Abstract
BACKGROUND
Retrospective studies have shown that statins decrease the rate and severity of exacerbations, the rate of hospitalization, and mortality in chronic obstructive pulmonary disease (COPD). We prospectively studied the efficacy of simvastatin in preventing exacerbations in a large, multicenter, randomized trial.
METHODS
We designed the Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD Exacerbations (STATCOPE) as a randomized, controlled trial of simvastatin (at a daily dose of 40 mg) versus placebo, with annual exacerbation rates as the primary outcome. Patients were eligible if they were 40 to 80 years of age, had COPD (defined by a forced expiratory volume in 1 second [FEV1] of less than 80% and a ratio of FEV1 to forced vital capacity of less than 70%), and had a smoking history of 10 or more pack-years, were receiving supplemental oxygen or treatment with glucocorticoids or antibiotic agents, or had had an emergency department visit or hospitalization for COPD within the past year. Patients with diabetes or cardiovascular disease and those who were taking statins or who required statins on the basis of Adult Treatment Panel III criteria were excluded. Participants were treated from 12 to 36 months at 45 centers.
RESULTS
A total of 885 participants with COPD were enrolled for approximately 641 days; 44% of the patients were women. The patients had a mean (±SD) age of 62.2±8.4 years, an FEV1 that was 41.6±17.7% of the predicted value, and a smoking history of 50.6±27.4 pack-years. At the time of study closeout, the low-density lipoprotein cholesterol levels were lower in the simvastatin-treated patients than in those who received placebo. The mean number of exacerbations per person-year was similar in the simvastatin and placebo groups: 1.36±1.61 exacerbations and 1.39±1.73 exacerbations, respectively (P = 0.54). The median number of days to the first exacerbation was also similar: 223 days (95% confidence interval [CI], 195 to 275) and 231 days (95% CI, 193 to 303), respectively (P = 0.34). The number of nonfatal serious adverse events per person-year was similar, as well: 0.63 events with simvastatin and 0.62 events with placebo. There were 30 deaths in the placebo group and 28 in the simvastatin group (P = 0.89).
CONCLUSIONS
Simvastatin at a daily dose of 40 mg did not affect exacerbation rates or the time to a first exacerbation in patients with COPD who were at high risk for exacerbations. (Funded by the National Heart, Lung, and Blood Institute and the Canadian Institutes of Health Research; STATCOPE ClinicalTrials.gov number, NCT01061671.)
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States.1 It is characterized by acute exacerbations that are associated with increased hospitalizations and costs of care, worsened quality of life, and increased mortality.2–9 Effective therapies for the treatment or prevention of exacerbations are limited.
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors that reduce the risks of acute cardiac events and death.10–13 Although widely used for their lipidlowering effects, statins are also reported to have antiinflammatory effects.14–19 Multiple retrospective studies have shown that statins are beneficial in COPD. Reported benefits include reductions in rates of hospitalization (for COPD or any other reason), lung-function decline, the need for mechanical ventilation, and death.20–24 However, except for one small, single-center, randomized study,25 all the studies that have shown beneficial effects of statins in patients with COPD have been retrospective. The Prospective Randomized Placebo-Controlled Trial of Simvastatin in the Prevention of COPD Exacerbations (STATCOPE) was a prospective, multicenter trial conducted by the National Heart, Lung, and Blood Institute (NHLBI) COPD Clinical Research Network to examine the effect of daily treatment with simvastatin for at least 12 months (range, 12 to 36) on the rate of exacerbations among patients with moderate-to-severe COPD and no other indications for statin treatment.
METHODS
STUDY DESIGN AND OVERSIGHT
In this randomized, parallel-group, placebo-controlled trial, participants were randomly assigned in a 1:1 ratio to receive simvastatin orally at a dose of 40 mg or an identical-appearing placebo once daily. Participants were recruited from 45 sites (29 sites in the United States and 16 in Canada). Written informed consent was obtained from all participants. The institutional review board at each center approved the study protocol. The complete protocol, including methods and the statistical analysis plan, is available with the full text of this article at NEJM.org. The study drugs, simvastatin and placebo, were purchased, prepared, and supplied by Temple University School of Pharmacy Current Good Manufacturing Practices Services. All the authors assume responsibility for the data and analyses, the adherence of the study to the protocol, and the completeness and accuracy of the reported findings.
PARTICIPANT POPULATION
We enrolled patients, 40 to 80 years of age, with a clinical diagnosis of moderate-to-severe COPD, defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria26 (a ratio of forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] of <70% after bronchodilator use and an FEV1 of <80% of the predicted value after bronchodilator use). Participants were former or current smokers with a lifetime cigarette consumption of 10 or more pack-years who also met at least one of the following criteria within the year before enrollment: use of supplemental oxygen, receipt of systemic glucocorticoids or antibiotic agents for respiratory problems, or presentation to the emergency department or hospitalization for COPD exacerbation.
We excluded patients who were already receiving statins; those who should have been receiving statins according to the Adult Treatment Panel III risk stratification27; those who were receiving drugs that are contraindicated with statins; those who had active liver disease, alcoholism, or allergy; and those who were unable to take statins. Patients who met the criteria for statin treatment were excluded from the study on the basis of established guidelines.27 Before patients were enrolled, lipid levels were measured after the patient had fasted for 12 hours.
