38.1 %; Wang et al. 2007), walleye pollack (37.5 %; Yan et al. 2008), grass carp (34.9 %; Zhang et al. 2007), and largefin longbarbel catfish (34.7 %; Zhang et al. 2009), respectively.
Ultraviolet spectra
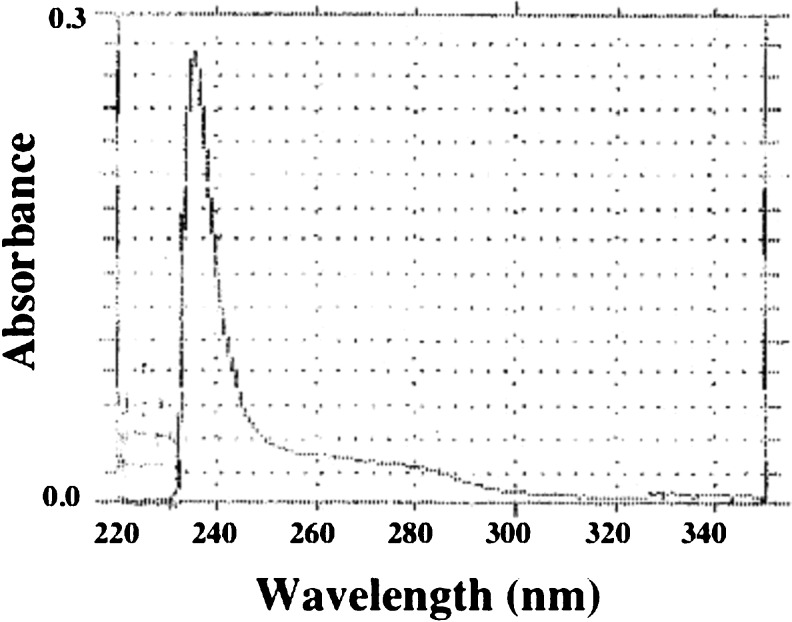
In general, there is no obvious absorption for collagen sample in the near ultraviolet region at 280 nm because of non existence of tryptophan residue in collagen. The maximum ultraviolet absorption spectra of the PSC from the emu skins was investigated. As a result, the distinct absorption of it was obtained near 235.1 nm (Fig. 2). Due to low content of phenyl alanine and tyrosine there are no absorption bands between 250 and 290 nm (Table 1 and Fig. 2). This was in accordance with the characteristic absorption of collagen. Li et al. (2004) reported that the collagen from the bullfrog skins has absorption near 236 nm. Moreover, other researchers reported the maximum absorptions of the collagens from the skins as follows: channel catfish; 232 nm (Liu et al. 2007) and largefin longbarbel catfish; 233 nm (Zhang et al. 2009).
Fig. 2.
Ultraviolet spectra of the PSC from emu skins
Peptide mapping
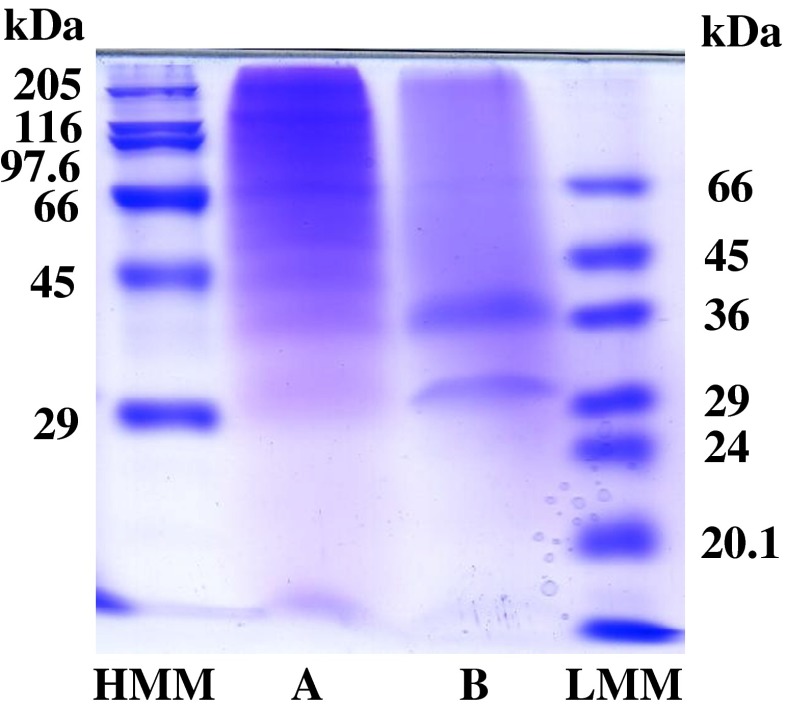
After the digestion by lysyl endopeptidase, the denatured collagen samples were applied to SDS-PAGE using a 10 % gel and the patterns of the peptide fragment were compared with those from the porcine skins. As shown in Fig. 3, varying degradation peptides with different molecular weights were obtained. Peptides with molecular weight of about 205, 125, 100, and 76 kDa were the dominant products of the collagen from the porcine skins. On the other hand, PSC from the emu skins showed the main bands with the molecular weights of about 37 and 31 kDa. Thus, the electrophoretic pattern of the PSC from the emu skins was different from that from the porcine skins. PSC from the emu skins was more susceptible to hydrolysis by lysyl endopeptidase than collagen from the porcine skins.
