Abstract
Effect of nisin on biochemical and microbial quality and shelf life of vacuum packaged rainbow trout (Oncorhynchus mykiss) during 16 days storage at 4 °C was investigated. According to the obtained results, nisin treated fish showed lower (p < 0.05) and acceptable biochemical (Peroxide value, thiobarbituric acid-index, pH, and total volatile base nitrogen) and bacteriological (total viable counts, psychrotrophic viable counts, and lactic acid bacteria) attributes up to 16 days storage at 4 °C compared with those treated without nisin. Furthermore, FAs composition analysis indicated that presence of the nisin preserved nutritional quality of fish lipid, so that nisin treated samples contained higher percentage (p > 0.05) of essential FAs such as eicosapentaenoic acid and docosahexaenoic acid. This study concluded that treatment of the vacuum packaged rainbow trout with nisin resulted in improvement of quality and extension of shelf life of the fish from 12 to 16 days at 4 °C.
Keywords: Rainbow trout, Nisin, Biochemical indices, Bacteriological indices, Fatty acids, Shelf life
Introduction
Rainbow trout (Oncorhynchus mykiss) belongs to the Salmonidae and is one of the main fish species farmed in Iran. The demand for rainbow trout in Iran and other country markets has grown significantly over the past decade and this could be due to its desirable characteristics (taste, aroma, white flesh) resulting in a high-quality product and nutritional value (Oraei et al. 2010).
Generally, various food preservation techniques have been used to improve the microbial safety and increase the shelf life of fish and shellfish, including freezing, chemical preservation, salting, and smoking (Anvari et al. 2012; Oraei et al. 2010). Between the mentioned techniques, salting and smoking are using to preserve up to 70 % of the total fish catch in developing countries (Anvari et al. 2012). However, previous researches demonstrated that these two traditional preservation methods lead to increased lipid oxidation (especially polyunsaturated fatty acids), caused from heating and brining during processing, and subsequently, decreasing nutritional value of the product (Anvari et al. 2012; Stolyhow et al. 2006). Therefore, there is a clear necessity for development of new technologies and effective preservation methods which permit shelf-life extension of seafood or fishery products.
In the recent years, the most of attentions on food preservation are associated with the presence of lactic acid bacteria (LAB) as a new natural preservative in the food products. LAB are the microorganisms that humans have used for many years to make variety of processed foods. Later it has been recognized that the preservation effect result from the antimicrobial action of bacteriocins as well as metabolites, such as hydrogen peroxide and lactic acid, produced by LAB (Caplice and Fitzgerls 1999). The bacteriocins produced by Gram positive bacteria like LAB are made up of small peptides, 3–6 KDa, in size (Nes et al. 1996). Nisin is the most extensively identified bacteriocin of antimicrobial proteins produced by LAB. Recent researches have been suggested that nisin is suitable biological food preservative because of its inhibitory effect on Gram positive bacteria and food borne pathogens (Suganthi et al. 2012). Furthermore, it is non-toxic, and can be quickly digested (Suganthi et al. 2012).
Nisin is the only bacteriocin to have found a widespread application in related food industries. For instance, in fishery industries, several studies have been done to characterize influence of this biopreservative agent (either alone or in combination with other preservatives) on improvement quality of fishes (Nykanen et al. 2000; Elotmani and Assobhei 2004; Langroudi et al. 2011; Ghomi et al. 2011), fish products (Raju et al. 2003), and shrimp (Shirazinejad et al. 2010). In this regard, some limited studies considered effect of the nisin on microbial and chemical quality of smoked or filleted rainbow trout (Nykanen et al. 2000; Kisla and Unluturk 2004). To the best of our knowledge, however, less information is available about nisin treatment of rainbow trout under other different processing conditions. Thus, the objective of this study was to investigate the effect of nisinon shelf life and fish quality of gutted rainbow trout under vacuum packaging at 4 °C, which is common condition for storage of the fish in retail centers and supermarkets in Iran and some other countries, by bacteriological and biochemical assessments and also, fatty acids composition analysis.
Materials and methods
Sample preparation
Thirty farmed rainbow trout (O. mykiss; average weight and length: 300 ± 5 g and 27 ± 1.4 cm, respectively) were provided from a fish farm in the north of Iran. The fish were manually gutted and washed by tap water at room temperature (20 ± 2 °C). A stock solution (0.2 % w/w) of nisin (Serva-Nurk, produced from Lactococcus lactis, Art number: 30413) was solved in 0.02 N HCl with pH 5.0 (immediately prior to adding to fish) and sprayed to the fish until whole surface and inside of sample became wet. The final concentration of nisin in the fish was approximately 100 μg/g (Hampikyan and Ugur 2007). In control group, samples were only sprayed with distilled water. All fish samples (treated without or with nisin) were packaged in barrier pouches (100 g per pouch) at room temperature (20 ± 2 °C). Pouches were vacuum-packaged and heat sealed using a BOSS model N84 mini-pack vacuum sealer (BOSS, Italy) and then stored under refrigeration (4 ± 0.5 °C) for 16 days. Biochemical, bacteriological, and fatty acids composition analyses (n = 3) were performed on days of 0, 4, 8, 12, and 16 days.
Biochemical assessments
Peroxide value (PV) and thiobarbituric acid-index (TBA-i) were determined according to Egan et al. (1997) method and results expressed as meq O2/kg muscle and mg malondialdehyde (MDA)/kg fish flesh, respectively. pH was determined according to the method of Masniyom et al. (2005) by using digital pH meter (Multiline P4 WTW, Singapore) at room temperature. Total volatile basic nitrogen (TVB-N) value was measured by the Goulas and Kontominas (2005) method and expressed as mg N/100 g of muscle.
Bacteriological assessments
Samples of 10 g of fish flesh (without skin) were taken, then mixed with 90 ml of 0.1 % peptone water, and finally, homogenized in a stomacher (Lab Blender FR 602, Fater Rizpardaz, Tehran, Iran) for 1 min. In all cases, serial dilutions from the microbial extracts were prepared in 0.1 % peptone water. Enumeration of total viable counts (TVC) and psychrotrophic viable counts (PVC) were determined on Tryptic soy agar (TSA, pH 7.3, Merck VM267758 425, Darmstadt, Germany) after incubation period of 2 days at 35 °C or 10 days at 4 °C, respectively (Arashisar et al. 2004; Kilinc et al. 2007). Lactic acid bacteria (LAB) were enumerated on de Man Rogosa and Sharp agar (MRS, pH 6.2, Quelab, 879843, Montreal, Canada) incubated anaerobically at 30 °C for 2 days (Hampikyan and Ugur 2007). Bacteriological data were transformed into logarithms of the number of colony-forming units program (log cfu/g).
