Abstract
This study is undertaken to determine the storage stability of Chemlali extra-virgin olive oil (EVOO) in different containers such as clear and dark glass bottles, polyethylene (PE) and tin containers. The different oil samples were stored under light at room temperature. Quality parameters monitored during a 6-month-storage period included: acidity, peroxide value (PV), spectrophotometric indices (K232 and K270), chlorophyll and carotene pigments, fatty acids and sterol compositions, total phenols, Rancimat induction time as well as sensory evaluation. Tin containers and dark glass bottles recorded the lowest oxidation values. In addition, oil packed in tin containers and dark glass bottles showed better physicochemical and organoleptic characteristics than that stored in clear glass bottles and PE containers. A significant decrease (p < 0.05) in the antioxidant contents (carotenes, chlorophylls and total phenols) was observed in the oil stored in the clear glass bottles and PE containers. Such results proved that the storage of oil in tin containers and dark glass bottles appeared most adequate, and showed a gradual loss of quality during storage, especially in PE containers and clear glass bottles. This study has shown that the best packaging materials for the commercial packing of Chemlali extra-virgin olive oil are tin containers and dark glass bottles.
Keywords: Chemlali extra-virgin olive oil, Storage time, Packaging materials, Oil quality
Introduction
Olive oil is a very versatile product. Long known to many generations in the Mediterranean world as essential to their health and diet, it is now widely appreciated around the world for its nutritional, health and sensory properties.
The olive oil sector plays an important role in the Tunisian economy, providing both employment and export revenue. Indeed, with an annual production of 170,000 t, Tunisia is the world’s fourth largest producer of olive oil.
Virgin olive oil is a product widely produced and consumed throughout the ages in the Mediterranean cuisine and is highly appreciated for its delicious taste and aroma, as well as for its nutritional properties (Moldao-Martins et al. 2004).
The nutritional benefits are primarily related to the fatty acid composition, mainly due to both the high content of oleic acid and the balanced ratio of saturated and polyunsaturated fatty acids. In addition, olive oil presents considerable amounts of natural antioxidants and is considered important in the prevention of many diseases (Bouaziz et al. 2010; Huang and Sumpio 2008; Ruiz-Canela and Martínez-González 2011).
This excellent quality of extra-virgin olive oil is the culmination of a process that begins with the tree and finishes in the bottle. Thus, it is necessary to care for each step of the process and the factors that can affect its commercial life (oxygen, light, temperature, etc.), leading to deterioration in quality as a consequence of oxidative and hydrolytic degradations (Psomiadou and Tsimidou 2002a, b). Like other products that are produced in a limited period of time, but that are consumed throughout the year, it must be stored, and these storage and packing conditions determine the commercial life of the olive oil (Méndez and Falqué 2007; Hrncirik and Fritsche 2005; Kiritsakis and Dugan 1984; Zanoni et al. 2005).
Oxidation constitutes a major factor for the quality deterioration of olive oil. The oxidation rate depends on a number of factors including the availability of oxygen and the presence of light and temperature. Auto-oxidation, which is oxidation in the absence of light, follows a free radical mechanism where, initially, the absorption of oxygen results in the formation of hydroperoxides. These labile compounds further decompose to produce a complex mixture of volatile compounds such as aldehydes, ketones, hydrocarbons, alcohols and esters responsible for the deterioration of olive oil flavor termed “oxidative rancidity” (Frankel 2005; Morales et al. 1997). In turn, when olive oils are exposed to light, photo-oxidation occurs through the action of natural photosensitizers (i.e. chlorophyll), which react with triplet oxygen to form the excited state singlet oxygen. Thus, protection from direct light is required for commercial edible oils (Bradley and Min 1992; Khan and Shahidi 1999).
Packaging can directly influence olive oil quality by protecting the product from both oxygen and light. The shelf life of the oils exposed to intense artificial light and diffused daylight is shorter than that of oils kept in the dark. Moreover, the storage temperature, the use of nitrogen atmosphere and the reduction of the oxygen in the headspace volume can appreciably control quality changes during storage time. The balance between stability and autoxidation should be controlled, and the optimum storage conditions should be well known (at high, low or RT, exposure to air and light) as well as the container type for the storage (polyethylene terephthalate, PET, Tetra-Brik1, clear or brown glass bottles) (Samaniego-Sánchez et al. 2012; Tawfik and Huyghebaert 1999).
The stability of extra-virgin olive oil is primarily due to its fatty acid composition and the antioxidant activity of its polyphenols and tocopherols. The major degradative aspects of storage involve the oxidation of fats, especially in the presence of trace metals.
The most significant factors affecting the olive oil quality after processing and during storage are environmental: temperature, exposure to light and contact with oxygen (Allen 1994; Pristouri et al. 2010). Much work has been done on the effect of storage conditions and packaging materials on olive oil quality. In this context, Pagliarini et al. (2000) studied the stability of extra-virgin olive oil by different types of bottles and under different commercial conditions and proved that it was not significantly influenced by different controlled bottling, transport and storage conditions in supermarkets.
Commonly, extra-virgin olive oil is packaged in glass, tin or plastic containers. The primary advantage for the first two is their impermeability to gases, but the glass and plastic containers have some disadvantages as they favour photo-oxidation.
The aim of this study is to investigate the change in Chemlali extra-virgin olive oil quality, according to the type of container and storage time, based on the analysis of such parameters as acidity, peroxide value (PV), spectrophotometric indices (K232 and K270), chlorophyll and carotene pigments, fatty acids and sterol compositions, total phenols, Rancimat induction time as well as sensory evaluation.
Materials and methods
Olive sampling and oil extraction
The olive oil samples were obtained from Tunisian quality-assured industrial oil mills during the 2008/2009 crop season. The samples stability was analyzed under diffused light and room temperature. Materials which have been used for olive oil packaging are glass (clear and dark), plastics (polyethylene “PE”) and metal (tin internally covered with a suitable varnish). The reference sample was processed immediately after extraction, while the other lots were stored in containers of 5 L during 25, 50, 75 and 180 days at room temperature. This study was carried out over a period of 6 months after the extraction of Chemlali oil.
Phenolic compound extraction
An amount of 4 g of the oil sample was added to 2 mL of n-hexane and 4 mL of a methanol/water (60/40, v/v) solution in a 20 mL centrifuge tube. After vigorous mixing, they were centrifuged for 3 min. The hydroalcoholic phase was collected, and the hexanic phase was re-extracted twice with 4 mL of methanol/water (60/40, v/v) solution each time. Finally, the hydroalcoholic fractions were combined, washed with 4 mL of n-hexane to remove the residual oil then concentrated and dried by evaporative centrifuge in vaccum at 35 °C.
Analytical methods
Quality index determination
The determination of free acidity, peroxide value and specific absorbance at 232 and 270 nm (K232 and K270) was determined according to the official methods described by the International Olive Council (COI 2010).
Chlorophyll and carotenoid determination
Chlorophyll and carotenoid compounds (mg.kg−1 of oil) were determined at 670 and 470 nm, respectively, in cyclohexane using the specific extinction values, by the method of Mínguez-Mosquera et al. (1991)
where A is the absorbance and d is the spectrophotometer cell thickness (1 cm).