Fig. 3.
Peptide mapping of lysyl endopeptidase digests of the collagens from the skins of porcine and emu. (HMM): high molecular marker; (A): porcine skin collagen; (B): emu skin PSC; (LMM): low molecular marker
Subunit composition
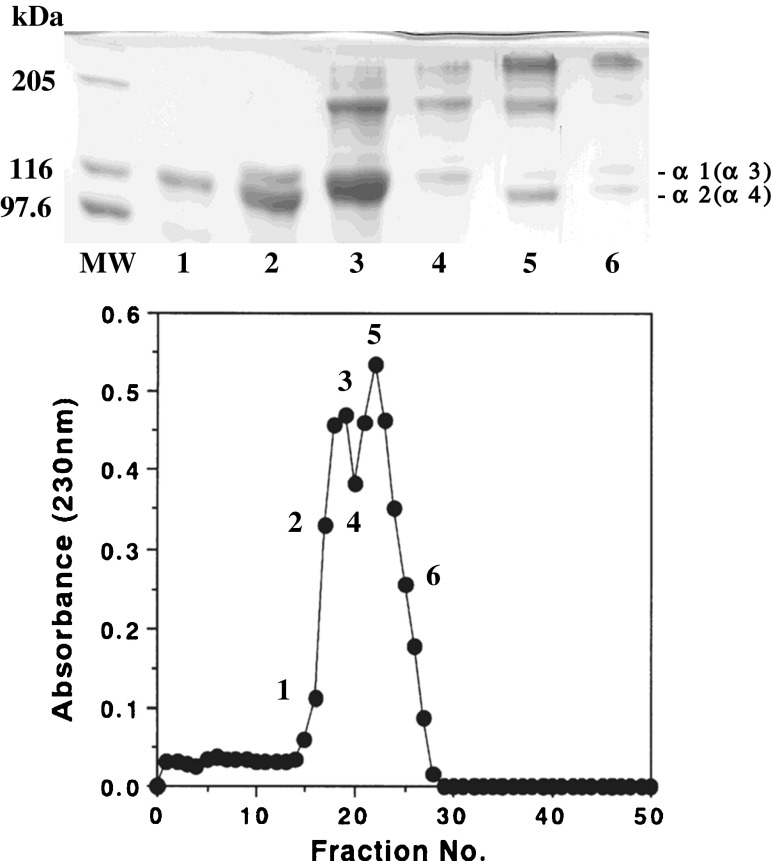
The subunit composition of the PSC from the emu skins was investigated by applying the denatured collagen to the CM-Toyopearl 650 M column chromatography. The chromatographic fractions as indicated by the numbers were analyzed by SDS-PAGE using 7.5 % gel. This was resolved in two protein fractions, consisted an α chain as a major component (Fig. 4). That is, these chains were α1 in fractions 1–4, α2 in fractions 2 and 3, and α3 in fractions 5 and 6, as indicated by the number. Interestingly, this collagen contained a large quantity of a fourth subunit that was previously designated α4 in fractions 5 and 6 as indicated by the number. This suggested that the PSC from the emu skins was a heterotetramer with a chain composition of α1α2α3α4. Nagai et al. (2000) studied the collagen from the mesogloea of rhizostomous jellyfish (Rhopilema asamushi) and then reported the existence of α4 subunit in this collagen. Kimura et al. (1987) reported that only eel skin collagen among some teleosts was quite unique and its collagen contained a fourth subunit α4.
Fig. 4.
