Abstract
Coriander (Coriandrum sativum L.), is an annual herb in the Apiaceae family which disperses in Mediterranean and Middle Eastern regions. The Coriander essential oil has been used in food products, perfumes, cosmetics and pharmaceutical industries for its flavor and odor. In Iran, fruits of Coriander used in pickle, curry powders, sausages, cakes, pastries, biscuits and buns. The aim of this study was to investigate microwave radiation effects on quality, quantity and antimicrobial activity of essential oil of Coriander fruits. The essential oils were obtained from the Coriander fruits by hydrodistillation (HD) and Microwave-assisted hydrodistillation (MAHD) then, the oils were analyzed by GC and GC-MS. Antimicrobial activities of essential oils were evaluated against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans by microdilution method. The results indicated that the HD and MAHD essential oils (EO) were dominated by monoterpenoids such as linalool, geranyl acetate and γ-terpinene. The major compound in both EO was linalool which its amount in HD and MAHD was 63 % and 66 %, respectively. The total amount of monoterpenes hydrocarbons in HD EO differ significantly with the amount in MAHD EO (12.56 % compare to 1.82 %). HD EO showed greater activity against Staphylococcus aureus and Candida albicans than MAHD EO. Moreover, their activities against Ecoli and P. aeruginosa were the same with Minimum Inhibitory Concentration (MIC) 0.781 and 6.25 μL mL−1, respectively. By using MAHD method, it was superior in terms of saving energy and extraction time, although the oil yield and total composition decrease by using this method.
Keywords: Coriandrum sativum L, Essential oil, GC-MS, Microwave, Hydrodistillation, Antimicrobial
Introduction
Coriander (Coriandrum sativum L.), is an annual herb from the Apiaceae family which disperses in Mediterranean and Middle Eastern regions (Darughe et al. 2012). In Iran, it is mainly distributed in Tabriz, Damavand, Ghazvin, Yazd, Bushehr, Kerman (Zargari 1991). The essential oil of Coriander has been used in food products, perfumes, cosmetics and pharmaceutical industries for its flavor and odor (Darughe et al. 2012). The seeds of this plant have been used as antispasmodic, appetizer, carminative, diuretic and to treat nausea, seasonal fever, convulsion, insomnia and anxiety (Ramezani et al. 2009; Rajeshwari and Andallu 2011). Furthermore, Coriander is applied to treat cough, bronchitis, dysentery, diarrhea, gout, rheumatism, intermittent fevers and as antiedemic, antiseptic (Duke et al. 2002; Sahib et al. 2012; Varier 1994). There are reports that show the leaves of Coriander have been used as an aphrodisiac (Bruneton 1995). In Iran, leaves and fruits of Coriander are traditionally used for relief of nervousness and insomnia (Emamghoreishi and Heidari-Hamedani 2006) and also fruits used in pickle, curry powders, sausages, cakes, pastries, biscuits and buns (Zargari 1991). Literature reviews show that there are some reports on the chemical composition of essential oil of C. sativum were collected from Iran (Ghannadi and Sadeh 1999; Nejad Ebrahimi et al. 2010) and other countries (Matasyoh et al. 2009; Bhuiyan et al. 2009). There are some studies of antimicrobial activity of Coriander essential oil against different species of Candida (Begnami et al. 2010), gram-positive and negative bacteria (Elgayyar et al. 2001; Tajkarimi et al. 2010). Furthermore, in one study antimicrobial and antioxidant activities of C. sativum essential oil was surveyed (Baratta et al. 1998). In previous study, hypoglycemic activity of C. sativum was investigated and the results indicated that Coriander possesses antihyperglycaemic, insulin releasing and insulin-like activity (Gray and Flatt 1999). The hypolipidemic activity of Coriander seeds has been reported by Ertas et al., that this plant could decrease saturated fatty acid contents (palmitic and stearic acids) in the blood (Ertas et al. 2005).
