Abstract
Annona muricata is a naturally occurring edible plant with wide array of therapeutic potentials. In India, it has a long history of traditional use in treating various ailments. The present investigation was carried out to characterize the phytochemicals present in the methanolic and aqueous leaf extracts of A. muricata, followed by validation of its radical scavenging and DNA protection activities. The extracts were also analyzed for its total phenolic contents and subjected to HPLC analysis to determine its active metabolites. The radical scavenging activities were premeditated by various complementary assays (DRSA, FRAP and HRSA). Further, its DNA protection efficacy against H2O2 induced toxicity was evaluated using pBR322 plasmid DNA. The results revealed that the extracts were highly rich in various phytochemicals including luteolin, homoorientin, tangeretin, quercetin, daidzein, epicatechin gallate, emodin and coumaric acid. Both the extracts showed significant (p < 0.05) radical scavenging activities, while methanolic extract demonstrated improved protection against H2O2-induced DNA damage when compared to aqueous extract. A strong positive correlation was observed for the estimated total phenolic contents and radical scavenging potentials of the extracts. Further HPLC analysis of the phyto-constituents of the extracts provides a sound scientific basis for compound isolation.
Keywords: Annona muricata, ROS, DNA protection, HPLC, TPC
Introduction
Natural products being a fertile source of therapeutic agents for the pharmaceutical industry have provided effective metabolites for disease treatment and management. Large amount of research is being carried out to study various ethno-based compounds in curing both infectious and non-infectious diseases. The drug discovery from plant resources is an area pertinent to complementary and alternative medicine which provides a basis for isolation of individual and potentially effective bioactive compounds (Rajkumar et al. 2012). Out of the 250,000–500,000 plant species on the earth, only a small percentage has been studied chemically and pharmacologically for their potent therapeutic effects (Borris 1996).
A great deal of literature reports are available to demonstrate that the plant products are highly rich sources of a variety of biologically active compounds with antioxidant potentials which can mitigate excessive ROS (reactive oxygen species), generated by normal physiological processes and various exogenous factors (Craig 1999; Gunjan et al. 2009). These excessive increases of ROS can lead to oxidative stress, that can cause tissue damage by means of DNA, protein and lipid damage and hence there is a necessity to take dietary rich antioxidant compounds that are needed in assisting the body to neutralize these free radicals (Nilima and Hande 2011). Many of the plant compounds have already been reported to inhibit the toxic effects of increasing ROS levels in the cellular components (Rajdeep et al. 2007; Gunjan et al. 2010). Hence, the screening of pharmacologically bioactive compounds to mitigate the effects of excess ROS continues to search for a novel compound which possesses better activity with comparatively lesser side effects.
Annona species commonly known as ‘Custard-Apple’ belongs to the family Annonaceae and was cultivated in many tropical countries all over the world, for its edible fruits. Among these, Annona muricata L. (Soursop or Graviola) is a naturally occurring plant seen in Central America and in Southern part of India, traditionally used to treat various ailments. Fruits and fruit juice of A. muricata were taken internally to treat worms and parasites, fever, to increase mother’s milk after child birth and as an astringent for diarrhoea and dysentery (Baskar et al. 2007). Apart from these, it has been used in many tropical African countries for an array of human ailments, especially for parasitic infections. In India, the root, bark and leaf of this plant was being used for centuries, as anthelmintic and antiphlogistic agents, while its flowers and fruit pods were used as remedies for catarrh and the unripe fruit of the plant is an astringent, and was used in the treatment of intestinal atony and for scurvy (Watt and Breyer-Brandwijk 1962). The leaves of the plant are found to be anti-spasmodoic, hypotensive and are rich in annonaceous acetogenins (Yuan et al. 2003). The n-butanolic leaf extracts of this plant was also reported to protect normal cells and selectively destroy cancer cells (Cijo et al. 2012). Further, an in vivo study has demonstrated the protective effects of aqueous extract of A. muricata in diabetic-induced rats (Adewole and Ojewole 2009).
Being such a prominent herbal resource, the active metabolites present in methanolic or aqueous extracts of A. muricata, to the best of our knowledge, have not been studied so far. Hence, the present study was carried out to analyse the antioxidant, DNA protective efficacy and various bio-active compounds present in these extracts by means of phytochemical and HPLC analysis.