After STATCOPE began on March 4, 2010, the Food and Drug Administration (FDA) issued a warning (on June 8, 2011) against the concomitant use of amlodipine or high doses of verapamil with simvastatin.28 This announcement resulted in discontinuation of the study drug in participants from both study groups who were receiving amlodipine or high-dose verapamil (16 patients in the simvastatin group and 20 in the placebo group). The STATCOPE data and safety monitoring board subsequently recommended the exclusion of patients with diabetes, according to the medical history or a glycated hemoglobin level of more than 6.5%, which resulted in discontinuation of the study treatment in 28 participants (14 in each group).
OUTCOMES
The primary outcome was the effect of simvastatin on the exacerbation rate, which was defined as the number of exacerbations per person-year. Secondary outcomes included the time to the first exacerbation, the severity of exacerbations, the number of acute cardiovascular events, quality of life, and changes in spirometric variables.
Exacerbations were defined as an increase in severity or new onset of two or more respiratory symptoms (cough, sputum, wheezing, dyspnea, or chest tightness) lasting for at least 3 days and requiring treatment with antibiotics or systemic glucocorticoids.26 Exacerbation severity was graded according to the following scale: mild (required only home management, with or without contact with a health care provider), moderate (required a visit to an emergency department), severe (required hospitalization), and very severe (required intubation and mechanical ventilation).
STUDY VISITS
Spirometric variables (FVC and FEV1) and disease-specific and general quality of life (assessed with the use of the St. George’s Respiratory Questionnaire [SGRQ]29 and the Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36],30 respectively) were measured at baseline and at 12, 24, and 36 months (and at the final visit if that did not coincide with an annual visit). Scores on the SGRQ range from 0 to 100, with lower scores indicating better functioning and with a minimal clinically important difference of 4 points.29 The SF-36 is a self-administered general health questionnaire that generates scores across eight health dimensions. Scores range from 0 to 100, with higher scores indicating better health on each dimension.30 Clinically important changes in the SF-36 score are increases of 5 to 10 points in specific domains.31
Spirometry was performed according to American Thoracic Society–European Respiratory Society guidelines.32 Data are presented as the percent of reference predicted values, with the use of prediction equations from Hankinson et al.33 Only data obtained after the patient had used a bronchodilator were analyzed.
Throughout the trial, participants were followed at clinic visits every 3 months and by means of monthly telephone contacts during months without a clinic visit. During each contact, participants were queried about the interim development of exacerbations and study-drug adherence.
INTERIM ANALYSIS, STOPPING GUIDELINES, AND MONITORING PLAN
The data and safety monitoring board met approximately every 6 months to review recruitment and follow-up rates, study-drug adherence, safety, and efficacy results. Reviews of outcome data at the meetings involved multiple statistical testing performed on a set of accumulating data, with the use of a sequential monitoring plan that was based on the alpha spending approach of Lan and DeMets.34
The formal guidelines for stopping the study owing to futility were not part of the monitoring plan, but as specified in the protocol, the results of analyses that were based on stochastic curtailment were presented to the data and safety monitoring board. At the March 2013 meeting, there were no indications of a treatment effect; however, the board members voted to continue the study. At the October 2013 meeting, with 878 participants having undergone randomization, the primary outcome was the same in the two study groups (COPD exacerbation rate, 1.33 exacerbations per person-year in each group). The power of the study, which was conditional on these observed rates, was 5%. There were no treatment effects in any subgroup. The rates of moderate and severe exacerbations did not differ significantly between the two study groups, and the numbers of deaths were similar (27 deaths in the simvastatin group and 26 in the placebo group). The data and safety monitoring board therefore voted to stop STATCOPE for futility.
STATISTICAL ANALYSIS
Sample-size and power considerations were based on the annual rate of exacerbations as the primary outcome. We estimated that the placebo group would have an average of 1.54 exacerbations per person-year and that simvastatin would reduce this rate by 15%. The estimated rate for the placebo group was based on the observed exacerbation rate in the placebo group of a previous trial of azithromycin conducted by the COPD Clinical Research Network.8
We based our sample-size calculations on a two-sided alpha level of 0.05, a study power of 90%, the expected number of exacerbations per person-year in the placebo group (1.54), the expected number of exacerbations per person-year in the simvastatin group (1.54 × 0.85 = 1.31), a mean duration of follow-up of 2 years, and an equal probability of assignment to the placebo or simvastatin group. We calculated that we would need to enroll 911 patients; assuming a 10% loss to follow-up, the total sample-size estimate was 1002 patients.
An important secondary outcome was the time to the first exacerbation. Assuming that simvastatin treatment would reduce the exacerbation rate by 15% and adjusting for the 10% loss to follow-up, we estimated that 1126 patients would be required in order to provide the study with 90% power for this secondary outcome. Following FDA recommendations and guidance from the data and safety monitoring board for the exclusion of patients who were receiving certain drugs and the exclusion of patients with diabetes, respectively, we projected that 74 additional participants would be needed, for a final study population of 1200 patients.