The main techniques to obtain essential oils (EO) from the medicinal herbs are hydrodistillation (HD), steam distillation, steam and water distillation, maceration, expression. Among these techniques, HD has been the most common method to extract the essential oils from plants. The HD method has several drawbacks such as long extraction time, high energy use and so on. Hence, in order to increase the extraction yield, save energy and time extraction, new approaches is improving, in recent years, the using of Microwave-assisted hydrodistillation (MAHD) method is increasing, especially for extraction (Golmakani and Rezaei 2008; Khanavi et al. 2013). By using microwave energy, the materials reach their boiling point rapidly, so leading to short extraction or distillation time and save energy (Khanavi et al. 2013).
Based on using Coriander as flavor and condiment in food products, the aim of this study was to investigate microwave radiation effects on quality, quantity and antimicrobial activity of essential oil of Coriander fruits.
Materials and methods
Hydrodistillation (HD)
The Coriander fruits (100 g) and 1,000 ml distilled water placed in a round bottom flask and connected to a Clevenger-type apparatus. Hydrodistillation was compeleted for 4 h after boiling. Oil yield of the sample was calculated on a moisture free basis. The oil was dried over anhydrous sodium sulphate and kept at 4 °C in the sealed brown vial until required.
Microwave-assisted hydrodistillation (MAHD)
The oil was obtained from the fruits of Coriander by hydrodistillation for 40 min using a Clevenger type apparatus placed in a modified microwave oven (OM-5224, P.R.C). The oil was dried over anhydrous sodium sulphate and kept at 4 °C in the sealed brown vial until required.
GC and GC-MS spectrometry
Analytical gas chromatography (GC) was carried out using a Shimadzu 15A gas chromatograph with capillary column HP-5 ms (60 m × 0.25 mm, ft 0.25 mm); carrier gas, He; split ratio 1:25 and using a flame ionisation detector (FID). The column temperature was programmed at 60 °C for 3 min and then it was heated to 260 °C at a rate of 5 °C min−1 and the temperature was then kept constant at 260 °C for 15 min. Gas chromatography mass spectroscopy (GC/MS) was performed on a HP 68900 with a HP 5973 quadrupole detector, on capillary column HP-5 ms (5 % phenyl methyl siloxane) (60 m × 0.25 mm; ft 0.25 mm); carrier gas, He; flow rate, 1 mL min−1. The column was held at 60 °C for 3 min and programmed up to 260 °C at the rate of 5 °C min−1, then kept constant at 260 °C for 15 min.
The MS was operated at 70 eV ionization energy. Retention indices were calculated using the retention time of n-alkanes that were injected after the oil at the same chromatographic conditions. Quantitative data were obtained from the electronic integration of the FID peak areas. The components of the oils were identified by comparison of their mass spectra and retention indices with Wiley library and those published in the literature (Adams 1995).
Antimicrobial activity
Antimicrobial activity of the essential oil was determined against gram-positive (Staphylococcus aureus ATCC 6538) and gram-negative (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027), bacteria and Candida albicans by broth microdilution method with visible growth observed by using 96 U-shaped wells plates (NCCLS 2006). After 24 h of incubation at 35 °C, 25 °C the microdilution plates were tested for the absence or presence of visible growth in comparison with that of the growth in drug-free control well. Each experiment was repeated three times with similar results. The endpoint of Minimum Inhibitory Concentration (MIC) is the lowest concentration of the compound at which the test strain does not demonstrate visible growth.
Statistical analysis
Comparisons between the major components of two methods were made using t-tests. Statistically significance level was set at p < 0.05.
Results
The chemical composition of the essential oil obtained from C. sativum in two methods (Microwave-assisted hydrodistillation and hydrodistillation) are represented together with the retention indices in Table 1, the oils yields of the plant was determined as 0.1 % and 0.2 % v/w in MAHD and HD respectively. The GC–MS analyses of both samples revealed the presence of a total of 32 components. Twenty-seven compounds were identified in the hydrodistilled oil (clevenger-type apparatus) which accounted for 90.95 % of the total oil composition. This oil was dominated by monoterpenoids such as, linalool, γ-terpinene, α-pinene and p-cymene. In the same way, 27 compounds were identified from the microwave extracted oil which accounted for 88.97 % of the total oil composition. The MAHD EO was dominated by monoterpenoids such as linalool and geranyl acetate. The structures of main components are shown in Fig. 1.