Materials and methods
Chemicals and reagents
Thiobarbituric acid (TBA), gallic acid, ascorbic acid, trichloroacetic acid (TCA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Dulbecco’s phosphate buffered saline (PBS) (Ca+2/Mg+2 free) and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Himedia Laboratories Pvt. Ltd. (India). Folin and Ciocalteau’s phenol reagent was procured from Sisco Research Laboratories Pvt. Ltd., Mumbai, India. pBR322 plasmid DNA was purchased from Medox Biotech India Pvt. Ltd. (India). The remaining chemicals and solvents used were of standard analytical and HPLC grade respectively.
Plant material: collection, processing and extraction
A. muricata (leaves) was collected in the month of October, 2010 from Teeose nursery, Thrichur District, Kerala, India, and identified by Dr. E. M. Muralidharan, Scientist E-II, Kerala Forest Research Institute, Peechi, Kerala, India. Voucher specimen is maintained at our laboratory for future references (VIT/SBST/CCL/2010/October/08). The collected healthy leaves were screened for contamination and shade dried after thorough washing. The leaves were then grinded in a mechanical mixer-grinder and the powder was used for extraction.
The plant leaf powder was extracted in a Soxhlet apparatus using methanol and water as solvents [sample (g): methanolic/aqueous) = 1:6 ratio]. The crude extracts obtained were concentrated at 40 °C under reduced pressure (72 mbar) with a Rotavapor R-215 (BUCHI Labortechnik AG, Switzerland) to yield dry extracts. Concentrated extracts were then stored in a vacuum desiccator at room temperature until further use.
Estimation of bioactive compounds
Phytochemical screening
Semi-quantitative phytochemical screening of the extracts were carried out using the protocols described by Treas and Evans (1989) with minor modifications. The extracts were analysed for the presence of flavonoids, saponins, terpenoids, reducing sugars, cardiac glycosides, steroids, tannins, phlobatannins, anthraquinones and oil.
Quantification of total phenolics
Total phenolic content (TPC) of the extracts were analysed using the Folin-Ciocalteau reagent method described by Rajkumar et al. (2011). To 50 μl of each extract concentrations (25, 50, 100, 200 and 400 μg), 2.5 ml of Folin-Ciocalteau reagent (1/10 dilution) and 2 ml of 7.5 % Na2CO3 (w/v) solution were added and incubated at 45 °C for 15 min and the absorbance was read at 765 nm using a Cary 50 UV–Vis spectrophotometer (Varian, Inc., CA, USA) using Na2CO3 solution (2 ml of 7.5 % Na2CO3 in 2.55 ml of distilled water) as blank. Gallic acid was used as a standard, and the results were expressed as GAE (Gallic acid equivalence) in μg.
Estimation of radical scavenging potentials
DPPH• radical scavenging assay (DRSA)
The DPPH• radical scavenging assay was performed as described by Brand-Williams et al. (1995) with few modifications. Various concentrations of the extracts (25, 50, 100, 200 and 400 μg) dissolved in methanol and aqueous solvents were added with 3 ml of DPPH• solution (0.1 mM in ethanol) and mixed vigorously. The mixture was incubated in dark at room temperature for 30 min and the absorbance was recorded at 517 nm using a Cary 50 UV–Vis spectrophotometer (Varian, Inc., CA, USA). Ascorbic acid (expressed in μg) was used as positive control. The level of percentage scavenging of DPPH• by the extracts were calculated following the formula:
Ferric reducing antioxidant property (FRAP)
FRAP assay was done according to the protocol of Benzie and Strain (1996) with some modifications. The stock solutions prepared were 300 mM acetate buffer (3.1 g C2H3NaO2.3H2O and 16.8 ml C2H4O2; pH 3.6), TPTZ solution (10 mM TPTZ in 40 mM HCl) and 20 mM FeCl3.6H2O solution. Working FRAP solution was prepared freshly by mixing 25 ml of acetate buffer, 2.5 ml TPTZ solution and 2.5 ml of FeCl3.6H2O solution. The mixture is then warmed at 37 °C. 150 μl of individual extract solution (containing 25, 50, 100, 200 and 400 μg respectively) were mixed with 2.85 ml of FRAP solution and incubated in dark for 30 min. Absorbance was read at 593 nm. Percentage Fe3+ reduction (to Fe2+) were calculated by a FeSO4 standard calibration curve. Percentage scavenging was also evaluated in ascorbic acid equivalence (AAE) in μg.