Fatty acid determination (GC)
The fatty acid composition of the Chemlali oil was determined by gas chromatography (GC) as fatty acid methyl esters (FAMEs). FAMEs were prepared by saponification/methylation with sodium methylate according to the EEC 2568/1991. A chromatographic analysis was performed in a Shimadzu set 17 A Series II gas chromatography equipped with a split/split-less injector (T = 230 °C) and flame ionization detector (FID) (T = 250 °C). A capillary column (stabilwax, Restek, length 50 m, internal diameter 0.32 mm and film thickness 0.25 μm) was used. The column temperature was isothermal at 180 °C. Fatty acids were identified by comparing retention times with standard compounds. Ten fatty acids were considered in this study. These were palmitic (16:0), hypogeic (16:1n-9) + palmitoleic (16:1n-7), margaric (17:0), margaroleic (17:1), stearic (18:0), oleic (18:1n-9) + Z-vaccenic (18:1n-7), linoleic (18:2), linolenic (18:3), arachidic (20:0) and gondoic (20:1n-9) acids expressed as percentages of fatty acid methyl esters.
Iodine values (IV) were calculated from the fatty acid percentages using the following formula:
Oxidative susceptibility (OS) of oils was estimated according to Cert et al. (1996) by means of the formula:
Sterol determination
The analysis of sterolic fractions extracted from olive oil was determined according to the method adopted by EEC 2568/1991. Briefly, 5 g of olive oil from Chemlali variety were added to α-cholestanol, used as internal standard and saponified with potassium hydroxide solution in ethanol (2 N). After 1 h of boiling, 100 mL of water were added and the extraction of the unsaponifiable fraction was carried out by 200 mL of ethyl ether 20 mg of which was dissolved in 0.5 mL of chloroform, then separated by Silica gel plate chromatography. The elution was achieved by a mixture of hexane and ether (65/35, v/v) then the plate was pulverized by a solution of 2,7-dichlorofluorescein (0.2 % in the ethanol), and then the band corresponding to sterols was scraped. Sterols recovered from the plate were dissolved in chloroform and filtered through a paper filter. The solvent was evaporated under N2 and the sterols were transformed into trimethylsilyl ethers by the addition of pyridine-hexamethyldisilizane-trimethylchlorosilane (9:3:1, v/v/v) (Supelco, Bellefonte, USA), left for 15 min, and then centrifuged. The mixture was analyzed by gas chromatography using a chromatograph Shimadzu set 17 A equipped with a capillary column (30 m length, 0.32 mm, 0.25 μm film thickness) coated with a stationary phase (stabilwax, Restek, Rtx-5MS) and a flame-ionisation detector. The injector was operated in splitless mode. The operating conditions were as follow: carrier gas: N2 at 1 mL.min−1; column temperature: 260 °C; injector and detector temperature: 280 °C and 290 °C, respectively; injection volume: 5 μL. The compounds were quantified by adding an internal pattern (α-cholestanol).
Total phenols content
The total phenol content of the olive oil samples was determined using the Folin–Ciocalteu method (Bouaziz et al. 2010). Briefly, a 50 μL aliquot of the extracts was assayed with 250 μL Folin reagent and 500 μL sodium carbonate (20 %, w/v). The mixture was vortexed and diluted with water to a final volume of 5 mL. After incubation for 30 min at room temperature, the absorbance was read at 765 nm; total phenols were expressed as gallic acid equivalents (GAE) using a calibration curve of a freshly prepared gallic acid solution. For gallic acid, the absorbance curve versus concentration is described by the equation:
Evaluation of oxidative stability
Oxidative stability was measured with the Rancimat 743 apparatus (Metrohm X, Basel, Switzerland) according to Gutierrez (1989). Stability was expressed as the oxidation induction time (h), using an oil sample of 3.6 g warmed to 101.6 °C and an air flow of 10 L/h.
Sensory assessment
The sensory evaluation of Chemlali extra-virgin olive oil (EVOO) samples was carried out in accordance with the official method for the olive oil sensory assessment. A total of 15 mL of each sample was kept in standardized glasses at 29 ± 2 °C for 15 min and then evaluated by five assessors. Assessors were free to qualify EVOOs with their own sensory descriptors in addition to those described in the official method (Keceli and Gordon 2001).
Statistical analysis
The results were expressed as mean ± standard deviation (SD) of three measurements for the analytical determination. Significant differences between the values of all parameters were determined at p < 0.05 according to the one way ANOVA: Student Newman–Keuls test, using SPSS Statistics 17.0 for Windows.
A uniform hierarchical cluster analysis (HCA) methodology was applied on data. HCA performed using XLSTAT software for Windows (v.2013.2.03, Addinsoft, New York, USA). Each cluster was determined by the following parameters: Dissimilarity, Euclidean distance matrix and the Ward’s method, generating a dendrogram for the Chemlali olive oil samples.
Results and discussion
Physicochemical properties of stored oils
To determine the effect of the container type on the oil quality during storage time, the results of each parameter were compared with those obtained for the samples analyzed immediately after opening the original cans (controls: T = 0 day). The values of the initial acidity of the extra-virgin olive oils studied are below the maximum levels established by the Regulations EEC/2568/1991 and EEC/2472/1997 of the European Union Commission.
The effects of packaging material, storage at room condition and storage time on measured properties were determined. Because edible oils are subject to oxidative rancidity, packaging in plastics poses some problems. Interactions between oxygen and unsaturated fatty acids are the major cause of quality deterioration in vegetable oils during storage.
Changes in free fatty acids
After 75 days of storage (Fig. 1), a similar slight increase in acidity of the studied oil in the different containers was observed, although this was less marked in a tin, followed by a dark glass bottle. From 75 to 180 days of storage, the increase in acidity in all the containers was detected, but to a lesser degree in a tin container, followed by dark glass and clear glass bottles. The plastic container appeared to be the container in which the hydrolytic processes of the glycerides was most intense. Although the glass bottle does not allow the penetration of gases, it is sensitive to the action of light on the fatty acids, which also produces an increase in acidity. The evolution of this chemical parameter shows slightly lower values in the olive oils stored in tin containers than in dark glass bottles. These results agree with the study performed by Tawfik and Huyghebaert (1999) during 2 months that confirmed the increase of acidity in olive oil over time. A similar effect was reported by Méndez and Falqué (2007) after 3 and 6 months of storage.
Fig. 1.
