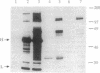
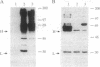
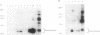Abstract
Proteins that bind to discrete domains of the Blk, Fyn, Lyn, and Btk protein tyrosine kinases were examined in pre-B cells that had not been subjected to any external stimulation, as well as in nonstimulated and antigen-receptor-ligated B cells. Proteins that bind to the Src homology 2 domains of Blk and Fyn were identified in B cells that had been activated with anti-IgM but were not identified in unstimulated B cells. A number of Blk and Fyn Src homology 2 domain-binding phosphoproteins were also observed in pre-B cells that had not been stimulated in vitro. The phosphoproteins seen in activated B cells potentially represent substrates that play a role in the pathway of antigen-receptor-mediated signaling. Distinct signaling pathways involving distinguishable kinase substrates may be relevant in pre-B-cell-receptor-mediated cell survival during ontogeny. These results indirectly support models that predict constitutive ligand-independent signaling by the pre-antigen receptor during lymphoid ontogeny.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil B. J., Pillai S. The omega/lambda 5 surrogate immunoglobulin light chain is expressed on the surface of transitional B lymphocytes in murine bone marrow. J Exp Med. 1991 Jan 1;173(1):111–116. doi: 10.1084/jem.173.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M. P., Perlmutter R. M. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989 Oct;1(1):66–74. [PubMed] [Google Scholar]
- Davidson W. F., Fredrickson T. N., Rudikoff E. K., Coffman R. L., Hartley J. W., Morse H. C., 3rd A unique series of lymphomas related to the Ly-1+ lineage of B lymphocyte differentiation. J Immunol. 1984 Aug;133(2):744–753. [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Ehlich A., Schaal S., Gu H., Kitamura D., Müller W., Rajewsky K. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993 Mar 12;72(5):695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Groettrup M., Ungewiss K., Azogui O., Palacios R., Owen M. J., Hayday A. C., von Boehmer H. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell. 1993 Oct 22;75(2):283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Lottspeich F., Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990 Dec;20(12):2795–2799. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Rolink A., Melchers F. A complex of glycoproteins is associated with VpreB/lambda 5 surrogate light chain on the surface of mu heavy chain-negative early precursor B cell lines. J Exp Med. 1993 Aug 1;178(2):469–478. doi: 10.1084/jem.178.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi H., Borgulya P., Scott B., Karjalainen K., Traunecker A., Kaufman J., von Boehmer H. Surface expression of the beta T cell receptor (TCR) chain in the absence of other TCR or CD3 proteins on immature T cells. EMBO J. 1991 Jan;10(1):93–100. doi: 10.1002/j.1460-2075.1991.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishihara K., Penninger J., Wallace V. A., Kündig T. M., Kawai K., Wakeham A., Timms E., Pfeffer K., Ohashi P. S., Thomas M. L. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993 Jul 16;74(1):143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992 Mar 12;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L. Cell cycle related heterogeneity of Ia antigen expression on a murine B lymphoma cell line:analysis by flow cytometry. J Immunol. 1981 Feb;126(2):626–631. [PubMed] [Google Scholar]
- Lassoued K., Nuñez C. A., Billips L., Kubagawa H., Monteiro R. C., LeBlen T. W., Cooper M. D. Expression of surrogate light chain receptors is restricted to a late stage in pre-B cell differentiation. Cell. 1993 Apr 9;73(1):73–86. doi: 10.1016/0092-8674(93)90161-i. [DOI] [PubMed] [Google Scholar]
- Levinson D. A., Campos-Torres J., Leder P. Molecular characterization of transgene-induced immunodeficiency in B-less mice using a novel quantitative limiting dilution polymerase chain reaction method. J Exp Med. 1993 Jul 1;178(1):317–329. doi: 10.1084/jem.178.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S. N., Desiderio S. SH2 domains of the protein-tyrosine kinases Blk, Lyn, and Fyn(T) bind distinct sets of phosphoproteins from B lymphocytes. J Biol Chem. 1993 Oct 25;268(30):22557–22565. [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. The omega and iota surrogate immunoglobulin light chains. Curr Top Microbiol Immunol. 1988;137:136–139. doi: 10.1007/978-3-642-50059-6_20. [DOI] [PubMed] [Google Scholar]
- Pillai S. Immunoglobulin transport in B cell development. Int Rev Cytol. 1991;130:1–36. doi: 10.1016/s0074-7696(08)61500-4. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Koyasu S., Nakayama K., Murphy K. M., Loh D. Y., Reinherz E. L., Alt F. W. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993 Feb 5;259(5096):822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. D., Sideras P., Smith C. I., Vorechovský I., Chapman V., Paul W. E. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993 Jul 16;261(5119):355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Yao X. R., Scott D. W. Antisense oligodeoxynucleotides to the blk tyrosine kinase prevent anti-mu-chain-mediated growth inhibition and apoptosis in a B-cell lymphoma. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7946–7950. doi: 10.1073/pnas.90.17.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]