Abstract
C-phycocyanin, a natural food colorant, is gaining importance worldwide due to its several medical and pharmaceutical applications. In the present study, aqueous two-phase extraction was shown to be an attractive alternative for the downstream processing of C-phycocyanin from Spirulina platensis. By employing differential partitioning, C-phycocyanin selectively partitioned to the polymer rich (top) phase in concentrated form and contaminant proteins to the salt rich (bottom) phase. This resulted in an increase in the product purity (without losing much of the yield) in a single step without the need of multiple processing steps. Effect of process parameters such as molecular weight, tie line length, phase volume ratio, concentration of phase components on the partitioning behavior of C-phycocyanin was studied. The results were explained based on relative free volume of the phase systems. C-phycocyanin with a purity of 4.32 and yield of about 79 % was obtained at the standardized conditions.
Keywords: Aqueous two-phase systems, C-phycocyanin, Relative free volume, Partition coefficient, Purification
Introduction
Aqueous two-phase extraction (ATPE) has been recognized by many researchers as a superior and versatile technique for the downstream processing of biomolecules (Walter et al. 1985; Diamond and Hsu 1992; Raghavarao et al. 1995, 1998) including natural colors (Rito-Palomares et al. 2001; Chethana et al. 2007). The major advantages of ATPE are high capacity and high yield, low process time and energy, and ease of scale up besides biocompatible environment.
Consumer awareness is growing in the recent times with regard to the importance of natural colors mainly due to their nutritious, pharmacological and health related advantages. As a result, applications of natural colors are increasing especially in food and cosmetic industry (Arad and Yaron 1992; Rito-Palomares et al. 2001; Patil et al. 2006). Phycocyanin is one of the important natural blue colorants finding food application in manufacturing of bubble gums, milky products, jelly, ice creams and beverages. Phycocyanin is also known for its antioxidant, anti-inflammatory and hepatoprotective effects (Romay and Gonzalez 2000; Reddy et al. 2000; Vadiraja and Madyastha 2000; Romay et al. 2003).
C-phycocyanin (CPC) is a blue colored biliprotein of blue green algae and was first reported by Lemberg (O’hEocha 1963). CPC comprises of a protein and chromophore, the protein moiety consists of alpha and beta sub-units of molecular weights in the range of 18,000 and 20,000 Da, respectively. This colorant is highly stable in the pH range of 5–8 and exhibits a strong red fluorescence when present in its native form.
Minkova et al. (2003) reported the purification of CPC from Spirulina (Arthrospira) fusiformis by a multistep procedure using rivanol in the ratio of 10:1 (v/v), followed by 40 % ammonium sulfate precipitation. The obtained purity and yield of phycocyanin were 4.3 and 43 %, respectively. Silveria et al. (2007) attempted extraction of CPC from Spirulina platensis using factorial design and response surface techniques, but did not succeed in achieving high purification (purity only ~0.46). Chromatographic method results in dilution of CPC pigment, needing an additional step for concentrating it to the desired level. Similarly, additional step of dialysis is required for the removal of salt after salt (ammonium sulfate) precipitation. Thus, the conventional purification of CPC involves many process steps and it is known that at each step there will be a loss of product yield (Zhang and Chen 1999; Patel et al. 2005; Chen et al. 2006; Soni et al. 2006; Patil et al. 2006). These problems can be eliminated to a large extent by using ATPE.
The purification of CPC from Spirulina maxima using ATPE was reported by Rito-Palomares et al. (2001). The purity of CPC obtained was low (2.4), which was further purified by ultrafiltration (purity 3.1 and yield 61 %) followed by ammonium sulfate precipitation to obtain a purity of 3.8. However, the overall yield was only about 30 % indicating a large scope for development of a protocol to achieve high purity without loosing the yield. ATPE was employed for downstream processing of CPC directly from cell homogenate of Spirulina platensis and the obtained purity was low (0.73) although the yield was high (90.34 %) (Narayan and Raghavarao 2007).
It may be noted that the purification procedure by ATPE is system specific and hence it needs to be standardized for Spirulina platensis. General protocol for ATPE (standardization of process parameters) was demonstrated with CPC as an example (Patil and Raghavarao 2007). However, in the present work higher purification of CPC was achieved without the need of multiple extraction or other processing steps. The results were explained based on the relative free volume of the phase systems.
Material and methods
Chemicals
Polyethylene glycol (PEG) (Molecular Weight (MW). 4000, 6000, 20000) was procured from SRL, Mumbai, India. PEG (MW 1500) was from Merck, Germany. Potassium di-hydrogen phosphate (KH2PO4) and di-potassium hydrogen phosphate (K2HPO4) were obtained from Ranbaxy chemicals, Punjab, India. All the chemicals used were of analytical grade.
