Abstract
Amylosin, a heat-stable channel-forming non-ribosomally synthesized peptide toxin produced by strains of Bacillus amyloliquefaciens isolated from moisture-damaged buildings, is shown in this paper to have immunotoxic and cytotoxic effects on human cells as well as antagonistic effects on microbes. Human macrophages exposed to 50 ng of amylosin ml−1 secreted high levels of cytokines interleukin-1β (IL-1β) and IL-18 within 2 h, indicating activation of the NLRP3 inflammasome, an integral part of the innate immune system. At the same exposure level, expression of IL-1β and IL-18 mRNA increased. Amylosin caused dose-dependent potassium ion efflux from all tested mammalian cells (human monocytes and keratinocytes and porcine sperm cells) at 1 to 2 μM exposure. Amylosin also inhibited the motility of porcine sperm cells and depolarized the mitochondria of human keratinocytes. Amylosin may thus trigger the activation of the NLRP3 inflammasome and subsequently cytokine release by causing potassium efflux from exposed cells. The results of this study indicate that exposure to amylosin activates the innate immune system, which could offer an explanation for the inflammatory symptoms experienced by occupants of moisture-damaged buildings. In addition, the amylosin-producing B. amyloliquefaciens inhibited the growth of both prokaryotic and eukaryotic indoor microbes, and purified amylosin also had an antimicrobial effect. These antimicrobial effects could make amylosin producers dominant and therefore significant causal agents of health problems in some moisture-damaged sites.
INTRODUCTION
Amylosin is a heat-stable 1,197-Da peptide toxin originally found to be produced by strains of Bacillus amyloliquefaciens isolated from moisture-damaged buildings (1, 2). B. amyloliquefaciens belongs to the Bacillus subtilis group, members of which have been found in high numbers in moisture-damaged buildings where occupants have experienced building-related ill health symptoms (3, 4). Amylosin forms cation-permeant ion channels with high selectivity for K+ in lipid membranes (5) and thus depolarizes the plasma membrane and the mitochondria inside live mammalian cells, disrupting cellular ion homeostasis, mitochondrial functions, and energy metabolism (1, 2). It has been suggested that amylosin causes health risks for people living or working in moisture-damaged buildings contaminated with amylosin-producing bacteria (1). Amylosin has also been shown to be produced by B. subtilis and Bacillus mojavensis strains connected to foodborne illness (6).
Moisture-damaged buildings harbor many kinds of fungi and bacteria, including producers of bioactive compounds (7, 8; reviewed in reference 9). The symptoms experienced by people living or working in moisture-damaged buildings vary (10), but they could at least partly result from activation of the innate immune system (11, 12). Symptoms such as acute inflammatory responses and pseudoallergic reactions may be a result of the activation of inflammasomes (12, 13). Inflammasomes are components of the innate immune system which activate inflammatory caspases, leading to cytokine release (14). Activation of the NLRP3 inflammasome (also known as NALP3 and cryopyrin), which stimulates the secretion of active cytokines interleukin-1β (IL-1β) and IL-18 by activating caspase-1 (15), has been shown to be dependent on K+ efflux (16, 17) and can be triggered by bacterial toxins (reviewed in references 17 and 18). Both IL-1β and IL-18 act as proinflammatory cytokines, activating signaling pathways in mammalian cells which lead to inflammation (19, 20, 21).
The purpose of this research was to study the detrimental effects of amylosin on cells of the human innate immune system in order to assess whether ill health symptoms experienced by people exposed to amylosin (via, e.g., inhaled air in moisture-damaged buildings, contaminated food, or commercial products produced using possible amylosin-producing bacterial strains) could result from the activation of the innate immune system. Human macrophages and peripheral blood mononuclear cells (PBMC) from healthy donors as well as human keratinocytes (HaCaT) and porcine spermatozoa were used as test cells to record the outcome of amylosin exposure. As toxicity endpoints, cytokine secretion, cytokine mRNA transcription, potassium efflux, and mitochondrial effects were measured. Effects of amylosin-producing B. amyloliquefaciens and purified amylosin on selected microorganisms isolated from moisture-damaged buildings were also assessed to see if the production of amylosin could offer a competitive advantage to producer strains.
MATERIALS AND METHODS
Microbial strains.
The following bacterial strains were used: Bacillus amyloliquefaciens strains 19b (HAMBI 2660; amylosin producer [1]) and IAM 1521 (HAMBI 2718), Bacillus cereus strains NS 58 (HAMBI 2450; cereulide producer [22]) and F4810/72 (DSM 4312; cereulide producer [23]), Bacillus megaterium Ne10 (DSM 17641), Williamsia muralis 140/96T (DSM 44343T), Mycobacterium murale MA113T (DSM 44340T), Sphingomonas aurantiaca MA101bT (DSM 14748T), Dietzia sp. strain MA147 (HAMBI 3595), and Bacillus sp. OS16 (isolated from hay dust). Chaetomium globosum MTAV35 (HAMBI 3336) is a toxigenic mold isolated from a microbially damaged indoor building environment involved in a serious human case (fatal). Strains were from the DSMZ Culture Collection (Braunschweig, Germany) or the HAMBI Culture Collection (University of Helsinki, Helsinki, Finland) except for Bacillus sp. OS16, which was from our laboratory collection. The strains were grown on tryptic soy agar (TSA; Scharlab SL, Barcelona, Spain) plates at 25 ± 3°C (mean ± standard deviation), except for S. aurantiaca, which was grown at 18°C.
Preparation of amylosin-containing and amylosin-free bacterial extracts.
Biomass of strains B. amyloliquefaciens 19b and IAM 1521 was harvested from TSA plates (grown for 4 days to 4 weeks) into glass vials prewashed with methanol. The vials were exposed to three freeze-thaw cycles. Methanol was then added (1 ml per 100 mg of biomass [wet weight]), after which the vials were sealed and held in a water bath at 100°C for 10 min. After cooling, the vials were agitated (200 rpm) overnight (at 20 to 22°C) and then centrifuged (3,800 rpm, 15 min), and the supernatants were transferred to preweighed glass ampoules. The methanol was then evaporated and the residue weighed and redissolved in methanol (10 mg [dry weight] ml−1). The biological activity of the extracts was assessed by using the boar sperm motility inhibition assay as described by Andersson et al. (24). The extracts were stored at −20°C.
Purification and quantification of amylosin.
Amylosin was purified by reversed-phase high-performance liquid chromatography (RP-HPLC) from a methanol extract of B. amyloliquefaciens 19b as described previously (6), except that the eluent was a gradient of A (0.1% formic acid) and B (methanol) from 70% B to 75% B in 10 min, to 85% B in 1 min, and then continued for 20 min by isocratic elution with 85% B at a flow rate of 1 ml min−1. Absorbance at a wavelength 365 nm was used for detection. Quantification of amylosin was done by a HPLC-UV method using amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) as a reference compound, as described by Mikkola et al. (2). HPLC-mass spectrometry (HPLC-MS) analysis of amylosin was done as described previously (6) using the HPLC conditions presented above.
Mammalian target cells.
Human macrophages were obtained by differentiating primary peripheral blood mononuclear cells (PBMC) isolated from the buffy coats of healthy blood donors. Cells were purified, allowed to differentiate, and cultured at a density of 0.75 × 106 to 1.5 × 106 monocytes ml−1 as described previously (13). The spontaneously immortalized human keratinocyte cell line HaCaT, which originated from adult human skin and exhibits normal differentiation (25), was cultivated in RPMI 1640 as described by Hoornstra et al. (26). Human monocyte-enriched PBMC were primary cells freshly isolated from buffy coats of healthy human blood donors (Finnish Red Blood Service, Helsinki, Finland) obtained with ethical permission, maintained for experiments in RPMI 1640 as described previously (26). Porcine spermatozoa were primary cells obtained from a commercial source (Figen Ltd., Tuomikylä Finland) as extended boar semen containing 3 ± 1 mM extracellular K+ as described by Hoornstra et al. (26).
