Abstract
The response surface methodology (RSM) was used to optimize the conditions for total flavonoid extraction from Scutellaria baicalensis Georgi. The influences of the ethanol concentration, extraction time, temperature, and the liquid–solid ratio on flavonoid yield were investigated. Based on ANOVA results, a second-order quadratic polynomial model could be applied to characterize the extraction process. The following optimal extraction conditions were identified: ethanol concentration, 52.98 %; extraction time, 2.12 h; extraction temperature, 62.46 °C; and liquid–solid ratio, 35.23. The predicted extraction yield was 19.437 mg/g when these optimal conditions were used. The proposed method was successfully employed to extract flavonoids from S. baicalensis.
Keywords: Scutellaria baicalensis Georgi, Flavonoid extraction, Response surface methodology, Optimization
Introduction
Scutellaria baicalensis Georgi (Labiatae), or Chinese Huangqin, is a widely used as herbal medicine in China and other East Asian countries (CPM 2010; Heo et al. 2009; Park et al. 2011). Its dried roots can treat inflammations, cancers, hepatitis, tumors, bronchitis, allergies, and arteriosclerosis (CPM 2010; Sun et al. 2008; Zhang et al. 2006). S. baicalensis has been reported to contain flavonoids, phenylethanoids, amino acids, and essential oils (Li et al. 2004; Liu et al. 2009, 2011; Sheng et al. 2009; Zhou et al. 1997). The main active components of S. baicalensis are the flavonoids, which have recently received much attention worldwide. These compounds have numerous reported medicinal properties such as antioxidant, antineurotoxic, anti-inflammation, antianxiety, antimutagenic, antiradical, anti-cancer, and anti-SARS coronavirus effects, as well as liver protection, hearing protection, neuroprotection, and spontaneous sleep–wake regulation (Chang et al. 2011; Chen et al. 2004; Heo et al. 2004; Himeji et al. 2007; Hui et al. 2002; Kang et al. 2010; Kim et al. 2009; Kimura and Sumiyoshi 2011; Lin et al. 2011; Shang et al. 2006; Wang et al. 2010; Woźniak et al. 2004; Zhao et al. 2006).
The orthogonal design methods have been widely applied in analytical procedures for optimizing to obtain higher extraction yields. However, such designs cannot measure the interactive effects among the variables (Baş and Boyacı 2007; Sheng et al. 2013). The response surface methodology (RSM) can overcome the disadvantages of the orthogonal design. RSM is a mathematical and statistical method for designing experiments, modeling, evaluating variable effects, and optimizing extraction conditions. This methology has been recently used to optimize chemical and physical processes (Najafi et al. 2012; Sheng et al. 2013; Wang et al. 2012, 2013; Xu et al. 2013; Yang et al. 2010).
In this study, the total flavonoids were extracted from S. baicalensis. The aim of the study was to understand the combined effect of the extraction parameters, including the ethanol concentration, extraction time, temperature, and the liquid–solid ratio by applying RSM. The response variables were examined based on the flavonoid yields under different operating conditions.
Materials and methods
Materials
S. baicalensis was locally purchased and verified by Professor Tiechen Cui (School of Life Science, Zhaoqing University). The voucher specimens were immediately deposited after the extraction of flavonoids. Rutin (No.100080-200707) was purchased from the Chinese Institute for the Control of Pharmaceutical and Biological Products. All the reagents were of analytical grade. The double distilled water was used for all the experiments. Analytical grade aluminum nitrate, ethanol, sodium hydroxide, and sodium nitrite were purchased from the Guangzhou Chemical Reagent Factory.
Extraction of total flavonoids
The plant samples (5.000 g) were used to extract flavonoids via reflux extraction by ethanol (School of Chemistry& Chemical Engineering of Zhaoqing University). The samples were vacuum-dried at 60 °C for 24 h and then finely ground to sieve through a 35/40 mesh (approximately 0.5 mm diameter). These samples were prepared in the solvent within given ranges for the temperature (25 °C to 95 °C), extraction time (0.5 h to 3.0 h), and liquid–solid rato (g/mL) (1:6 to 1:21) at different ethanol concentrations (0, 10 %, 30 %, 50 %, 70 %, and 90 %). The extraction solution was centrifuged at 3,000 rpm for 5 min and passed through a Xinhua filter (Hangzhou Xinhua Paper Industry Co., Ltd.). The filtrate was diluted to 100 mL prior to the measurement of the total flavonoid content.