Preparation of crude extract
Spirulina platensis was grown outdoor in open raceway ponds (70 m3) (Naidu et al. 1999). Freshly harvested biomass was washed thoroughly using deionised water to remove all the nutrients from the culture broth. The biomass was homogenized over a pressure range of 200–400 kg/cm2 for about 5 min to break the cells and centrifuged at 10,000 rpm for about 10 min to separate the released phycocyanin from cell debris. The crude extract was stored at 4–5 °C and used for the experiments.
Aqueous two-phase extraction
ATPE was carried out by adding predetermined quantities of polymer (PEG) and salt (potassium phosphate) from the reported phase diagrams (Albertsson 1986; Zaslavsky 1995) to the crude extract (making the total weight of the system 100 % on w/w basis) and mixing thoroughly using a magnetic stirrer for achieving equilibration. The phosphate salt was chosen as the phase forming salt because of its minimal adverse effects on the proteins. After the extraction, the phases were separated by gravity, the volumes of top and bottom phases of the system were noted and analyzed for the CPC and total protein concentration. The CPC was reported to be stable in pH range of 6.0 to 8.0 (Lauro and Francis 2000). The ATPE experiments were carried out at 25 ± 1 °C and pH 7.0 (by taking suitable combinations of potassium salts), in triplicates and the average values were reported.
Spectroscopic measurements
Absorption spectra of CPC were measured using UV-Visible spectrophotometer (Double beam spectrometer, Shimadzu, model UV-160A Japan). The concentration of total proteins and CPC were determined using the absorbance at 280 nm and 620 nm, respectively. The purity of CPC was defined as the ratio of absorbance at 620 nm to 280 nm, wherein A620 is the maximum absorbance of CPC and A280 is the absorbance of total proteins (Boussiba and Richmond 1979; Patel et al. 2005).
Gel electrophoresis
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) was carried out as described in Methods in Enzymology using 10 % polyacrylamide gel (Deutscher 1990). Electrophoresis was run at 50 V and 12.5 mA for about 3.5–4 h. The gel was stained with a solution containing coomassie brilliant blue R-250 (0.05 % w/v) in methanol (50 %, v/v) and acetic acid (12 %, v/v) and the gel was destained using the same buffer without the dye.
Analytical procedures
Tie line length
The tie line length (TLL) of the aqueous two-phase systems was calculated from the reported phase diagrams (Albertsson 1986; Zaslavsky 1995) according to the following equation
| 1 |
where Cpt and Cpb are PEG concentrations (% w/w) in the top and bottom phases, respectively and Cst and Csb are salt concentration (% w/w) in top and bottom phases, respectively.
Volume ratio
The phase volume ratio (Vr) is defined as the ratio of volumes of the top phase to that of the bottom phase.
| 2 |
where Vt and Vb are volumes of the top and bottom phases, respectively.
Partition coefficient
The partition coefficient (K) of the protein and CPC were calculated using the equation
| 3 |
where Ct and Cb are the equilibrium concentrations of protein and CPC in the top and bottom phases, respectively.
Yield in top phase
The yield of CPC (YT %) in top phase was calculated using the following equation
| 4 |
where VT and VI are volumes of the top phase and initial volume of the crude extract, respectively. CT and CI are the concentrations of CPC in the top phase and in the crude extract, respectively.
Relative free volume
Relative free volume of the phase was calculated using the following equation
| 5 |
where ρ is the density of the phase and ρ0 is the density of the reference solution (Eiteman and Gainer 1989; Grossman and Gainer 1988). The densities are measured in duplicates at 25 ± 1 °C using specific gravity bottle and the average values are reported.
Change in free volume
The change in free volume between the phases was estimated from the following equation (Eiteman and Gainer 1989; Grossman and Gainer 1988).
| 6 |
where Vf(top) and Vf(bottom) are the relative free volumes in top and bottom phases, respectively.
Statistical analysis
Significant differences between means were determined by t test (paired two samples for mean) using Microsoft Excel. Significance of differences was defined at p < 0.05
Results and discussion
The pH of the aqueous two-phase systems was kept constant at 7.0. During aqueous two-phase extraction, CPC preferentially partitioned to the top phase. Experiments were carried out by varying the process parameters such as molecular weight of phase forming polymer, tie line length, phase volume ratio and concentrations of phase forming polymer and salt in order to understand and optimize the partitioning behavior of CPC. The results are discussed in the following sections.