Hydroxyl radical scavenging Activity (HRSA)
The method of Klein et al. (1981) was employed for estimating hydroxyl radical scavenging activity of the extracts. Different concentrations of the extracts (25, 50, 100, 200 and 400 μg) were taken in individual test tubes containing 1 ml of iron-EDTA solution (0.13 % ferrous ammonium sulfate and 0.26 % EDTA), 0.5 ml of 0.018 % EDTA and 1 ml of 0.85 % (v/v) DMSO (in 0.1 M phosphate buffer, pH 7.4). To these test tubes, 0.5 ml of 0.22 % (w/v) ascorbic acid was and incubated in water bath at 85 ºC for 15 min. Post incubation, 1 ml of ice-cold trichloroacetic acid (17.5 % w/v) was added in each tube. 3 ml of Nash reagent (7.5 g of ammonium acetate, 300 μl glacial acetic acid and 200 μl acetyl acetone were mixed and made up to 100 ml with distilled water) was added to all the tubes and again incubated at room temperature for 15 min. Absorbance was measured at 412 nm and the results were expressed as ascorbic acid equivalence (AAE). Hydroxyl radical scavenging activity (%HRSA) was calculated by the following formula:
DNA damage protective activity
The potentials of the extracts to prevent DNA damage was tested by photolysing pBR322 plasmid DNA via UV radiation in the presence of H2O2 (Russo et al. 2001) and observing through agarose gel electrophoresis after loading the irradiated DNA. 1 μl of pBR322 DNA (200 μg/ml) and 50 μg of the plant extracts were added in individual polyethylene micro-centrifuge tubes. A tube as irradiated control was maintained separately without adding any of the extracts. All the tubes were added with 4 μl of 3 % H2O2, followed by direct exposure to UV radiation (300 nm) for 15 min by using a UV transilluminator (8000 μW/cm2). 1 μl aliquot of stock pBR322 plasmid DNA was placed in a separate tube and served as the non-irradiated control. The DNA samples were run on 1 % agarose gel and photographed with a Lourmat gel imaging system (Vilbar, France).
Characterization of phenolic compounds: HPLC analysis
HPLC analysis was carry out using a Waters 2487 HPLC system consisting of a dual λ detector and a Waters 1525 binary pump, and equipped with a Waters Symmetry® C18 column (5 mm, 4.6 × 50 mm) with Waters SentryTM universal guard column (5 mm, 4.6 × 20 mm) (Waters Corporation, Milford, MA, USA). Phenolic compounds in the methanolic and aqueous extract of A. muricata were identified using the phenolic reference standard for HPLC (Sakakibara et al. 2003). Gradient elution was performed at 35 ºC with solution A (50 mM sodium phosphate in 10 % methanol; pH 3.3) and solution B (70 % methanol) in the following gradient elution program: 0–15 min–100 % of Solution A; 15–45 min–70 % of Solution A; 45–65 min–65 % of Solution A; 65–70 min–60 % of Solution A; 70–95 min–50 % of Solution A; 95–100 min–0 % of Solution A. Flow rate was 1 ml min–1 and injection volume was 20 μl. Detection was examined at diverse wavelengths (around λ max) for various phenolic compounds, i.e. 250 nm for benzoic acids, isoflavones and most anthraquinones; 280 nm for some flavones, flavanones, catechins, theaflavins and some anthraquinones; 320 nm for cinnamic acids, most flavones and chalcones; 370 nm for flavonols; 510 nm for anthocyanins.
Statistical data analysis
All the analysis were carried out in triplicates. Data were presented as mean ± standard deviation (SD). Statistical analysis were performed by one-way ANOVA. MATLAB ver. 7.0 (Natick, MA, USA), GraphPad Prism 5.0 (San Diego, CA, USA) and Microsoft Excel 2007 (Roselle, IL, USA) were used for the statistical and graphical evaluations. Significant differences between groups were determined at p < 0.05. To evaluate relationships between experimental parameters, results were analyzed for correlation and tested for significance by Student’s t-test.
Results and discussion
Extract yield and total phenolics
The A. muricata plant leaf powder (50 g) yielded 3.56 g of methanolic and 7.5 g of aqueous crude extracts after sequential extraction. Phenolic compounds are the one of the most efficient antioxidants present in the plants and have been found to encounter activities related to stress in plants (Velioglu et al. 1998). Further, phenolic compounds are well known for their antioxidant, antimutagenic and anti-tumor activities (Othman 2007). Variations in the quantity of total phenolics in diverse concentrations of the extracts are presented in Table 1. Quantitative estimation proved that the extracts have considerably high constitutions of phenolic compounds with increasing concentration of extracts as presented by gallic acid equivalence (GAE).
Table 1.