Determination the content of total flavonoids
A standard colorimetric assay was applied to measure the amount of total flavonoids, with slight modifications. Briefly, 1 mL of the diluted flavonoid extract, 5 mL of 60 % (v/v) ethanol, and 0.3 mL of 5 % (w/v) sodium nitrite were mixed for 6 min, and then 0.3 mL of 10 % aluminium chloride (w/v) was added. After another 6 min of mixing, 4 mL of 1 mol/L sodium hydroxide was added to the extraction mixture. A final volume of 10 mL was obtained by adding distilled water to the extract. The solution was left to stand for 15 min. The absorption at 507 nm was then measured using a UV–vis 916 spectrophotometer (GBC Scientific Equipment Pty Ltd., Australia) against the same mixture, without using the sample as a blank. The calibration curve ranged from 24 to 52 μg/mL (y = 11.994x + 0.0298, where y is the absorbance and x is the concentration of the sample; R2 = 0.9998).
Experimental design and statistical analysis
Several parameters affect the total flavonoid yield. Previous trials showed that the ethanol volume, extraction temperature, extraction time, and liquid–solid ratio have significant effects on the yield of total flavonoid extraction (Liu et al. 2009). Given the preliminary results, a central composite rotatable design (CCRD) was used to investigate the effects of four independent variables, namely, the ethanol volume (X1), extraction time (X2), temperature (X3), and the solid–liquid ratio (X4) on the yield of flavonoids (Y). The independent variables were designated as “+1,” “0,” and “−1” for high, intermediate, and low values, respectively. The coded and corresponding uncoded levels of the independent variables used in the CCRD design are listed in Table 1. The complete design consisted of 29 experimental runs, including 5 replications of the center points.
Table 1.
Independent variables and their levels used in the RSM design
| Levels | |||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ethanol concentration (%) (A) | 50 | 60 | 70 |
| Extraction time (h) (B) | 1.5 | 2 | 2.5 |
| Extraction temperature (°C) (C) | 50 | 60 | 70 |
| liquid–solid ratio (mL/g) (D) | 20 | 30 | 40 |
A second-degree polynomial model was used to explain the behavior of the system based on the experimental data. The nonlinear computer-generated quadratic model is stated in the following equation:
| 2 |
where Y is the response and β0 is a constant. βi, βii, and βij are the linear, quadratic, and interactive coefficients, respectively; Xi and Xj are the levels of the independent variables.
The Design-Expert software (trial version 8.0.4, Stat-Ease Inc., Minneapolis, USA) was utilized to perform this operation for the experimental design, multiple regression analysis (R2), ANOVA, and the numerical optimization of the response surface regression (RSREG) procedure. The practical yield was obtained under the optimal conditions.
Results and discussion
Single factor analysis method
Effect of ethanol concentration on the total flavonoid yield
Different concentrations of ethanol solutions (40 %, 50 %, 60 %, 70 %, and 80 %) were used to study the effect of ethanol on extraction performance. The procedures were conducted at 60 °C for 2 h with a liquid-material ratio of 30:1 (mL/g).
The flavonoid yields were significantly affected by the ethanol concentrations (Fig. 1a). The yield was highest when 60 % ethanol was used as the extraction solvent. The yield increased with the increasing ethanol solutions concentration when the concentration of ethanol was less than 60 %. However, the yield decreased within the range of 60 % to 100 % ethanol. Therefore, 60 % ethanol was used as the center point for the RSM experiment.