Fatty acids composition assessment
For fatty acid (FA) composition analysis, the lipid was extracted from 1 g of minced samples with chloroform: methanol (2:1 v/v) (Bligh and Dyer 1959). The extracted lipids were methyl estered by BF3 and then fatty acid methyl esters (FAME) was recovered by n-hexane (Metcalfe et al. 1966). FAME samples were analyzed using a Philips PU 4400 gas chromatograph equipped with a fused silica capillary column BPX – 70 (25 m × 0.32 mm, film thickness 0.25 μm) and a FID detector. The carrier gas was helium. The run method was through a temperature gradient from 160 up to 230 °C with an increase rate of 1.5 °C/min. Initial times, final times, and total run time were 0, 15, and 50 min, respectively. FA identification was accomplished by comparison of sample peak retention times with those of FAMEs external and internal standards (PUFA NO. 1, #47033, Supelco). FA composition was calculated from the total identified FA area and the values are averages of at least three injections of each sample.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA). Normal distribution and homoscedasticity was evaluated using Colmogorov-Smirnov and Leven test, respectively. All statistical analysis were tested at the 0.05 level of probability, using the software SPSS 11.5 for windows. The relationship between chemical and microbiological analysis were evaluated by a simple linear correlation. Data were analyzed using the Statistica® 6.0 data analysis software system.
Results and discussion
Assessment of biochemical spoilage
Lipid oxidation indices
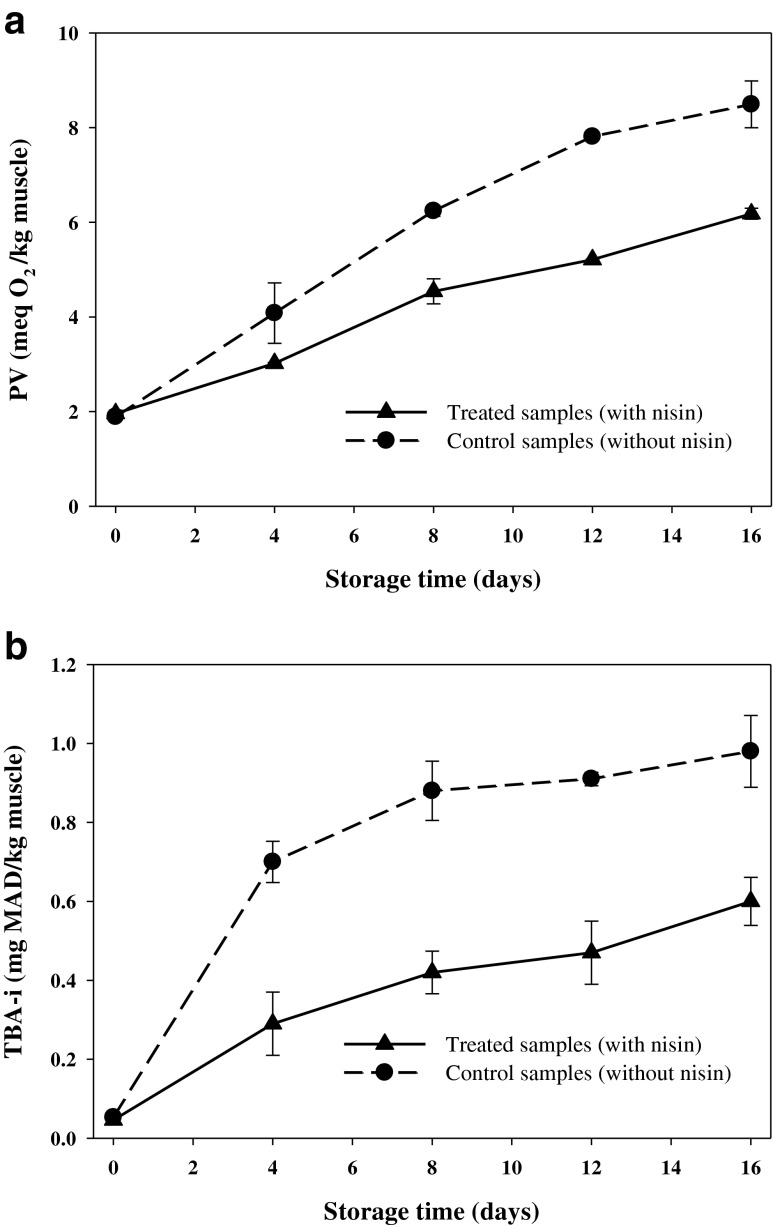
Lipid oxidative rancidity has been long recognized as a main cause of seafood and food spoilage. PV and TBA-i values are commonly used as indexes which representing the rate of lipid oxidation occurring in the seafoods (Rahman et al. 2009). Accordingly, changes in the PV values of the vacuum packaged rainbow trout treated with nisin or control samples (without nisin) during 16 days storage at 4 °C are shown in Fig. 1(a). The initial PV values (0 day) for the control and nisin-treated fish averaged 0.053 and 0.046 (meq O2/kg muscle), respectively. At this stage, which is named as slow oxidation period, some intracellular energy sources such as ATP offering electrons whereby presenting antioxidative properties and as soon as these energy sources are consumed, slow oxidation period ends up followed by a rapid increase in peroxide level (Layrisse and Matches 1984). Min et al. (2008) compared the susceptibility of meats from different animal species to lipid oxidation during storage at 4 °C. They concluded that the contents of myoglobin and the endogenous antioxidant capacities (ferric ion reducing capacity and radical scavenging activity) play the primary role for the differences in susceptibility of raw meats to lipid oxidation. These previous findings could explain significant enhancement (p < 0.05) of the PV values in both fish groups during storage time in our study. The presence of higher levels of peroxides in the control samples could have accelerated oxidation, as lipid hydroperoxides will be decomposed to reactive alkoxyl radicals in the presence of heme iron or trace metals. This result is in agreement with the Rezaei and Hosseini (2008) findings, who were considered lipid quality of farmed rainbow trout during chilled storage. In contrast, the nisin-treated rainbow trout exhibited lower lipid oxidation (p < 0.05) during whole preservation period, which might be attributed to metal ion chelating or scavenge of reactive oxygen species caused from antioxidative activity of the nisin (Lin and Yen 1999). At the end of storage time (16th day), the PV values of the both groups remained within the ranges (up to 10–20 meq O2/kg muscle) required for human consumption (Lakshmanan 2000). In addition to antioxidative activity of the nisin in the treated rainbow trout, limited oxygen availability in the vacuum packaged samples could give reason for low lipid oxidation during storage time in all fish, especially in the controls.
Fig. 1.