CM-Toyoperal 650 M column chromatography of denatured emu skin PSC. A 1.0 × 6.0 column of CM-Toyopearl 650 M was equilibrated with 20 mM sodium acetate buffer (pH 4.8) containing 6 M urea, and maintained at 37 °C. The PSC (10.0 mg) was dissolved in 5 ml of the same buffer, denatured at 45 °C for 30 min, and then eluted from the column with a linear gradient of 0 to 0.15 M NaCl at a flow rate of 0.8 ml/min. The fractions indicated by the numbers were examined by SDS-PAGE using 7.5 % gel
Recently, Nagai and co-researchers have studied the collagens from the aquatic organisms and their application for industrial purposes. They reported that the diversity existed in the subunit composition of the collagens from these species: diamondback squid outer skin PSC (α1α2α3; Nagai 2004), paper nautillus outer skin PSC (α1α2α3, Nagai and Suzuki 2002a), cuttlefish outer skin PSC [(α1)2α2; Nagai et al. 2001], and ocellate puffer fish skin PSC [(α1)2α2; Nagai and Suzuki 2002b], respectively. Senaratne et al. (2006) studied the collagen from the brown backed toadfish skins (Lagocephalus gloveri) and characterized. The collagen had a chain composition of α1α2α3 heterotrimer. Other researchers also studied the PSCs from the bullfrog skins (Li et al. 2004), channel catfish (Liu et al. 2007), and grass carp (Zhang et al. 2007), but did not investigate the subunit composition of these collagens. On the other hand, we recently isolated collagen from the common minke whale unesu by pepsin treatment and characterized (Nagai et al. 2008). This collagen consisted of a (α1)2α2 heterotrimer, similar to that of mammalian collagen, such as the collagen from the porcine skins.
Denaturation temperature
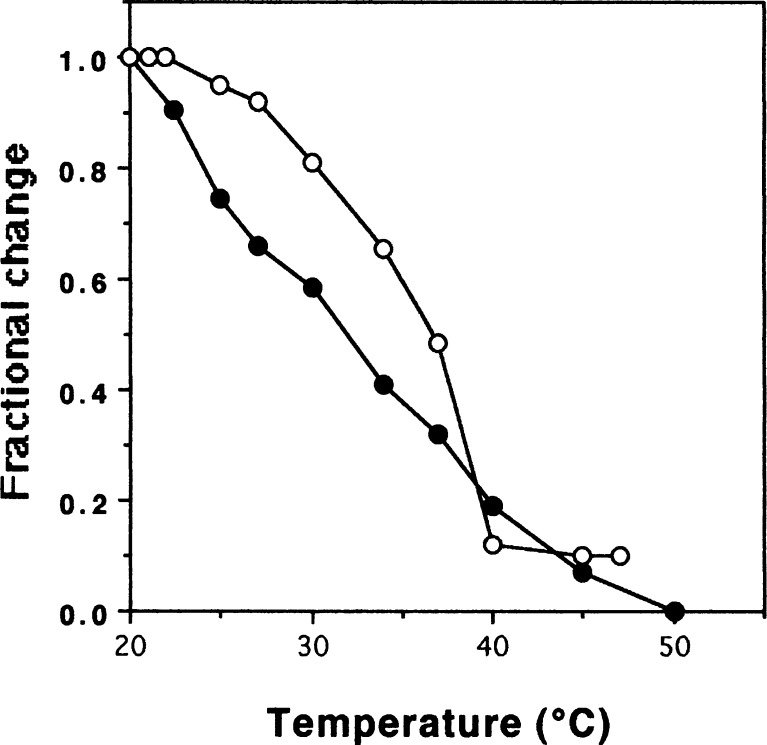
The denaturation temperature of the PSC from the emu skins was calculated from the thermal denaturation curve, since the heat transformation of the collagen is interpreted as disintegration of triple helical structure of the collagen into random coils. For comparison, the denaturation temperature of the collagen from the porcine skins was also measured. As a result, it was calculated that the denaturation temperature of the PSC was about 31.5 °C (Fig. 5). This value was about 5.5 °C lower than that of the collagen from the porcine skins. By the literature of Nagai and co-researchers, the denaturation temperatures of the collagens from aquatic organisms were as follows: fish skin (28.0 °C, Nagai and Suzuki 2002b), octopus outer skin (27.0 °C, Nagai and Suzuki 2002a), cuttlefish outer skin (27.0–27.5 °C, Nagai 2004; Nagai et al. 2001), exumbrella and mesogloea from jellyfishes (26.0–28.8 °C, Nagai et al. 1999, 2000), respectively. In recent years, Nagai et al. (2008) reported that the denaturation temperature of the collagen from common minke whale unesu was calculated to be about 31.5 °C. Other researchers reported the denaturation temperatures of the collagens as follows: bullfrog skin PSC (30.3 °C, Li et al. 2004), brown backed toadfish skin PSC (28.0 °C, Senaratne et al. 2006), channel catfish skin ASC (32.5 °C, Liu et al. 2007), grass carp skin PSC (28.4 °C, Zhang et al. 2007), and largefin longbarbel catfish skin ASC and PSC (32.1 °C and 31.6 °C, Zhang et al. 2009). Generally, it is known that the denaturation temperatures of the collagens from land animals are higher than those from aquatic organisms. This is correlated with their environmental and body temperatures.