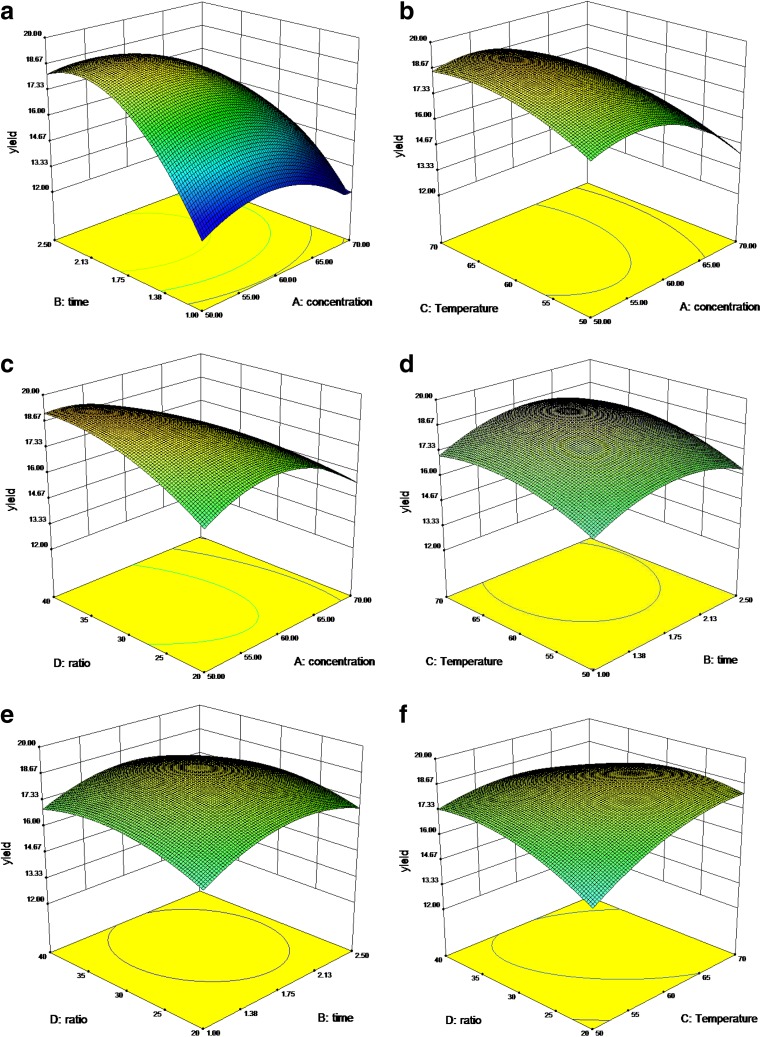
Fig. 1.
Response surface (3D) showing the effect of different extraction parameters (X1: ethanol concentration, %; X 2: extraction time, h; X3: extraction temperature, °C; X4: liquid–solid ratio, mL/g) added on the response
Effect of extraction time on the total flavonoid yield
The extract yield for the total flavonoids increased with the increasing extraction time of 0.5 h to 2.0 h (Fig. 1b). The yield peaked at 2.0 h before it significantly decreased. Therefore, the optimal extraction time was determined to be 2.0 h.
Effect of temperature on the total flavonoids yield
The extraction yield gradually rose with the increasing temperature, reached a maximum at 60 °C, and finally dropped at the range from 60 to 90 °C (Fig. 1c). However, much higher temperatures could cause activity loss, promote the degradation of thermosensitive compounds, and increase the solubility of impurities. Therefore, the optimal temperature was considered to be 60 °C.
Effect of the liquid–solid ratio on the total flavonoid yield
The liquid–solid ratio is an important variable. Flavonoids cannot be completely extracted if this ratio is too high, whereas the process costs would increase if the ratio is too low. The effect of varying the ratio of liquids to solids (20:1, 30:1, 40:1, 50:1, and 60:1) was investigated (Fig. 1d). The extraction yield was greatly increased when this ratio increased from 20:1 to 30:1 but subsequently decreased with higher liquid–solid ratios. Therefore, we chose a liquid–solid ratio of 30:1 as the center point for the RSM experiment.
Analysis of the model
The extraction yield of the total flavonoids from S. baicalensis was optimized with the RSM approach based on the single-factor experiment values. The experiments were randomized, as described in detail in Table 2. Given the multiple regression analysis of the experimental data, the coefficients of the independent variables produced the following second-order polynomial stepwise equation:
| 3 |
where X1, X2, X3, and X4 are the coded values of the ethanol concentration, extraction time, temperature, and the liquid–solid ratio, respectively.
Table 2.