Changes in PV (a) and TBA-i (b) contents of vacuum packaged rainbow trout treated with or without nisin during 16 days storage at 4 °C
The TBA-i test measures secondary products of lipid oxidation, malondialdehyde and like products. The higher TBA-i value of seafood products has been associated with lower consumer acceptance for these products (Ke et al. 1984). Ke et al. (1984) proposed that TBA-i values for seafoods below 0.58 mg MDA/kg were perceived as not rancid; 0.58–1.51 mg MDA/kg slightly rancid, but acceptable; and above 1.51 mg MDA/kg were perceived as rancid. In the present study, despite of significant increase (p < 0.05) in the TBA-i values of the control and nisin-treated samples during preservation (Fig. 1(b)), the obtained values at 16th day, 0.98 and 0.60 mg MDA/kg for the control and treated rainbow trout, respectively, were in acceptable range for human consumption. Comparison between two groups revealed lower TBA-i values (p < 0.05) in the treated fish, which can be related to less initial lipid oxidation of the samples resulted from presence of the nisin. Almost similar results on TBA-i values were obtained by Seddigh Jasour et al. (2011), who considered effect of α-Tocopheryl Acetate, as an antioxidant agent, on lipid quality of filleted rainbow trout during 12 days storage at 4 °C.
Evaluation of pH
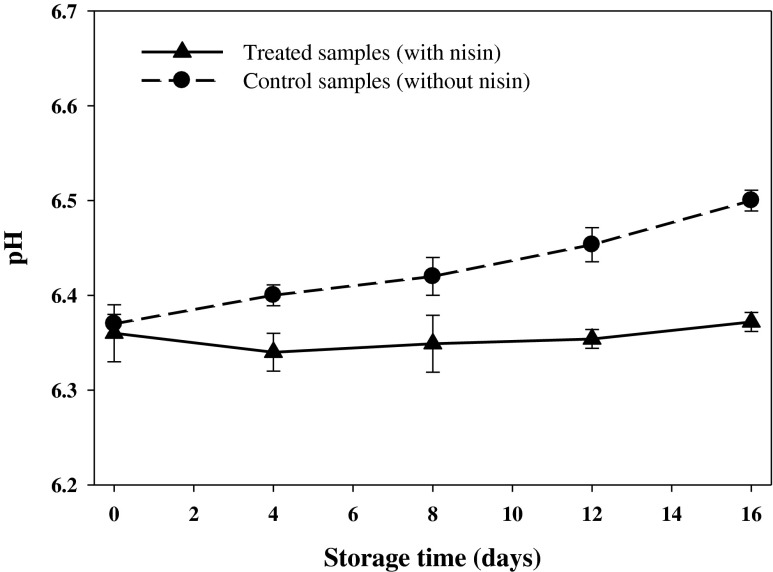
The pH is one of the most critical factors affecting microbial growth and spoilage of foods and seafoods. The pH of live fish muscle is close to the value 7.00, however, chilled fish pH can vary from 6.0 to 6.5 depending on fish species and other factors (Simeonidou et al. 1998). The pH of the rainbow trout muscle treated without or with nisin at day 0 of the storage at 4 °C were 6.37 and 6.36, respectively, which were in agreement with previous findings. The pH values increased to 6.50 (p > 0.05) by the end of the storage period for the control samples (Fig. 2), presumably due to production of trimethylamine and other volatile bases caused from the bacterial spoilage (Sallam et al. 2007). Comparison of the pH values did not reveal any significant difference (p > 0.05) between the two groups, however, lower values were observed in the treated samples, which could be related to inhibition of bacterial spoilage and consequently, less production of basic amines caused from antibacterial activity of the nisin. The slight increase (p > 0.05) in pH of the treated fish over the storage period could have resulted from buffering capacity of the rainbow trout muscle.
Fig. 2.
Changes in pH of vacuum packaged rainbow trout treated with or without nisin during 16 days storage at 4 °C
Evaluation of total volatile basic nitrogen (TVB-N)
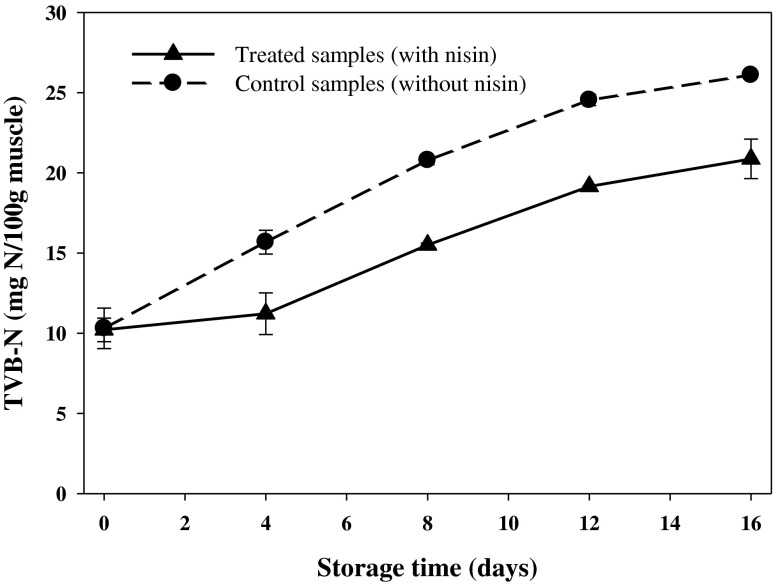
TVB-N is one of the most widely used methods to estimate the degree of decomposition of fish. In the present study, TVB-N content of all samples are depicted in Fig. 3. The initial TVB-N values for the control and treated rainbow trout were 10.31 and 10.21 mg N/100 g muscle, respectively, which were similar to the values reported by Gimenez et al. (2002) and Arashisar et al. (2004), who investigated shelf life of filleted rainbow trout packaged in vacuum and gas mixture conditions stored at 1 and 4 °C, respectively. In this study, the TVB-N content of muscle in the both groups of fish increased (p < 0.05) throughout the storage and reached to 26.10 and 20.87 mg N/100 g muscle for those treated without and with nisin, respectively, at day 16. This phenomenon probably caused from the deamination of amino-acids, formation of NH3, and other volatile amines during storage time (Arashisar et al. 2004). Given that no limit for acceptability of rainbow trout has been determined by Decision 95/149 (EU 1995), Gimenez et al. (2002) recommended a value of 25 mg N/100 g muscle as the highest acceptable level. In this study, all TVB-N values remained below this limit of acceptability throughout the entire storage period at 4 °C with the exception of control rainbow trout for which a value of 26.10 mg N/100 g muscle was obtained on day 16. Statistical analysis revealed significant differences (p < 0.05) on the obtained TVB-N values between the control and nisin-treated fish during storage (except day 0), probably due to higher bacterial decomposition of fish flesh in the controls. During storage, decomposition of nitrogenous compounds leads to an increase in pH in the fish flesh, which may be partially correlated to the production of alkaline compounds (Sallam et al. 2007). This was in line with our results, where enhancement of the TVB-N was followed by increase in the pH (Fig. 2).