Table 1.
Chemical composition of essential oil of Coriandrum sativum L. extracted by hydrodistillation and microwave distillation
| No. | Compound | RT a(HD) | RT (MW) | RI b(HD) | RI (MW) | %HD | %MW |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 11.46 | 11.33 | 931 | 929 | 3.46 | 0.15 |
| 2 | β-Pinene | 13.40 | – | 971 | – | 0.63 | – |
| 3 | β-Myrcene | 14.24 | – | 989 | – | 0.27 | – |
| 4 | Decene | – | 14.76 | – | 1000 | – | 0.29 |
| 5 | p-Cymene | 15.98 | 15.95 | 1023 | 1015 | 2.94 | 0.30 |
| 6 | γ-Terpinene | 17.92 | 17.78 | 1062 | 1058 | 5.26 | 1.37 |
| 7 | Linalool | 22.15 | 21.51 | 1146 | 1133 | 66.29 | 63.27 |
| 8 | Camphor | 23.39 | 22.61 | 1171 | 1155 | 0.56 | 0.28 |
| 9 | Citronella | 23.51 | 22.89 | 1173 | 1161 | 0.21 | 0.24 |
| 10 | Borneol | 24.05 | 23.53 | 1185 | 1174 | 0.23 | 0.11 |
| 11 | Terpineol-4-ol | 24.47 | 24.02 | 1193 | 1184 | 0.30 | 0.18 |
| 12 | α-Terpineol | 25.00 | 24.64 | 1204 | 1196 | 0.53 | 0.11 |
| 13 | Decanal | 25.47 | 25.21 | 1214 | 1209 | 0.25 | 0.19 |
| 14 | Citronella | 26.53 | 26.39 | 1237 | 1234 | 0.54 | 0.51 |
| 15 | Carvone | 27.12 | 26.96 | 1249 | 1246 | 0.35 | 0.25 |
| 16 | Geraniol | 27.77 | 27.63 | 1264 | 1260 | 1.55 | 0.91 |
| 17 | Decenal | – | 27.77 | – | 1263 | – | 0.18 |
| 18 | Decanol | 28.33 | – | 1275 | – | 0.11 | – |
| 19 | Bornyl acetate | – | 28.87 | – | 1287 | – | 0.13 |
| 20 | Undecanal | 29.79 | – | 1307 | – | 0.08 | – |
| 21 | Myrtenyl acetate | 30.59 | 30.62 | 1325 | 1326 | 0.11 | 0.15 |
| 22 | Citeral dimethoxy | 31.77 | 31.86 | 1352 | 1354 | 0.10 | 0.19 |
| 23 | Neryl acetate | 32.24 | 32.35 | 1363 | 1365 | 0.12 | 0.17 |
| 24 | Geranyl acetate | 33.43 | 33.49 | 1390 | 1391 | 0.06 | 8.49 |
| 25 | Dodecanal | 34.17 | 34.30 | 1407 | 1415 | 0.12 | 0.34 |
| 26 | Caryophyllene | 34.72 | 34.82 | 1420 | 1427 | 0.08 | 0.15 |
| 27 | Dodecenal | 36.72 | 36.85 | 1469 | 1476 | 2.12 | 2.90 |
| 28 | Dodecanoic acid | 40.66 | – | 1567 | – | 0.08 | – |
| 29 | Tetradecanol | 44.40 | 44.67 | 1665 | 1672 | 0.12 | 0.26 |
| 30 | Tetradecanoic acid | – | 48.93 | – | 1790 | – | 2.89 |
| 31 | Tetradecanoic acid ethyl ester | 49.09 | – | 1665 | – | 4.56 | – |
| 32 | Hexadecanoic acid | – | 55.56 | – | 1990 | – | 4 |
| 33 | Osthole | – | 60.23 | – | 2144 | – | 1.29 |
| Monoterpenes hydrocarbons | 12.56 | 1.82 | |||||
| Monoterpenes oxygenated | 70.95 | 74.99 | |||||
| Sesquiterpenes hydrocarbons | 0.08 | 0.15 | |||||
| Others | 7.44 | 12.16 | |||||
| Total | 91.03 | 89.3 |
a RT retention time
b RI retention index on HP-6890 with reference to n-alkanes injected after the oil at the same chromatographic conditions
Fig. 1.