Effect of polymer molecular weight
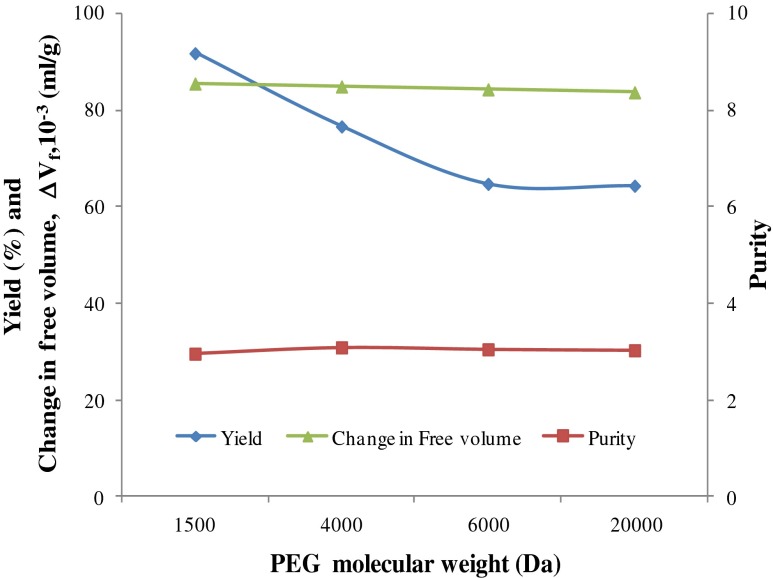
In order to study the effect of polymer molecular weight, ATPE experiments were carried out using PEG/potassium phosphate system by varying the molecular weight of PEG, while maintaining a constant total phase composition (7 % PEG and 20 % potassium phosphate, w/w) and volume ratio ~0.3. It can be seen from Fig. 1 that the purity of CPC remained constant (3.0) with an increase in the molecular weights of the PEG (1500 to 20000), while the yield decreased. This could not directly be related to the marginal change in free volume (∆Vf) observed (Fig. 1). Hence in order to arrive at the suitable molecular weight of PEG for CPC partitioning, it was thought desirable to study the effect of the total phase composition for a given molecular weight of PEG.
Fig. 1.
Effect of PEG molecular weight on yield, purity and change in free volume (∆Vf)
Effect of tie line length (TLL)
In order to study the effect of phase composition, ATPE experiments were carried out using PEG/potassium phosphate systems of different TLL and different molecular weights of PEG (1500, 4000 and 6000). Results are given in Table 1. In PEG 1500 system, the partition coefficient of CPC as well as total protein increased with an increase in TLL, which had resulted in a decrease in purity of CPC (p < 0.05). The relative free volume (Vf) in the top phase remained almost constant while that the relative free volume in the bottom phase decreased with an increase in TLL (%) and as an overall result the change in free volume (∆Vf) increased from 41.6 × 10−3 to 89.7 × 10−3 ml/g (Table 1). As a result of this, the contaminant proteins partitioned along with CPC to the top phase, which in turn decreased the purity of CPC. The maximum purity of CPC observed in case of PEG 1500 was 2.84 with a yield of ~100 % at TLL 17 %.
Table 1.
Effect of tie line length (TLL, %) on the change in free volume and partitioning
| PEG molecular weight (Da) | TLL (%) | Phase volume ratio, Vr (−) | Change in free volume, ∆Vf (10−3 ml/g) | KCPC | KTP | Purity of CPC (−) | Yield of CPC (%) |
|---|---|---|---|---|---|---|---|
| 1500 | 17 | 1.05 | 41.6 | 28.9 | 1.1 | 2.84 ± 0.2a | ~100 a |
| 21 | 1.07 | 60.7 | 51.3 | 1.3 | 2.71 ± 0.1b | 96.8 ± 0.2b | |
| 24 | 1.20 | 89.0 | 44.00 | 2.4 | 1.47 ± 0.09c | 90.1 ± 0.7c | |
| 4000 | 10 | 0.64 | 37.36 | 13.9 | 1.1 | 3.56 ± 0.07a | 92.5 ± 0.2a |
| 14 | 0.59 | 38.03 | 16.7 | 1.4 | 3.37 ± 0.02 a | 94.4 ± 08b | |
| 19 | 0.53 | 42.92 | 19.1 | 1.7 | 3.26 ± 0.08 a | 90.0 ± 0.7c | |
| 23 | 0.27 | 106.06 | 37.7 | 2.5 | 3.30 ± 0.02 a | 67.5 ± 0.2d | |
| 30 | 0.14 | 109.32 | 39.7 | 6.4 | 2.13 ± 0.07 b | 67.0 ± 0.9d | |
| 6000 | 16 | 0.77 | 76.7 | 2.5 | 2.5 | 2.52 ± 0.02a | 52.5 ± 0.6a |
| 20 | 0.50 | 78.9 | 5.2 | 3.6 | 2.14 ± 0.05a | 67.6 ± 0.9b | |
| 24 | 0.31 | 83.4 | 27.0 | 9.4 | 2.51 ± 0.08a | 65.3 ± 0.8c |
Means of the same column followed by different letters differ significantly (p < 0.05), Purity of CPC in crude extract was 1.0