Fig. 3.
Changes in TVB-N content of vacuum packaged rainbow trout treated with or without nisin during 16 days storage at 4 °C
Assessment of bacteriological spoilage
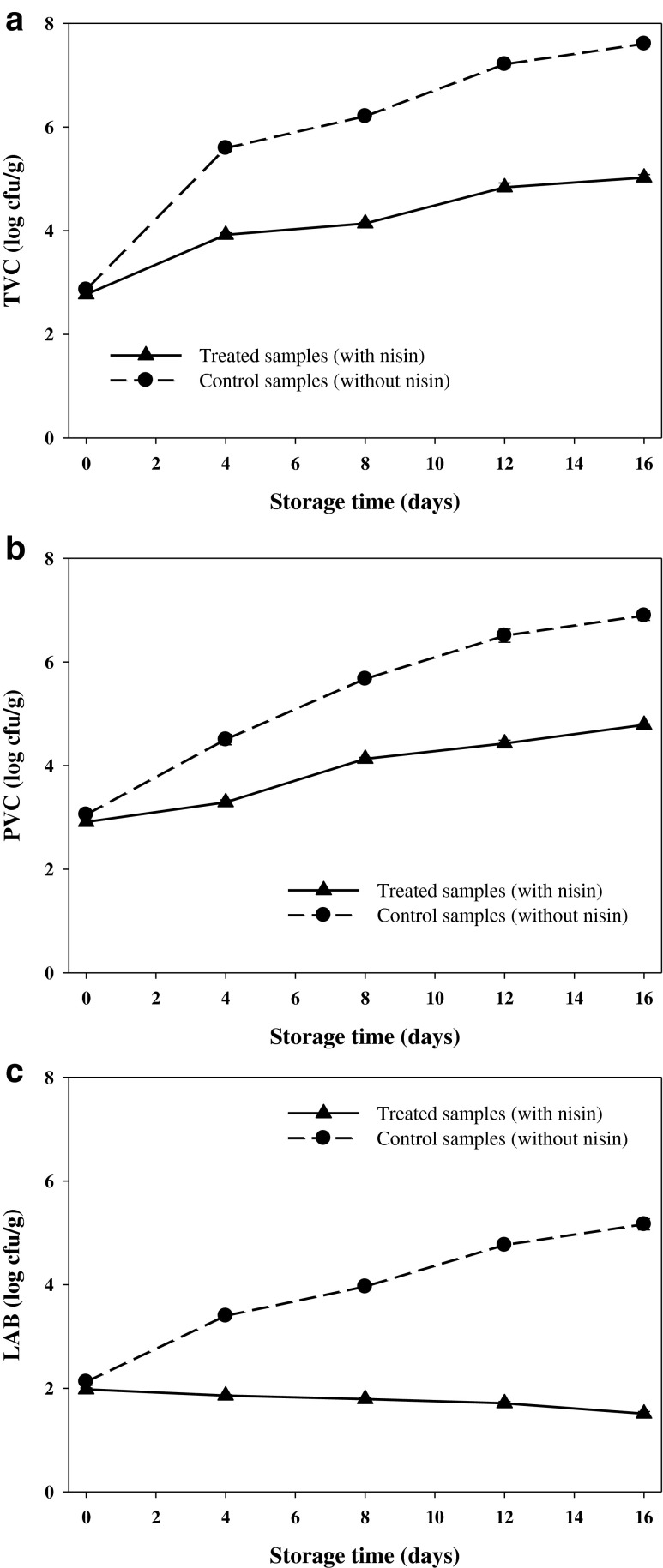
Microorganisms are the main cause of spoilage of most seafood products. However, only a few members of the microbial community, the specific spoilage organisms, lead to the offensive off-flavours related with seafood spoilage. During storage, the microbial flora changes owing to different abilities of the microorganisms to tolerate the preservation conditions (Gram and Dalgaard 2002). In the present research, changes in microflora of the vacuum packaged rainbow trout as a function of nisin-treatment during 16 days storage at 4 °C are shown in Fig. 4(a–c). Notwithstanding it is widely accepted that the initial microbial load of freshwater fish varies depending on water conditions and temperature, most of previous works on different freshwater fish species (tilapia, striped bass, rainbow trout, and silver perch) suggested bacterial counts of 102–106 cfu/g (Chytiri et al. 2004). In our work, initial total viable counts (TVC) of 2.86 log cfu/g for the control samples and 2.77 log cfu g−1 for the treated samples (Fig. 4(a)) were indicative of high fish quality. Moreover, the initial mesophilic counts (2.86 and 2.77 log cfu/g) found in the control or nisin-treated fish were in close agreement with results reported by Lyhs et al. (2001) for gravad vacuum packaged rainbow trout stored at 3 and 8 °C. The TVC of 7 log cfu/g has been used to determine the end of microbiological shelf-life of fresh fish (ICSMF 1986), while Mohan et al. (2008) recommend 3 × 106 cfu/g. In this study, an arbitrary value for TVC of 7 log cfu/g was taken for the upper acceptability limit of fresh rainbow trout. The TVC values in the control group increased (p < 0.05) with increase of the storage time and exceeded the upper limit on day 12. In compare, lower values (p < 0.05) were detected in the treated fish, which might be due to antibacterial activity of the nisin and subsequently, bacterial growth inhibition. Nisin is active against Gram positive organisms including bacterial spores, but it is not generally active against Gram negative bacteria, yeasts and fungi (Hampikyan and Ugur 2007). This previous finding could explain non-significant (p > 0.05) enhancement in the TVC values of the treated samples observed during the preservation time.
Fig. 4.