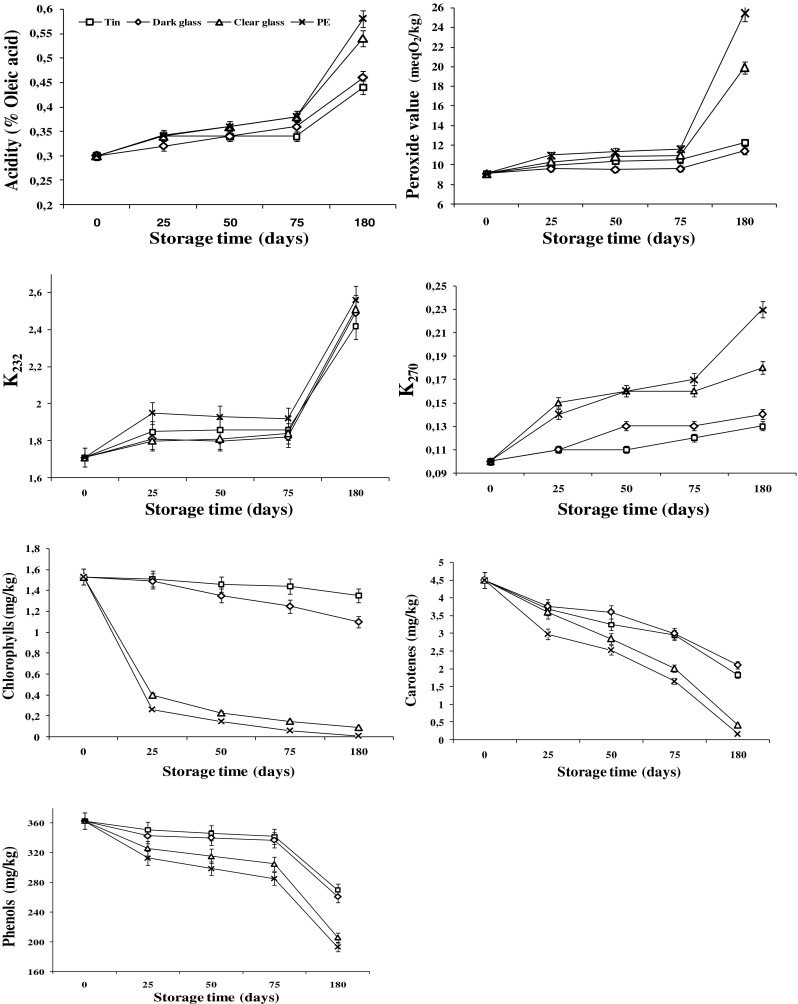
Changes in physic-chemical characteristics of Chemlali extra-virgin olive oil during storage time and in different containers; (□): tin; (◊): dark glass; (∆): clear glass; (x): polyethylene (PE). Error bars indicate the SD of the mean of three replicates (n = 3)
Changes in peroxide value (PV)
Hydroperoxide formation in a crude oil can serve as an indicator of the oxidative processes and in turn, of the oil quality. Thus, a rapid hydroperoxide formation demonstrates the initiation of the oxidative reactions that precede rancidity (Elez-Martinez et al. 2007).
The amount of PVs showed significant increases in oil stored in different packaging material (Fig. 1). It is clear that the EVOO packaged in the dark glass bottle gave the lowest PV, while oil packaged in the PE container gave the highest PV. The latter (25.40 meqO2/kg) was recorded for the oil sample packaged in a PE container after 6 months of storage. This is due to the combined effects of the relatively high permeability of PE to oxygen as well as the transmittance of light. The oil that was filled in dark glass bottles and stored at room conditions under light showed the lowest deterioration PV of 11.41 meqO2/kg after 6 months of storage. It should be noted that the quality of EVOO is highly affected by the ability of the container to exclude light and oxygen, which further retards oxidative changes.
According to Baiano et al. (2005), this behaviour can be explained if it is accepted that there was an initial increase in hydroperoxides (odorless, flavorless compounds, produced during the primary step of oxidation) and that they successively broke down into aldehydes and ketones, which are responsible for off-flavors (secondary oxidation). Non-volatile compounds such as oligopolymers and cyclic compounds are also produced by the breakdown of hydroperoxides. These results are in accordance with the study performed by Méndez and Falqué (2007) during 3 and 6 months that confirmed the variation of PV in olive oil over time.
Changes in K232 and K270 parameters
The specific extinctions at 232 and 270 nm, which revealed the oxidative deterioration and purity of the oils, are shown in Fig. 1. In PE material, K232, an indicator of the formation of hydroperoxide and conjugated dienes, increased in value starting from the 10th day. K270, a good indicator of the secondary phase of oxidation because it is related to the presence of final products such as trienes or unsaturated carbonyl compounds which account for the characteristic flavor of an oxidized oil, showed the same trends (Gertz and Klostermann 2000).
The values of the K270 and K232 coefficients are between 0.10 and 0.23 for K270, and between 1.71 and 2.56 for K232, within the limits permitted by legislation during 6 months of storage. However, the EVOO stored in a tin container and in a dark glass bottle showed lower levels of absorbance at 270 nm than in the other containers and there was a lesser degree of oxidation (secondary) in the tin container.
An increase in oxidation occurred in the samples stored in clear glass and PE plastic containers due to the increase in the number of compounds resulting from the degradation of the hydroperoxides, which was confirmed by an increase of the K270 coefficient (Gasparoli et al. 1991; Olías-Jiménez and Gutiérrez-González-Quijano 1971). But, compounds susceptible to primary oxidation were still found in the samples, corroborated by the values of the K232 coefficient, which increased in all the samples stored in clear glass and PE plastic containers. Higher values of K232 coefficient were found in the olive oils stored in PE than in the rest of the containers. This could be due to the joint action of light and the permeability of this type of container to oxygen, which catalyses the oxidation reaction and the material from which the container was prepared as previously reported (Kiritsakis and Dugan 1984).
The lesser oxidative alteration of EVOO in the dark glass bottles and tin containers could be related to the antioxidant effect of the natural pigments acting synergistically with the phenols. It is worth noting that the index K232 increases with the PV while the decrease in the PV is followed by the increase in K270. It is also characteristic that K232 and K270 present the max value in different times. Glass acts as a barrier to oxygen avoiding the loss of certain components that deteriorate under its presence but it allows the direct action of light on the olive oil and this could promote oxidative rancidity as a consequence of its sensibility to photo-oxidation.
Therefore, the storage of extra-virgin olive oil in PE containers, under the action of both light and even small oxygen permeability could not be suggested as the most appropriate means for maintaining its quality.
Finally, as a consequence of the results reported herein, the packaging material should ensure protection from light in order to maintain the olive oil quality, especially when the oil is stored under the studied commercial conditions. These results are in agreement with the study performed by Dabbou et al. (2011) after 3 and 6 months that confirmed the variation of K232 and K270 in olive oil over time. It was reported that K232 and K270 varied after 3 months of olive oil storage in different containers.
Changes in chlorophylls and carotenes contents
Photooxidation of olive oil in the presence of naturally occurring chlorophyll pigments produces singlet oxygen, which acts with unsaturated fatty acids and produces fatty acid hydroperoxides. This photooxidation results in a change in color and because of the formation of hydroperoxide decomposition products, it develops undesirable odor and flavor constituents (Rahmani and Saari-Csallany 1998).
Carotenoids are effective inhibitors of photo-oxidation by quenching singlet oxygen and triplet excited states of photosensitizers. The physical quenching mechanism of carotenoids is based on their low singlet energy state, which facilitates the acceptance of energy from singlet oxygen. Chlorophyll is also functioned as a photosensitizer resulting in the rapid oxidation of the oil and the added components and loss of color (Psomiadou and Tsimidou 2002a).
Chlorophylls and carotenoids play an important role in oxidative stability due to their antioxidant nature in the dark and prooxidant activity in the light and are mainly responsible with pheophytins for the colour of virgin olive oil that varies from yellow-green to greenish gold (Criado et al. 2008).
Changes in the concentrations of chlorophylls and carotenes in the extra-virgin olive oil samples during storage for 6 months in tin, PE, clear and dark glass containers are shown in Fig. 1. The lowest chlorophyll concentrations were observed in oil stored in PE container and clear glass bottle. In fact, the oil stored in these containers exhibited a reduced intensity of the chlorophyll pigments during 6 months of experiment, with a very small reduction in the oil stored in other containers (tin and dark glass).
The values of the chlorophylls and carotenes concentrations are between 1.53 mg/kg to 0.01 mg/kg for chlorophylls, and between 4.5 mg/kg and 0.17 mg/kg for carotenes, during 6 months of storage. However, the EVOO stored in a tin container and in a dark glass bottle showed lower decrease in chlorophylls and carotenes concentrations than in the other containers. The intensity of the chlorophyll and carotenes levels decreased in all of the stored samples, to the extent which depended on the storage conditions (time and type of container).