In PEG 4000 system the partition coefficient of CPC as well as total proteins increased with an increase in TLL thus decreasing the purity of CPC in the top phase. CPC purity of 3.5 with a yield of 92.5 % was observed at low TLL (10 %). With an increase in TLL, the relative free volume (Vf) in the bottom phase decreased from −0.129 (at 10 % TLL) to −0.206 ml/g (at 30 % TLL), whereas the decrease in relative free volume in the top phase was very less (−0.091 to −0.096 ml/g). As an overall result, the change in free volume (∆Vf) increased with an increase in TLL (Table 1). However, volume of the extracting phase decreased as the volume ratio decreased with an increase in TLL (%). Accordingly, the CPC yield decreased considerably with an increase in TLL. On the other hand, the purity of CPC remained practically constant (except at very high TLL), since both CPC and total proteins partitioned to top phase (indicated by increase in both KCPC and KTP) with an increase in TLL.
In case of PEG 6000 system, the partitioning behavior of CPC and total proteins at different TLL was observed to be similar to that of PEG 1500 and 4000. However, the purity of CPC obtained was 2.52.
The PEG 4000/potassium phosphate showed the highest purity when compared to that of PEG (1500, 6000)/potassium phosphate systems. This is due to the differential partitioning of CPC and total proteins to the top and bottom phases, respectively. Hence, the effect of other parameters such as phase volume ratio, concentration of polymer and salt (which in turn changes the ∆Vf) was studied employing PEG 4000/potassium phosphate system.
Effect of phase volume ratio
The effect of phase volume ratio (0.22, 0.54 and 1.25) on partitioning was studied for PEG 4000/potassium phosphate system (TLL 14 %). The results are shown in Table 2. The partition coefficient of CPC increased with an increase in phase volume ratio. The maximum CPC purity of 3.4 was observed at lower volume ratio (0.2). However, the obtained yield was low (40 %) since the volume of the extracting phase (top) was low and most of the phycocyanin partitioned to the bottom phase. The yield of CPC increased with an increase in the volume ratio (as the volume of the extracting phase increased), while purity of CPC decreased (p < 0.05), since the other proteins (contaminating) also partitioned to the top phase along with CPC.
Table 2.
Effect of phase volume ratio on change in free volume and partitioning
| Phase volume ratio, Vr (−) | Change in free volume, ∆Vf (10−3 ml/g) | KCPC | KTP | Purity of CPC (−) | Yield of CPC (%) |
|---|---|---|---|---|---|
| 0.22 | 24.5 | 4.66 | 1.51 | 3.39 ± 0.1a | 40.1 ± 0.9a |
| 0.54 | 27.0 | 6.39 | 1.09 | 2.30 ± 0.8b | 77.4 ± 0.6b |
| 1.25 | 31.1 | 22.32 | 3.08 | 2.47 ± 0.4c | 88.0 ± 0.8c |
Means of the same column followed by different letters differ significantly (p < 0.05), Purity of CPC in crude extract was 1.0
From Table 2, it can be observed that the change in free volume (∆Vf) increased only marginally with an increase in phase volume ratio (since the total phase composition chosen on the same TLL which eventually results in the same phase composition of the polymer rich top and salt rich bottom phases). As a result, purity and yield of CPC was observed to depend mainly on the volume of the extracting phase (in other words volume ratio).
Effect of polymer concentration
Partitioning of CPC was studied at different polymer concentrations of PEG 4000 by maintaining the process parameters like pH (7.0) and salt concentration (15 % w/w) constant. The results are shown in Table 3.
Table 3.