Central composite design for independent variables and their response
| Runs | A (Ethanol concentration (%)) |
B (Time (h)) |
C (Temperture (°C)) |
D (Liquid–solid ratio (mL/g) |
Yield (mg/g) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 1 | 13.950 |
| 2 | 0 | 1 | 0 | −1 | 17.746 |
| 3 | 0 | 0 | 0 | 0 | 18.649 |
| 4 | 1 | 1 | 0 | 0 | 15.243 |
| 5 | 0 | 1 | −1 | 0 | 15.488 |
| 6 | −1 | 1 | 0 | 0 | 17.984 |
| 7 | 1 | −1 | 0 | 0 | 14.842 |
| 8 | 0 | 0 | −1 | −1 | 15.583 |
| 9 | 1 | 0 | 0 | −1 | 13.494 |
| 10 | 0 | 0 | 1 | 1 | 17.147 |
| 11 | 0 | 0 | 0 | 0 | 18.354 |
| 12 | 0 | −1 | 0 | 1 | 16.689 |
| 13 | 0 | 0 | 0 | 0 | 19.180 |
| 14 | −1 | 0 | −1 | 0 | 17.899 |
| 15 | −1 | 0 | 0 | −1 | 15.466 |
| 16 | −1 | 0 | 1 | 0 | 18.354 |
| 17 | 0 | 1 | 1 | 0 | 17.329 |
| 18 | 0 | −1 | 0 | −1 | 17.104 |
| 19 | 0 | 0 | 0 | 0 | 19.90 |
| 20 | −1 | 0 | 0 | 1 | 20.396 |
| 21 | 0 | −1 | −1 | 0 | 15.618 |
| 22 | 0 | −1 | 1 | 0 | 17.289 |
| 23 | 0 | 0 | −1 | 1 | 16.727 |
| 24 | −1 | −1 | 0 | 0 | 15.688 |
| 25 | 1 | 0 | 1 | 0 | 14.892 |
| 26 | 0 | 0 | 0 | 0 | 16.733 |
| 27 | 0 | 0 | 1 | −1 | 18.951 |
| 28 | 1 | 0 | −1 | 0 | 14.962 |
| 29 | 0 | 1 | 0 | 1 | 16.542 |
ANOVA was applied to evaluate the significance of the regression coefficients of the response surface quadratic polynomial model. If a model has a significant regression and a non-significant lack of fit, it is said to be well fitted to the experimental results. The lack of fit (p > 0.05) was not significant (Table 2). Thus, few unknown factors can affect the experiment results. The model with R2 > 0.75 was considered acceptable (Yang et al. 2010). The polynomial model (p < 0.05) and the correlation coefficient (R2 > 0.75) demonstrated that the regression model was suitable for the actual situation. A relationship between the flavonoid yield and the studied extraction conditions was revealed. Therefore, the obtained regression model could optimize and satisfactorily predict the conditions for maximum yield. A, A2, and B2 significantly affected the extraction yield, which demonstrated that the influence factors did not have a simple linear or quadratic relationship (Table 3). However, the analysis showed that the studied factors influence the total flavonoid yield in the following order: ethanol concentration > extraction temperature > liquid–solid ratio > extraction time.
Table 3.
Results of the ANOVA to the response surface quadratic model
| Source | Sum of squares | Degree of freedom | Mean square | F-value | P-value | Significant |
|---|---|---|---|---|---|---|
| Model | 65.0378 | 14 | 4.6456 | 3.2072 | 0.0185 | significant |
| A(Concentration) | 28.2256 | 1 | 28.2256 | 19.4867 | 0.0006 | * |
| B (Time) | 0.8019 | 1 | 0.8019 | 0.5536 | 0.4692 | |
| C (Temperature) | 4.9216 | 1 | 4.9216 | 3.3978 | 0.0866 | |
| D (Ratio) | 0.8045 | 1 | 0.8045 | 0.5554 | 0.4685 | |
| AB | 0.8978 | 1 | 0.8978 | 0.6198 | 0.4442 | |
| AC | 0.0689 | 1 | 0.0689 | 0.0476 | 0.8305 | |
| AD | 5.0042 | 1 | 5.0042 | 3.4548 | 0.0842 | |
| BC | 0.0072 | 1 | 0.0072 | 0.0050 | 0.9447 | |
| BD | 0.1556 | 1 | 0.1556 | 0.1075 | 0.7479 | |
| CD | 2.1727 | 1 | 2.1727 | 1.5000 | 0.2409 | |
| A2 | 16.8061 | 1 | 16.8061 | 11.6028 | 0.0043 | * |
| B2 | 7.2970 | 1 | 7.29704 | 5.0378 | 0.0415 | * |
| C2 | 3.4190 | 1 | 3.41903 | 2.3606 | 0.1467 | |
| D2 | 3.9617 | 1 | 3.9617 | 2.7351 | 0.1204 | |
| Residual | 20.2784 | 14 | 1.44846 | |||
| Lack of fit | 14.7102 | 10 | 1.47102 | 1.0567 | 0.5233 | not significant |
| Pure error | 5.5682 | 4 | 1.39206 | |||
| Cor total | 85.3162 | 28 |
R 2 = 0.7623, Adj. R 2 = 0.5246, Adeq Precision = 6.1282, CV = 7.15 %
a P < 0.01 highly significant; 0.01 < P < 0.05 significant; P > 0.05 not significant
Analysis of response surface
The extraction yield can also be predicted from the three-dimensional (3D) response surface and two-dimensional (2D) contour plots of the different variables influencing the flavonoid yield. The 3D response surface graphically revealed the sensitivity of the response value towards the change in the variable. The 2D contour plot illustrated the significant coefficients between the different variables. The independent variables were obtained by keeping the other two factors at center values (Figs. 1 and 2). The response surface plots illustrated the magnitude of the response values. The steeper plots implied that the response value had a greater effect on the extraction conditions. Otherwise, the observed influences were slight (Bezerra et al. 2008).