Chlorophylls are present in olive oils and are the responsible for the greenish coloration of certain olive oils. Those pigments are also important in olive oil stability. Carotenes are present too in olive oil and are responsible for its yellow coloration. The behaviour of these pigments versus oxidation process was similar that of chlorophylls as shown in Fig. 1 but chlorophylls losses were more noticeable than those related to carotenoid, which reaffirmed the findings of Guil-Guerrero and Urda-Romacho (2009).
Another study (Kiritsakis 1991) carried out on olive oil exposed to light showed significant decreases in chlorophylls and carotenes contents in oil sample.
It should be noted that the quality of olive oil is highly affected by the ability of the container to exclude oxygen and light. Tin and dark glass proved to be quite adequate in preserving the quality of the Chemlali extra-virgin olive oil and avoid oxidation up to 6 months when stored in the light and at room temperature.
Changes in fatty acids composition
Lipid oxidation is a major deteriorative reaction affecting edible oils and fats and consequently of primary concern to processors and consumers. Unsaturated lipids are particularly susceptible to oxidation during processing and storage via autoxidation and photosensitized oxidation (Marfil et al. 2008).
The initial content of fatty acids of all the samples is within the established limits. Olive oil fatty acid composition for tin, PE, clear glass and dark glass bottles are given in Table 1 according to storage time. The fatty acid contents fell within the intervals required by the EU (European Union) regulations in all the analysed olive oil samples.
Table 1.
Effect of packaging materials and storage time on fatty acid composition
| Fatty acids (%) | Fresh oil % initial | Tin after 75 days | Tin after 180 days | DG after 75 days | DG after 180 days | CG after 75 days | CG after 180 days | PE after 75 days | PE after 180 days |
|---|---|---|---|---|---|---|---|---|---|
| Palmitic acid | 16.59 ± 0.33 * | 16.69 ± 0.25 * | 16.75 ± 0.21 * | 16.69 ± 0.29 * | 16.78 ± 0.26 * | 16.87 ± 0.15 * | 16.98 ± 0.18 * | 16.93 ± 0.19 * | 17.06 ± 0.27 * |
| Hypogeic + Palmitoleic acids | 2.01 ± 0.04 * | 2.00 ± 0.03 * | 2.01 ± 0.03 * | 2.00 ± 0.03 * | 1.99 ± 0.03 * | 2.07 ± 0.02 ** | 2.11 ± 0.02 ** | 2.08 ± 0.02 ** | 2.13 ± 0.02 ** |
| Heptadecanoic acid | 0.04 ± 0.00 * | 0.04 ± 0.00 * | 0.04 ± 0.00 * | 0.04 ± 0.00 * | 0.04 ± 0.00 * | 0.04 ± 0.00 * | 0.05 ± 0.00 ** | 0.04 ± 0.00 * | 0.05 ± 0.00 ** |
| Heptadecenoic acid | 0.07 ± 0.00 * | 0.06 ± 0.00 * | 0.06 ± 0.00 * | 0.06 ± 0.00 * | 0.06 ± 0.00 * | 0.06 ± 0.00 * | 0.05 ± 0.00 ** | 0.07 ± 0.00 * | 0.06 ± 0.00 * |
| Stearic acid | 2.42 ± 0.05 * | 2.42 ± 0.04 * | 2.45 ± 0.03 * | 2.46 ± 0.04 * | 2.54 ± 0.04 ** | 2.45 ± 0.02 * | 2.48 ± 0.03 * | 2.47 ± 0.03 * | 2.50 ± 0.02 ** |
| (Z)-Vaccenic + Oleic acids | 61.59 ± 1.23 * | 61.58 ± 0.92 * | 61.55 ± 0.77 * | 61.57 ± 1.08 * | 61.52 ± 0.95 * | 61.51 ± 0.55 * | 61.48 ± 0.65 * | 61.45 ± 0.68 * | 61.39 ± 0.98 * |
| Linoleic acid | 16.36 ± 0.32 * | 16.31 ± 0.24 * | 16.23 ± 0.20 * | 16.30 ± 0.29 * | 16.20 ± 0.25 * | 16.13 ± 0.15 * | 15.98 ± 0.17 * | 16.09 ± 0.18 * | 15.93 ± 0.25 * |
| Linolenic acid | 0.48 ± 0.01 * | 0.47 ± 0.01 * | 0.48 ± 0.01 * | 0.46 ± 0.01 ** | 0.45 ± 0.01 ** | 0.44 ± 0.00 ** | 0.42 ± 0.00 ** | 0.45 ± 0.00 ** | 0.44 ± 0.00 ** |
| Arachidic acid | 0.35 ± 0.01 * | 0.35 ± 0.01 * | 0.35 ± 0.00 * | 0.34 ± 0.01 * | 0.35 ± 0.01 * | 0.36 ± 0.00 * | 0.38 ± 0.00 ** | 0.36 ± 0.00 * | 0.37 ± 0.00 ** |
| Gondoïc acid | 0.09 ± 0.00 * | 0.08 ± 0.00 * | 0.08 ± 0.00 * | 0.08 ± 0.00 * | 0.07 ± 0.00 ** | 0.07 ± 0.00 ** | 0.07 ± 0.00 ** | 0.06 ± 0.00 ** | 0.07 ± 0.00 ** |
| ((Z)-Vaccenic + Oleic)/Linoleic | 3.76 | 3.78 | 3.79 | 3.78 | 3.80 | 3.81 | 3.85 | 3.82 | 3.85 |
| ∑ SFA (%) | 19.40 ± 0.39 * | 19.50 ± 0.29 * | 19.59 ± 0.24 * | 19.53 ± 0.34 * | 19.71 ± 0.31 * | 19.72 ± 0.18 * | 19.89 ± 0.21 * | 19.80 ± 0.22 * | 19.98 ± 0.29 * |
| ∑ MUFA (%) | 63.76 ± 1.27 * | 63.72 ± 0.95 * | 63.70 ± 0.80 * | 63.71 ± 1.11 * | 63.64 ± 0.98 * | 63.71 ± 0.57 * | 63.71 ± 0.67 * | 63.66 ± 0.70 * | 63.65 ± 1.00 * |
| ∑ PUFA (%) | 16.84 ± 0.34 * | 16.78 ± 0.25 * | 16.71 ± 0.21 * | 16.76 ± 0.29 * | 16.65 ± 0.26 * | 16.57 ± 0.15 * | 16.40 ± 0.17 * | 16.54 ± 0.18 * | 16.37 ± 0.25 * |
| ∑ UFA (%) | 80.60 ± 1.61 * | 80.50 ± 1.20 * | 80.41 ± 1.01 * | 80.47 ± 1.40 * | 80.29 ± 1.24 * | 80.28 ± 0.72 * | 80.11 ± 0.84 * | 80.20 ± 0.88 * | 80.02 ± 1.25 * |
| ∑ MUFA/∑ PUFA | 3.78 | 3.79 | 3.81 | 3.80 | 3.82 | 3.84 | 3.88 | 3.84 | 3.88 |
| IV (%) | 88.37 | 88.23 | 88.10 | 88.18 | 87.91 | 87.83 | 87.51 | 87.74 | 87.42 |
| OS (%) | 847.96 | 844.67 | 842.05 | 843.21 | 837.64 | 833.56 | 824.81 | 832.71 | 824.50 |
Each value represents the mean of three determinations (n = 3) and three independent experiments. Different symbols (*, **) within the same line indicate significant differences (p < 0.05) between the fresh oil and each oil group in both storage periods (75 and 180 days)
DG dark glass, CG clear glass, PE polyethylene, SFA saturated fatty acids, PUFA polyunsaturated fatty acids, MUFA monounsaturated fatty acids, UFA unsaturated fatty acids, IV iodine value, OS oxidative susceptibility
Main fatty acids in the Chemlali fresh oil include palmitic acid (16.59 %), oleic acid (61.59 %), linoleic acid (16.36 %) and linolenic acid (0.48 %), yet slight alterations were detected in the amounts of the mentioned fatty acids (Table 1) after storage in the all containers at room temperature.