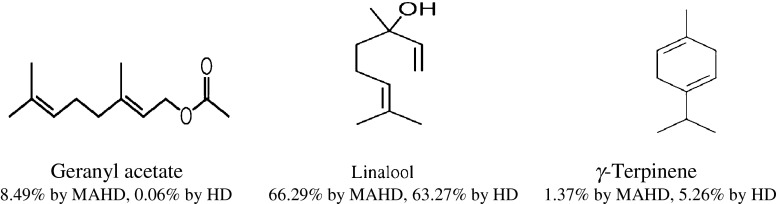
Main components in the essential oils of coriander
The broth microdilution method was used to determine the antimicrobial activity of MAHD and HD Coriander EO against gram-positive and gram-negative bacteria and Candida, the results are shown in Table 2, HD EO showed greater activity against S. aureus and C. albicans than MAHD EO, significantly (P < 0.05). Moreover, their activity against Ecoli and P. aeruginosa were the same (MICs = 0.781 and 6.25 μL mL−1, respectively).
Table 2.
Minimum inhibitory concentration (MIC) of coriander essential oil against selected bacteria and Candida albicans
| MICa | ||||
|---|---|---|---|---|
| Microorganism | MAHDb essential oil | Classical HDb essential oil | Gentamycin | Nystatin |
| Staphylococcus aureus | 6.25 | 3.125 | 0.62 | – |
| Escherichia coli | 0.781 | 0.781 | 0.009 | – |
| Pseudomonas aeruginosa | 6.25 | 6.25 | 2.49 | – |
| Candida albicans | 0.39 | 0.097 | – | 0.02 |
aMIC was determined by broth micro dilution method and expressed in μL mL−1
b MAHD microwave-assisted hydrodistillation, HD hydrodistillation
Discussion
According to results in current study, the major compound in both EO was linalool which its amount in HD and MAHD was 66.27 ± 0.23 % and 63.29 ± 0.38 %, respectively. Significant difference was observed in the linalool content of MAHD EO and HD EO (P < 0.05). The total amount of monoterpene hydrocarbons in HD EO differ significantly with the amount in MAHD EO (12.56 ± 0.11 % compare to 1.82 ± 0.03 %) (P < 0.05). Moreover there were a difference between oxygenated monoterpenes content in MAHD EO and HD EO (74.99 ± 0.61 % and 70.95 % ± 0.67 respectively) (P < 0.05). The content of geranyl acetate in MAHD EO (8.49 ± 0.31 %) increased compared to HD EO (0.065 ± 0.00), significantly (P < 0.05).
A critical observation of the oil compositions revealed that higher amounts of oxygenated monoterpenes are present in the essential oil isolated by MAHD (74.99 %) in comparison with the oil extracted by HD (70.95 %). MAHD method was important in terms of saving energy and extraction time (40 min compared to 240 min in HD method) and the amount of oxygenated monoterpenes which play the great role in the essential oil properties increased, although the oil yield and total composition decrease by using this method. The EO of Coriander showed antimicrobial and antifungal activity and HD EO showed more potent anti-Candida and anti- Staphylococcus than MAHD EO.