Total phenolic contents of methanolic and aqueous extracts of A. muricata
| Concentration (μg) | Gallic acid equivalence (GAE) ± SD (μg) | |
|---|---|---|
| Methanolic extract | Aqueous extract | |
| 25 | 07.7 ± 0.11* | 06.9 ± 0.08* |
| 50 | 10.2 ± 0.14* | 08.4 ± 0.09* |
| 100 | 14.8 ± 0.40* | 10.3 ± 0.06* |
| 200 | 22.8 ± 0.25* | 14.0 ± 0.09* |
| 400 | 36.2 ± 0.36* | 19.1 ± 0.14* |
*GAE ± SD with significant difference between treatment groups at p < 0.05 (n = 3)
Phytochemicals identified
Plant polyphenols have been studied thoroughly for decades, to explore its potentials against number of diseases related to oxidative stress and free radical-induced damage, such as cardiovascular, neurodegenerative diseases, cancer, diabetes, autoimmune disorders and other inflammatory diseases (Fridovich 1999). The data obtained from phytochemical screening revealed the presence of polyphenols including tannins, steroids, cardiac glycosides, flavonoids, terpenoids, reducing sugars and anthraquinones which are presented in Table 2.
Table 2.
Phytochemical screening of methanolic and aqueous leaf extracts of A. muricata
| Phytochemical tests | Methanolic | Aqueous |
|---|---|---|
| Saponins | – | – |
| Flavonoids | ++ | + |
| Terpenoids | + | – |
| Tannins | ++ | ++ |
| Steroids | + | + |
| Phlobatannins | – | – |
| Oil | + | ++ |
| Cardiac glycosides | + | – |
| Reducing sugars | – | + |
| Anthraquinones | + | – |
+Mild presence
++Strong presence
−Absence
Antioxidant estimations
DRSA
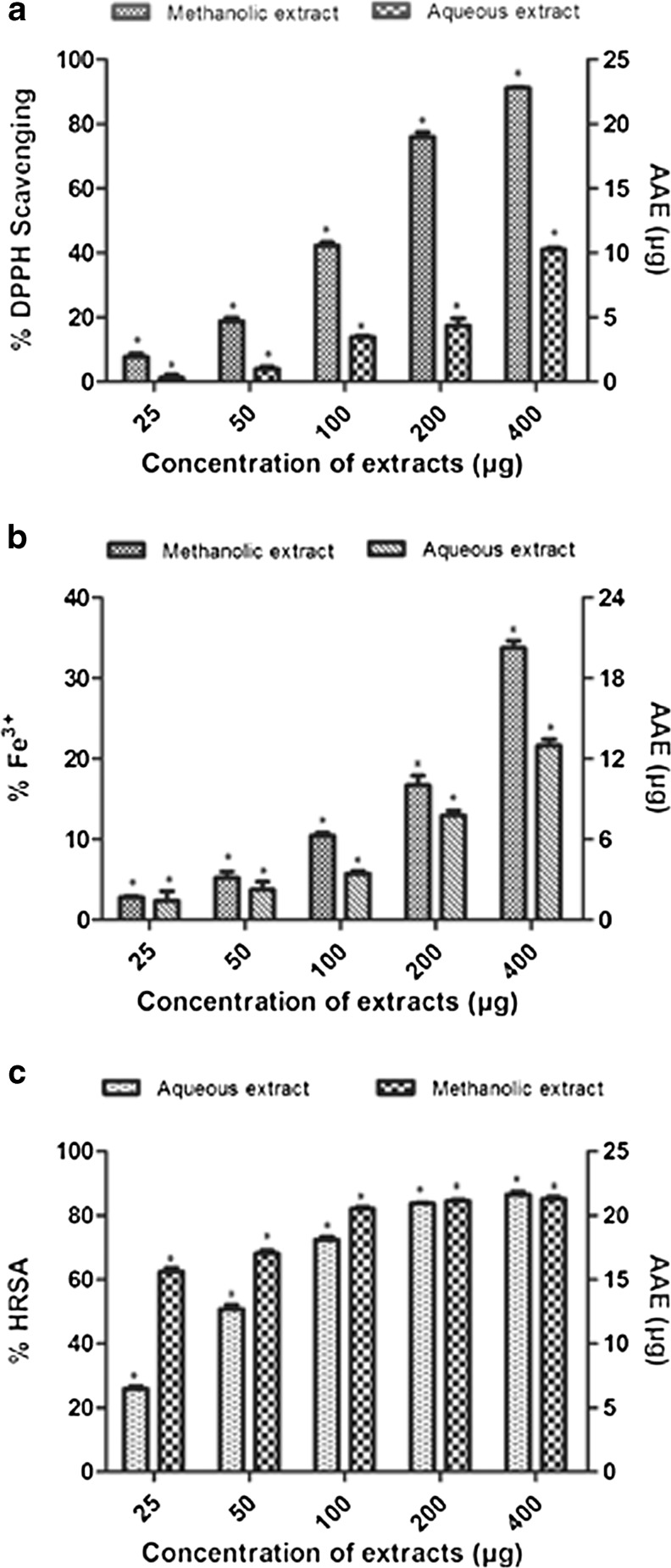
DPPH is a kind of unstable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule which is widely used to investigate radical scavenging activity of the leaf extracts (Blois 1958). Quantitative analysis revealed significant scavenging of free radicals by both extracts, in a dose-dependent manner and this may be attributed to their electron donating ability (Fig. 1a). Qualitatively, methanolic extract showed increased scavenging potentials of free radicals with an IC50 value of 119 μg, than the aqueous extract (IC50 > 400 μg). The results also reveal that the extracts might prevent reactive radical species from damaging biomolecules in susceptible biological and food systems (Halliwell et al. 1995).