Fig. 5.
Thermal denaturation curve of emu skin PSC. The denaturation temperature was measured by viscosity in 0.1 M acetic acid. The incubation time at each temperature was 30 min. Collagen concentration 0.03 %; (white circle): porcine skin collagen; (black circle): emu skin PSC
ATR-FTIR spectroscopy analysis
With the advancement of Fourier transform infrared and the introduction of ATR devices in the past few years, ATR-FTIR spectroscopy is one of the most versatile techniques for the quick identification and characterization of collagen because it gathers information from any type of sample.
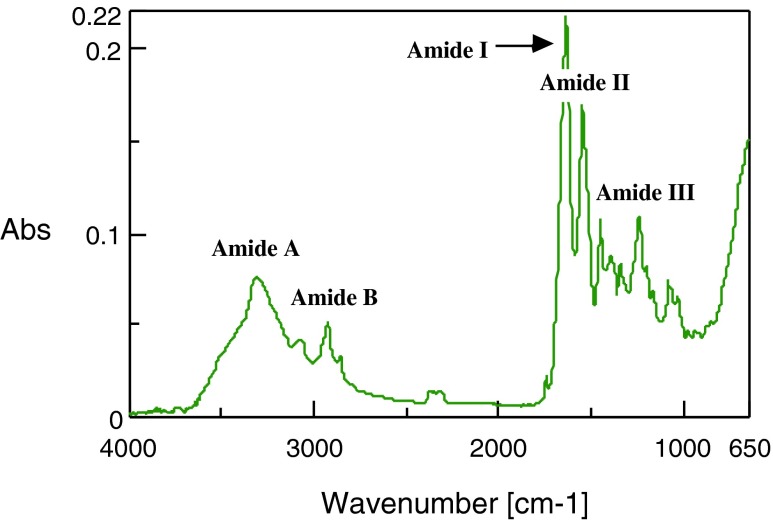
The ATR-FTIR spectrum of the PSC from the emu skins is shown in Fig. 6. The amide I band with its characteristic frequencies in the range of 1,600–1,700 cm−1, associated with the stretching vibrations of the carbonyl group (C = O bond) along the polypeptide backbone (Payne and Veis 1988), is a sensitive marker of the peptide secondary structure (Surewicz and Mantsch 1988). The amide I, II, and III bands of the PSC were found at 1,633, 1,541, and 1,237 cm−1, respectively (Fig. 6). The first two bands indicate C = O stretching and N-H bending vibrations. The band at 1,237 cm−1 cannot be for C-H stretch (Payne and Veis 1988). The amide A band is associated with the N-H stretching frequency. Doyle et al. (1975) reported a free N-H stretching vibration occurs in the range of 3,400 to 3,300 cm−1, and when the NH group of a peptide is involved in hydrogen bond, the position is shifted to lower frequencies, usually around 3,300 cm−1. The band at 3,309 cm−1 indicates the existence of hydrogen bonds in the PSC from the emu skins. On the other hand, it is known that amide B band is related to asymmetrical stretch of CH2 (Muyonga et al. 2004). The band was observed at 2,925 cm−1. These results indicate the existence of helical arrangements of the PSC from the emu skins. This is a similar spectral pattern to those of other species collagens (Li et al. 2004; Liu et al. 2007; Muyonga et al. 2004; Nagai et al. 2008; Singh et al. 2011; Wang et al. 2007; Yan et al. 2008; Zhang et al. 2009). On the other hand, a weak 1,741 cm−1 peak cannot be due to C-H stretching for PSC, that was in agreement with the findings of Singh et al. (2011) and Nagai et al. (2008), but was different from those of Nagai et al. (2011).