All the patients who underwent randomization were followed until the end of the study. The final analysis was performed on an intention-to-treat basis. COPD exacerbation rates in the two study groups were compared with the use of a rate ratio. The independence of individual exacerbations was ensured by considering participants to have had two separate exacerbations if the onset dates were at least 14 days apart. Exacerbation rates in each group and the between-group differences were analyzed with the use of negative binomial regression modeling and time-weighted intention-to-treat analyses with adjustments of confidence intervals for between-participant variation (overdispersion).
Secondary outcome measures were assessed at baseline and at 12, 24, and 36 months (and at the final visit if it did not coincide with an annual visit). Analyses of the time to the first exacerbation and the rate of death from any cause were performed with the use of Kaplan–Meier methods, with survival curves for the probability of remaining outcome-free in the two groups as a function of time from randomization. Curves were compared with the use of the log-rank test. Continuous outcome measures, including absolute and relative changes in FEV1 and FVC, dyspnea assessment, and SGRQ scores were computed as rates of change over time, from baseline to the date of the last observation.
RESULTS
ENROLLMENT AND FOLLOW-UP
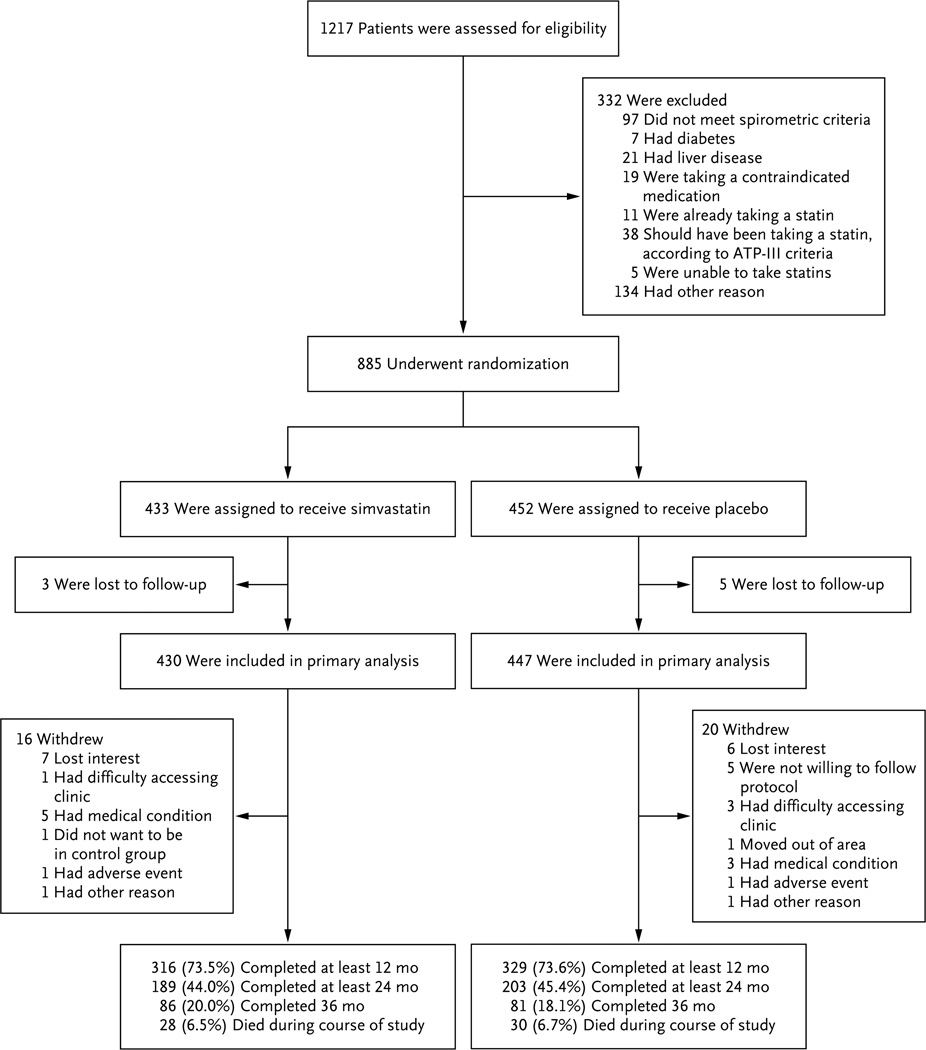
Screening for the study, randomization, and follow-up of the participants are shown in Figure 1. Enrollment began March 4, 2010, and the last participant was enrolled on October 11, 2013. Participants were followed for a mean (±SD) of 641±354 days in the simvastatin group and 639±351 days in the placebo group.
Figure 1. Screening, Randomization, and Study Completion.
ATP-III denotes Adult Treatment Panel III.
CHARACTERISTICS OF THE PARTICIPANTS
The demographic and baseline clinical characteristics of the participants are shown in Table 1 (for complete demographic and baseline clinical data, see Table S1 in the Supplementary Appendix, available at NEJM.org). We randomly assigned 885 participants with COPD to the two study groups. A total of 44% of the patients were women. The patients had a mean age of 62.2±8.4 years, an FEV1 that was 41.6±17.7% of the predicted value, and a smoking history of 50.6±27.4 pack-years. There were no significant differences between the two groups with respect to any characteristics at baseline. Follow-up visit information was not available for 8 of the 885 participants (0.9%), so 430 patients in the simvastatin group and 447 in the placebo group were included in the primary analysis.