Macrophage stimulation.
Prior to stimulation, human macrophages were primed for 3 h with lipopolysaccharide (LPS; Escherichia coli O111:B4; Sigma-Aldrich, St. Louis, MO, USA) diluted in Dulbecco's phosphate-buffered saline (DPBS) without calcium and magnesium (Lonza, Basel, Switzerland) and used at a final concentration of 1 μg ml−1. For stimulation, predetermined amounts of cell-free bacterial extracts, purified toxins, or control substances were added into the stimulation medium, which was macrophage-SFM (serum-free medium; Invitrogen, Carlsbad, CA, USA) containing l-glutamine and supplemented with 10 ng ml−1 GM-CSF (granulocyte-macrophage colony-stimulating factor; ImmunoTools, Friesoythe, Germany), or in Gibco RPMI (Roswell Park Memorial Institute) 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM alanyl-l-glutamine (UltraGlutamine 1; Lonza, Basel, Switzerland) when cell culture supernatants were collected for Western blot analysis. Both stimulation media were supplemented with 50 U penicillin ml−1 and 50 μg streptomycin ml−1 (Pen Strep; Invitrogen, Carlsbad, CA, USA). After stimulation for 2 to 18 h, supernatants and cells were collected from the plates and stored at −70°C. Roridin A (Sigma-Aldrich, St. Louis, MO, USA), known to stimulate cytokine production in human macrophages (13), was used as a positive reference compound for macrophage stimulation. All stimulations were carried out in flat-bottom sterile plastic cell culture microtiter plates.
Cytokine detection methods.
Concentrations of cytokines in the supernatants collected from the stimulated macrophage cultures were determined with enzyme-linked immunosorbent assays (ELISA) according to the manufacturers' instructions. IL-1β was analyzed using the human IL-1β Eli-Pair assay (Nordic BioSite, Täby, Sweden), and IL-18 was detected with the human IL-18 matched antibody pairs for ELISA kit (eBioscience, San Diego, CA, USA).
IL-1β and IL-18 secretion levels were analyzed by Western blotting from concentrated human macrophage culture supernatants collected after 4 h of stimulation. Supernatants (4 ml per sample) were concentrated to ca. 100 μl by using Amicon Ultra-15 tubes (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Five-microliter aliquots of concentrated cell supernatants were separated on 12% precast sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Mini-Protean precast gels; Bio-Rad, Hercules, CA, USA) at 200 V for 30 min and transferred onto Immobilon-P transfer membranes (Millipore, Billerica, MA, USA) with the PowerPac 3000 system (Bio-Rad, Hercules, CA, USA) at +4°C and 100 V for 1 h. The Precision Plus protein dual color standard (Bio-Rad, Hercules, CA, USA) was used as a molecule weight marker. Blotted membranes were stained with Ponceau red to confirm transfer of protein samples, washed three times with PBS containing 0.05% Tween 20 for 10 min, and blocked for 45 min in PBS containing 5% skimmed milk. Membranes were then stained overnight with previously described anti-IL-1β and anti-Il-18 primary antibodies (27, 28) at +4°C and subsequently washed three times with PBS containing 0.05% Tween 20 for 10 min. The membranes were incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako A/S, Glostrup, Denmark) for 1 h at 20 ± 2°C and then visualized using the Immobilon Western chemiluminescence HRP substrate kit (Millipore, Billerica, MA, USA) and the ImageQuant LAS 4000 imager (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Detection of cytokine mRNA transcription.
IL-1β and IL-18 mRNA transcription levels were detected using a quantitative real-time reverse transcription-PCR (qRT-PCR) assay as described previously (13).
Assay of potassium ion efflux from intact mammalian cells.
Assays of potassium efflux from the human PBMC and HaCaT cells as well as the porcine spermatozoa were performed as described previously (26). Briefly, cells were pelleted by centrifugation, resuspended in isotonic, sodium phosphate-buffered K+-free medium, and placed in a measurement cuvette provided with magnetic stirring, temperature control (24°C), and a potassium ion-selective electrode (NIKO-ANALIT, Moscow, Russia) linked to PC recording software (Record 4; IBC, Pushchino, Russia). The concentration of K+ in the extracellular medium was recorded once per second. The electrode signal was calibrated by adding 100 μM KCl into the cuvette at the end of each run, as done in a previous study (29). The isotonic (300 mOsm kg−1) K+-free medium contained 150 mM NaCl, 5 mM NaH2PO4, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose, and 10 mM HEPES (pH adjusted to 7.2 with Trizma base). Alamethicin (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive reference compound.
Assessment of toxic effects on mammalian cells.
The effects of toxins on boar sperm motility were investigated by using the boar sperm motility assay as described previously (24).
Testing for mitochondrial effects of amylosin inside live human cells was done with HaCaT cells seeded to a density of 4 × 104 cells ml−1 in RPMI 1640 medium (Lonza, Verviers, Belgium) supplemented with 2 mM glutamine, 10% FBS, 50 IU of penicillin, and 50 μg of streptomycin (Sigma, St. Louis, MO, USA) ml−1. The cells were grown for 48 h in 8-well, flat-bottom glass chamber slides prior to toxin exposure. The assays were performed by adding RP-HPLC-purified amylosin to the wells and incubating for up to 48 h. After exposure, the cells were stained with the fluorogenic transmembrane potential (ΔΨ) responsive dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, dissolved in dimethyl sulfoxide; Invitrogen, Carlsbad, CA, USA) and propidium iodide (dead stain; 2.4 mg ml−1 in water; Invitrogen, Carlsbad, CA, USA) as described previously (26). All assays were done in triplicate.
Assessment of antimicrobial growth inhibition.
The growth-inhibitory effects of amylosin-producing B. amyloliquefaciens 19b against bacteria and fungi were tested by coculturing the strains on TSA plates for 10 days at 20 ± 2°C. Growth and inhibition were examined visually.
To assess the growth-inhibitory effects of RP-HPLC-purified amylosin, other lipopeptides known to be produced by B. amyloliquefaciens (surfactin, iturin, and fengycin) (30), and purified cereulide on bacterial cultures, B. megaterium Ne10 and Dietzia sp. MA147 were exposed to the substances for 24 h in broth culture. Cereulide was purified from Bacillus cereus F4810/72 as described previously (31). Fengycin and iturin were from Aibi Lipopeptides (Gembloux, Belgium), and surfactin was from Sigma-Aldrich (St. Louis, MO, USA). The bacterial target strains were grown in TSB (Scharlab SL, Barcelona, Spain) for 18 h (B. megaterium Ne10) or 40 h (Dietzia sp. MA147), after which the viable counts on TSA plates were 5 × 107 to 5 × 108 CFU ml−1 and the turbidity of the cultures was 1,000 (filter 520; Klett-Summerson turbidometer). For exposure, 10 μl of each target strain broth culture was used to inoculate 2 ml of TSB, leading to suspensions with viable counts and turbidities of 105 to 106 CFU ml−1 and <50, respectively. These culture suspensions were then exposed to the test substances at concentrations from 0.001 μg to 100 μg ml−1 TSB. Prior to being added to the culture suspensions, the pure substances were dissolved in methanol and heated in a water bath (80°C) for 10 min, and the bioactivity of the methanol solutions was verified in the boar sperm motility assay, using a 30-min exposure time as described previously (24). TSB with the methanol extracts of the test substances only was used as a sterility control of the test substances, and pure methanol, without a target strain, was used as an internal negative control (with no bacterial growth indicated by no increase in turbidity). Methanol only, together with the target strains, was used as a positive control (no inhibition of bacterial growth, as indicated by the maximal turbidity value after 24 h).