EVOO Storage for 6 months in relation to the type of container had no significant (p > 0.05) effect on the sums of fatty acids under the same storage conditions. In fact, fatty acids profiles were slightly modified throughout the storage period (Gutierrez and Fernández 2002).
As regards the content of palmitic acid, the highest values were found for the samples of oils stored in PE conatiner. The high percentage of oleic acid is noticeable, which is the predominant acid (Table 1). The analyses performed after 6 months of storage showed that the percentage of the analysed fatty acids revealed slight variations with respect to the initial composition in all the containers.
The oil samples stored in the different packaging materials did not show significant (p > 0.05) differences after 6 months of storage, as regard oleic and linoleic acids. These results are in accordance with those of a previous report (Frega et al. 1999) which confirmed the stability of the fatty acid composition during 3 months of storage. Similar results were reported by Gutierrez and Fernández (2002) after 6 months of storage.
The data showed that the amount of unsaturated fatty acids (UFA) decreased slightly (p > 0.05) during 6 months of storage at room temperature, whereas the amount of saturated fatty acids (SFA) increased not significantly (p > 0.05) in all the packaging materials. It also showed that the amount of oxidative susceptibility (OS) decreased gradually during storage at room temperature in all containers. The ratio ((Z)-Vaccenic + Oleic)/Linoleic) increased slightly in all packaging materials after 75 days of storage and continued to increase a little after 180 days.
It should be noted that all the values of saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), unsaturated fatty acids (UFA) and iodine value (IV) showed slight variations (p > 0.05) in all containers throughout the storage period of the Chemlali olive oil.
Changes in sterol composition
Sterols are important nonglyceridic constituents of oil and are widely used to check authenticity. Every vegetable oil has a specific sterol composition. Thus, they have a great importance in adulteration detection. These chemical components are also used for varietal characterizations (Lukic et al. 2013), and are reported to be indicators of the best period of harvest (Fiorino and Nizzi-Grifi 1991).
The high rate of apparent β-Sitosterol was noticeable, with 95.29 % before storage (Table 2). It showed a slight decrease but not significant (p > 0.05) for the oil stored in all containers after both storage periods (75 and 180 days). Δ-7-Avenasterol and ∑ (Erythrodiol + Uvaol) rates remained nearly constant (p > 0.05) for the oils stored in the tin container and the dark glass bottle after 75 and 180 days whereas they showed significant decreases (p < 0.05) in the other containers after the same storage periods.
Table 2.
Effect of packaging materials and storage time on the sterolic composition
| Sterols (%) | Fresh oil % initial | Tin after 75 days | Tin after 180 days | Dark glass after 75 days | Dark glass after 180 days | Clear glass after 75 days | Clear glass after 180 days | PE after 75 days | PE after 180 days |
|---|---|---|---|---|---|---|---|---|---|
| Cholesterol | 0.17 ± 0.00 * | 0.20 ± 0.01 ** | 0.21 ± 0.01 ** | 0.19 ± 0.01 ** | 0.20 ± 0.01 ** | 0.16 ± 0.01 * | 0.18 ± 0.01 * | 0.21 ± 0.02 ** | 0.22 ± 0.00 ** |
| Campesterol + Campestanol | 3.24 ± 0.06 * | 3.35 ± 0.10 * | 3.45 ± 0.05 ** | 3.43 ± 0.06 ** | 3.47 ± 0.05 ** | 3.82 ± 0.05 ** | 3.89 ± 0.04 ** | 3.82 ± 0.03 ** | 3.98 ± 0.04 ** |
| Stigmasterol | 0.36 ± 0.01 * | 0.39 ± 0.01 ** | 0.40 ± 0.02 ** | 0.39 ± 0.01 ** | 0.41 ± 0.01 ** | 0.45 ± 0.01 ** | 0.49 ± 0.01 ** | 0.48 ± 0.00 ** | 0.51 ± 0.01 ** |
| Chlerosterol | 1.16 ± 0.01 * | 1.17 ± 0.01 * | 1.15 ± 0.01 * | 1.17 ± 0.01 * | 1.15 ± 0.01 * | 1.15 ± 0.01 * | 1.14 ± 0.02 * | 1.15 ± 0.01 * | 1.18 ± 0.02 * |
| β-Sitosterol | 83.65 ± 1.74 * | 83.54 ± 2.60 * | 83.19 ± 1.16 * | 83.42 ± 1.46 * | 83.27 ± 1.28 * | 83.19 ± 0.97 * | 83.09 ± 0.87 * | 83.04 ± 0.79 * | 82.85 ± 0.78 * |
| Sitostanol | 0.39 ± 0.01 * | 0.38 ± 0.00 * | 0.37 ± 0.01 ** | 0.37 ± 0.00 ** | 0.37 ± 0.00 ** | 0.38 ± 0.00 * | 0.37 ± 0.00 ** | 0.38 ± 0.00 * | 0.38 ± 0.00 * |
| Δ5-Avenasterol | 9.38 ± 0.11 * | 9.28 ± 0.18 * | 9.58 ± 0.21 * | 9.35 ± 0.15 * | 9.46 ± 0.26 * | 9.23 ± 0.22 * | 9.28 ± 0.17 * | 9.40 ± 0.14 * | 9.32 ± 0.15 * |
| Δ5,24-Stigmastenol | 0.71 ± 0.00 * | 0.74 ± 0.01 ** | 0.75 ± 0.01 ** | 0.73 ± 0.01 ** | 0.72 ± 0.00 ** | 0.75 ± 0.01 ** | 0.76 ± 0.01 ** | 0.74 ± 0.00 ** | 0.76 ± 0.02 ** |
| Δ-7-Stigmastenol | 0.32 ± 0.01 * | 0.31 ± 0.01 * | 0.29 ± 0.01 ** | 0.32 ± 0.00 * | 0.34 ± 0.01 ** | 0.31 ± 0.01 * | 0.29 ± 0.01 ** | 0.27 ± 0.00 ** | 0.31 ± 0.01 * |
| Δ-7-Avenasterol | 0.62 ± 0.01 * | 0.64 ± 0.02 * | 0.61 ± 0.01 * | 0.63 ± 0.01 * | 0.61 ± 0.01 * | 0.56 ± 0.00 ** | 0.51 ± 0.01 ** | 0.51 ± 0.00 ** | 0.49 ± 0.01 ** |
| Apparent β-Sitosterola | 95.29 ± 1.87 * | 95.11 ± 2.80 * | 95.04 ± 1.40 * | 95.04 ± 1.63 * | 94.97 ± 1.55 * | 94.70 ± 1.21 * | 94.64 ± 1.07 * | 94.71 ± 0.94 * | 94.49 ± 0.97 * |
| Erythrodiol + Uvaol | 1.56 ± 0.03 * | 1.54 ± 0.04 * | 1.58 ± 0.02 * | 1.52 ± 0.02 * | 1.59 ± 0.02 * | 1.63 ± 0.01 ** | 1.66 ± 0.02 ** | 1.65 ± 0.01 ** | 1.70 ± 0.01 ** |
| Total sterols (mg/kg) | 1,565 ± 30.67 * | 1,562 ± 46.08 * | 1,559 ± 20.26 * | 1,561 ± 26.84 * | 1,558 ± 25.55 * | 1,554 ± 20.04 * | 1,549 ± 17.81 * | 1,551 ± 15.35 * | 1,546 ± 16.08 * |
Each value represents the mean of three determinations (n = 3) and three independent experiments. Different symbols (*, **) within the same line indicate significant differences (p < 0.05) between the fresh oil and each oil group in both storage periods (75 and 180 days)
PE polyethylene
a[Chlerosterol + β-Sitosterol + Sitostanol + Δ5-Avenasterol + Δ5,24-Stigmastadienol]
The Campesterol and Campestanol rates increased significantly (p < 0.05) after 75 and 180 days of storage in all containers except the tin container they showed slight decreases but not significant (p > 0.05) after 75 days of storage.