Table 1.
Characteristics of the Patients.*
| Characteristic | Simvastatin (N = 433) |
Placebo (N = 452) |
|---|---|---|
| Age — yr | 62.2±8.5 | 62.3±8.4 |
| Female sex — no. (%) | 184 (42.5) | 203 (44.9) |
| FEV1 after bronchodilator use — liters (% of predicted value)† | 1.2±0.6 (41.5±17.8) | 1.2±0.6 (41.6±17.6) |
| FEV1:FVC — %† | 44.4±12.6 | 44.3±13.5 |
| GOLD stage — no./total no. (%)‡ | ||
| 2 | 142/430 (33.0) | 145/449 (32.3) |
| 3 | 142/430 (33.0) | 163/449 (36.3) |
| 4 | 146/430 (34.0) | 141/449 (31.4) |
| Smoking history — pack-yr | 50.0±26.1 | 51.2±28.7 |
| Current smoking — no. (%) | 133 (30.7) | 143 (31.6) |
| COPD medication — no. (%) | ||
| Inhaled glucocorticoids only | 14 (3.2) | 12 (2.7) |
| Long-acting muscarinic antagonist only | 29 (6.7) | 35 (7.7) |
| Long-acting beta2-agonist only | 10 (2.3) | 10 (2.2) |
| Inhaled glucocorticoids and long-acting muscarinic antagonist | 5 (1.2) | 9 (2.0) |
| Inhaled glucocorticoids and long-acting beta2-agonist | 71 (16.4) | 89 (19.7) |
| Long-acting muscarinic antagonist and long-acting beta2-agonist | 25 (5.8) | 23 (5.1) |
| Inhaled glucocorticoids, long-acting muscarinic antagonist, and long-acting beta2-agonist | 223 (51.5) | 228 (50.4) |
| None | 56 (12.9) | 46 (10.2) |
| Entry criteria — no. (%) | ||
| Acute COPD exacerbation requiring hospitalization or ED visit within previous 12 mo | 216 (49.9) | 238 (52.7) |
| Systemic glucocorticoid or antibiotic use within previous 12 mo | 367 (84.8) | 382 (84.5) |
| Use of supplemental oxygen within previous 12 mo | ||
| All patients who met this criterion | 198 (45.7) | 222 (49.1) |
| Patients who met only this criterion | 48 (11.1) | 53 (11.7) |
Plus–minus differences are means ±SD. There were no significant differences between the simvastatin and placebo groups, and there was no imbalance regarding race between the two groups. COPD denotes chronic obstructive pulmonary disease, ED emergency department, FEV1 forced expiratory volume in 1 second, and FVC forced vital capacity.
Spirometric measurements were available for 430 patients in the simvastatin group and 449 in the placebo group.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging system is used to assess the severity of lung disease, with stage 4 indicating the most severe disease.
COPD EXACERBATIONS
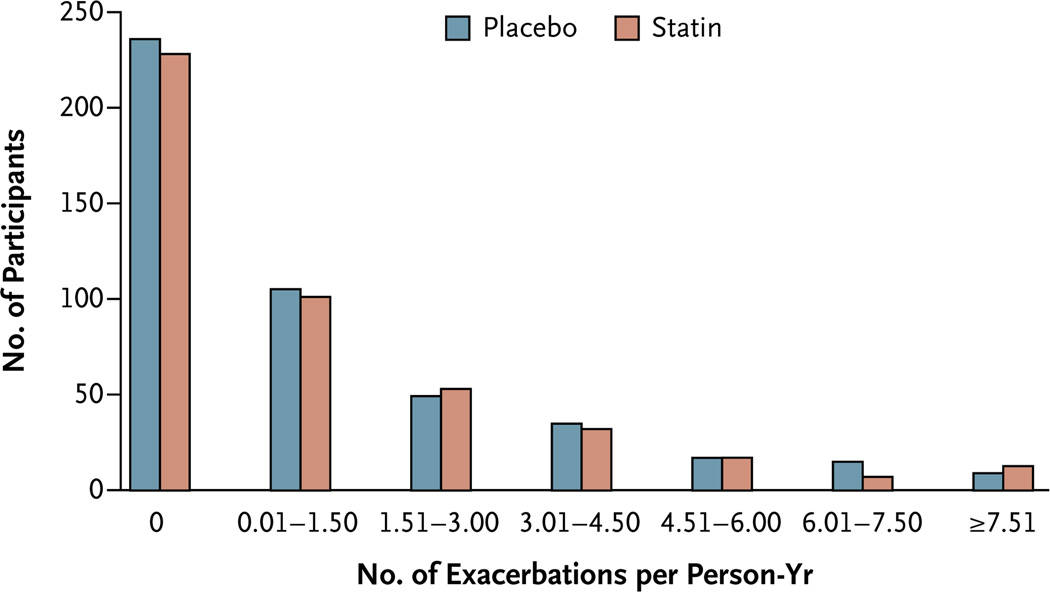
A total of 1982 acute COPD exacerbations occurred during the study: 965 exacerbations among the 430 patients in the simvastatin group and 1017 exacerbations among the 447 patients in the placebo group. The COPD exacerbation rates were similar in the simvastatin and placebo groups: 1.36±1.61 exacerbations per person-year and 1.39±1.73 exacerbations per person-year, respectively (P = 0.54) (Fig. 2). We found no effect of sex on the exacerbation rate.