Effect of polymer concentration on change in free volume and partitioning
| Total phase composition (%, w/w) | Phase volume ratio, Vr (−) | Change in free volume, ∆Vf (10−3 ml/g) | KCPC | KTP | Purity of CPC (−) | Yield of CPC (%) | |
|---|---|---|---|---|---|---|---|
| Polymer | Salt | ||||||
| 9 | 15 | 0.45 | 54.29 | 37.22 | 3.29 | 3.54 ± 0.2a | 78.6 ± 0.7a |
| 12 | 15 | 0.95 | 70.42 | 48.86 | 1.45 | 3.78 ± 0.5b | 74.0 ± 0.2b |
| 15 | 15 | 1.22 | 89.80 | 41.80 | 1.90 | 2.71 ± 0.4c | 74.9 ± 0.5 b |
| 20 | 15 | 1.60 | 107.54 | 43.70 | 2.22 | 1.94 ± 0.8d | 71.1 ± 0.9 c |
Means of the same column followed by different letters differ significantly (p < 0.05), Purity of CPC in crude extract was 1.0
The polymer concentration of 9 % (w/w) resulted in a CPC purity of 3.5. At 12 % (w/w) polymer concentration, the purity of CPC increased to 3.8 (with a yield of 74.0 %) due to the differential partitioning of CPC and contaminating proteins into opposite phases. Further increase in the concentration of polymer (15 %, w/w), the purity of CPC decreased since the contaminating proteins also partitioned to the top phase as volume of the extracting phase increased. At a very high concentration of PEG (20 %, w/w), the yield and purity of CPC decreased as the proteins precipitated at the interface.
Relative free volume in top phase and bottom phase decreased with an increase in polymer concentration. However, the rate of decrease in bottom phase was much higher when compared to that in the top phase and as a result, the change in free volume (∆Vf) increased (Table 3). This decrease in relative free volume (Vf) in the bottom phase facilitated the partitioning of contaminant proteins to the top phase thus decreasing the purity of CPC (p < 0.05). The yield of CPC remained almost constant initially and later decreased with increasing polymer concentration, perhaps as the top phase reaching the solubility limits for CPC.
Effect of salt concentration
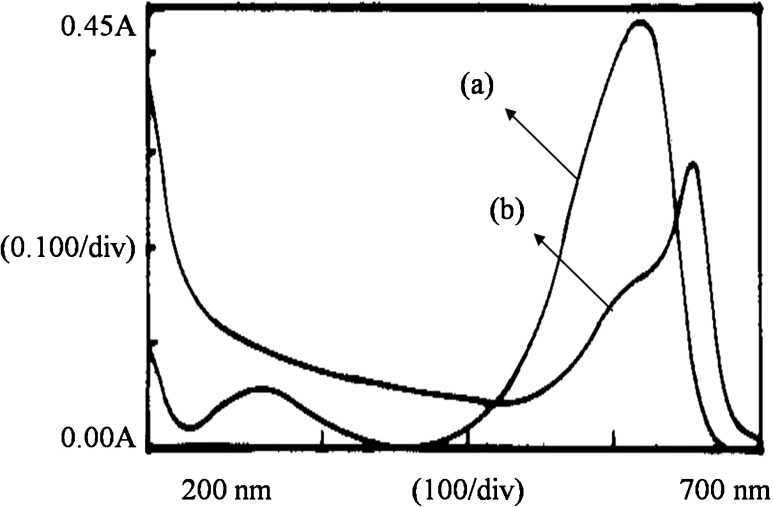
Partitioning of CPC at different salt concentrations of potassium phosphate was studied while maintaining the process parameters like pH (7.0) and PEG concentration (6 %, w/w) constant. From Table 4 it can be observed that at higher salt concentration, CPC and the contaminant proteins partitioned to the top phase, since the change in free volume (∆Vf) increased with an increase in salt concentration. Hence, the purity of CPC decreased with an increase in the salt concentration (p < 0.05). The maximum purity of 4.32 and yield of about 79 % in the top phase were observed at 15 % (w/w) salt concentration. Figure 2 shows the absorption spectra of top and bottom phases wherein the differential partitioning of CPC and contaminant proteins was highest.
Table 4.
Effect of salt concentration on change in free volume and partitioning
| Total phase composition (%, w/w) | Phase volume ratio, Vr (−) | Change in free volume, ∆Vf (10−3 ml/g) | KCPC | KTP | Purity of CPC (−) | Yield of CPC (%) | |
|---|---|---|---|---|---|---|---|
| Polymer | Salt | ||||||
| 6 | 15 | 0.25 | 42.68 | 37.39 | 3.80 | 4.32 ± 0.3a | 78.83 ± 0.4 a |
| 6 | 20 | 0.21 | 73.34 | 186.34 | 7.11 | 3.28 ± 0.5 b | 78.52 ± 0.2 a |
| 6 | 25 | 0.19 | 103.36 | 503.70 | 8.95 | 2.65 ± 0.2 c | 75.31 ± 0.5b |
Means of the same column followed by different letters differ significantly (p < 0.05), Purity of CPC in crude extract was 1.0
Fig. 2.