The previous studies showed that linalool was the main compound on the EO Coriander collected in Serbia (Samojlik et al. 2010) and Romania (Tsagkli et al. 2012) which agrees with our research that linalool was major component in the MAHD and HD EO. In the same way, Singh et al. investigated the EO chemical composition of Coriander from India and found that the major compounds were linalool (75.3 %), geranyl acetate (8.1 %) and α-pinene (4.1 %) (Singh et al. 2006). There is a report about the MAHD and HD EO of Coriander in which, their results were similar to our research. In MAHD essential oil the content of geranyl acetate increased while, monoterpene hydrocarbons content decreased. Furthermore oxygenated monoterpenes increased in MAHD EO (Kosar et al. 2005). In another study, microwave radiation effects on quality and quantity essential oil of Mangifera indica L. were investigated that, the results indicated content of oxygenated monoterpenes were more than HD EO, so, these studies showed oxygenated components appeared to increase in MAHD essential oils (Wang et al. 2010).
Linalool is the major component in the MAHD and HD EO which has been found to have antimicrobial activity against various microbes (Carson and Riley 1995) and it inhibits the spore germination and fungal growth by the mechanism of respiratory suppression of aerial mycelia (Lahlou and Berrada 2001). Geranyl acetate which is the second major component in the MAHD EO, has antifungal activity and anti-inflammatory effect (José Goncalvesa et al. 2011).
The antimicrobial activity of the essential oil could be contribute to the presence of active compounds, such as linalool (Koutsoudaki et al. 2005), α-pinene and β-pinene (Dorman and Deans 2000), p-cymene and γ-terpinene (Xianfei et al. 2007).
In the study conducted by Matasyoh, the Coriander leaves EO showed antibacterial and antifungal activity against C. albicans, gram positive and gram negative bacteria, also its major components were aldehydes and alcohols which accounted for 55.5 % and 36.3 % of the oil respectively, and linalool was accounted for 0.32 % of the oil (Matasyoh et al. 2009). This study could show us that other components of EO also have the capacity to act against microbe and also, showed the content of linalool in the Coriander leaves EO is less than the Coriander fruits EO, but aldehydes and alcohols were more than fruits EO. Following of this fact, aldehydes and alcohols could show antimicrobial activity and these components had various effects against microorganism which is related to the functional group present and hydrogen bonding parameters. In the same way dodecenal accounted for 2.12 % and 2.90 % in HD and MAHD EO respectively, is an aldehyde which could consider as one of the antimicrobial active ingredient of Coriander EO (Kubo et al. 2004). In previous studies synergistic antimicrobial activity between Coriander essential oil and different antimicrobial drugs (Cefoperazone, Chloramphenicol, Ciprofloxacin, Gentamicin, Tetracycline, Piperacillin and Amphotericin B) was investigated (Duarte et al. 2012; Silva et al. 2011) according to these studies, Coriander essential oil had synergistic antimicrobial effects with antimicrobial drugs. The essential oil of Coriander had fungicidal ability with inhibition of germ tube formation and synergetic effect with Amphotericin B (Silva et al. 2011). Moreover, it was shown that the antimicrobial activity of Coriander is not only for its linalool, but also interaction between different components in essential oil led to antimicrobial activity (Begnami et al. 2010).
Conclusions
In MAHD method, time extraction is significantly shorter than HD method. The MAHD with lower energy consumption than HD is offered instead traditional methods. Therefore, considering the operation cost MAHD could be carried out using half of the expenses required by HD. However the antimicrobial activity of EO obtained by HD against Staphylococcus aureus and Candidia albicans is greater than MAHD. The MAHD yielded less percentage of γ-terpinene, α-pinene and p-cymene compared to HD, therefore, HD is more efficient method for obtaining essential oil of Coriander. Furthermore the important compound of Coriander EO as linalool is not significantly different in both method quantitatively (63 % compare to 66 %).