Figure 2. Acute Exacerbations of Chronic Obstructive Pulmonary Disease per Person-Year, According to Study Group.
The mean (±SD) number of exacerbations per person-year were similar in the simvastatin and placebo groups: 1.36±1.61 exacerbations per person-year and 1.39±1.73 exacerbations per person-year, respectively.
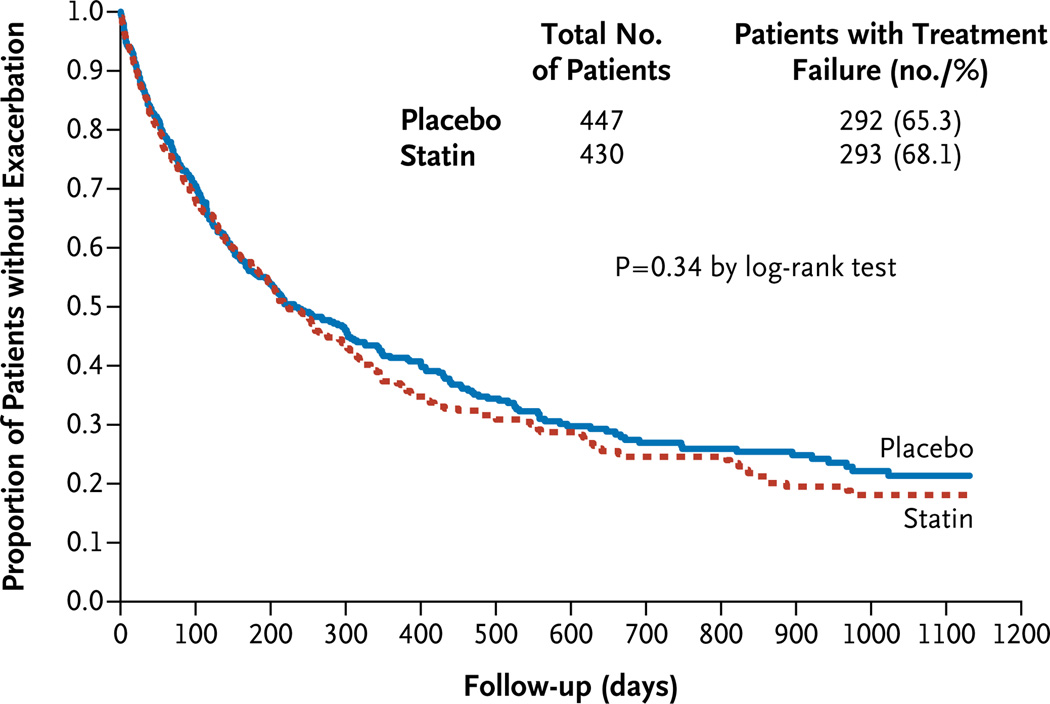
The median number of days to the first exacerbation was also similar in the two groups: 223 days (95% confidence interval [CI], 195 to 275) in the simvastatin group and 231 days (95% CI, 193 to 303) in the placebo group (P = 0.34) (Fig. 3). The time to the first exacerbation in the simvastatin group was slightly shorter for men than for women, but this difference did not reach statistical significance (P =0.09) (Fig. S1 in the Supplementary Appendix).
Figure 3. Effect of Simvastatin on the Time to the First Acute Exacerbation of Chronic Obstructive Pulmonary Disease.
There were no significant between-group differences in the time to the first exacerbation. The median time to the first exacerbation was 223 days (95% CI, 195 to 275) in the simvastatin group and 231 days (95% CI, 193 to 303) in the placebo group.
The severity of the exacerbations was not affected by the study-drug assignment or sex. The number and severity of exacerbations in the simvastatin and placebo groups are shown in Tables S2 and S3 in the Supplementary Appendix, and rates of exacerbation according to severity are shown in Table S4 in the Supplementary Appendix.
A total of 296 of 877 participants (33.8%) had three or more exacerbations during the study (155 patients in the placebo group and 141 in the simvastatin group; the difference was not significant). The study-drug assignment had no effect on the frequency of exacerbations (Fig. S2 in the Supplementary Appendix). There was no effect of study-drug assignment on the percentage of patients who received antibiotic or glucocorticoid therapy for an exacerbation (Table S8 in the Supplementary Appendix). Smoking status, study center, patient’s age, severity of airflow obstruction (i.e., GOLD stage), and use or nonuse of oxygen did not affect the outcomes (Fig. S3 in the Supplementary Appendix).
SECONDARY OUTCOMES
There was no effect of simvastatin on lung function as assessed spirometrically. Likewise, simvastatin had no effect on the general or respiratoryspecific quality of life, as assessed by the SF-36 and SGRQ, respectively (Table S5 in the Supplementary Appendix).