Fig. 1.
a. Scavenging potentials of free radicals (DPPH) in a dose-dependent manner with AAE (in μg) as standard, b. Percentage Fe3+ reducing potential (FRAP) and c. Percentage hydroxyl radical scavenging activity (HRSA) of methanolic and aqueous leaf extracts of A. muricata with AAE (in μg) as standard. n = 3, * p < 0.05. (AAE ascorbic acid equivalence)
FRAP
The potential of extracts to reduce ferric (III) iron to ferrous (II) iron were determined by FRAP reagent (Wong et al. 2006; Alothman et al. 2009). The reducing properties of plant extracts, via hydrogen atom donation, are generally attributed to the presence of reductones, which exert an antioxidant action by breaking the free radical chains (Gordon 1990; Pin-Der-Duh 1998). Figure 1b showed the reducing activity of both extracts compared with AAE. The methanolic extract demonstrated improved activity when compare to the aqueous extract.
HRSA
The •OH scavenging activity was estimated by generating the hydroxyl radicals using ascorbic acid-iron EDTA. The •OH formed by the oxidation will react with dimethyl sulfoxide (DMSO) to form formaldehyde, which provides a convenient method to detect hydroxyl radicals, by treatment with Nash reagent (Singh et al. 2002). In biological systems, this hydroxyl radical is produced in an enormous amount and has the capacity to join nucleotides in DNA and cause strand breakage, which contributes to carcinogenesis, mutagenesis and cytotoxicity. It is capable of damaging almost every molecule found in living cells (Hochestein and Atallah 1988). Further, these radicals are proven to initiate lipid peroxidation process quickly, extracting hydrogen atoms from unsaturated fatty acids (Kappus 1991). In this study, both the extracts were found to be effective in quenching OH radical; the methanolic extract having a higher potential than the aqueous one, albeit the latter demonstrated a steeper dosage-dependent curve in comparison to the former (Fig. 1c). However, at higher concentration of 400 μg both the extracts exhibited about 85 % of •OH scavenging activity.
Different antioxidant compounds may act through different mechanisms; consequently, one method is not sufficient to completely evaluate the antioxidant capacity of plant based extracts (Gulcin et al. 2009). Hence, the present study utilized three various tests to evaluate antioxidant potentials of the two extracts. The results of the DPPH•, FRAP and HRSA assays proved a dosage-dependent increase in the antioxidant potentials over different ranges with distinct extract specific efficiencies. The methanolic extract exhibited significant scavenging potentials of ROS when compared to aqueous extract in all the analytical experiments carried out. This could possibly be due to the presence of different phytochemicals being eluted out in the two different solvents. This diversity in antioxidant potential shown in these experiments may also be due to the stereoselectivity of radicals or the differential solubility of the extracts in the testing systems (Yu et al. 2002; Gunjan et al. 2009). Similar antioxidant results from the leaves of other Annona species reported previously by Baskar et al. (2007), also confirm the radical scavenging potentials of the plant. Flavonoids and tannins are phenolic compounds and the presence of these compounds in the extracts might be responsible for the free radical scavenging effects observed. Supportive evidence for this aspect is provided by the strength of the significant statistical correlation between the TPC of the extracts and the free radical scavenging activity observed (Table 3) for various assays at p < 0.05.
Table 3.