Fig. 6.
Fourier transform infrared spectra of emu skin PSC
This percentage of secondary structural components, such as α-helix, β-sheet, β-turn, and others (random coil structure), of the PSC from the emu skins were calculated. Sarver and Krueger (1991) investigated the protein secondary structure using FTIR and analyzed the resulting spectra to construct a database. So we tried to analyze the spectra of the PSC from the emu skins using an IR Protein Secondary Structure Analysis Program (JASCO Co.). As a result, the percentage of these components of the PSC was 9 % α-helix, 35 % β-sheet, 18 % β-turn, and 20 % others. For comparison, the percentage of the collagen from the porcine skins was calculated as follows: 9 % α-helix, 51 % β-sheet, 13 % β-turn, and 15 % others. This suggests that the sheet structure in the collagen of the porcine skins is different from the PSC from the emu skins.
Conclusion
Based on the studies above, the collagen from the emu skins was successfully prepared by the digestion of pepsin for 4 days. This collagen did not possess telopeptide chains as major portion of antigenic sites in collagen. The yield of the collagen was fairly high. This collagen contained a large quantity of an unique and fourth subunit that was previously designated α4. By the physicochemical investigation of the collagen, we suggest that a large quantity of emu skins as by-products have potential as a good alternative source of high-quality collagen for industrial purposes in the foods, cosmetics, and pharmaceutical and biomedical fields.
References
- Cliché S, Amiot J, Avezard C, Gariépy C. Extraction and characterization of collagen with or without telopeptides from chicken skin. Poult Sci. 2003;82:503–509. doi: 10.1093/ps/82.3.503. [DOI] [PubMed] [Google Scholar]
- Doyle BB, Bendit EG, Blout ER. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers. 1975;14:937–957. doi: 10.1002/bip.1975.360140505. [DOI] [PubMed] [Google Scholar]
- Jaroenviriyapap T, Vittayanont M. Type and content chondroitin sulphate and collagen in poultry tracheas. Asian J Food Agro-Ind. 2009;2:974–980. [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of Brownstripe red snapper (Lutjanus vitta) Food Chem. 2005;93:475–484. doi: 10.1016/j.foodchem.2004.10.026. [DOI] [Google Scholar]
- Kimura S, Ohno Y, Miyauchi Y, Uchida N. Fish skin type I collagen: wide distribution of α3 subunit in teleosts. Comp Biochem Physiol. 1987;88B:27–34. doi: 10.1016/0305-0491(87)90074-5. [DOI] [PubMed] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus) Food Chem. 2005;89:363–372. doi: 10.1016/j.foodchem.2004.02.042. [DOI] [Google Scholar]
- Li H, Liu BL, Gao LZ, Chen HL. Studies on bullfrog skin collagen. Food Chem. 2004;84:65–69. doi: 10.1016/S0308-8146(03)00167-5. [DOI] [Google Scholar]
- Liu HY, Li D, Guo SD. Studies on collagen from the skin of channel catfish (Ictalurus punctaus) Food Chem. 2007;101:621–625. doi: 10.1016/j.foodchem.2006.01.059. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) Food Chem. 2004;85:81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Nagai T. Collagen from diamondback squid (Thysanoteuthis rhombus) outer skin. Z Naturforsch. 2004;59c:271–275. doi: 10.1515/znc-2004-3-426. [DOI] [PubMed] [Google Scholar]
- Nagai T, Suzuki N. Isolation of collagen from fish waste material-skin, bone and fins. Food Chem. 2000;68:277–281. doi: 10.1016/S0308-8146(99)00188-0. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Preparation and partial characterization of collagen from paper nautilus (Argonauta argo, Linnaeus) outer skin. Food Chem. 2002;76:149–153. doi: 10.1016/S0308-8146(01)00255-2. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes) Food Chem. 2002;78:173–177. doi: 10.1016/S0308-8146(01)00396-X. [DOI] [Google Scholar]
- Nagai T, Ogawa T, Nakamura T, Ito T, Nakagawa H, Fujiki K, Nakao M, Yano T. Collagen of edible jellyfish exumbrella. J Sci Food Agric. 1999;79:855–858. doi: 10.1002/(SICI)1097-0010(19990501)79:6<855::AID-JSFA299>3.0.CO;2-N. [DOI] [Google Scholar]