After 24 h of incubation at 21 ± 2°C and 120 rpm, the turbidity of the exposed target strain cultures was measured (filter 520; Klett-Summerson turbidometer). To verify the identity and purity of the exposed strains and to obtain postexposure viable counts, aliquots from the exposed broth cultures were cultured on TSA plates. The identity of the target strains was concluded from the characteristic color of the colonies: deep red colonies indicated Dietzia sp. MA147, and white colonies of extremely big (3- by 2-μm) endospore-forming cells indicated B. megaterium Ne10. The turbidity and viable counts of the exposed cultures were compared to the turbidity and viable counts measured before exposure started. Inhibition of growth was detected as a decrease in the turbidity and viable count compared to those of the respective methanol controls. Inhibition of bacterial growth caused by a certain concentration of the test substance was calculated using the following equation: I = 100% − [(Gi − G0)/Gmax − G0) × 100], with I representing inhibition, Gi the turbidity or CFU ml−1 after exposure and incubation for 24 h, G0 the turbidity or CFU ml−1 prior to exposure and incubation, and Gmax the turbidity or CFU ml−1 of the positive control after incubation for 24 h. The 50% effective concentration (EC50) was calculated as the amount of a test substance (in μg ml−1 TSB) causing ≥50% inhibition of growth of the target strain.
RESULTS
Amylosin-containing extract of B. amyloliquefaciens stimulates secretion of cytokines IL-1β and IL-18 in human macrophages.
To test if an amylosin-containing extract of B. amyloliquefaciens can trigger cytokine secretion in human macrophages, LPS-primed and nonprimed macrophages were exposed to extracts prepared from the biomass of B. amyloliquefaciens strains 19b (amylosin producer) and IAM 1521 (amylosin nonproducer). After exposure, the concentrations of cytokines IL-1β and IL-18 in the macrophage culture supernatants were analyzed via an ELISA. Figure 1 shows that when macrophages primed with LPS were exposed to the extract of the amylosin-producing strain 19b containing ≥50 ng amylosin ml−1, the secretion of IL-1β was activated. The same amylosin concentration increased the secretion of IL-18 from both nonprimed and LPS-primed macrophages, with a higher level of secretion from the LPS-primed cells. The extract of B. amyloliquefaciens IAM 1521, a strain that does not produce amylosin, stimulated no secretion of IL-1β in the LPS-primed or the nonprimed macrophages and had only a slight effect on the secretion of IL-18 in the macrophages primed with LPS. These observations indicated that amylosin, contained in the extract of strain 19b, was the cause of the increased cytokine secretion. The results also showed that exposure to the amylosin-containing bacterial extract of B. amyloliquefaciens 19b dose dependently stimulated cytokine secretion in the human macrophages.
FIG 1.
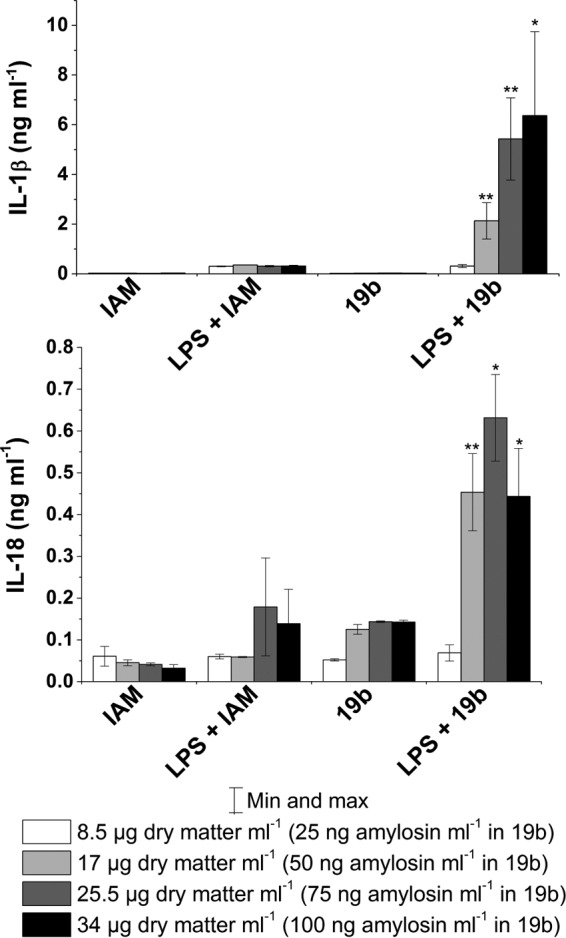
Amylosin-containing extract of B. amyloliquefaciens 19b stimulates dose-dependent secretion of cytokines IL-1β and IL-18 in LPS-primed human macrophages. Macrophages obtained by differentiation from pooled monocytes extracted from human blood (three separate healthy donors for each experiment; total of six donors) were exposed for 18 h to extracts prepared from biomass of amylosin-producing B. amyloliquefaciens 19b and from amylosin-nonproducing B. amyloliquefaciens IAM 1521. The macrophages were exposed to methanol-soluble biomass as well as amylosin in the extract of B. amyloliquefaciens 19b in the amounts indicated in the figure. The label LPS indicates that the macrophages were primed with 1 μg ml−1 LPS for 3 h prior to exposure to the bacterial extracts. IL-1β and IL-18 levels were determined by ELISA. Results are expressed as means ± minimum and maximum values. The results obtained with 19b are marked with asterisks to indicate statistical difference from the corresponding IAM result (unpaired two-tailed Student's t test). *, P ≤ 0.05; **, P ≤ 0.01.
Activation kinetics of cytokine secretion caused by the amylosin-containing bacterial extract.
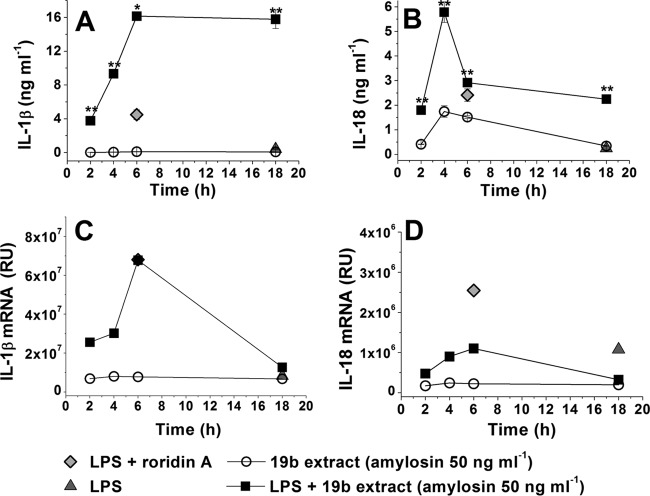
To determine the activation kinetics of the cytokine secretion induced by the amylosin-containing extract from B. amyloliquefaciens 19b, human macrophages were exposed to a set concentration (17 μg methanol-soluble compound [dry weight] containing 50 ng amylosin ml−1) of the extract for 2 to 18 h. After exposure, the concentrations of secreted IL-1β and IL-18 in the macrophage culture supernatants were determined by ELISA, and total RNA was extracted from the macrophages to determine the amounts of IL-1β and IL-18 mRNA by real-time qRT-PCR. The results in Fig. 2 show that exposure to the amylosin-containing bacterial extract from strain 19b stimulated the secretion of cytokines IL-1β and IL-18, as well as the expression of IL-1β and IL-18 mRNA, in LPS-primed human macrophages within 2 h. Maximal transcription of IL-1β and IL-18 mRNA was reached within 4 to 6 h, and maximal secretion of these cytokines occurred within 6 h. Western blot analysis of concentrated macrophage culture supernatants collected after 4 h of exposure confirmed that processing and secretion of active IL-1β and IL-18 had occurred in the exposed cells (results not shown). We concluded that the amylosin-containing extract of B. amyloliquefaciens 19b triggered high levels of both transcription and secretion of cytokines IL-1β and IL-18 within 2 h of exposure.