Correlations between experimental results (TPC with DPPH•, FRAP and HRSA) tested for significance at p < 0.05
| Correlation | Extracts | R2 (p < 0.05) |
|---|---|---|
| TPC & DPPH | Methanolic | 0.957 |
| Aqueous | 0.986 | |
| TPC & FRAP | Methanolic | 0.996 |
| Aqueous | 0.995 | |
| TPC & HRSA | Methanolic | 0.932 |
| Aqueous | Not significant |
R2 denotes coefficient of determination
DNA damage protective activity
Antioxidants are found to play an important role in protecting DNA from various ROS mediated damages. Extracts from medicinal plants are reported to protect DNA and their protective nature is attributed to the presence of antioxidant components (Attaguile et al. 2000). The antioxidant/prooxidant activity of phytocompounds depends on factors such as chelating behavior, pH, and solubility characteristics (Decker 1997; Sindhu and Emilia 2005). Further, the protective nature of the plant extracts was tested by exposing pBR322 plasmid DNA to H2O2 and UV radiation along with 50 μg of the extracts. This approach was based on an earlier report emphasizing the need to explore naturally occurring antioxidant rich plants to mitigate H2O2-induced toxicity (Gunjan et al. 2010), and can be correlated with protection conferred against hydroxyl radicals. Supercoiled circular DNA (scDNA) was found to be absent in the H2O2 treated control sample (Ct) (Fig. 2). Both extracts rendered significant protection against DNA damage of which Am (methanolic extract of A. muricata) showed a banding pattern identical to Control (Co), which inferred that Am might have a high efficiency of DNA damage inhibition. Aa (Aqueous extract of A. muricata) showed very fainter band when compared to Am. However, the precise compounds which are accountable for this activity in the extracts were yet to be investigated.
Fig. 2.
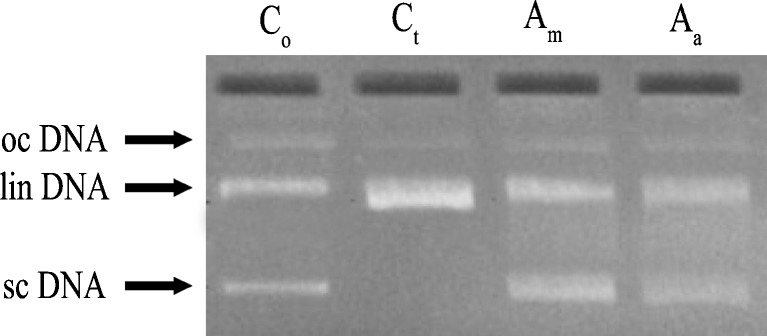
Protective effect of A. muricata extracts (50 μg) on supercoiled DNA (plasmid pBR322) against oxidative damage caused by UV exposed H2O2 -induced toxicity. Co = untreated non-irradiated DNA (control). Ct = untreated UV-irradiated DNA (control). Am = UV-irradiated methonolic extract treated. Aa = UV-irradiated aqueous extract treated
HPLC analysis
Many bio-active components including acetogenins, cyclohexapeptide flavonoids, aporphine alkaloids, glycoside and squamoline were isolated previously from the bark and seeds of this plant (Hopp et al. 1998; Alassane et al. 2004). However, major phenolic compounds present in methanolic and aqueous extracts of A. muricata leaves were not reported earlier and hence determined by HPLC analysis. A library of the analytical characteristics (λ max, retention time, determining λ, slope and limit calibration) of more than 100 phenolic standards established by Sakakibara et al. (2003) was used as reference for compound identification and our values were compared with the reported standard retention time values. Table 4 showed the phenolic compounds identified in these extracts along with the respective wave length in nm (λa).
Table 4.
Major phenolic compounds identified in methanolic and aqueous extracts of A. Muricata by HPLC
| A. muricata aqueous extracts | λa (nm) | A. muricata methanolic extract | λa (nm) |
|---|---|---|---|
| Flavones | Flavones | ||
| Luteolin | 320 | Luteolin | 320 |
| Homoorientin | 320 | Homoorientin | 320 |
| Tangeretin | 320 | ||
| Isoflavonols | Flavonols | ||
| Genistein | 250 | Quercetin | 370 |
| Glycitein | 250 | ||
| Flavanones | Isoflavones | ||
| (+) – Taxifolin | 280 | Daidzein | 250 |
| (+) – Catechin | 280 | ||
| (-) –Gallocatechin | 280 | Cinnamic acids | |
| (-) –Epicatechin gallate | 280 | Coumarid acid | 320 |
| Anthraquinones | Isoferulic acid | 320 | |
| Emodin | 250 |
awavelength for determination (Sakakibara et al. 2003)
Phenolic compounds present in the plants are reported to be the major phytochemicals responsible for antioxidant activity (Javanmardi et al. 2003). Luteolin, homoorientin, tangeretin, quercetin, daidzein, epicatechin gallate, emodin and coumaric acid were present in higher amounts when compare to other compounds as identified from the HPLC data. Among these, luteolin, quercetin, epicatechin gallate and emodin were already isolated from many plants and known for their significant antioxidant activities (Molina et al. 2003; Franklin et al. 2004; Henning et al. 2005). The extracts also contained unknown compounds as evident from the HPLC data whose characterization would serve to further evaluate the beneficial properties of this plant.