Absorption spectrum of top and bottom phase of PEG/potassium phosphate system (total phase composition: 6/15 %, w/w): a Top phase; b Bottom phase
The change in free volume (∆Vf) with an increase in salt concentration is shown in Table 4. Significant increase in the change in free volume (∆Vf) (from 42.68 × 10−3 to 103.3 × 10−3 ml/g) with increasing salt concentration resulted in increased partitioning of CPC as well as contaminating proteins to the top phase, thus reducing the purity. However, corresponding increase in yield was not observed because volume of the extracting phase as well as the volume ratio decreased with increasing salt concentration, resulting in precipitation of CPC. This can be seen even by the significant increase in the partition coefficients of CPC and contaminant proteins with an increase in salt concentration.
Patel et al. (2005) attempted the purification of CPC from Spirulina sp. employing ATPE followed by UF and ammonium sulphate precipitation which resulted in purity of 3.8 with about 30 % overall yield. Benavides and Rito-Palomares (2005) reported purification of CPC from Spirulina maxima with a purity of 3.4 with 74 % yield after third stage ATPE. The purification of CPC form Spirulina platensis using ion exchange chromatography was reported by Silveiria et al. (2007) with a purity of 3.4 and yield of 77.3 %. Singh et al. (2009) demonstrated the purification of CPC form Phormidium ceylanicum employing ultrafiltration followed by ion exchange chromatography which resulted in a purity of 4.15 and 63.5 % yield. In the current study purity of 4.32 and yield of about 79 % of CPC were obtained in a single step ATPE.
SDS-PAGE
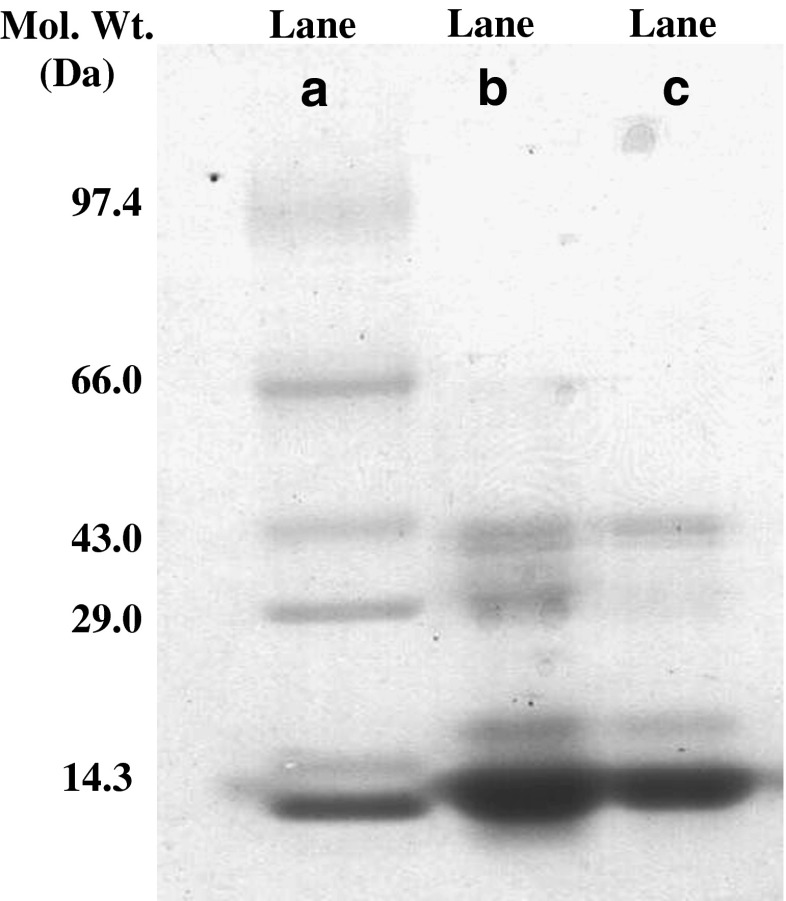
The molecular weights of the CPC subunits were confirmed using SDS-PAGE. The purified CPC obtained from ATPE was subjected to gel electrophoresis as shown in Fig. 3. Lane A is the standard molecular weight marker and Lane B is crude extract and Lane C is the purified CPC in top phase. The CPC shows two bands α and β in the range of 18 and 20 KDa each. It can be observed that, during ATPE the majority of the contaminant proteins present in the crude extract were partitioned to the bottom phase resulted in an increase in purity of CPC in top phase hence, reduced number of bands in lane C compared to crude sample (Lane B). Other bands are visible in lane C apart from CPC (Fig. 3) indicating the presence of some other contaminant proteins.
Fig. 3.