Changes in a TVC, b PVC, and c LAB counts of vacuum packaged rainbow trout treated with or without nisin during 16 days storage at 4 °C
Psychrotrophic viable counts (PVC) of all samples showed increasing trends with increase of storage time at 4 °C (Fig. 3(b)). The PVC of the control increased rapidly (p < 0.05) from an initial value of 3.05 to 6.89 log cfu/g within 16 days and was generally higher than the nisin-treated rainbow trout (p < 0.05). However, the PVC value obtained at day 16 for the control was below the limit of acceptability of 7 log cfu/g, presumably due to absence of oxygen under the vacuum packaging and production of CO2 in the rainbow trout flesh. Study of Lyhs et al. (2001) on microbiological quality and shelf-life of vacuum-packaged gravad rainbow trout stored at 3 and 8 °C revealed that when vacuum-packaging is used, due to bacterial metabolism, carbon dioxide levels in the gaseous phase gradually increase inside the package, thereby presumably influencing the growth of the bacteria. Moreover, it has been found that CO2 exhibit an inhibitory effect mainly against psychrotrophic aerobic Gram-negative bacteria (e.g. Pseudomonas and Shewanella spp), the main spoilage microflora of proteinaceous raw food such as fish when preserved under refrigeration (Gimenez et al. 2002). Masniyom et al. (2002) reported that CO2 enters into mass action equilibrium for enzymatic decarboxylation, leading to inhibition of the metabolic activity of the bacterial flora. Beside of inhibitory effect of carbon dioxide on bacterial growth, antibacterial activity of the nisin in the treated rainbow trout could describe lower PVC values in this group compare to the control. Basically, the antibacterial activity of nisin is based on pore formation in the cytoplasmic membrane of target bacteria (mainly psychrotrophic Gram-positive bacteria) through dissipation of the proton motive force, which resulting in an efflux of low molecular-weight solutes, such as amino acids and potassium ions (Hampikyan and Ugur 2007).
Lactic acid bacteria (LAB), as Gram-positive pathogens, are facultative anaerobic bacteria that can grow under both anaerobic and aerobic conditions (Mexis et al. 2009). Mexis et al. (2009) demonstrated that LAB were part of the natural microflora of fresh rainbow trout fillets. In the present work, initial LAB count (Fig. 4(c)) in the rainbow trout treated without and with nisin were 2.12 and 1.97 log cfu/g, respectively, which were in general agreement with the results of Lyhs et al. (2001) and Mexis et al. (2009), who considered microbial spoilage of slices and fillet of rainbow trout, respectively, stored under refrigeration condition. With increase of storage time, LAB counts in the control samples progressively increased (p < 0.05) and reached to 5.16 log cfu/g at day 16, while reverse trend was observed for those treated with the nisin, where LAB counts decreased (p > 0.05) and reached to 1.51 log cfu/g at the end day. As mentioned before, the bactericidal activity of nisin mostly influenced on Gram-positive bacteria, therefore, the observed trend in LAB counts of the treated samples could be expectable. Based on the results obtained from bacteriological analysis in the present study, it was found that nisin had important effect to preserve the microbial quality of the rainbow trout under vacuum packaging at 4 °C.
Assessment of fatty acid composition
Nowadays, it is demonstrated that fatty acids of fish lipids are the most useful for human health because they are rich in long chain ω-3 polyunsaturated fatty acid (PUFA), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Stolyhow et al. 2006). The fatty acids (FA) composition of fish tissue can be affected by diet, size, age, reproductive cycle, salinity, temperature, season and geographical location (Zlatanos and Laskaridis 2007). Moreover, composition and amount of lipids in fish and shellfish products can be influenced under different processing procedures and storage conditions (Anvari et al. 2012). Results obtained by analysis of fatty acids in muscle tissue lipids of the vacuum packaged rainbow trout treated without and with nisin are shown in Tables 1 and 2, respectively. Comparison between the main groups of the FAs in the both groups of samples revealed that monounsaturated fatty acid (MUFAs) had the highest percentage of the lipids followed by PUFAs and saturated fatty acid (SFAs) in order (Tables 1 and 2). This finding was in contrast with the results obtained by Blanchet et al. (2005) and Timberg et al. (2011), who investigated fatty acid composition of farmed rainbow trout. They reported that PUFAs had the highest proportion of FAs in the lipid composition of the farmed rainbow trout. These differences between our results and previous findings might be caused from composition of fish feed, environmental conditions of farming, fish age, and even feed additives. During 16 days storage at 4 °C, all of the vacuum packaged samples showed decreasing trends in percentage of the major FAs. Based on statistical analyses, MUFAs and PUFAs exhibited impressive reduction (p < 0.05) with increase of storage time, whereas no significant difference in SFAs values achieved during storage was revealed.
Table 1.
Fatty acid composition (% of total fatty acids) of vacuum packaged rainbow trout treated without nisin (control) during 16 days storage at 4 °Ca
| Fatty acids | Storage time (days) | ||||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | |
| C14 | 0.96 ± 0.08 | 0.93 ± 0.08 | 0.96 ± 0.06 | 1.10 ± 0.05 | 1.40 ± 0.15 |
| C15 | ndb | 0.13 ± 0.03 | 0.03 ± 0.00 | nd | nd |
| C16 | 12.63 ± 0.61 | 12.12 ± 0.18 | 12.76 ± 0.21 | 12.33 ± 0.50 | 12.03 ± 1.72 |
| C16:1 | 1.90 ± 0.10 | 1.06 ± 0.72 | 2.76 ± 0.33 | 2.53 ± 0.32 | 2.10 ± 0.26 |
| C18 | 4.06 ± 3.83 | 4.00 ± 0.02 | 2.29 ± 0.34 | 2.00 ± 0.23 | 1.93 ± 0.08 |
| C18:1 | 45.33 ± 1.35 | 44.60 ± 0.72 | 33.30 ± 5.77 | 30.26 ± 5.20 | 29.73 ± 3.62 |
| C18:2 ω6 | 19.90 ± 0.72 | 19.66 ± 0.38 | 17.76 ± 0.43 | 15.98 ± 1.60 | 15.16 ± 2.56 |
| C18:3 ω3 | 0.40 ± 0.20 | 0.50 ± 0.05 | 0.43 ± 0.03 | 0.60 ± 0.05 | 0.50 ± 0.05 |
| C20 | 3.25 ± 0.05 | 3.93 ± 0.21 | 3.83 ± 0.16 | 3.80 ± 0.30 | 3.86 ± 0.48 |
| C20:4 ω6 | 0.67 ± 0.20 | 0.53 ± 0.08 | 0.50 ± 0.10 | 0.43 ± 0.14 | 0.43 ± 0.04 |
| C22 | 0.40 ± 0.02 | 0.20 ± 0.02 | 0.33 ± 0.03 | 0.50 ± 0.05 | 0.56 ± 0.07 |
| C20:5 (EPA) | 0.70 ± 0.05 | 0.5 ± 0.33 | 0.66 ± 0.03 | 0.56 ± 0.02 | 0.54 ± 0.05 |
| C24 | 0.33 ± 0.07 | 0.26 ± 0.08 | 0.16 ± 0.03 | 0.30 ± 0.05 | 0.15 ± 0.06 |
| C22:5 ω3 | 0.36 ± 0.03 | 0.33 ± 0.03 | 0.36 ± 0.03 | 0.33 ± 0.03 | 0.40 ± 0.05 |
| C22:6 (DHA) | 5.23 ± 0.18 | 4.86 ± 0.20 | 4.60 ± 0.49 | 4.43 ± 0.72 | 4.23 ± 0.80 |
| SFA | 21.63 ± 4.79a | 21.57 ± 2.10b | 20.36 ± 3.21bc | 20.03 ± 4.00c | 19.93 ± 1.96c |
| MUFA | 47.23 ± 5.25a | 45.66 ± 1.11b | 36.06 ± 4.67c | 32.79 ± 3.51d | 31.83 ± 5.00d |
| PUFA | 27.26 ± 1.22a | 26.38 ± 3.98b | 24.31 ± 5.44c | 22.33 ± 5.21d | 21.26 ± 1.77e |
| EPA+DHA | 5.93 ± 1.20a | 5.36 ± 1.08b | 5.26 ± 0.78bc | 4.99 ± 0.94bc | 4.77 ± 0.84c |
| ω3/ω6 | 0.32 ± 0.04a | 0.30 ± 0.03a | 0.33 ± 0.01a | 0.36 ± 0.05a | 0.36 ± 0.08a |
| PUFA/SFA | 1.26 ± 0.30a | 1.22 ± 0.06a | 1.20 ± 0.07a | 1.11 ± 0.41a | 1.06 ± 0.32a |
aDifferent letters within each row represent a significant difference (p < 0.05)
b nd non detected
Table 2.