References
- Adams RP. Identification of essential oil components by gas chromatography/ mass spectroscopy. Carol Stream: Allured Publishing Corporation; 1995. [Google Scholar]
- Baratta MT, Dorman HJD, Deans SG, Biondi DM, Ruberto G. Chemical composition, antimicrobial and antioxidative activity of laurel, sage, rosemary, oregano and Coriander essential oils. J Essent Oil Res. 1998;10:618–627. doi: 10.1080/10412905.1998.9700989. [DOI] [Google Scholar]
- Begnami AF, Duarte MCT, Furletti V, Rehder VLG. Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro. Food Chem. 2010;118:74–77. doi: 10.1016/j.foodchem.2009.04.089. [DOI] [Google Scholar]
- Bhuiyan NI, Begum J, Sultana M. Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh J Pharmacol. 2009;4:150–153. doi: 10.3329/bjp.v4i2.2800. [DOI] [Google Scholar]
- Bruneton J. Pharmacognosy, phytochemistry, medicinal plants. Paris: Lavoisier; 1995. [Google Scholar]
- Carson CF, Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca acternifonlia. J Appl Bacteriol. 1995;78:264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- Darughe F, Barzegar M, Sahari MA. Antioxidant and antifungal activity of Coriander (Coriandrum sativum L.) essential oil in cake. IFRJ. 2012;19:1253–1260. [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbial. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ferreira S, Silva F, Domingues FC. Synergistic activity of Coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine. 2012;19:236–238. doi: 10.1016/j.phymed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Duke JA, Bogenschutz-Godwin MJ, Pu Celliar J, Duke PAK (2002) Handbook of medicinal herbs. Boca Raton
- Elgayyar M, Draughon FA,Golden DA, Mount JR (2001) Antimicrobial activity of essential oils from plants against selected patogenic and sarophytic microorganism. J Food Protect 1019–1024 [DOI] [PubMed]
- Emamghoreishi M, Heidari-Hamedani G. Sedative-hypnotic activity of extracts and essential oil of Coriander seeds. Iran J Med Sci. 2006;31:22–27. [Google Scholar]
- Ertas ON, Guler T, Cftc M, Dalklc B, Ylmaz O. The effect of a dietary supplement Coriander seeds on the fatty acid composition of breast muscle in Japanese quail. Recl Med Vet. 2005;156:514–518. [Google Scholar]
- Ghannadi A, Sadeh D. Volatile constituents of the fruit of Coriandrum sativum L. from Isfahan. DARU. 1999;7:12–14. [Google Scholar]
- Golmakani MT, Rezaei K. Comparison of microwave-assisted hydrodistillation with the traditional hydrodistillation method in the extraction of essential oils from Thymus vulgaris L. Food Chem. 2008;109:925–930. doi: 10.1016/j.foodchem.2007.12.084. [DOI] [PubMed] [Google Scholar]
- Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti- diabetic plant Coriandrum sativum (Coriander) Br J Nutr. 1999;81:203–209. doi: 10.1017/S0007114599000392. [DOI] [PubMed] [Google Scholar]
- José Goncalvesa M, Teresa Cruzb M, Cristina Tavaresc A, Cavaleiroa C, Celeste Lopesb M, Canhotoc J, Salgueiroa L. Composition and biological activity of the essential oil from Thapsia minor, a new source of geranyl acetate. Ind Crop Prod. 2011;35:166–171. doi: 10.1016/j.indcrop.2011.06.030. [DOI] [Google Scholar]
- Khanavi M, Hajimehdipoor H, Emadi F, Kalantari Khandani N. Essential oil compositions of Thymus kotschyanus Boiss. Obtained by hydrodistillation and microwave oven distillation. J Essent Oil Bear Plants. 2013;16:117–122. doi: 10.1080/0972060X.2013.764159. [DOI] [Google Scholar]