FIG 2.
Time course of the onset of the secretion of cytokines IL-1β and IL-18 and the expression of IL-1β and IL-18 mRNA in LPS-primed human macrophages in response to amylosin-containing extract from B. amyloliquefaciens 19b. Macrophages obtained by differentiation from pooled monocytes extracted from human blood (as described for Fig. 1) were exposed for 2 to 18 h to an amylosin-containing extract of B. amyloliquefaciens 19b. After exposure, secreted IL-1β and IL-18 levels in the supernatants were determined by ELISA, and total RNA was extracted from the macrophages and used to synthesize cDNA, which was analyzed by real-time qRT-PCR to detect IL-1β and IL-18 mRNA. (A and B) Concentrations of secreted IL-1β (A) and IL-18 (B), expressed as the mean ± minimum and maximum values. (C and D) Amounts of IL-1β mRNA (C) and IL-18 (D) mRNA, representing the results of two independent experiments. The measured relative units (RU) represent the fold change in gene expression normalized to a reference gene (18S rRNA) and in relation to a no-template control calibrator. In all figures, LPS indicates that macrophages were primed with 1 μg ml−1 LPS for 3 h prior to exposure. Roridin A was used as a positive reference compound. The cytokine measurement results obtained with 19b are marked with asterisks to indicate statistical differences from the LPS control result (unpaired two-tailed Student's t test). *, P ≤ 0.05; **, P ≤ 0.01.
Preparation of purified amylosin.
Amylosin was purified and quantified from methanol extracts of B. amyloliquefaciens 19b by using HPLC and identified using HPLC-MS. Figure 3A shows the HPLC-UV chromatogram of purified amylosin at 365 nm, and Fig. 3B shows single- and double-charged sodium adducts [M+Na]+ and [M+2Na]2+ of amylosin (17.5 min), with m/z values of 1219.6 and m/z 621.4, respectively, corresponding to the mass of amylosin, 1,196 Da. The thus-purified amylosin was used for further experiments.
FIG 3.
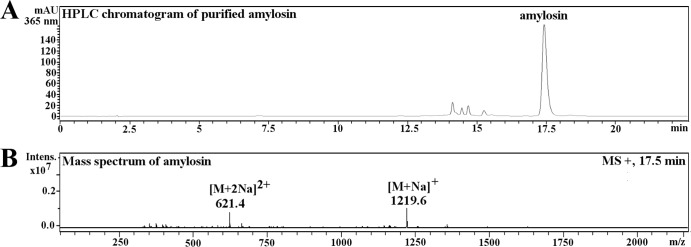
HPLC-UV chromatogram and the mass spectrum of amylosin purified from the methanol extract of strain B. amyloliquefaciens 19b. (A) HPLC-UV elution profile (365 nm) of purified amylosin (17.5 min). (B) Single- and double-charged sodium adducts [M+Na]+ and [M+2Na]2+ of amylosin (17.5 min) at m/z 1219.6 and m/z 621.4, respectively.
Amylosin stimulates secretion of IL-1β and IL-18 in human macrophages.
LPS-primed and nonprimed human macrophages were exposed to amylosin purified from biomass extracts of B. amyloliquefaciens 19b (purity of the amylosin preparation shown in Fig. 3). The amounts of secreted IL-1β and IL-18 were measured from the macrophage culture supernatants by ELISA. Figure 4 shows that secretion of IL-1β from macrophages primed with LPS was activated by exposure to 100 ng amylosin ml−1. The same concentration of amylosin activated secretion of IL-18 in both nonprimed and LPS-primed macrophages. The level of IL-1β secretion from the macrophages was similar to that induced by exposure to 100 ng ml−1 of the mycotoxin roridin A, a known activator of cytokine secretion (13). These results showed that cytokine secretion by human macrophages was stimulated by exposure to purified amylosin in a dose-dependent manner.
FIG 4.
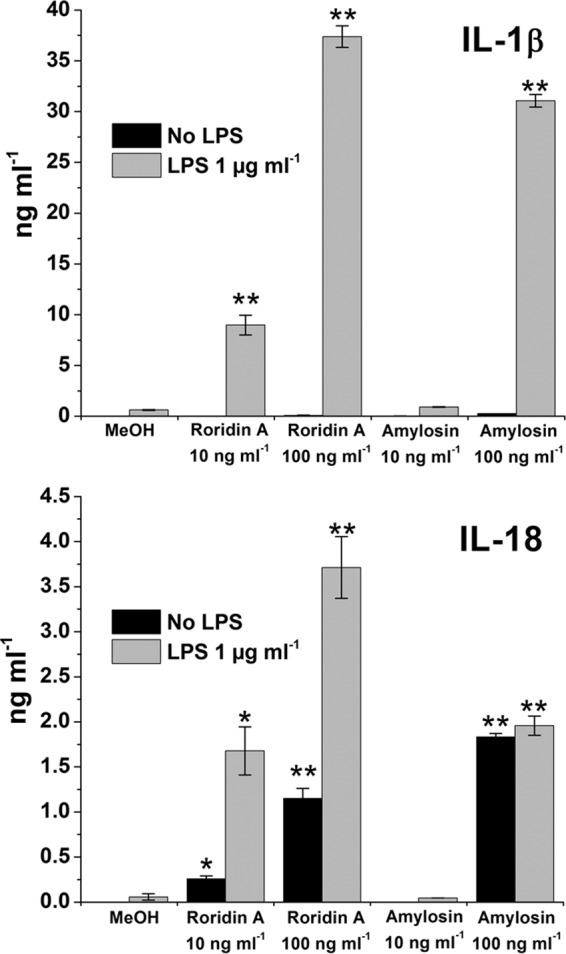
Purified amylosin stimulates dose-dependent secretion of cytokines IL-1β and IL-18 in LPS-primed human macrophages. Macrophages obtained by differentiation from pooled monocytes extracted from human blood (three separate healthy donors) were exposed for 18 h to 10 or 100 ng ml−1 purified amylosin or roridin A (used as a reference compound). LPS indicates priming of cells with 1 μg LPS ml−1 for 3 h prior to toxin exposure, and MeOH indicates the vehicle (methanol) control. IL-1β and IL-18 levels were determined by ELISA. The measurement results obtained are marked with asterisks to indicate statistical differences from the LPS and MeOH control results (unpaired two-tailed Student's t test) *, P ≤ 0.05; **, P ≤ 0.01. Results are expressed as means ± minimum and maximum values and are representative of two independent experiments.
Amylosin causes dose-dependent potassium ion efflux from mammalian cells.