Fatty acid composition (% of total fatty acids) of vacuum packaged rainbow trout treated with nisin during 16 days storage at 4 °Ca
| Fatty acids | Storage time (days) | ||||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | |
| C14 | 1.53 ± 0.23 | 1.45 ± 0.02 | 1.46 ± 0.08 | 1.55 ± 0.28 | 1.05 ± 0.08 |
| C15 | ndb | nd | 0.13 ± 0.06 | nd | nd |
| C16 | 12.60 ± 2.47 | 12.55 ± 0.02 | 12.40 ± 0.36 | 13.68 ± 1.18 | 13.57 ± 0.11 |
| C16:1 | 3.13 ± 0.31 | 2.95 ± 0.00 | 2.29 ± 0.30 | 2.15 ± 0.25 | 2.75 ± 0.31 |
| C18 | 2.80 ± 1.36 | 2.72 ± 0.36 | 2.56 ± 0.08 | 1.10 ± 0.46 | 1.95 ± 0.89 |
| C18:1 | 46.42 ± 9.93 | 45.55 ± 0.54 | 44.66 ± 9.94 | 43.40 ± 3.23 | 41.65 ± 11.98 |
| C18:2 ω6 | 20.19 ± 1.93 | 20.05 ± 0.43 | 19.53 ± 5.59 | 19.45 ± 6.03 | 18.25 ± 0.14 |
| C18:3 ω3 | 1.00 ± 0.32 | 0.90 ± 0.05 | 1.53 ± 1.18 | 0.70 ± 0.00 | 1.00 ± 0.00 |
| C20 | 4.73 ± 0.83 | 4.70 ± 0.02 | 4.65 ± 1.06 | 4.30 ± 0.23 | 4.20 ± 0.05 |
| C20:4 ω6 | 0.46 ± 0.08 | 0.52 ± 0.04 | 0.56 ± 0.03 | 0.55 ± 0.02 | 0.40 ± 0.17 |
| C22 | 0.33 ± 0.03 | 0.22 ± 0.04 | 0.30 ± 0.00 | 0.05 ± 0.02 | 0.15 ± 0.08 |
| C20:5 (EPA) | 0.30 ± 0.17 | 0.52 ± 0.10 | 0.56 ± 0.03 | 0.50 ± 0.11 | 0.45 ± 0.08 |
| C24 | 0.46 ± 0.12 | 0.36 ± 0.06 | 0.30 ± 0.00 | nd | 0.15 ± 0.02 |
| C22:5 ω3 | 0.46 ± 0.03 | 0.42 ± 0.01 | 0.33 ± 0.06 | 0.40 ± 0.00 | 0.55 ± 0.02 |
| C22:6 (DHA) | 6.06 ± 1.01 | 5.70 ± 0.10 | 5.27 ± 0.18 | 5.13 ± 0.46 | 5.01 ± 0.11 |
| SFA | 22.35 ± 1.87a | 22.06 ± 0.24b | 21.80 ± 0.86b | 20.68 ± 0.28c | 21.07 ± 0.73c |
| MUFA | 49.55 ± 3.49a | 48.50 ± 1.15b | 46.95 ± 0.54c | 45.55 ± 1.31d | 44.40 ± 0.60e |
| PUFA | 28.47 ± 0.72a | 28.11 ± 0.46a | 27.78 ± 0.83a | 26.73 ± 2.70b | 25.66 ± 0.20c |
| EPA+DHA | 6.36 ± 1.03a | 6.22 ± 1.10a | 5.83 ± 0.20b | 5.63 ± 0.57b | 5.46 ± 0.20c |
| ω3/ω6 | 0.37 ± 0.22a | 0.36 ± 0.03a | 0.38 ± 0.97a | 0.33 ± 1.21a | 0.37 ± 0.09a |
| PUFA/SFA | 1.27 ± 0.08a | 1.27 ± 0.00a | 1.27 ± 0.19a | 1.29 ± 0.31a | 1.21 ± 0.04a |
aDifferent letters within each row represent a significant difference (p < 0.05)
b nd non detected
It has been demonstrated that DHA and EPA have preventive effects on human coronary artery disease (Osibona et al. 2009). In the present study, initial values (day 0) of EPA+DHA for the control and treated groups were 5.93 and 6.36 %, respectively. These two long-chain polyunsaturated fatty acids are susceptible compounds to oxidation and their content into lipid composition of fish products can be changed by processing and preservation conditions (e.g., temperature, presence of preservatives, and duration of storage) (Sampaio et al. 2006). This finding could explained reduction in EPA+DHA (p < 0.05) values during 16 days storage at 4 °C in our study. Moreover, larger values (p > 0.05) in the treated samples might be due to the antioxidant activity of the Nisin, which inhibited higher lipid oxidation in the muscle tissue of the rainbow trout (Fig. 1(a)).