- Nagai T, Worawattanamateekul W, Suzuki N, Nakamura T, Ito T, Fujiki K, Nakao M, Yano T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi) Food Chem. 2000;70:205–208. doi: 10.1016/S0308-8146(00)00081-9. [DOI] [Google Scholar]
- Nagai T, Yamashita E, Taniguchi K, Kanamori N, Suzuki N. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas) Food Chem. 2001;72:425–429. doi: 10.1016/S0308-8146(00)00249-1. [DOI] [Google Scholar]
- Nagai T, Suzuki N, Nagashima T. Collagen from common minke whale (Balaenoptera acutorostrata) unesu. Food Chem. 2008;111:296–301. doi: 10.1016/j.foodchem.2008.03.087. [DOI] [PubMed] [Google Scholar]
- Nagai T, Suzuki N, Tanoue Y, Kai N, Nagashima T. Characterization of acid-soluble collagen from skins of surf smelt (Hypomesus pretiosus japonicus Brevoort) Food Nutr Sci. 2011;1:59–66. doi: 10.4236/fns.2010.12010. [DOI] [Google Scholar]
- Owusu-Apenten RK. Food protein analysis. Quantitative effects on processing. New York: Marcel Dekker, Inc; 2002. pp. 1–43. [Google Scholar]
- Payne KJ, Veis A. Fourier transform IR spectroscopy of collagen and gelatin solutions: deconvolution of the amide I band for conformational studies. Biopolymers. 1988;27:1749–1760. doi: 10.1002/bip.360271105. [DOI] [PubMed] [Google Scholar]
- Sarver RW, Jr, Krueger WC. Protein secondary structure from Fourier transform infrared spectroscopy: a data base analysis. Anal Biochem. 1991;194:39–100. doi: 10.1016/0003-2697(91)90155-M. [DOI] [PubMed] [Google Scholar]
- Senaratne LS, Park P-J, Kim S-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour Technol. 2006;97:191–197. doi: 10.1016/j.biortech.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Shao CH, Avens JS, Schmidt GR, Maga JA. Functional, sensory, and microbiological properties of restructured beef and emu steaks. J Food Sci. 1999;64:1052–1054. doi: 10.1111/j.1365-2621.1999.tb12280.x. [DOI] [Google Scholar]
- Singh P, Benjakul S, Maqsood S, Kishimura H. Isolation and characterization of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus) Food Chem. 2011;124:97–105. doi: 10.1016/j.foodchem.2010.05.111. [DOI] [Google Scholar]
- Snowden JM, Whitehouse MW. Anti-inflammatory activity of emu oils in rats. Inflammopharmacology. 1997;5:127–132. doi: 10.1007/s10787-997-0021-x. [DOI] [PubMed] [Google Scholar]
- Surewicz WK, Mantsch HH. New insight into protein secondary structure from resolution enhanced infrared spectra. Biochim Biophys Acta. 1988;952:115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Swatschek D, Schatton W, Kellermann J, Muller WEG, Kreuter J. Marine sponge collagen: isolation, characterization and effects on the skin parameters surface—pH, moisture and sebum. Eur J Pharm Biopharm. 2002;53:107–113. doi: 10.1016/S0939-6411(01)00192-8. [DOI] [PubMed] [Google Scholar]
- Wang L, An X, Xin Z, Zhao L, Hu Q. Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella) J Food Sci. 2007;72:E450–E455. doi: 10.1111/j.1750-3841.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Yan M, Li B, Zhao X, Ren G, Zhuang Y, Hou H, Zhang X, Chen L, Fan Y. Characterization of acid-soluble collagen from the skin of walleye pollack (Theragra chalcogramma) Food Chem. 2008;107:1581–1586. doi: 10.1016/j.foodchem.2007.10.027. [DOI] [Google Scholar]
- Zhang ZK, Li GY, Shi B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J Soc Leather Technol Chem. 2006;90:23–28. [Google Scholar]
- Zhang Y, Liu W, Li G, Shi B, Miao Y, Wu X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella) Food Chem. 2007;103:906–912. doi: 10.1016/j.foodchem.2006.09.053. [DOI] [Google Scholar]
- Zhang M, Liu W, Li G. Isolation and characterization of collagens from the skin of largefin longbarbel catfish (Mystus macropterus) Food Chem. 2009;115:826–831. doi: 10.1016/j.foodchem.2009.01.006. [DOI] [Google Scholar]