- Kosar M, Ozek T, Goger F, Kurkcuoglu M, Can Baser KH. Comparison of microwave-assisted hydrodistillation and hydrodistillation methods for the analysis of volatile secondary metabolites. Pharm Biol. 2005;43:491–495. doi: 10.1080/13880200500220136. [DOI] [Google Scholar]
- Koutsoudaki C, Krsek M, Rodger A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia. J Agric Food Chem. 2005;53:7681–7685. doi: 10.1021/jf050639s. [DOI] [PubMed] [Google Scholar]
- Kubo I, Fujita KI, Kubo A, Nihei KI, Ogura T. Antibacterial activity of Coriander volatile compounds against Salmonella cholerasuis. Agric Food Chem. 2004;52:3329–3332. doi: 10.1021/jf0354186. [DOI] [PubMed] [Google Scholar]
- Lahlou M, Berrada R. Composition and niticidal activity of essential oils of three chemotypes of Rosmarinas officinalis L. Pharm Biol. 2001;4:207–210. [Google Scholar]
- Matasyoh JC, Maiyo ZC, Ngure RM, Chepkorir R. Chemical composition and antimicrobial activity. Food Chem. 2009;113:526–529. doi: 10.1016/j.foodchem.2008.07.097. [DOI] [Google Scholar]
- NCCLS . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. PA: Author, Wayne; 2006. [Google Scholar]
- Nejad Ebrahimi S, Hadian J, Ranjbar H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat Prod Res. 2010;24:1287–1294. doi: 10.1080/14786410903132316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshwari U, Andallu B. Medicinal benefits of Coriander (Coriandrum Sativum L) Spatula DD. 2011;1:51–58. doi: 10.5455/spatula.20110106123153. [DOI] [Google Scholar]
- Ramezani S, Rasouli F, Solaimani B. Changes in essential oil content of Coriander (Coriandrum sativum L.) aerial parts during four phonological stages in Iran. Jeobp. 2009;12:683–689. [Google Scholar]
- Sahib NG, Anwar F, Gilani AH, Hamid AA, Saari N, Alkharfy KM (2012) Coriander (Coriandrum sativum L.): a potential source of high-value components for functional foods and nutraceuticals- a review. Phytother Res [DOI] [PubMed]
- Samojlik I, Lakić N, Mimica-Dukić N, Daković-Svajcer K, Bozin B. Antioxidant and hepatoprotective potential of essential oils of Coriander (Coriandrum sativum) J Agric Food Chem. 2010;58:8848–8853. doi: 10.1021/jf101645n. [DOI] [PubMed] [Google Scholar]
- Silva F, Ferreira S, Duarte A, Mendonça DI, Domingues FC. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine. 2011;19:42–47. doi: 10.1016/j.phymed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Singh G, Maurya S, De Lampasona MP, Catalan CAN. Studies on the essential oils, part 41. Chemical composition, antifungal, antioxidant and sprout suppressant activities of Coriander (Coriandrum sativum) essential oil and its oleoresin. Flavour Fragr J. 2006;21:472–479. doi: 10.1002/ffj.1608. [DOI] [Google Scholar]
- Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- Tsagkli A, Hancianu M, Aprotosoaie C, Cioanca O, Tzakou O. Volatile constituents of Romanian Coriander fruit. Rec Nat Prod. 2012;6:156–160. [Google Scholar]
- Varier PS. Coriandrum sativum in Indian medicinal plants: a compendium of 500 species. Chennai: Orient Longman, LtD; 1994. [Google Scholar]
- Wang H, Liu Y, Wei S, Yan Z, Lu K. Comparison of microwave-assisted and conventional hydrodistillation in the extraction of essential Oils from Mango (Mangifera indica L.) flowers. Molecules. 2010;15:7715–7723. doi: 10.3390/molecules15117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianfei X, Xiaoqiang C, Shunying Z, Guolin Z. Chemical composition and antimicrobial activity of essential oils of Chaenomeles speciosa from China. Food Chem. 2007;100:1312–1315. doi: 10.1016/j.foodchem.2005.12.011. [DOI] [Google Scholar]
- Zargari A. Medicinal plants. Tehran: Tehran University Publications; 1991. [Google Scholar]