ADVERSE EVENTS
The most common adverse events, aside from exacerbations, were pneumonia and other respiratory and cardiovascular events. The rates of all nonfatal adverse events (the number of serious adverse events per person-year) were similar in the simvastatin and placebo groups (0.63±1.56 events and 0.62±1.48 events, respectively; P>0.20 for all comparisons), except for nonfatal serious adverse events involving the gastrointestinal tract, which were more frequent with simvastatin than with placebo (in 30 patients [0.05 events per personyear] vs. 17 patients [0.02 events per person-year], P = 0.02) (Table 2). There were 28 deaths in the simvastatin group and 30 in the placebo group (P = 0.89 by life-table analysis).
Table 2.
Nonfatal Serious Adverse Events and Fatal Events, According to Study Group.
| Event | Simvastatin (N = 430) |
Placebo (N = 447) |
P Value |
|---|---|---|---|
| Nonfatal serious adverse event — no. of events/person-yr | |||
| Acute exacerbation | 0.32 | 0.32 | 0.99 |
| Respiratory event | |||
| Pneumonia | 0.01 | <0.01 | 0.56 |
| Chronic bronchitis | 0.02 | 0.02 | 0.53 |
| Other | 0.01 | 0.01 | 0.75 |
| Cancer | |||
| Lung cancer | 0.02 | 0.03 | 0.81 |
| Other | 0.03 | 0.03 | 0.21 |
| Cardiovascular event | |||
| Cardiac event | 0.04 | 0.01 | 0.35 |
| Other | 0.03 | 0.08 | 0.23 |
| Gastrointestinal tract event | 0.05 | 0.02 | 0.02 |
| Other | 0.11 | 0.11 | 0.99 |
| Total | 0.63 | 0.62 | 0.96 |
| Fatal event — no. of patients (%) | |||
| Acute exacerbation | 6 (1.4) | 5 (1.1) | 0.72 |
| Other respiratory event | |||
| Pneumonia | 1 (0.2) | 1 (0.2) | 0.99 |
| Chronic bronchitis | 0 | 2 (0.4) | 0.16 |
| Other | 6 (1.4) | 4 (0.9) | 0.49 |
| Cancer | |||
| Lung cancer | 3 (0.7) | 4 (0.9) | 0.73 |
| Other | 0 | 1 (0.2) | 0.33 |
| Cardiovascular event | |||
| Heart | 0 | 1 (0.2) | 0.33 |
| Other | 4 (0.9) | 3 (0.7) | 0.67 |
| Gastrointestinal tract event | 0 | 3 (0.7) | 0.09 |
| Other | 8 (1.9) | 6 (1.3) | 0.56 |
| Total | 28 (6.5) | 30 (6.7) | 0.89 |
PERMANENT DISCONTINUATION OF STUDY DRUG AND STUDY WITHDRAWAL
The most common reason for discontinuation of the study drug was the use of incompatible medications; other common reasons were a diagnosis of diabetes and the FDA warning about additional incompatible medications.28 A small number of participants had myalgias or abnormal laboratory values, but the rates did not differ according to study-drug assignment (Table S6 in the Supplementary Appendix). A total of 36 participants (4.1%) declined to continue participation for various reasons, all of which were unrelated to the studydrug assignment (Table S7 in the Supplementary Appendix).
LIPID LEVELS
Total cholesterol, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, and triglyceride levels were similar in the simvastatin and placebo groups at baseline; the LDL cholesterol levels were 113.9±28.2 mg per deciliter (2.95±0.73 mmol per liter) and 114.4±27.6 mg per deciliter (2.96±0.71 mmol per liter), respectively. At 1 year after randomization, high-density lipoprotein cholesterol levels in the simvastatin group had increased by 2.5 mg per deciliter (0.06 mmol per liter), whereas the levels in the placebo group had decreased by 0.5 mg per deciliter (0.01 mmol per liter) (P = 0.01); LDL cholesterol levels had decreased by 33.2 mg per deciliter (0.86 mmol per liter) in the simvastatin group, as compared with 6.6 mg per deciliter (0.17 mmol per liter) in the placebo group (P<0.001). (Complete data on lipid levels are provided in Table S9 in the Supplementary Appendix.)
DISCUSSION
In this large, prospective, multicenter, randomized trial, we found that 40 mg of daily simvastatin had no effect on the frequency of exacerbations, the time to the first exacerbation, or the severity of exacerbations in patients with moderate-to-severe COPD who were at high risk for exacerbations. Simvastatin also had no effect on lung function or on general or disease-specific quality of life. The rates of serious adverse events were low and similar in the two study groups. These data show that daily use of 40 mg of simvastatin has no role in preventing exacerbations in moderate-to-severe COPD.