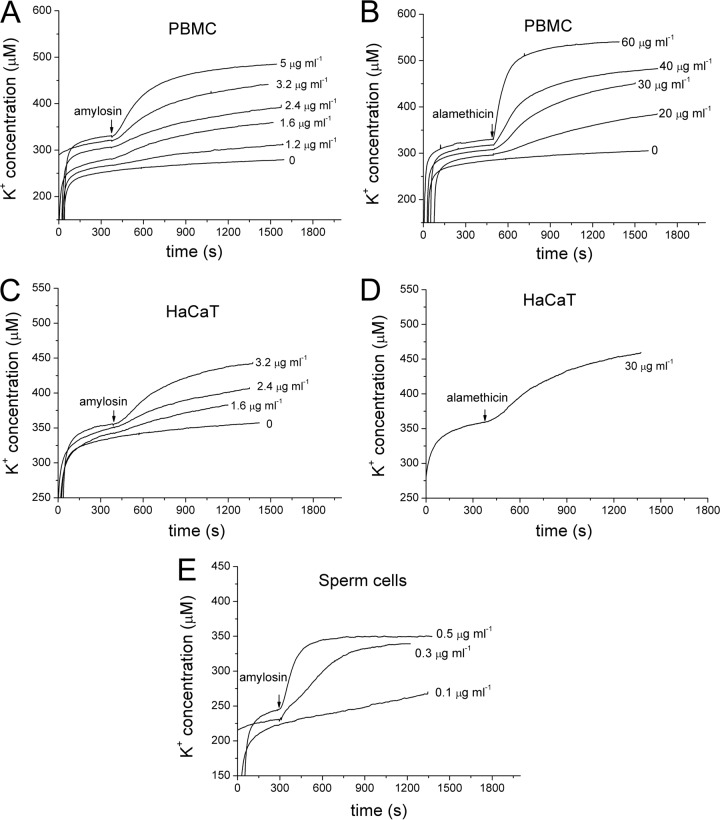
To assess if amylosin causes potassium efflux from live cells, leakage of K+ ions was measured from three types of mammalian cells: PBMC, HaCaT, and porcine spermatozoa. The cells were placed in potassium-free isotonic medium in a measurement cuvette, and the change of extracellular [K+] in response to the addition of 0 to 5 μg ml−1 amylosin (purity of the amylosin preparation shown in Fig. 3) was recorded in real time with a K+-selective electrode. The K+ efflux induced by purified amylosin was compared to that induced by 0 to 60 μg ml−1 of the pore-forming antibiotic alamethicin. Amylosin caused the efflux of potassium ions in all three cell types (Fig. 5A, C, and E) at exposure concentrations approximately 1/10 of those required for alamethicin to produce similar magnitudes of K+ efflux (Fig. 5B and D). The results showed that low concentrations (≤5 μg ml−1) of purified amylosin caused dose-dependent efflux of K+ in human somatic cells (PBMC and HaCaT) as well as porcine spermatozoa.
FIG 5.
Purified amylosin causes dose-dependent potassium ion efflux from human primary PBMC, cultured keratinocytes (HaCaT), and porcine spermatozoa. Washed PBMC (20 × 106 cells ml−1) (A and B), HaCaT cells (6 × 106 cells ml−1) (C and D), or spermatozoa (54 × 106 cells ml−1) (E) were placed in isotonic potassium-free medium in a measurement chamber. The traces show the extracellular [K+] before and during exposure to the indicated concentrations of amylosin (A, C, and E) or alamethicin (B and D), recorded with a K+-selective electrode (one reading per second). The time points of amylosin and alamethicin additions are indicated by arrows.
The measurement results summarized in Fig. 5 allowed the calculation of the extent and kinetics of the amylosin-induced efflux of K+ ions from the human cells (PBMC and HaCaT) and porcine spermatozoa. The measurement chamber (1 ml) contained 20 × 106 PBMC (cell diameter, 7 μm) or 6 × 106 HaCaT cells (cell diameter, 30 μm). The summed cell volumes in the cuvette were thus ∼4 μl (PBMC) or ∼80 μl (HaCaT). Assuming the intracellular [K+] to be 150 mM (32), the summed potassium stores of the cells in the measurement chamber were 0.5 μmol (PBMC) or 12 μmol (HaCaT). A dose of 5 μg of amylosin into the PBMC holding chamber (1 ml) provoked an efflux Δ[K+] of 200 nmol within 900 s, i.e., 10 nmol of K+ discharged per 106 PBMC (Fig. 5A). A dose of 3.2 μg of amylosin into the HaCaT cell holding chamber (1 ml) provoked an efflux Δ[K+] of 80 nmol within 900 s, i.e., 13 nmol discharged per 106 HaCaT cells (Fig. 5C). The amounts discharged within 15 min of exposure to amylosin represented 40% and 1% of the cellular K+ stores of the PBMC and HaCaT cells, respectively. As shown in Fig. 5E, the porcine spermatozoa responded to amylosin exposure by an instant, massive efflux of K+. The measurement chamber contained 54 × 106 sperm cells ml−1, with a summed cell volume of ∼0.6 μl (11 μm3 per sperm cell [33]) and summed intracellular store of 90 nmol K+. Addition of 0.5 μg of amylosin into the chamber (1 ml) provoked an efflux Δ[K+] of 85 nmol within 300 s (Fig. 5E), depleting the spermatozoan intracellular K+ stores. Exposure to 0.3 μg amylosin ml−1 also depleted the K+ stores of the spermatozoa, but less rapidly. The experimental evidence thus strongly indicated that exposure to low concentrations (0.3 to 5 μg ml−1, i.e., 0.3 to 5 μM) of purified amylosin instantly (within seconds) caused a major efflux of cytoplasmic K+ into the extracellular fluid from primary and cultured human and porcine cells.
Exposure to low concentrations (≤2 μM) of amylosin depolarizes human and porcine cellular membrane potentials (ΔΨ) and impairs motility of porcine spermatozoa.
Amylosin provoked leakage of K+ ions from primary human PBMC and cultured keratinocytes (HaCaT), as shown in Fig. 5, in a medium which was isotonic (300 mOsm kg−1) but potassium-free, to avoid overloading the potassium electrodes used to measure the K+ efflux. Blood plasma contains 3.5 to 4 mM K+ (32). Since the intracellular [K+] is high (150 mM) (32), any major leakage of K+ from the cells should affect the cellular transmembrane potentials. To observe this, monolayers of human keratinocytes (HaCaT) were exposed to purified amylosin in tissue culture medium with a close-to-physiological [K+] (in RPMI 1640, 5 mM K+). To visualize changes in membrane potential, the cells were stained with the membrane-permeant ΔΨ-responsive dye JC-1. JC-1 emits orange fluorescence in membranes with ΔΨ of >140 mV and green fluorescence when ΔΨ is ≤100 mV. As a second indicator, the membrane-nonpermeant dye propidium iodide (death stain) was used to reveal any damage to the cell membrane permeability barrier. Figure 6 shows examples of the outcome of amylosin exposure in physiological [K+] medium. Epifluorescence microscopic views of the double-stained HaCaT cell monolayers showed that exposure to 0.8 μg of amylosin ml−1 for 24 h completely depolarized all cellular membranes, including the mitochondria. This occurred while the cell membrane permeability barrier, which prevents the influx of propidium iodide, was preserved (no purple-red fluorescing cells visible in Fig. 6). Therefore, exposure to amylosin depolarized the cells without damaging the cell membrane and making it permeable to propidium iodide. This result matches well with the results shown in Fig. 5, where measurable K+ efflux from the HaCaT cells was observed within 20 min (1,200 s [Fig. 5C]) upon exposure to 1.6 μg of amylosin ml−1. Combining these results, we concluded that exposure to 1 to 2 μg of amylosin ml−1 (corresponding to 1 to 2 μM) made the HaCaT cells leaky to K+ ions under physiological ionic conditions. Similar results were obtained with the human primary PBMC (data not shown).
FIG 6.