The data showed a significant (p < 0.05) increase that was observed only in Stigmasterol rate in all containers after both storage periods. The increase in the Stigmasterol rate could be explained by the hydrolysis of the β-Sitosterol, the major sterol in olive oil, and has a clear influence in the loss of the sensory scores of oils. These results are in good agreement with those of Gutierrez and Fernández (2002) who have found that the sterolic composition remains constant during storage, and only a significant (p < 0.05) increase is detected in stigmasterol rate for the oil stored at 30 °C for 6 months.
Changes in total phenols contents
Polyphenols compounds are naturally present in olive oils and are the major compounds responsible for the stability of the oil during storage. Recent interest in olive phenols has greatly increased because of their antioxidant and free radical scavenging abilities, associated with the potential benefits for human health, and the high oxidative stability they confer to the resulting olive oil during storage (Conde et al. 2008).
Total phenols as affected by packaging material and storage periods are present in Fig. 1. The decrease of phenolic concentration with storage time depends on the type of container. Indeed, the total phenols content decrease leads to the typical bitter taste and the pungent note of fresh EVOO decreases in intensity. This finding is in harmony with other research works showing that during storage, phenols undergo qualitative and quantitative modifications due to decomposition and oxidation reactions (Esti et al. 2009; Dabbou et al. 2011).
After 75 days of storage, a significant degradation of phenolic compounds was detected when oil was stored in the PE container from 363 to 285 mg kg−1 and from 363 to 305 mg kg−1 in the clear glass bottle. However, in the other materials, it decreased slightly from 363 to 342 mg kg−1 in the tin container and from 363 to 337 mg kg−1 in the dark glass bottle during the same period of storage.
The results obtained after 180 days of storage also indicated that the nature of the material had a notable influence on phenolic content. Indeed, the phenolic concentrations decreased drastically in the PE container from 363 to 193 mg/kg and from 363 to 206 mg/kg in the clear glass bottle. This fact could be attributed to the joint action of light and the permeability of the PE container to the oxygen that catalyzes the oxidation reaction (Vekiari et al. 2007; Bouaziz et al. 2008). However, the phenomenon was less pronounced in the oil stored in the tin container and the dark glass bottle. In these samples, phenolic compounds content attained 270 and 261 mg/kg, respectively after 6 months of storage.
Changes in oxidative stability
Oxidative stability is one of the main parameter for estimating extra-virgin olive oil quality and evaluating the susceptibility of the oil to oxidative degeneration, which is the main cause of damage. The oxidative induction time values were highly variable among the samples analyzed (Table 3). The lowest stability value (11.32 h) of Chemlali extra-virgin olive oil in the PE container was due to the lower level in phenols (193 mg.kg−1). However, the highest stability value was registered in the oil stored in the tin container, which can be explained by its richness in total phenol content (270 mg.kg−1). A linear relationship exists between the phenolic content and the oxidative stability of the two olive oil samples as previously described (Bouaziz et al. 2008).
Table 3.
Effect of packaging materials and storage time on the oxidative stability by Rancimat method
| Tin | Dark glass | Clear glass | PE | |
|---|---|---|---|---|
| Stability (h) T = 0 day | 31.08 ± 1.03 a | 31.08 ± 1.03 a | 31.08 ± 1.03 a | 31.08 ± 1.03 a |
| Stability (h) T = 180 days | 28.45 ± 1.42 b | 27.00 ± 0.64 b | 16.85 ± 0.76 c | 11.32 ± 0.97 d |
Each value represents the mean of three determinations (n = 3) and three independent experiments. Values with different letters (a, b, c, d) differ significantly p < 0.05. Values with a same letter (b) do not differ significantly p > 0.05
PE polyethylene
Sensory assessment
Sensory characteristics are used to define virgin olive oil quality. In fact, virgin olive oil is characterized by a unique flavour, which represents one of the most important qualitative aspects of olive oil, and plays a major role in consumer approval. A full description of the organoleptic characteristics of the oil is only obtainable through sensory analysis.
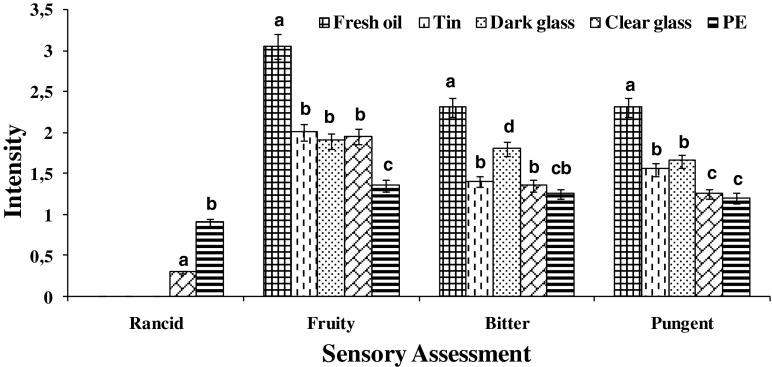
The intensity of positive and negative attributes was evaluated during the storage of olive oil. No defects were detected in the fresh oil sample (Fig. 2). Also a light intensity of the positive attributes was recorded. These results are consistent with the International Olive Oil Council (IOOC) standard that allows us to classify the oil as extra virgin. After the storage of Chemlali olive oil in the four types of packaging for 75 days, no defects were observed except a slight decrease in the intensity of positive attributes (fruity, bitter and pungent) for oil packaged in the dark glass bottle and the tin container, which was more pronounced in the clear glass bottle and the PE container. This decrease could be caused by the degradation of chlorophyll pigments by light (photooxidation) and the degradation of the phenolic compounds responsible for the bitter taste in oils. These results are in accordance with those of a previous report (Albi and Gutierrez 1991) which showed a slight variation of positive attributes of the oil for 3 months with a decrease in the median of fruity from 3.20 to 2.80.
Fig. 2.
Evolution of the organoleptic characteristics of Chemlali extra-virgin olive oil before storage and after 180 days of storage in different containers. Error bars indicate the SD of the mean of three replicates (n = 3). Bars with different letters differ significantly for the same parameter p < 0.05; PE polyethylene
The negative and positive attributes of the Chemlali extra-virgin olive oil samples stored after 180 days in different packaging materials are presented in Fig. 2. Quantitative descriptive analysis during storage at room temperature revealed the partial loss of positive attributes (fruity, bitter and pungent) and the appearance of one negative attribute (rancid), reaching a score of 0.90 for PE and 0.30 for clear glass at the end of the period. A significant decrease (p < 0.05) in the median of fruity was detected when the oil was stored in the PE container from 3.05 to 1.35. The median of bitter decreased significantly (p < 0.05) in all containers but slightly when the oil was stored in the dark glass bottle from 2.30 to 1.80. Finally, the median of pungent showed a significant decrease (p < 0.05) when the oil was stored in the PE container from 2.30 to 1.20 and from 2.30 to 1.25 in the clear glass bottle.