Our results differ from previously reported findings that statins prevent exacerbations, attenuate lung-function decline, and reduce mortality among patients with COPD.20–24 A retrospective analysis of a large Canadian database showed that statin therapy in patients with COPD who did not have cardiovascular disease was associated with large reductions in the rate of hospitalization due to COPD and cardiovascular morbidity and mortality.24 A matched cohort study involving 76,232 patients showed that among those who received a moderate statin dose (≥40 mg per day), the odds ratios for influenza-related death and pneumonia-related death were significantly reduced.22 Similar reductions in mortality were reported with statin use in population studies involving Japanese and Norwegian patients with COPD.35,36 Keddissi et al. compared the annual decline in the FVC and FEV1 according to statin use or nonuse in current and former smokers who were treated at a Veterans Affairs (VA) medical center.23 Study participants who used statins had smaller declines, as compared with nonusers, in FEV1 (P<0.001) and FVC (P<0.001). The VA Normative Aging Study showed that patients using statins had significantly less decline in FEV1, as compared with patients who did not use statins (P<0.001).20 The underuse of statins in persons with cardiac risk factors who have been included in retrospective studies may account in part for the differences between our findings and those previously reported.37
We chose to use simvastatin because it is one of the most effective HMG-CoA reductase inhibitors for lowering cardiovascular risk, it is widely available and affordable as a generic drug, and the literature documenting its pleiotropic actions is extensive.38,39 The antilipid effects of simvastatin are dose-dependent, as are the risks of side effects such as myopathy.28 The antiinflammatory effects of simvastatin are less dependent on the dose, with nearly maximal reductions in serum C-reactive protein (CRP) levels at a dose of 20 to 40 mg per day.40 We believed that antiinflammatory effects were more important than antilipid effects in producing the clinical benefits we were seeking. Thus, the balance of risk to potential benefit, as well as reports of clear benefits with respect to mortality with simvastatin at the 40-mg daily dose, led us to favor this lower dose over a dose of 80 mg per day (which has greater antilipid effects).41,42
Our study has some limitations. First, it was terminated early for futility with respect to a treatment effect. However, a thorough examination of the data by the STATCOPE data and safety monitoring board and the investigators showed no signal for a delayed effect of simvastatin on the exacerbation rate. Furthermore, we did not use a marker of systemic inflammation (serum CRP level) to screen patients for enrollment in the study, and this may have limited our ability to detect an effect of simvastatin in reducing the rate of exacerbations.43 Also, the participants in our study had moderate-to-severe airway obstruction, and it is unclear whether our observation that simvastatin was not beneficial would apply to patients with less impairment. Finally, new FDA recommendations regarding the interactions of simvastatin with other drugs were announced during the study period. These recommendations hindered recruitment to a study that was already impeded by high background statin use in clinical practice. Despite these recruitment challenges, relatively few participants withdrew from the study or discontinued the study drug. The low rate of withdrawal allowed us to conclusively evaluate the effect of simvastatin on the occurrence of exacerbations in our study population.
In conclusion, simvastatin at a dose of 40 mg daily, in addition to usual care, did not reduce the exacerbation rate or prolong the time to the first exacerbation among patients with moderate-to-severe COPD who were at high risk for exacerbations. Furthermore, simvastatin had no effect on lung function, quality of life, the rate of severe adverse events, or mortality. Thus, our data did not show a therapeutic benefit of statins in patients with moderate-to-severe COPD.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (U10 HL074407, U10 HL074408, U10HL074409, U10 HL074416, U10 HL074418, U10 HL074422, U10 HL074424, U10 HL074428, U10 HL074431, U10 HL074439, and U10 HL074441) and the Canadian Institutes of Health Research (115074).
APPENDIX
The authors’ full names and degrees are as follows: Gerard J. Criner, M.D., John E. Connett, Ph.D., Shawn D. Aaron, M.D., Richard K. Albert, M.D., William C. Bailey, M.D., Richard Casaburi, M.D., Ph.D., J. Allen D. Cooper, Jr., M.D., Jeffrey L. Curtis, M.D., Mark T. Dransfield, M.D., MeiLan K. Han, M.D., Barry Make, M.D., Nathaniel Marchetti, D.O., Fernando J. Martinez, M.D., Dennis E. Niewoehner, M.D., Paul D. Scanlon, M.D., Frank C. Sciurba, M.D., Steven M. Scharf, M.D., Ph.D., Don D. Sin, M.D., M.P.H., Helen Voelker, B.A., George R. Washko, M.D., Prescott G. Woodruff, M.D., and Stephen C. Lazarus, M.D.