SDS PAGE of C-phycocyanin purified by aqueous two-phase extraction: Lane a- Marker; Lane b- Crude extract; Lane c- Top phase
Conclusion
This study reports a process for the purification of the blue colorant CPC from Spirulina platensis by aqueous two-phase extraction. It has been shown that phase composition significantly affects the change in relative free volume (ΔVf) of the system which in turn affects the partitioning behavior and hence the purity as well as the yield of CPC. A purity of 4.32 of CPC with a yield of about 80 % in the top phase was achieved in a single extraction step without the need of multiple extraction or other processing steps.
Acknowledgments
We thank the Director, CFTRI, Mysore for his keen interest in separation processes. We also, thank Head, Plant Cell Biotechnology, CFTRI, Mysore for providing the Spirulina biomass. The authors, CS, CAN and MCM thank Council of Scientific and Industrial Research (CSIR) for the Senior Research Fellowship. The authors acknowledge the support of the Department of Biotechnology (DBT) Government of India (BT/PR-5286/PID), for providing financial support to this research work.
Nomenclature
- % TLL
Tie line length (%)
- % YT
Yield (%) in top phase
- ATPE
Aqueous two-phase extraction
- ATPSs
Aqueous two-phase systems
- CPC
C-phycocyanin
- KCPC
Partition coefficient of C-phycocyanin
- KTP
Partition coefficient of total proteins
- PEG
Poly (ethylene) glycol
- Vf
Relative free volume (ml/g)
- Vr
Phase volume ratio
- ∆Vf
Difference in relative free volume (ml/g)
- ρ
Density of the top/bottom phase (g/ml)
References
- Albertsson PÅ. Partition of cells particles and macromolecules. 3. New York: Wiley; 1986. [Google Scholar]
- Arad S, Yaron A. Natural pigments from red micro algae for use in food and cosmetics. Trends Food Ind. 1992;18:164–168. [Google Scholar]
- Benavides J, Rito-Palomares M. Potential aqueous two-phase processes for the primary recovery of colored protein from microbial origin. Eng Life Sci. 2005;5(3):259–266. doi: 10.1002/elsc.200420073. [DOI] [Google Scholar]
- Boussiba S, Richmond AE. Isolation and purification of phycocyanins from the blue green algae Spirulina platensis. Arch Microbiol. 1979;120:155–159. doi: 10.1007/BF00409102. [DOI] [Google Scholar]
- Chen T, Wong Y, Zheng W. Purification and characterization of selenium containing phycocyanin from selenium–enriched Spirulina platensis. Phytochem. 2006;67:2424–2430. doi: 10.1016/j.phytochem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Chethana S, Nayak CA, Raghavarao KSMS. Aqueous two-phase extraction for purification and concentration of betalains. J Food Eng. 2007;81:679–687. doi: 10.1016/j.jfoodeng.2006.12.021. [DOI] [Google Scholar]
- Deutscher M. Methods Enzymol. 1990;182:425. doi: 10.1016/0076-6879(90)82035-Z. [DOI] [PubMed] [Google Scholar]
- Diamond AD, Hsu JT. Aqueous two-phase systems for biomolecule separation. Adv Biochem Eng Biotechnol. 1992;47:89–133. doi: 10.1007/BFb0046198. [DOI] [PubMed] [Google Scholar]
- Eiteman MA, Gainer JL. The effect of relative free volume changes on partitioning in magnesium sulphate-poly (ethylene glycol) aqueous two-phase systems. Acta Biochim Biophys. 1989;992:125–127. doi: 10.1016/0304-4165(89)90058-5. [DOI] [Google Scholar]
- Grossman PD, Gainer JL. Correlation of aqueous two pahse partitioning of proteins with change in free volume. Biotechnol Prog. 1988;4(1):6–11. doi: 10.1002/btpr.5420040103. [DOI] [Google Scholar]
- Lauro GJ, Francis FJ. Natural food colorants: science and technology. New York: Marcel Dekker Publication; 2000. [Google Scholar]
- Minkova KM, Tchernov AA, Tchorbadjieva MI, Fournadjieva ST, Antova RE, Busheva MC. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J Biotechnol. 2003;102:55–59. doi: 10.1016/S0168-1656(03)00004-X. [DOI] [PubMed] [Google Scholar]
- Naidu AK, Sharada R, Monoj G, Khan MY, Mahadevaswamy M, Vishvanatha S, Murthy KN, Ravishankar GA, Leela S. Toxicity assessment of phycocyanin- a blue colorant from blue green alga Spirulina platensis. Food Biotechnol. 1999;13(1):56–66. doi: 10.1080/08905439609549961. [DOI] [Google Scholar]