Chemometric analysis
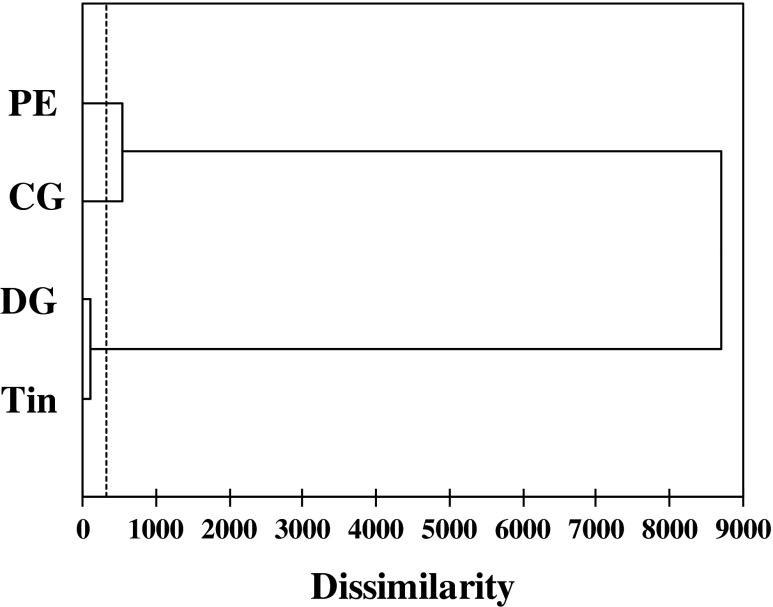
Hierarchical Cluster analysis (HCA) was performed in order to observe similarities or dissimilarities between the oil samples. The dissimilarity of different clusters was defined by the Euclidean distance matrix and calculated by Ward’s method. The results were presented in a dendrogram structure (Fig. 3), showing three distinct clusters based on the threshold value (323.06), with a high similarity between tin and dark glass (DG) containers (Dissimilarity coefficient D = 108.58). Whereas clear glass (CG) and polyethylene (PE) containers presented two distinct clusters with a high dissimilarity (D = 538.4) between them and the other group of containers.
Fig. 3.
Hierarchical cluster analysis (HCA) dendrogram of the all analyses performed. PE polyethylene, CG clear glass, DG dark glass
Conclusions
Tin containers and dark glass bottles, best packaging materials showed the greatest stability against oxidation and preserved their acceptability properties for at least 180 days at 20 °C. Our results clearly indicate which indices determine the loss of the extra quality during storage. Furthermore, this study demonstrated that the time beyond which the oils lose their extra quality can be predicted according to their initial stability. The Chemlali olive oil packed in tin containers and dark glass bottles can be successfully stored up to 6 months at ambient conditions without any appreciable change in its quality. The results of this study could be interesting for virgin olive oil packagers and marketers to estimate the caducity date of extra quality oil.
Acknowledgments
The authors would like to thank the “Ministère de l’Enseignement Supérieur et de la Recherche Scientifique, Tunisia” for the support of this research work. They also wish to extend their thanks to Mrs. Leila MAHFOUDHI, an English teacher at the Sfax Faculty of Science, for having proofread this paper and they are grateful to Dr. Mohamed MAKNI, for his precious help with statistical analyses.
Footnotes
Boutheina Gargouri and Akram Zribi contributed equally to this work.
References
- Albi MA, Gutierrez F. Study of the precision of an analytical taste panel for sensory evaluation of virgin olive oil. Establishment of criteria for the elimination of abnormal results. J Sci Food Agric. 1991;54:254–267. doi: 10.1002/jsfa.2740540212. [DOI] [Google Scholar]
- Allen JC. In: Rancidity in foods. 3. Allen JC, Hamilton RJ, editors. London: Springer; 1994. [Google Scholar]
- Baiano T, Gomes F, Caponio F. A comparison between olive oil and extra-virgin olive oil used as covering liquids in canned dried tomatoes: hydrolytic and oxidative degradation during storage. Int J Food Sci Technol. 2005;40:829–834. doi: 10.1111/j.1365-2621.2005.00998.x. [DOI] [Google Scholar]
- Bouaziz M, Fki I, Jemai H, Ayadi M, Sayadi S. Effect of storage on refined and husk olive oils composition: stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008;108(1):253–262. doi: 10.1016/j.foodchem.2007.10.074. [DOI] [Google Scholar]
- Bouaziz M, Jemai H, Khabou W, Sayadi S. Oil content, phenolic profiling and antioxidant potential of Tunisian olive drupes. J Sci Food Agric. 2010;90:1750–1758. doi: 10.1002/jsfa.4013. [DOI] [PubMed] [Google Scholar]
- Bradley DG, Min DB. Singlet oxygen oxidation of foods. Crit Rev Food Sci Nutr. 1992;31:211–236. doi: 10.1080/10408399209527570. [DOI] [PubMed] [Google Scholar]
- Cert A, Alba J, Leon-Camacho M, Moreda W, Perez-Camino MC. Effects of talc addition and operating mode on the quality and oxidative stability of virgin olive oils obtained by centrifugation. J Agric Food Chem. 1996;44:3930–3934. doi: 10.1021/jf9603386. [DOI] [Google Scholar]
- Conde C, Delrot S, Gerosa H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J Plant Physiol. 2008;165:1545–1562. doi: 10.1016/j.jplph.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Criado MN, Romero MP, Casanovas M, Motilva MJ. Pigment profile and colour of monovarietal virgin olive oils from Arbequina cultivar obtained during two consecutive crop seasons. Food Chem. 2008;110:873–880. doi: 10.1016/j.foodchem.2008.02.075. [DOI] [PubMed] [Google Scholar]
- Dabbou S, Gharbi I, Dabbou S, Brahmi F, Nakbi A, Hammami M. Impact of packaging material and storage time on olive oil quality. Afr J Biotechnol. 2011;10(74):16937–16947. [Google Scholar]
- EC (1991) Commission Regulation EC no 2568/91 July 11, 1991. Official EC Journal L 248 of September 5, 1991, pp 0001–0082
- EC (1997) Commission Regulation EC no 2472/97 December 12, 1997. Official EC Journal L 341 of December 12, 1997, pp 0025–0039
- Elez-Martinez P, Soliva-Fortuny R, Martin-Belloso O. Oxidative rancidity in avocado purée as affected by α-tocopherol, sorbic acid and storage atmosphere. Eur Food Res Technol. 2007;226:295–300. doi: 10.1007/s00217-006-0539-y. [DOI] [Google Scholar]
- Esti M, Contini M, Moneta E, Sinesio F. Phenolics compounds and temporal perception of bitterness and pungency in extra-virgin olive oils: changes occurring throughout storage. Food Chem. 2009;113:1095–1100. doi: 10.1016/j.foodchem.2008.08.076. [DOI] [Google Scholar]
- Fiorino P, Nizzi-Grifi F. Maturation des olives et variations de certains composants de l’huile. Olivae. 1991;35:25–33. [Google Scholar]
- Frankel EN. Lipid oxidation. 2. Bridgewater: Barnes, P.J. & Associates, the Oily Press; 2005. [Google Scholar]
- Frega N, Mozzon M, Lercker G. Effects of free fatty acids on oxidative stability of vegetable oil. J Am Oil Chem Soc. 1999;76(3):325–329. doi: 10.1007/s11746-999-0239-4. [DOI] [Google Scholar]
- Gasparoli A, Taormina F, Fedeli E. Storage of virgin olive oil in the light: effect on absorbance at 268 nm. Riv Ital Sostanze Grasse. 1991;68:615–622. [Google Scholar]