The authors’ affiliations are as follows: the Department of Pulmonary and Critical Care Medicine, Temple University School of Medicine, Philadelphia (G.J.C., N.M.); Department of Biostatistics, School of Public Health (J.E.C.), Data Coordinating Center (J.E.C., H.V.), and Department of Pulmonary, Allergy, Critical Care, and Sleep Medicine (D.E.N.), University of Minnesota, Minneapolis, and Department of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester (P.D.S.) — all in Minnesota; Ottawa Hospital Research Institute, University of Ottawa, Ottawa (S.D.A.), and Department of Medicine and University of British Columbia James Hogg Research Centre, Institute for Heart and Lung Health, University of British Columbia, Vancouver (D.D.S.) — both in Canada; Department of Medicine, Denver Health Medical Center (R.K.A.), and Division of Pulmonary Critical Care and Sleep Medicine, National Jewish Health (B.M.) — both in Denver; Department of Pulmonary Allergy and Critical Care Medicine (W.C.B., M.T.D.) and the Pulmonary Section (J.A.D.C.), University of Alabama at Birmingham and Birmingham Veterans Affairs Medical Center, Birmingham; Harbor–UCLA Research and Education Institute, Los Angeles (R.C.); Department of Pulmonary Medicine, Veterans Affairs Medical Center and University of Michigan (J.L.C.), and Department of Pulmonary and Critical Care Medicine, University of Michigan Health System (M.K.H., F.J.M.) — all in Ann Arbor; Department of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh Medical Center, Pittsburgh (F.C.S.); Department of Pulmonary and Critical Care Medicine, University of Maryland, Baltimore (S.M.S.); Department of Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, Boston (G.R.W.); and Department of Pulmonary and Critical Care Medicine, University of California, San Francisco, San Francisco (P.G.W., S.C.L.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalal AA, Christensen L, Liu F, Riedel AA. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341–349. doi: 10.2147/COPD.S13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Druss BG, Marcus SC, Olfson M, Pincus HA. The most expensive medical conditions in America. Health Aff (Millwood) 2002;21(4):105–111. doi: 10.1377/hlthaff.21.4.105. [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 8.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–698. doi: 10.1056/NEJMoa1104623. [Erratum, N Engl J Med 2012; 366: 1356.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [Erratum, Lancet 2003; 361: 1660.] [DOI] [PubMed] [Google Scholar]
- 10.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 11.Johnson BA, Iacono AT, Zeevi A, Mc-Curry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med. 2003;167:1271–1278. doi: 10.1164/rccm.200205-410OC. [DOI] [PubMed] [Google Scholar]
- 12.Mortensen EM, Copeland LA, Pugh MJ, et al. Impact of statins and ACE inhibitors on mortality after COPD exacerbations. Respir Res. 2009;10:45. doi: 10.1186/1465-9921-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 14.Kinlay S, Schwartz GG, Olsson AG, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108:1560–1566. doi: 10.1161/01.CIR.0000091404.09558.AF. [DOI] [PubMed] [Google Scholar]
- 15.Inoue I, Goto S, Mizotani K, et al. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci. 2000;67:863–876. doi: 10.1016/s0024-3205(00)00680-9. [DOI] [PubMed] [Google Scholar]
- 16.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferns GA. Differential effects of statins on serum CRP levels: implications of recent clinical trials. Atherosclerosis. 2003;169:349–351. doi: 10.1016/s0021-9150(03)00191-6. [DOI] [PubMed] [Google Scholar]
- 18.Bellosta S, Via D, Canavesi M, et al. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–1678. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 19.Balk EM, Lau J, Goudas LC, et al. Effects of statins on nonlipid serum markers associated with cardiovascular disease: a systematic review. Ann Intern Med. 2003;139:670–682. doi: 10.7326/0003-4819-139-8-200310210-00011. [DOI] [PubMed] [Google Scholar]
- 20.Alexeeff SE, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blamoun AI, Batty GN, DeBari VA, Rashid AO, Sheikh M, Khan MA. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int J Clin Pract. 2008;62:1373–1378. doi: 10.1111/j.1742-1241.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- 22.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 23.Keddissi JI, Younis WG, Chbeir EA, Daher NN, Dernaika TA, Kinasewitz GT. The use of statins and lung function in current and former smokers. Chest. 2007;132:1764–1771. doi: 10.1378/chest.07-0298. [DOI] [PubMed] [Google Scholar]
- 24.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Lee TM, Chen CC, Shen HN, Chang NC. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci (Lond) 2009;116:497–505. doi: 10.1042/CS20080241. [DOI] [PubMed] [Google Scholar]
- 26.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 27.Bethesda, MD: National Heart, Lung, and Blood Institute; Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): final report 2014. ( http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf). [Google Scholar]
- 28.FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. 2014 Mar; ( http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm#TableofSimvastatinDose Limitations).
- 29.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther. 2000;22:1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31.Wyrwich KW, Finn SD, Tierney WM, Kroenke K, Babu AN, Wolinsky FD. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease. J Gen Intern Med. 2003;18:196–202. doi: 10.1046/j.1525-1497.2003.20203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 33.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 34.Lan KKG, DeMets DL. Design and analysis of group sequential tests based on the type 1 error spending rate function. Biometrika. 1987;74:149–154. [Google Scholar]
- 35.Ishida W, Kajiwara T, Ishii M, et al. Decrease in mortality rate of chronic obstructive pulmonary disease (COPD) with statin use: a population-based analysis in Japan. Tohoku J Exp Med. 2007;212:265–273. doi: 10.1620/tjem.212.265. [DOI] [PubMed] [Google Scholar]
- 36.Søyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29:279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 37.Andell P, Koul S, Martinsson A, et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart. 2014;1:e000002. doi: 10.1136/openhrt-2013-000002. ( http://openheart.bmj.com/content/1/1/e000002.full.pdf). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 39.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 40.Li JJ, Chen MZ, Chen X, Fang CH. Rapid effects of simvastatin on lipid profile and C-reactive protein in patients with hypercholesterolemia. Clin Cardiol. 2003;26:472–476. doi: 10.1002/clc.4960261008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 42.MAAS Investigators. Effect of simvastatin on coronary atheroma: the Multicentre Anti-Atheroma Study (MAAS) Lancet. 1994;344:633–638. [PubMed] [Google Scholar]
- 43.Lahousse L, Loth DW, Joos GF, et al. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm Pharmacol Ther. 2013;26:212–217. doi: 10.1016/j.pupt.2012.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.