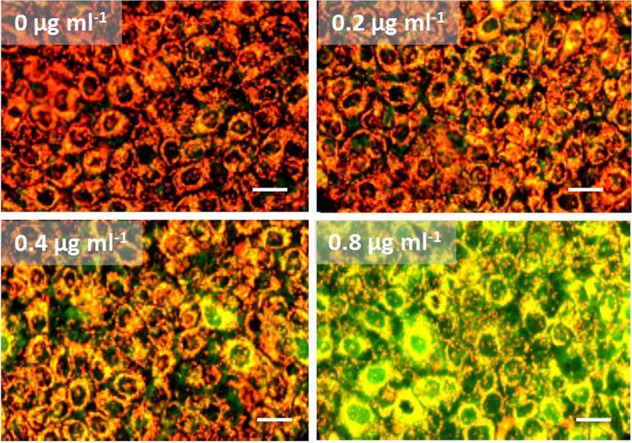
Purified amylosin causes a dose-dependent loss in the cellular transmembrane potential (ΔΨ) in human keratinocytes (HaCaT). Monolayers of HaCaT cells were exposed to 0, 0.2, 0.4, or 0.8 μg of purified amylosin ml−1 for 24 h and then double-stained with the membrane-permeant ΔΨ-responsive dye JC-1 and the membrane-nonpermeant dye propidium iodide. The orange fluorescence in JC-1-stained cells indicates a high membrane potential (ΔΨ, >140 mV), and green fluorescence indicates a dissipated membrane potential (ΔΨ, <100 mV). The 0 μg ml−1 data are for the vehicle-only control (≤1% [vol/vol] methanol). None of the cells displayed purple-red fluorescence following propidium iodide staining (death staining). The images are representative of three independent microscopic views. Bar, 30 μm.
The motility of spermatozoa is known to be a highly sensitive indicator of damage to energized mitochondria (34). We therefore tested the effect of the amylosin preparation, used for the cytokine emission results shown in Fig. 1 and 2, and purified amylosin on porcine spermatozoa. It was found that exposure to 0.0022 μg of purified amylosin ml−1 inhibited the motility of 27 × 106 porcine spermatozoa within 2 days. Exposure to a biomass extract of B. amyloliquefaciens 19b at a concentration of 0.0024 μg of amylosin ml−1 along with 0.8 μg of methanol-soluble biomass (dry weight) ml−1 gave a similar result, whereas a similarly prepared extract of B. amyloliquefaciens IAM1521 (amylosin nonproducer) did not affect sperm motility, even at an exposure concentration of 50 μg of methanol-soluble biomass (dry weight) ml−1. These results showed that amylosin blocked sperm motility at very low exposure concentrations (0.002 μg ml−1), both in a crude bacterial extract and as a purified substance.
Amylosin-producing B. amyloliquefaciens and purified amylosin inhibit microbial growth.
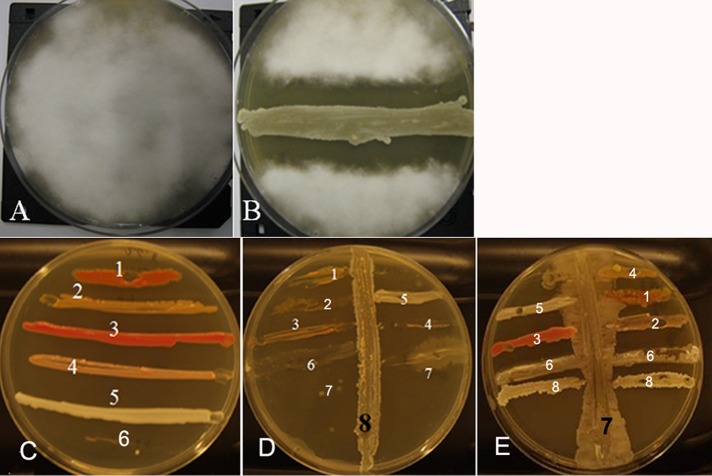
The effect of amylosin-producing B. amyloliquefaciens 19b and purified amylosin on the growth of selected bacterial and fungal strains was investigated by coculturing on TSA plates and by measuring the change in turbidity and viable counts of broth cultures after exposure to amylosin. Figure 7A and B shows that B. amyloliquefaciens 19b, when inoculated on a TSA plate seeded with a spore suspension of Chaetomium globosum MTAV 35, a toxigenic fungal isolate from a moisture-damaged indoor space, prevented the growth of the mold up to a distance of 12 mm. Bacterial isolates from similarly troubled indoor spaces were inoculated across a streak of B. amyloliquefaciens 19b on a TSA plate. Figure 7D shows that the growth of the test bacteria was inhibited compared to that on a plate with no B. amyloliquefaciens 19b (Fig. 7C). In Fig. 7E, the sensitivities of same test bacteria were assayed on a plate streaked with B. cereus NS58, a strain which emits cereulide, which is known to cause efflux of K+ ions from live bacteria (22). As can be seen in Fig. 7E, B. cereus NS58 caused no or only minor growth inhibition of the test bacteria.
FIG 7.
Amylosin-producing B. amyloliquefaciens 19b, isolated from a microbially damaged indoor space, inhibited the growth of fungi and bacteria isolated from similarly damaged indoor spaces and plated on TSA plates. (A and B) Two TSA plates were inoculated with a spore suspension of Chaetomium globosum MTAV 35 (an indoor fungus) and grown for 1 day, after which a horizontal streak of B. amyloliquefaciens 19b was drawn across one of the plates (B). Upon further culturing (10 days), the spores of C. globosum MTAV 35 failed to germinate in the vicinity of the streaked B. amyloliquefaciens 19b. The inhibition zone was 12 mm wide. (C to E) Six bacterial strains were horizontally streaked on three TSA plates: 1, Williamsia muralis MA140/96T; 2, Mycobacterium murale MA113T; 3, Dietzia sp. MA147; 4, Sphingomonas aurantiaca MA101bT; 5, Bacillus sp. OS16; 6, Bacillus megaterium Ne10. Vertical streaks of B. amyloliquefaciens 19b (strain 8, amylosin producer) or Bacillus cereus NS58 (strain 7, cereulide producer) were drawn vertically across plates D and E, respectively.
B. amyloliquefaciens is also a known producer of bioactive lipopeptide toxins. The results in Table 1 show that amylosin was 100-fold-more inhibitory toward the growth of B. megaterium Ne10 and Dietzia sp. MA147 in broth culture than the other tested lipopeptides produced by B. amyloquefaciens or cereulide produced by Bacillus cereus. The motility of porcine spermatozoa was inhibited by amylosin also at concentrations 100- to 1,000-fold lower than the other lipopeptides. Based on the results in Fig. 7 and Table 1, amylosin thus appears to be active toward both prokaryotic and eukaryotic microbial cells.
TABLE 1.
Inhibitory effects of purified amylosin, other known products of B. amyloliquefaciens, and purified cereulide on growth of Bacillus megaterium and Dietzia sp., as well as effects on the motility of porcine spermatozoaa
| Parameter measure and target cells | EC50 (μg/ml) for: |
||||
|---|---|---|---|---|---|
| Amylosin | Fengycin | Iturin | Surfactin | Cereulide | |
| Inhibition of bacterial growth, 24-h exposure | |||||
| Bacillus megaterium Ne10 | 0.06 | ≥40 | 6 | >50 | >10 |
| Dietzia sp. MA147 | 0.06 | NA | 6 | 10 | >10 |
| Motility loss, 30-min exposure | |||||
| Porcine sperm cells | 0.1 | >100 | >250 | 16 | 0.002 |
The effects on B. megaterium Ne10 and Dietzia sp. MA147 are expressed as the substance concentration (EC50) that blocked growth in broth culture (TSB), measured by turbidometry and viable count. The effect on porcine sperm cells was determined based on the concentration needed to block motility of >50% of the cells in the sperm extender. The negative control was vehicle only (1% [vol/vol] methanol).