The ω3/ω6 ratio has been suggested to be a useful indicator for comparing the relative nutritional values of fish oils (Cengiz et al. 2010). It was suggested that a ratio between 0.2 and 1.6 would constitute a healthy human diet (Cengiz et al. 2010). In this work, all of the samples had the ω3/ω6 ratio within the recommended ratio over whole of storage time. Statistical analyses revealed that storage time and treatment of rainbow trout without or with nisin did not have any significant (p > 0.05) influence on the ω3/ω6 values. A minimum value of PUFA/SFA ratio recommended is 0.45 (HMSO 1994), which is lower than those obtained from all of the samples studied during 16 days storage. This result indicated that the vacuum packaged rainbow trout treated without or with nisin were rich in PUFAs after 16 days storage at 4 °C. However, the obtained values for the treated fish were slightly higher than those of the control (p > 0.05).
Conclusion
The biochemical and bacteriological spoilage indices followed by fatty acids composition of the vacuum packaged rainbow trout during 16 days storage at 4 °C were analyzed, to understand influence of nisin, as a biopreservative agent, on fish quality and shelf life of the rainbow trout. The vacuum packaged samples were better preserved under treatment with nisin, maintaining acceptable biochemical attributes (PV, TBA-i, pH, and TVB-N) up to 16 day storage at 4 °C, whereas, those treated without nisin (control) showed significantly higher values (p < 0.05), so that TVB-N was exceeded the upper limit of acceptability for human consumption at day 16 of the storage. The results obtained from bacteriological indices (TVC, PVC, and LAB) analyses were in close agreement with the biochemical findings, where the controls exhibited higher microbial flora (p < 0.05) and their TVC value reached to above the recommended values at day 12. A comparison of fatty acids composition between two groups of samples during whole of storage time demonstrated that the nisin-treated fish were contained higher percentage of essential fatty acids (EPA+DHA). Moreover, larger values of ω3/ω6 and PUFA/SFA in the treated rainbow trout confirmed their higher nutritional value than the controls. Totally, as the best result in the use of the nisin, it can be expressed that the shelf life of the vacuum packaged rainbow trout treated with nisin could be extended to 16 days compared with 12 days for those treated without nisin, which presumably attributed to bactericidal and antioxidant activity of the biopreservative.
References
- Anvari M, Rezaei M, Kim SM (2013) Effects of previous gutting on biochemical changes and profile of long-chain polyunsaturated fatty Acids in cold-smoked kutum (Rutilus frisii kutum) stored at room temperature (25 ± 2 C). J Food Biochem 37:742–747
- Arashisar S, Hisar O, Kaya M, Yanik T. Effect of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorynchus mykiss) fillets. Int J Food Microbiol. 2004;97:209–214. doi: 10.1016/j.ijfoodmicro.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Lucas M, Julienc P, Morind R, Gingrasa S, Dewailly É. Fatty acid composition of wild and farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) Lipids. 2005;40:529–531. doi: 10.1007/s11745-005-1414-0. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. C J Biochem Phys. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Caplice E, Fitzgerls GF. Food fermentation role of microorganism in food production and preservation. Int J Food Microbiol. 1999;50(2):131–149. doi: 10.1016/S0168-1605(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Cengiz Eİ, Ünlü E, Başhan GR. Fatty acid composition of total lipids in muscle tissues of nine freshwater fish from the River Tigris (Turkey) Turk J Biol. 2010;34:433–438. [Google Scholar]
- Chytiri S, Chouliara I, Savvaidis IN, Kontominas MG. Microbiological, chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol. 2004;21:157–165. doi: 10.1016/S0740-0020(03)00059-5. [DOI] [Google Scholar]
- Egan H, Kirk RS, Sawyer R. Pearson’s chemical analysis of food. 9. UK: Longman Scientific and Technical; 1997. pp. 609–634. [Google Scholar]
- Elotmani F, Assobhei O. In vitro inhibition of microbial flora of fish by nisin and lactoperoxidase system. Lett Appl Microbiol. 2004;38:60–65. doi: 10.1046/j.1472-765X.2003.01441.x. [DOI] [PubMed] [Google Scholar]
- EU Decision 95 ⁄ 149 ⁄ EC. Total volatile basic nitrogen TVBN limit values for certain categories of fishery products and specifying the analysis methods to be used. Off J. 1995;L097:84–87. [Google Scholar]
- Ghomi MR, Nikoo M, Heshmatipour Z, Amir jannati A, Ovissipour M, Hashemi M, Faghani Langroudi H, Hasandoost M, Jadiddokhani D. Effects of sodium acetate and nisin on microbial and chemical changes and fatty acid composition of grass carp Ctenopharyngodon idella during refrigeration storage. J Food Saf. 2011;31:169–175. doi: 10.1111/j.1745-4565.2010.00281.x. [DOI] [Google Scholar]
- Gimenez B, Roncales P, Beltran J. Modified atmosphere packaging of filleted rainbow trout. J Sci Food Agric. 2002;82:1154–1159. doi: 10.1002/jsfa.1136. [DOI] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93:511–520. doi: 10.1016/j.foodchem.2004.09.040. [DOI] [Google Scholar]
- Gram L, Dalgaard D. Fish spoilage bacteria – problems and solutions. Curr Opin Biotechnol. 2002;13:262–266. doi: 10.1016/S0958-1669(02)00309-9. [DOI] [PubMed] [Google Scholar]
- Hampikyan H, Ugur M. The effect of nisin on L. monocytogenes in Turkish fermented sausages (sucuks) Meat Sci. 2007;76:327–332. doi: 10.1016/j.meatsci.2006.11.014. [DOI] [PubMed] [Google Scholar]
- HMSO . Nutritional aspects of cardiovascular disease (report on health and social subjects’ No. 46) London: HMSO; 1994. [PubMed] [Google Scholar]
- ICSMF . Microorganisms in Food 2, Sampling of microbiological analysis. Principle and specific applications. 2. Oxford: Blackwell Science; 1986. [DOI] [PubMed] [Google Scholar]
- Ke PJ, Cervantes E, Robles-Martinez C. Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation spectrophotometric method. J Sci Food Agric. 1984;35:1248–1254. doi: 10.1002/jsfa.2740351117. [DOI] [Google Scholar]