- Gertz C, Klostermann S. A new analytical procedure to differentiate virgin or non-refined from refined vegetable fats and oils. Eur J Lipid Sci Technol. 2000;102:329–336. doi: 10.1002/(SICI)1438-9312(200005)102:5<329::AID-EJLT329>3.0.CO;2-#. [DOI] [Google Scholar]
- Guil-Guerrero JL, Urda-Romacho J. Quality of extra virgin olive oil affected by several packaging variables. Grasas Aceites. 2009;60(2):125–133. doi: 10.3989/gya.043308. [DOI] [Google Scholar]
- Gutierrez F. Determinacion de la estabilidade oxidativa de aceites de oliva virgenes: comparacion entre del metodo A.O.M. yel metodo Rancimat. Grasas Aceites. 1989;40:1–5. [Google Scholar]
- Gutierrez F, Fernández JL. Determinant parameters and components in the storage of virgin olive oil. Prediction of storage time beyond which the oil is no longer of extra quality. J Agric Food Chem. 2002;50:571–577. doi: 10.1021/jf0102158. [DOI] [PubMed] [Google Scholar]
- Hrncirik K, Fritsche S. Relation between the endogenous antioxidant system and the quality of extra virgin olive oil under accelerated storage conditions. J Agric Food Chem. 2005;53:2103–2110. doi: 10.1021/jf048363w. [DOI] [PubMed] [Google Scholar]
- Huang CL, Sumpio BE. Olive oil, the Mediterranean diet, and cardiovascular health. J Am Coll Surg. 2008;207:407–416. doi: 10.1016/j.jamcollsurg.2008.02.018. [DOI] [PubMed] [Google Scholar]
- International Olive Council, COI/T,15/NC n°3 Rev 5, November (2010) Principe de Vergara, 154, 28002, Madrid, España
- Keceli T, Gordon MH. The antioxidant activity and stability of the phenolic fraction of green olives and extra virgin olive oil. J Sci Food Agric. 2001;81:1391–1396. doi: 10.1002/jsfa.959. [DOI] [Google Scholar]
- Khan MA, Shahidi F. Rapid oxidation of commercial extra virgin olive oil stored under fluorescent light. J Food Lipids. 1999;6(4):331–339. doi: 10.1111/j.1745-4522.1999.tb00154.x. [DOI] [Google Scholar]
- Kiritsakis A. Olive oil. Champaign: AOCS; 1991. [Google Scholar]
- Kiritsakis A, Dugan LR. Effect of selected storage conditions and packaging materials on olive oil quality. J Am Oil Chem Soc. 1984;61(12):1868–1870. doi: 10.1007/BF02540820. [DOI] [Google Scholar]
- Lukic M, Lukic I, Krapac M, Sladonja B, Pilizota V. Sterols and triterpene diols in olive oil as indicators of variety and degree of ripening. Food Chem. 2013;136(1):251–258. doi: 10.1016/j.foodchem.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Marfil R, Cabrera-Vique C, Giménez R, Bouzas PR, Martinez O, Sanchez JA. Metal content and physicochemical parameters used as quality criteria in virgin argan oil: influence of the extraction method. J Agric Food Chem. 2008;56:7279–7284. doi: 10.1021/jf801002w. [DOI] [PubMed] [Google Scholar]
- Méndez AI, Falqué E. Effect of storage time and container type on the quality of extra-virgin olive oil. Food Control. 2007;18:521–529. doi: 10.1016/j.foodcont.2005.12.012. [DOI] [Google Scholar]
- Mínguez-Mosquera MI, Rejano L, Gandul A, Sanchez BH, Garrido J. Color-pigment correlation in virgin olive oil. J Am Oil Chem Soc. 1991;68:322–337. doi: 10.1007/BF02660592. [DOI] [Google Scholar]
- Moldao-Martins M, Beirao-da-Costa S, Neves C, Cavaleiro C, Salgueiro L, Beirao-da Costa ML. Olive oil flavoured by the essential oils of Mentha x piperita and Thymus mastichina L. Food Qual Prefer. 2004;15:447–452. doi: 10.1016/j.foodqual.2003.08.001. [DOI] [Google Scholar]
- Morales MT, Rios JJ, Aparicio R. Changes in the volatile composition of virgin olive oil during oxidation: flavors and off-flavors. J Agric Food Chem. 1997;45:2666–2673. doi: 10.1021/jf960585+. [DOI] [Google Scholar]
- Olías-Jiménez JM, Gutiérrez-González-Quijano R. Canning of olive oil in commercial type containers. IV. Conservation in glass, tin, PVC and polyethylene containers. Kreis test and oils color evolution. Grasas Aceites. 1971;22:81–85. [Google Scholar]
- Pagliarini E, Zanoni B, Giovanelli G. Predictive study on Tuscan extra virgin olive oil stability under several commercial conditions. J Agric Food Chem. 2000;48:1345–1351. doi: 10.1021/jf990372v. [DOI] [PubMed] [Google Scholar]
- Pristouri G, Badeka A, Kontominas MG. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control. 2010;21:412–418. doi: 10.1016/j.foodcont.2009.06.019. [DOI] [Google Scholar]
- Psomiadou E, Tsimidou M. Stability of virgin olive oil. 1. Autoxidation studies. J Agric Food Chem. 2002;50:716–721. doi: 10.1021/jf0108462. [DOI] [PubMed] [Google Scholar]
- Psomiadou E, Tsimidou M. Stability of virgin olive oil. 2. Photo-oxidation studies. J Agric Food Chem. 2002;50:722–727. doi: 10.1021/jf010847u. [DOI] [PubMed] [Google Scholar]
- Rahmani M, Saari-Csallany A. Role of minor constituents in the photooxidation of virgin olive oil. J Am Oil Chem Soc. 1998;75:837–843. doi: 10.1007/s11746-998-0234-1. [DOI] [Google Scholar]
- Ruiz-Canela M, Martínez-González M. Olive oil in the primary prevention of cardiovascular disease. Maturitas. 2011;68(3):245–250. doi: 10.1016/j.maturitas.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Samaniego-Sánchez C, Oliveras-López JM, Quesada-Granados JJ, Villalón-Mir M, López G, Serrana H. Alterations in picual extra virgin olive oils under different storage conditions. Eur J Lipid Sci Technol. 2012;114(2):194–204. doi: 10.1002/ejlt.201100191. [DOI] [Google Scholar]
- Tawfik MS, Huyghebaert A. Interaction of packaging materials and vegetable oils: oil stability. Food Chem. 1999;64:451–459. doi: 10.1016/S0308-8146(97)00202-1. [DOI] [Google Scholar]
- Vekiari SA, Papadopoulou P, Kiritsakis A. Effects of processing methods and commercial storage conditions on the extra virgin olive oil quality indexes. Grasas Aceites. 2007;58(3):237–242. doi: 10.3989/gya.2007.v58.i3.178. [DOI] [Google Scholar]
- Zanoni B, Bertuccioli M, Rovellini P, Marotta F, Mattei A. A preliminary approach to predictive modelling of extra virgin olive oil stability. J Sci Food Agric. 2005;85:1492–1498. doi: 10.1002/jsfa.2135. [DOI] [Google Scholar]