DISCUSSION
The results of our research show that the K+ ionophoric bacterial toxin amylosin causes many types of adverse effects in human cells and therefore could potentially explain symptoms of potassium homeostasis disruption (35, 36) in people exposed to it, even at low concentrations, in moisture-damaged indoor spaces. Exposure to a concentration down to 300 ng amylosin ml−1 was shown here to be sufficient to generate a major potassium efflux from live cells, similar to that shown previously for isolated mitochondria (2). Therefore, exposure to extremely low concentrations of amylosin may initiate in vivo potassium leakage from human cells (intracellular concentration of potassium of 150 mM [32]) down the concentration gradient toward that of blood plasma (potassium concentration, 3.5 to 5.0 mM [32]). Important cells of the human innate immune system, namely, freshly isolated PBMC, representing the precursors of macrophages, and keratinocytes (HaCaT), responded within minutes (Fig. 5) to micromolar exposure to amylosin. Based on the results in the present paper (Fig. 1, 2, and 4), exposure to even smaller amounts of amylosin may be expected to activate the NLRP3 inflammasome, leading to cytokine release from macrophages and consequently activation of cellular innate immunity responses. Although microbial pore-forming toxins have been previously reported as activators of the NLRP3 inflammasome (18), this is the first report of a channel-forming bacterial toxin stimulating cytokine release from human primary macrophages. To our knowledge, amylosin is the first bacterial toxin reported to cause activation of the NLRP3 pathway possibly solely via potassium efflux, thus potentially leading to human illness. Interestingly, reported cases of B. amyloliquefaciens causing human illness have involved environmental exposures, namely, contaminated indoor air (1, 2) or food (6), whereas human infection by B. amyloliquefaciens has not been reported.
In recent years, there has been increased focus on inflammasomes, which are cytoplasmic components of the innate immune system; they are responsible for activating many types of inflammatory responses. Inflammasomes were first described only a decade ago (14) but have since been shown to have a significant role in the activation of inflammation and inflammatory diseases (37). The NLRP3 inflammasome has been most widely studied and is linked to several autoinflammatory and autoimmune diseases (17, 38, 39), and several bacterial toxins have been reported to trigger activation of this inflammasome (reviewed in references 17 and 18). Recently, NLRP3 inflammasome activation has also been connected to lung diseases, including asthma (40). Microbial growth in moisture-damaged buildings and the consequent exposure of occupants to microbes and microbe-associated biological agents (e.g., cell components and microbial toxins) are accepted as causes of various adverse health effects, including asthma, allergies, and other symptoms connected with the functioning of the immune system (41). For example, exposure to moisture-damaged indoor environments is reportedly associated with a 30 to 50% increase in various respiratory and asthma-related symptoms (42). The common trigger in these illnesses could be the activation of the NLRP3 inflammasome and the consequent secretion of the proinflammatory cytokines IL-1β and IL-18, driven by potassium ion efflux (16). As our results showed that amylosin causes potassium efflux from both primary and cultured mammalian cells, it appears likely that the secretion of active forms of IL-1β and IL-18, stimulated by exposure to amylosin, is a result of the activation of the NLRP3 inflammasome. Amylosin is a moderately lipophilic molecule with a log Kow (octanol-water partition coefficient) between 3 and 4. This estimate is based on its RP-HPLC retention, where surfactin-containing iso-C12 fatty acid (log Kow, 4.05) and amphotericin B (log Kow, 2.46) were used as references (43). Therefore, inhaled amylosin could induce activation of alveolar macrophages. Secreted IL-1β and IL-18 affect many immune functions and are significant in both local and systemic inflammation (21). Therefore, the release of active cytokines caused by exposure of cells of the innate immune system to amylosin could explain some of the inflammatory symptoms occupants of moisture-damaged buildings display.
Our results also showed that amylosin-producing B. amyloliquefaciens 19b can inhibit the growth of several bacteria with very different cell wall compositions as well as growth of indoor fungi. In addition to amylosin, B. amyloliquefaciens 19b produces surfactin and antifungal fengycin (2, 43). Strains of B. amyloliquefaciens producing several antibacterial and antifungal linear and cyclic lipopeptides (e.g., fengycins, iturins, and surfactins) have also been isolated from, e.g., marine environments, mangrove forests, and plant rhizospheres (30, 44, 45). These reports together with our results suggest that the simultaneous production of several antimicrobial substances by B. amyloliquefaciens strains is widespread in nature and offers a competitive advantage to the producer microbe. In addition, our results showed that amylosin inhibited the growth of several taxonomically unrelated bacteria at concentrations lower than those described for any of the other antibacterial compounds produced by strains of B. amyloliquefaciens, which could enhance the competitive advantage of amylosin-producing strains. Strains of B. amyloliquefaciens producing antibacterial and antifungal compounds may have many potential commercial and therapeutic applications. Our results suggest that moisture-damaged buildings and dust may contain microbes producing bioactive compounds suitable for commercial development. The symptoms of damp building-related illness are most likely due to the combined effect of exposure to many different microbes and bioactive compounds (41). However, the suggested competitive advantage could make amylosin producers dominant in moisture-damaged materials when the surrounding conditions are suitable for their growth, possibly making these strains a significant cause of ill health symptoms experienced by occupants of moisture-damaged buildings.
Our results showed that amylosin was more effective at inducing potassium efflux from human cells and porcine spermatozoa than alamethicin (Fig. 5), which is commonly used as a model compound for ion channel formation. In addition, amylosin caused depolarization of mitochondria in human keratinocytes (HaCaT) in a similar way as reported for cereulide (26). Cereulide has a positive charge and is highly lipophilic (log Kow, 6 [46]). The log Kow of amylosin is estimated to be 3 to 4 (43), making it less lipophilic than cereulide or paenilide, a toxin with higher hydrophobicity than cereulide that induces potassium efflux from mammalian cells and mitochondria and that is produced by Paenibacillus tundrae (29). This could explain why amylosin acts as a potassium efflux channel (2), leading to high levels of potassium ion release down the concentration gradient, instead of acting as a potassium ionophore and passing through the mitochondrial membrane as cereulide and paenilide do (29, 31). Interestingly, cereulide has been reported to have no effect on the IL-1β production of monocytes (47), whereas amylosin was shown in the current study to have a strong activating effect on human macrophages, indicating that the potassium efflux-related activation of the NLRP3 inflammasome and subsequent cytokine secretion require a channel-based flow of potassium out of the cell.
Strains of B. amyloliquefaciens are of commercial interest due to the many bioactive compounds they produce. The U.S. Food and Drug Administration lists B. amyloliquefaciens as a food additive producer with “generally recognized as safe” (GRAS) status (48), and the European Food Safety Authority (EFSA) has proposed B. amyloliquefaciens for “qualified presumption of safety” (QPS) status (49). Several patents have been approved that cover the use of strains of B. amyloliquefaciens for the production of antifungal and antibacterial substances for use in the food and feed industries (e.g., U.S. patent applications 20100143316 A1 and 20110274673 A1). However, it appears that the possible coproduction of substances with adverse effects toward human cells has not been fully considered. Human consumption of l-tryptophan commercially produced by a genetically engineered strain of B. amyloliquefaciens has been reported to have caused an epidemic of eosinophilia-myalgia syndrome (50), but the causative agent for this epidemic has not been identified with certainty (51). Based on our results, the use of B. amyloliquefaciens to commercially produce enzymes and antimicrobial compounds could pose a risk to human health if the purity of the produced substances is not absolute.
ACKNOWLEDGMENTS
We acknowledge grants for this work from the Academy of Finland (CoE Photobiomics, grant 118637) and Finnish Work Environment Fund (grant Tsr 112134), as well as the Graduate School for Applied Biosciences (ABS) for a scholarship awarded to Stiina Rasimus-Sahari.
We thank Frederick A. Rainey for the 16S rRNA gene sequencing and identification of strain MA147 as a member of the genus Dietzia and Ossian Saris for the isolation of Bacillus sp. OS16. We also thank Leena Kuoppasalmi and Tiiu Arumäe for expert contributions with PBMC and HaCaT cell preparations.