- Kilinc B, Cakli S, Dincer T, Cadun A. Effects of phosphates treatment on the quality of frozen–thawed fish species. J Muscle Foods. 2007;20(4):377–391. doi: 10.1111/j.1745-4573.2009.00154.x. [DOI] [Google Scholar]
- Kisla D, Unluturk A. Microbial shelf life of rainbow trout fillets treated with lactic culture and lactic acid. Adv Food Sci. 2004;26:17–20. [Google Scholar]
- Lakshmanan PT. In: Quality assurance in seafood processing. Lyer TSG, Kandoran MK, Thomas M, Mathew PT, editors. Cochin: Society Fisher Techno (Indian); 2000. [Google Scholar]
- Langroudi HF, Soltani M, Kamali A, Ghomi MR, Hoseini SE, Benjakul S, Heshmatipour Z. Effect of Listeria monocytogenes inoculation, sodium acetate and nisin on microbiological and chemical quality of grass carp Ctenopharyngodon idella during refrigeration storage. Afr J Biotechnol. 2011;10:8484–8490. [Google Scholar]
- Layrisse ME, Matches JR. Microbiological and chemical changes of spotted shrimp (Pandulus platyceros) stored under modified atmospheres. J Food Protec. 1984;47:453–457. doi: 10.4315/0362-028X-47.6.453. [DOI] [PubMed] [Google Scholar]
- Lin NY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47:1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- Lyhs U, Lahtinen J, Fredriksson-Ahomaa M, Hyytia-Trees E, Elfing K, Korkeala H. Microbiological quality and shelf-life of vacuum-packaged ‘gravad’ rainbow trout stored at 3 and 8 °C. Int J Food Microbiol. 2001;70:221–230. doi: 10.1016/S0168-1605(01)00548-7. [DOI] [PubMed] [Google Scholar]
- Masniyom P, Benjakul S, Visessanguan W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J Sci Food Agric. 2002;82:873–880. doi: 10.1002/jsfa.1108. [DOI] [Google Scholar]
- Masniyom P, Soottawat B, Visessanguan W. Combination effect of phosphate and modified atmosphere on quality and shelf-life extension of refrigerated seabass slices. JFST. 2005;38:745–756. [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR (1996) Rapid preparation of fatty acids esters from lipids for gas chromatographic analysis. Analytical Chem 38:524–535
- Mexis SF, Chouliara E, Kontominas MG. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009;26:598–605. doi: 10.1016/j.fm.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Min B, Nam KC, Cordray J, Ahn DU. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. JFS. 2008;73(6):439–446. doi: 10.1111/j.1750-3841.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Mohan CO, Ravishankar CN, Srinivasagopal K. Effect of O2 scavenger on the shelf-life of catfish (Pangasius sutchi) steaks during chilled storage. J Sci Food Agric. 2008;88:442–448. doi: 10.1002/jsfa.3105. [DOI] [Google Scholar]
- Nes IF, Bao Diep D, Havarstein LS, Brurberg MB, Eijsink V, Holo H. Biosynthesis of bacteriocins of lactic acid bacteria. A Van Leeuw. 1996;70:113–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- Nykanen A, Weckman K, Lapvetelainen A. Synergistic inhibition of Listeria monocytogenes on cold-smoked rainbow trout by nisin and sodium lactate. Int J Food Microbiol. 2000;61:63–72. doi: 10.1016/S0168-1605(00)00368-8. [DOI] [PubMed] [Google Scholar]
- Oraei M, Motalebi AA, Hoseini E, Javan S. Effect of Gamma irradiation and frozen storage on microbial quality of Rainbow trout (Oncorhynchus mykiss) fillet. IJFS. 2010;10(1):75–84. [Google Scholar]
- Osibona AO, Kusemiju K, Akande GR. Fatty acid composition and amino acid profile of two freshwater species, African catfish (Clarias gariepinus) and tilapia (Tilapia zillii) AJFAND. 2009;9:608–621. [Google Scholar]
- Rahman MS, Al-Belushi RM, Guizani N, Al-Saidi GS, Soussi B. Fat oxidation in freeze-dried grouper during storage at different temperatures and moisture contents. Food Chem. 2009;114:1257–1264. doi: 10.1016/j.foodchem.2008.11.002. [DOI] [Google Scholar]
- Raju CV, Shamsunder BA, Udupa KS. The use of nisin as a preservative in fish sausage stored at ambient temperature (28 ± 2 °C) and refrigerated (6 ± 2 °C) temperature. Intl J Food Sci Technol. 2003;38:171–185. doi: 10.1046/j.1365-2621.2003.00663.x. [DOI] [Google Scholar]
- Rezaei M, Hoseeini SF. Quality assessment of farmed rainbow trout (oncorhynchus mykiss) during chilled storage. J Food Sci. 2008;73:93–96. doi: 10.1111/j.1750-3841.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Sallam KI, Ahmed AM, Elgazzar MM, Eldaly EA. Chemical quality and sensory attributes of marinated Pacific saury (Cololabis saira) during vacuum-packaged storage at 4 °C. Food Chem. 2007;102:1061–1070. doi: 10.1016/j.foodchem.2006.06.044. [DOI] [Google Scholar]
- Sampaio GR, Bastos DHM, Soares RAM, Queiroz YS, Torres EAFS. Fatty acids and cholesterol oxidation in salted and dried shrimp. Food Chem. 2006;95:344–351. doi: 10.1016/j.foodchem.2005.02.030. [DOI] [Google Scholar]
- Seddigh Jasour M, Zakipour Rahimabadi E, Ehsani A, Rahnama M, Arshadi A. Effects of refrigerated storage on fillet lipid quality of rainbow trout (Oncorhynchus mykiss) supplemented by α-tocopheryl acetate through diet and direct addition after slaughtering. J Food Process Technol. 2011;2(5):1–5. [Google Scholar]
- Shirazinejad AR, Noryati I, Rosma A, Darah I. Inhibitory effect of lactic acid and nisin on bacterial spoilage of chilled shrimp. World Acad Sci Eng Technol. 2010;65:163–167. [Google Scholar]
- Simeonidou S, Govaris K, Vareltzis K. Quality assessment of seven Mediterranean fish species during storage on ice. Food Res Int. 1998;30(7):479–484. doi: 10.1016/S0963-9969(98)00008-8. [DOI] [Google Scholar]
- Stolyhow A, Kolodziejska I, Sikorski ZE. Long chain polyunsaturated fatty acids in smoked Atlantic mackerel and Baltic sprats. Food Chem. 2006;94:589–595. doi: 10.1016/j.foodchem.2004.11.050. [DOI] [Google Scholar]
- Suganthi V, Selvarajan E, Subathradevi C, Mohanasrinivasan V. Lantibiotic nisin: natural preservative from Lactococcus lactis. IRJP. 2012;3(1):13–19. [Google Scholar]
- Timberg L, Kuldjärv R, Koppel K, Paalme T. Rainbow trout composition and fatty acid content in Estonia. Agron Res. 2011;9:495–500. [Google Scholar]
- Zlatanos S, Laskaridis K. Seasonal variation in the fatty acid composition of three Mediterranean fish-sardine (Sardina pilchardus), anchovy (Engraulis encrasicholus) and picarel (Spicara smaris) Food Chem. 2007;103:725–728. doi: 10.1016/j.foodchem.2006.09.013. [DOI] [Google Scholar]