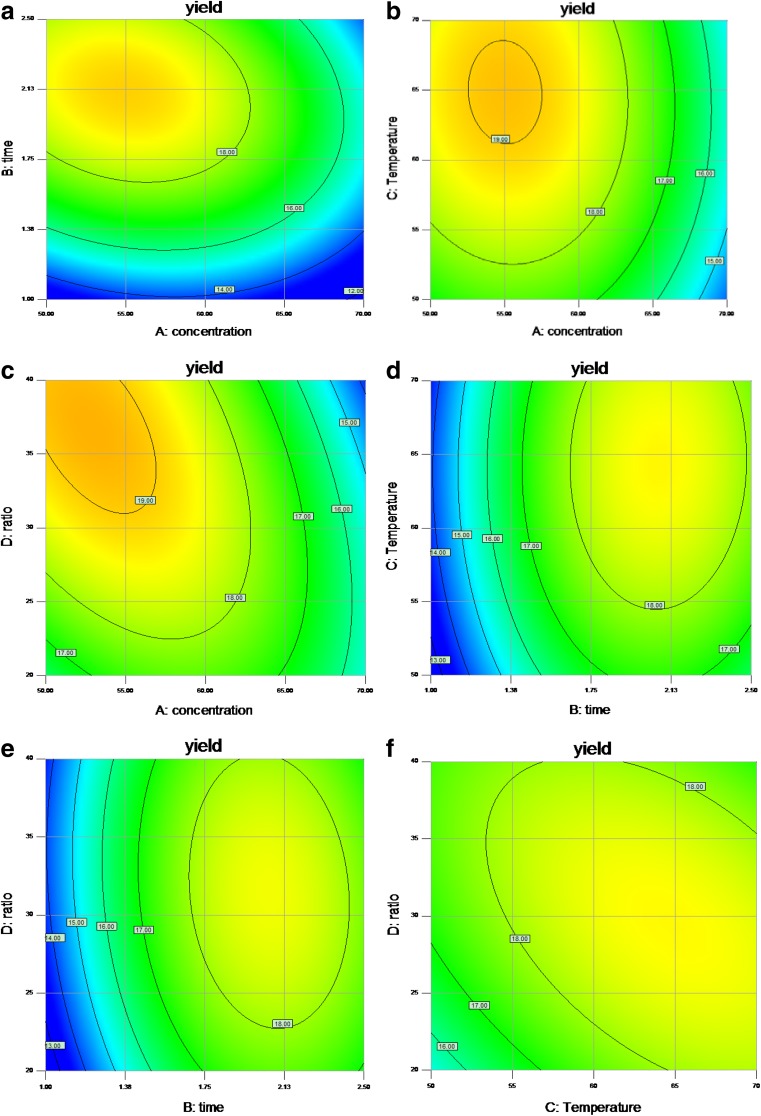
Fig. 2.