- Narayan AV, Raghavarao KSMS. Extraction and purification of C-phycocyanin from Spirulina platensis employing aqueous two phase systems. Int J Food Eng. 2007;3(4):1556–3758. doi: 10.2202/1556-3758.1105. [DOI] [Google Scholar]
- O’hEocha C. Spectral properties of the phycobiliproteins. Biochem. 1963;2(2):375–382. doi: 10.1021/bi00902a034. [DOI] [PubMed] [Google Scholar]
- Patel A, Mishra S, Pawar R, Ghosh PK. Purification and characterization of C-phycocyanin from cyanobacterial species of marine and fresh water habitat. Protein Expr Purif. 2005;40:248–255. doi: 10.1016/j.pep.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Patil G, Raghavarao KSMS. Aqueous two-phase extraction for purification of C-phycocyanin. Biochem Eng J. 2007;34:156. doi: 10.1016/j.bej.2006.11.026. [DOI] [Google Scholar]
- Patil G, Chethana S, Sridevi AS, Raghavarao KSMS. Method to obtain high purity of C-phycocyanin. J Chromatogr A. 2006;1127:76–81. doi: 10.1016/j.chroma.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Raghavarao KSMS, Rastogi NK, Gowthaman MK, Karanth NG. Aqueous two-phase extraction for the downstream processing of enzymes/proteins. Adv Appl Microbiol. 1995;41:97–171. doi: 10.1016/S0065-2164(08)70309-5. [DOI] [Google Scholar]
- Raghavarao KSMS, Guinn M, Todd P. Recent developments in aqueous two-phase extraction in bioprocessing. Sep Purif Meth. 1998;27:1–50. doi: 10.1080/03602549809351638. [DOI] [Google Scholar]
- Reddy CM, Vadiraja BB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM. Selective inhibition of cyclo- oxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun. 2000;277:599–603. doi: 10.1006/bbrc.2000.3725. [DOI] [PubMed] [Google Scholar]
- Rito-Palomares M, Nunez L, Amador DJ. Practical application of aqueous two-phase systems for the development of a prototype process for C-phycocyanin recovery from Spirulina maxima. J Chem Technol Biotechnol. 2001;76:1273–1280. doi: 10.1002/jctb.507. [DOI] [Google Scholar]
- Romay C, Gonzalez R. Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J Pharmacol. 2000;52:367–368. doi: 10.1211/0022357001774093. [DOI] [PubMed] [Google Scholar]
- Romay C, Gonzalez R, Ledon N, Remirez D, Rimbaw V. C-phycocyanin: a biliprotein with antioxidant, anti-inflamatory and neuro-protective effects. Curr Protein Pept Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- Silveiria ST, Quines LK, Burkert CAV, Kalil SJ. Separation of phycocyanin from Spirulina platensis using ion exchange chromatography. Bioprocess Biosyst Eng. 2007;31(5):477–482. doi: 10.1007/s00449-007-0185-1. [DOI] [PubMed] [Google Scholar]
- Silveria ST, Burkert JFM, Costa JAV, Burkert CAV, Kalil SJ. Optimization of phycocyanin extraction from Spirulina platensisi using factorial design. Bioresour Technol. 2007;98:1629–1634. doi: 10.1016/j.biortech.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Singh NK, Parmar A, Madamwar D. Optimization of medium components for increased production of C-phycocyanin from Phormidium ceylanicum and its purification by single step process. Bioresour Technol. 2009;100(4):1663–1669. doi: 10.1016/j.biortech.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Soni B, Klavadia B, Trivedi U, Madamwar D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata- isolated from the rocky shores of Bet- Dwarka, Gujrat, India. Process Biochem. 2006;41:2017–2023. doi: 10.1016/j.procbio.2006.04.018. [DOI] [Google Scholar]
- Vadiraja BB, Madyastha KM. C-phycocyanin: a potent peroxyl radical scavenger in-vivo and in-vitro. Biochem Biophys Res Commun. 2000;275:20–25. doi: 10.1006/bbrc.2000.3270. [DOI] [PubMed] [Google Scholar]
- Walter H, Brooks DE, Fisher D. Partitioning in aqueous two-phase systems: theory, methods, uses and applications to biotechnology. Orlando: Academic; 1985. [Google Scholar]
- Zaslavsky BY. Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. New York: Marcel Dekkar Inc; 1995. [Google Scholar]
- Zhang YM, Chen FA. Simple method for efficient separation and purification from Sprulina platensis. Biotechnol Tech. 1999;13:601–603. doi: 10.1023/A:1008914405302. [DOI] [Google Scholar]