Conclusion
The study concludes that, the methanolic extract of A. muricata has the promising therapeutic compounds which possess significant radical scavenging and DNA protection activities, in a dose-dependent manner thereby providing an evidence for the development of an edible medicine. These promising results warrant further purification, characterization and isolation of the individual bio-active compounds from this plant for the better evaluation of its relative antioxidant properties.
Acknowledgment
We are thankful to the management of VIT University, Vellore, Tamil Nadu, India, for providing the necessary infrastructure for successful accomplishment of this research work. The authors also thank Mr. Biju, Teeose nursery, Mannuthy, Thrissur, Kerala, for providing the sample (leaves of Annona muricata) which is used in this study.
Conflict of interest
The authors declare that there are no conflicts of interest.
Abbreviations
- % DRSA
Percentage DPPH radical scavenging activity
- % HRSA
Percentage hydroxyl scavenging activity
- AAE
Ascorbic acid equivalence
- ANOVA
Analysis of variance
- BHT
Butylated hydroxytoluene
- DPPH
2, 2-diphenyl-1-picrylhydrazyl
- EDTA
Ethylene diamine tetraacetic acid
- FRAP
Ferric reducing antioxidant property
- GAE
Gallic acid equivalence
- HPLC
High performance liquid chromatography
- ROS
Reactive oxygen species
- SD
Standard deviation
- TBA
Thiobarbituric acid
- TCA
Trichloroacetic acid
- TPTZ
2, 4, 6-tripyridyl-s-triazin
- TPC
Total phenolic content
References
- Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement. 2009;6:30–41. doi: 10.4314/ajtcam.v6i1.57071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alassane W, Yanjun Z, Christelle C, Jean-Paul B, Jean-Louis P, Bernard B. Annomuricatin C, a novel cyclohexapeptide from the seeds of Annona muricata. C R Chim. 2004;7:981–988. doi: 10.1016/j.crci.2003.12.022. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Attaguile G, Russo A, Campisi A, Savoca F, Acquaviva R, Ragusa N, Vanella A. Antioxidant activity and protective effect on DNA cleavage of extracts from Cistus incanus L. and Cistus monspeliensis L. Cell Biol Toxicol. 2000;16:83–90. doi: 10.1023/A:1007633824948. [DOI] [PubMed] [Google Scholar]
- Baskar R, Rajeswari V, Kumar TS. In vitro antioxidant studies in leaves of Annona species. Indian J Exp Biol. 2007;45:480–485. [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Borris RP. Natural product research. Perspective from a major Pharmaceutical company. J Ethnopharmacol. 1996;51:29–38. doi: 10.1016/0378-8741(95)01347-4. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Cijo GV, Naveen KDR, Rajkumar V, Suresh PK, Ashok KR. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona Muricata Linn. in normal and immortalized human cell lines. Asian Pac J Can Prev. 2012;13:699–705. doi: 10.7314/APJCP.2012.13.2.699. [DOI] [PubMed] [Google Scholar]
- Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70:491S–499S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55:396–407. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Franklin V, Yrene D, Karla C. Antioxidant and scavenging activity of emodin, aloe-emodin, and rhein on free-radical and reactive oxygen species. Pharm Biol. 2004;42:342–348. doi: 10.1080/13880200490519613. [DOI] [Google Scholar]
- Fridovich I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann N Y Acad Sci. 1999;893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. New York: App Sci; 1990. pp. l–18. [Google Scholar]
- Gulcin I, Elias R, Gepdiremen A, Taoubi K, Koksal K. Antioxidant secoiridoids from fringe tree (Chionanthus virginicus L.) Wood Sci Technol. 2009;43:195–212. doi: 10.1007/s00226-008-0234-1. [DOI] [Google Scholar]
- Gunjan G, Rajkumar V, Ashok KR, Lazar M. Therapeutic potential of polar and non-polar extracts of cyanthillium cinereum in vitro. Evid Based Compl Alt Med. 2009 doi: 10.1093/ecam/nep155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunjan G, Rajkumar V, Ashok KR, Lazar M. Aqueous extract of Phyllanthus amarus inhibits chromium(VI)-induced toxicity in MDA-MB-435S cells. Food Chem Toxicol. 2010;48:396–401. doi: 10.1016/j.fct.2009.10.028. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol. 1995;33:601–617. doi: 10.1016/0278-6915(95)00024-V. [DOI] [PubMed] [Google Scholar]