Contour plots (2D) showing the effect of different extraction parameters (X1: ethanol concentration, %; X 2: extraction time, h; X3: extraction temperature, °C; X4: liquid–solid ratio, mL/g) added on the response Y
The response surface and contour plots for the influence of the ethanol concentration and extraction time on the flavonoid yield are shown in Figs. 1a and 2a. The extraction time exhibited a significant effect on the flavonoid yield, whereas the effect of the ethanol concentration was weaker. The yield was increased when the ethanol concentration increased from 50 to 53 % and the extraction time increased from 1.5 to 2.12 h. However, prolonging the extraction time and further increasing the ethanol concentration appeared to be disadvantageous for the extract yield. Prolonging the extraction time may cause flavonoid decomposition and consequently decrease the yield. By contrast, increasing the ethanol concentration may change the solvent polarity and increase the amount of impurities. These results suggest that the ethanol concentration and the extraction time had a quadratic effect on the response, whereas the mutual interactions between the ethanol concentration and the extraction time were not significant.
The effects of the extraction temperature and the ethanol concentration on the flavonoid yield are shown in Figs. 1b and 2b. When the ethanol concentration was lower than 53 %, the yield was positively correlated to the increasing ethanol concentration. The results indicated that the highest yield could be produced when extraction was performed at 62 °C with 53 % ethanol.
The combined effect of the ethanol concentration and the liquid–solid ratio on the extraction yield is indicated by Figs. 1c and 2c. The extraction yield increased linearly when the ethanol concentration increased from 50 to 53 %, but the yield decreased when the ethanol concentration exceeded 53 %. The liquid–solid ratio had a less significant effect on the yield. The mutual interactions of the ethanol concentration with the liquid–solid ratio were similarly not significant.
Both the extraction time and the temperature exhibited weak effects on the flavonoid yield (Figs. 1d and 2d). The extraction yield increased when the temperature ranged from 50 to 62 °C, but the yield decreased when the temperature was higher than 62 °C. The interaction effect of the extraction time with the temperature on the flavonoid yield was not significant.
The effects of the extraction time and the liquid–solid ratio on the flavonoid yield are shown in Figs. 1e and 2e. A lower liquid–solid ratio produced lower yield. The yield increased when the liquid–solid ratio changed from 20 to 35, but decreased thereafter. The influence of the extraction time on yield was less significant than the liquid–solid ratio. The flavonoid yield was improved by prolonging the extraction time from 1.5 to 2.12 h, but it decreased thereafter. The interaction effect of the extraction time with the liquid–solid ratio and the temperature on the flavonoid yield was not significant.
The effects of the extraction temperature and the liquid–solid ratio are given in Figs. 1f and 2f. Lower temperatures induced lower yields. As the temperature rose from 50 to 62 °C, the yield was increased. However, the yield decreased when the temperature rose beyond 62 °C. The influence of the temperature on the yield was more significant than that of the liquid–solid ratio. The flavonoid yield was improved by prolonging the extraction time from 1.5 to 2.12 h; after which, the yield was decreased.
Defining priority factors
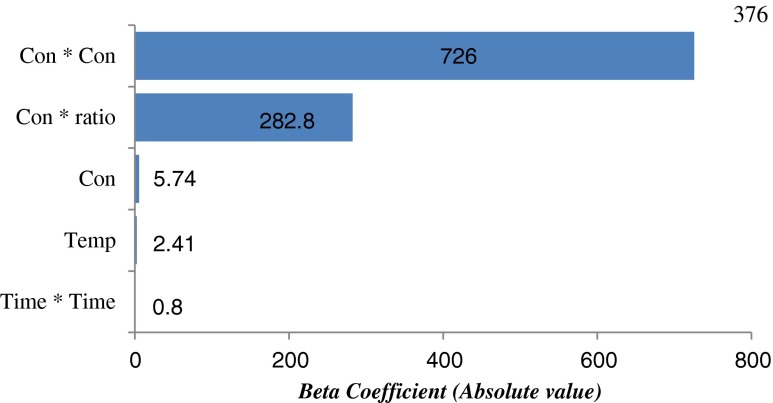
Priority factors were needed to be defined definitely to improve the yield of total flavonoids from S. baicalensis based on the established model. Pareto chart of the standardized coefficients corresponding to the independent variable and their interactions with statistical significance (p < 0.05) was applied for investigating the relative contribution (Fig. 3) (dos Santos et al. 2012; Wang et al. 2014). The result showed the effects of the independent variable and their interactions were ranked as A2 >> AD >> A > C > B2.
Fig. 3.
Analysis of Pareto chart of the standardized effects for the flavonoid yield
Optimization of extraction parameters
The selected variables were further reduced for optimization using Design-Expert. The software identified the following optimum conditions: ethanol concentration, 52.98 %; extraction time, 2.12 h; extraction temperature, 62.46 °C; and liquid–solid ratio, 35.23. Under the abovementioned conditions, the predicted extraction yield was 19.437 mg/g.
Conclusion
This study investigated the effects of the ethanol concentration, extraction time, temperature, and the liquid–solid ratio on the yield of flavonoid extraction from S. baicalensis. The extraction parameters were optimized using RSM and the second-order polynomial regression model. The yield was significantly increased under the optimized conditions.
Acknowledgments
This work received financial support from Science and Technology Planning Project of Guangdong Province (2011B020314011), Natural Science Foundation of Guangdong Province (S2011010004004), and Science and Technology innovation Project of Zhaoqing city (2012G25 and 2013F013).
References
- Baş D, Boyacı IH. Modeling and optimization I: usability of response surface methodology. J Food Eng. 2007;78:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Chang HH, Yi PL, Cheng CH, Lu CY, Hsiao YT, Tsai YF, Li CL, Chang FC. Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep–wake regulation. J Ethnopharmacol. 2011;135:359–368. doi: 10.1016/j.jep.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, Cheng VCC, Tsui WHW, Lee TSW, Peiris JSM, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPM (China Pharmacopoeia Committee) Pharmacopoeia of the People’s Republic of China, 2010 ed. Beijing: China Medical Science Press; 2010. [Google Scholar]
- dos Santos TC, Palma Gomes DP, Ferreira Bonomo RC, Franco M. Optimisation of solid state fermentation of potato peel for the production of cellulolytic enzymes. Food Chem. 2012;133:1299–1304. doi: 10.1016/j.foodchem.2011.11.115. [DOI] [Google Scholar]
- Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY. Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid β protein-induced neurotoxicity. J Agric Food Chem. 2004;52:4128–4132. doi: 10.1021/jf049953x. [DOI] [PubMed] [Google Scholar]
- Heo H, ShinY CW, Choi Y, Kim H, Kwon YK. Memory improvement in ibotenic acid induced model rats by extracts of Scutellaria baicalensis. J Ethnopharmacol. 2009;122:20–27. doi: 10.1016/j.jep.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Himeji M, Ohtsuki T, Fukazawa H, Tanaka M, Yazaki S, Ui S, Nishio K, Yamamoto H, Tasaka K, Mimura A. Difference of growth-inhibitory effect of Scutellaria baicalensis-producing flavonoid wogonin among human cancer cells and normal diploid cell. Cancer Lett. 2007;245:269–274. doi: 10.1016/j.canlet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Hui KM, Huen MSY, Wang HY, Zheng H, Sigel E, Baur R, Ren H, Li ZW, Wong JTF, Xue H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol. 2002;64:1415–1424. doi: 10.1016/S0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- Kang TH, Hong BN, Park C, Kim SY, Park R. Effect of baicalein from Scutellaria baicalensis on prevention of noise-induced hearing loss. Neurosci Lett. 2010;469:98–302. doi: 10.1016/j.neulet.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126:320–331. doi: 10.1016/j.jep.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sumiyoshi M. Effects of baicalein and wogonin isolated from Scutellaria baicalensis roots on skin damage in acute UVB-irradiated hairless mice. Eur J Pharmacol. 2011;66:124–132. doi: 10.1016/j.ejphar.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B. 2004;812:277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AMY, Ping YH, Chang GF, Wang JY, Chiu JH, Kuo CD, Chi CW. Neuroprotective effect of oral S/B remedy (Scutellaria baicalensis Georgi and Bupleurum scorzonerifolfium Willd) on iron-induced neurodegeneration in the nigrostriatal dopaminergic system of rat brain. J Ethnopharmacol. 2011;134:884–891. doi: 10.1016/j.jep.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Liu G, Ma J, Chen Y, Tian Q, Shen Y, Wang X, Chen B, Yao S. Investigation of flavonoid profile of Scutellaria bacalensis Georgi by high performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J Chromatogr A. 2009;1216:4809–4814. doi: 10.1016/j.chroma.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Liu G, Rajesh N, Wang X, Zhang M, Wu Q, Li S, Chen B, Yao S. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J Chromatogr B. 2011;879:1023–1028. doi: 10.1016/j.jchromb.2011.02.050. [DOI] [PubMed] [Google Scholar]