- Henning SM, Niu Y, Liu Y, et al. Bioavailability and antioxidant effect of epigallocatechin gallate administered in purified form versus as green tea extract in healthy individuals. J Nutr Biochem. 2005;16:610–616. doi: 10.1016/j.jnutbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hochestein P, Atallah AS. The nature of oxidant and antioxidant systems in the inhibition of mutation and cancer. Mutat Res. 1988;202:363–375. doi: 10.1016/0027-5107(88)90198-4. [DOI] [PubMed] [Google Scholar]
- Hopp DC, Alali FQ, Gu ZM, McLaughlin JL. Mono-THF ring annonaceous acetogenins from Annona squamosa. Phytochemistry. 1998;47:803–809. doi: 10.1016/S0031-9422(97)00822-4. [DOI] [PubMed] [Google Scholar]
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. doi: 10.1016/S0308-8146(03)00151-1. [DOI] [Google Scholar]
- Kappus H. Lipid peroxidation – mechanism and biological relevance. In: Aruoma OI, Halliwell B, editors. Free radicals and food additives. London: Taylor and Francis; 1991. pp. 59–75. [Google Scholar]
- Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulphoxide by hydroxyl radical generating system. Biochemistry. 1981;20:6006–6012. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- Molina MF, Sanchez-Reus I, Iglesias I, Benedi J. Quercetin, a flavonoid antioxidant, prevents and protects against ethanol-induced oxidative stress in mouse liver. Biol Pharm Bull. 2003;26:1398–1402. doi: 10.1248/bpb.26.1398. [DOI] [PubMed] [Google Scholar]
- Nilima S, Rajurkar HSM. Estimation of Phytochemical content and antioxidant activity of some selected traditional indian medicinal plants. Indian J Pharm Sci. 2011;73:146–151. doi: 10.4103/0250-474X.91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman A, Ismail A, Abdul NG, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–1530. doi: 10.1016/j.foodchem.2005.12.021. [DOI] [Google Scholar]
- Pin-Der-Duh X. Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free-radical active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Rajdeep C, Abhishek D, Susri R, Nilendu S, Ashok K, Giri KC. In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem Toxicol. 2007;46:740–751. doi: 10.1016/j.fct.2007.09.108. [DOI] [PubMed] [Google Scholar]
- Rajkumar V, Guha G, Kumar RA. Antioxidant and anti-cancer potentials of Rheum emodi rhizome extracts. Evid Based Complement Alternat Med. 2011 doi: 10.1093/ecam/neq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar V, Gunjan G, Ashok KR. Isolation and bioactivity evaluation of two metabolites from the methanolic extract of oroxylum indicum stem bark. Asian Pac J Trop Biomed. 2012;2:S7–S11. doi: 10.1016/S2221-1691(12)60120-8. [DOI] [Google Scholar]
- Russo A, Izzo AA, Cardile V. An Indian medicinal plants as antiradicals and DNA cleavage protectors. Phytomedicine. 2001;8:125–132. doi: 10.1078/0944-7113-00021. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- Sindhu M, Emilia AT. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2005;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakash GK. Studies on antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vivo models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Treas GE, Evans WC. Pharmacognosy. 13. London: Bailliere Tindall; 1989. pp. 176–180. [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of Southern and Eastern Africa. 2. London, UK: Churchill Livingstone; 1962. pp. 58–59. [Google Scholar]
- Wong C, Li H, Cheng KW, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- Yuan SS, Chang HL, Chen HW, et al. Annonacin, a mono-tetrahydrofuran acetogenin, arrests cancer cells at the G1 phase and causes cytotoxicity in a Baxand caspase-3-related pathway. Life Sci. 2003;72:2853–2861. doi: 10.1016/S0024-3205(03)00190-5. [DOI] [PubMed] [Google Scholar]