- Najafi NM, Tavakoli H, Abdollahzadeh Y, Alizadeh R. Comparison of ultrasound-assisted emulsification and dispersive liquid–liquid microextraction methods for the speciation of inorganic selenium in environmental water samples using low density extraction solvents. Anal Chim Acta. 2012;714:82–88. doi: 10.1016/j.aca.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Park KI, Park HS, Kang SR, Nagappan A, Lee DH, Kim JA, Han DY, Kim GS. Korean Scutellaria baicalensis water extract inhibits cell cycle G1/S transition by suppressing cyclin D1 expression and matrix-metalloproteinase-2 activity in human lung cancer cells. J Ethnopharmacol. 2011;133:634–641. doi: 10.1016/j.jep.2010.10.057. [DOI] [PubMed] [Google Scholar]
- Shang YZ, Miao H, Cheng JJ, Qi JM. Effects of amelioration of total flavonoids from stems and leaves of Scutellaria baicalensis georgi on cognitive deficits, neuronal damage and free radicals disorder induced by cerebral ischemia in rats. Biol Pharm Bull. 2006;29:805–810. doi: 10.1248/bpb.29.805. [DOI] [PubMed] [Google Scholar]
- Sheng J, Chen H, Shen L. Comparative study on selenium and amino acids content in leaves of planted and wild Scutellaria Baicalensis. Spectrosc Spectr Anal. 2009;29:211–213. [PubMed] [Google Scholar]
- Sheng ZL, Wan PF, Dong CL, Li YH. Optimization of total flavonoids content extracted from Flos Populi using response surface methodology. Ind Crop Prod. 2013;43:778–786. doi: 10.1016/j.indcrop.2012.08.020. [DOI] [Google Scholar]
- Sun Y, Bi S, Song D, Qiao C, Mu D, Zhang H. Study on the interaction mechanism between DNA and the main active components in Scutellaria baicalensis Georgi. Sensors Actuators B Chem. 2008;129:799–810. doi: 10.1016/j.snb.2007.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine. 2010;17:63–68. doi: 10.1016/j.phymed.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Y, Wei S, Yan Z. Application of response surface methodology to optimise supercritical carbon dioxide extraction of essential oil from Cyperus rotundus Linn. Food Chem. 2012;132:582–587. doi: 10.1016/j.foodchem.2011.10.075. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu Y, Chen G, Yue W, Liang Q, Wu Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason Sonochem. 2013;20:846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Zhang ZH, Li JB, Lin T, Pan YJ, Zhao Y. Modeling Vibrio parahaemolyticus inactivation by acidic electrolyzed water on cooked shrimp using response surface methodology. Food Control. 2014;36:273–279. doi: 10.1016/j.foodcont.2013.08.031. [DOI] [Google Scholar]
- Woźniak D, Lamer-Zarawska E, Matkowski A. Antimutagenic and antiradical properties of flavones from the roots of Scutellaria baicalensis georgi. Food Nahrung. 2004;48:9–12. doi: 10.1002/food.200200230. [DOI] [PubMed] [Google Scholar]
- Xu Q, Shen Y, Wang H, Zhang N, Xu S, Zhang L. Application of response surface methodology to optimise extraction of flavonoids from fructus sophorae. Food Chem. 2013;138:2122–2129. doi: 10.1016/j.foodchem.2012.11.099. [DOI] [PubMed] [Google Scholar]
- Yang L, Cao YL, Jiang JG, Lin QS, Chen J, Zhu L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J Sep Sci. 2010;33:1349–1355. doi: 10.1002/jssc.200900776. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Wang XY, Wang XR, Xu ZH, Liu Z, Ni Q, Chu XP, Qiu MF, Zhao AH, Jia W. Protective effect of flavonoids from Scutellaria baicalensis Georgi on cerebral ischemia injury. J Ethnopharmacol. 2006;108:355–360. doi: 10.1016/j.jep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li H, Gao Z, Gong G, Xu H. Effects of flavonoids extracted from Scutellaria baicalensis Georgi on hemin–nitrite–H2O2 induced liver injury. Eur J Pharmacol. 2006;536:192–199. doi: 10.1016/j.ejphar.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hirotani M, YoshiKawa T, Furuya T. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry. 1997;44:83–87. doi: 10.1016/S0031-9422(96)00443-8. [DOI] [Google Scholar]