REFERENCES
- 1.Mikkola R, Andersson MA, Grigoriev P, Teplova VV, Saris NE, Rainey FA, Salkinoja-Salonen MS. 2004. Bacillus amyloliquefaciens strains isolated from moisture-damaged buildings produced surfactin and a substance toxic to mammalian cells. Arch Microbiol 181:314–323. doi: 10.1007/s00203-004-0660-x. [DOI] [PubMed] [Google Scholar]
- 2.Mikkola R, Andersson MA, Teplova V, Grigoriev P, Kuehn T, Loss S, Tsitko I, Apetroaie C, Saris NE, Veijalainen P, Salkinoja-Salonen MS. 2007. Amylosin from Bacillus amyloliquefaciens, a K+ and Na+ channel-forming toxic peptide containing a polyene structure. Toxicon 49:1158–1171. doi: 10.1016/j.toxicon.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Thorne PS. 1993. Sump additives as a source of bioaerosols in a school building. Vet Hum Toxicol 35:141–143. [PubMed] [Google Scholar]
- 4.Andersson MA, Jääskeläinen EL, Teplova VV, Veijalainen P, Apetroaie C, Hoornstra D, Kroppenstedt RM, Salkinoja-Salonen MS. 2005. Indoor bacilli and streptomycetes produce substances toxic to mammalian cells, p 292–299. In Johanning E. (ed), Bioaerosols, fungi, bacteria, mycotoxins and human health: patho-physiology, clinical effects, exposure assessment, prevention and control in indoor environments and work. Fungal Research Group Foundation, Albany, NY. [Google Scholar]
- 5.Saris NE, Andersson MA, Mikkola R, Andersson LC, Teplova VV, Grigoriev PA, Salkinoja-Salonen MS. 2009. Microbial toxin's effect on mitochondrial survival by increasing K+ uptake. Toxicol Ind Health 25:441–446. doi: 10.1177/0748233709103405. [DOI] [PubMed] [Google Scholar]
- 6.Apetroaie-Constantin C, Mikkola R, Andersson MA, Teplova V, Suominen I, Johansson T, Salkinoja-Salonen M. 2009. Bacillus subtilis and B. mojavensis strains connected to food poisoning produce the heat stable toxin amylosin. J Appl Microbiol 106:1976–1985. doi: 10.1111/j.1365-2672.2009.04167.x. [DOI] [PubMed] [Google Scholar]
- 7.Peitzsch M, Sulyok M, Täubel M, Vishwanath V, Krop E, Borras-Santos A, Hyvärinen A, Nevalainen A, Krska R, Larsson L. 2012. Microbial secondary metabolites in school buildings inspected for moisture damage in Finland, The Netherlands and Spain. J Environ Monit 14:2044–2053. doi: 10.1039/c2em30195d. [DOI] [PubMed] [Google Scholar]
- 8.Täubel M, Sulyok M, Vishwanath V, Bloom E, Turunen M, Järvi K, Kauhanen E, Krska R, Hyvärinen A, Larsson L, Nevalainen A. 2011. Co-occurrence of toxic bacterial and fungal secondary metabolites in moisture-damaged indoor environments. Indoor Air 21:368–375. doi: 10.1111/j.1600-0668.2011.00721.x. [DOI] [PubMed] [Google Scholar]
- 9.Thrasher JD, Crawley S. 2009. The biocontaminants and complexity of damp indoor spaces: more than what meets the eyes. Toxicol Ind Health 25:583–615. doi: 10.1177/0748233709348386. [DOI] [PubMed] [Google Scholar]
- 10.Bornehag CG, Sundell J, Bonini S, Custovic A, Malmberg P, Skerfving S, Sigsgaard T, Verhoeff A, EUROEXPO. 2004. Dampness in buildings as a risk factor for health effects. EUROEXPO: a multidisciplinary review of the literature (1998-2000) on dampness and mite exposure in buildings and health effects. Indoor Air 14:243–257. doi: 10.1111/j.1600-0668.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirvonen MR, Huttunen K, Roponen M. 2005. Bacterial strains from moldy buildings are highly potent inducers of inflammatory and cytotoxic effects. Indoor Air 15(Suppl 9):S65–S70. doi: 10.1111/j.1600-0668.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolff CH. 2011. Innate immunity and the pathogenicity of inhaled microbial particles. Int J Biol Sci 7:261–268. doi: 10.7150/ijbs.7.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kankkunen P, Rintahaka J, Aalto A, Leino M, Majuri ML, Alenius H, Wolff H, Matikainen S. 2009. Trichothecene mycotoxins activate inflammatory response in human macrophages. J Immunol 182:6418–6425. doi: 10.4049/jimmunol.0803309. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of pro-IL-beta. Mol Cell 10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 15.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. 2004. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20:319–325. doi: 10.1016/S1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. 2013. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrilli V, Dostert C, Muruve DA, Tschopp J. 2007. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Freche B, Reig N, van der Goot FG. 2007. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin Immunopathol 29:249–260. doi: 10.1007/s00281-007-0085-0. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello CA. 1998. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci 856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. 2002. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 20(5 Suppl 27):S1–S13. [PubMed] [Google Scholar]
- 21.Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 22.Ekman JV, Kruglov A, Andersson MA, Mikkola R, Raulio M, Salkinoja-Salonen M. 2012. Cereulide produced by Bacillus cereus increases the fitness of the producer organism in low-potassium environments. Microbiology 158:1106–1116. doi: 10.1099/mic.0.053520-0. [DOI] [PubMed] [Google Scholar]
- 23.Turnbull PC, Kramer JM, Jorgensen K, Gilbert RJ, Melling J. 1979. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr 32:219–228. [DOI] [PubMed] [Google Scholar]
- 24.Andersson MA, Mikkola R, Rasimus S, Hoornstra D, Salin P, Rahkila R, Heikkinen M, Mattila S, Peltola J, Kalso S, Salkinoja-Salonen MS. 2010. Boar spermatozoa as a biosensor for detecting toxic substances in indoor dust and aerosols. Toxicol In Vitro 24:2041–2052. doi: 10.1016/j.tiv.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoornstra D, Andersson MA, Teplova VV, Mikkola R, Uotila LM, Andersson LC, Roivainen M, Gahmberg CG, Salkinoja-Salonen MS. 2013. Potato crop as a source of emetic Bacillus cereus and cereulide-induced mammalian cell toxicity. Appl Environ Microbiol 79:3534–3543. doi: 10.1128/AEM.00201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. 1999. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol 162:7322–7329. [PubMed] [Google Scholar]
- 28.Pirhonen J, Sareneva T, Julkunen I, Matikainen S. 2001. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur J Immunol 31:726–733. doi:. [DOI] [PubMed] [Google Scholar]
- 29.Rasimus S, Mikkola R, Andersson MA, Teplova VV, Venediktova N, Ek-Kommonen C, Salkinoja-Salonen M. 2012. Psychrotolerant Paenibacillus tundrae isolates from barley grains produce new cereulide-like depsipeptides (paenilide and homopaenilide) that are highly toxic to mammalian cells. Appl Environ Microbiol 78:3732–3743. doi: 10.1128/AEM.00049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez F, Castro M, Principe A, Borioli G, Fischer S, Mori G, Jofre E. 2012. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J Appl Microbiol 112:159–174. doi: 10.1111/j.1365-2672.2011.05182.x. [DOI] [PubMed] [Google Scholar]
- 31.Mikkola R, Saris NE, Grigoriev PA, Andersson MA, Salkinoja-Salonen MS. 1999. Ionophoretic properties and mitochondrial effects of cereulide - the emetic toxin of B. cereus. Eur J Biochem 263:112–117. doi: 10.1046/j.1432-1327.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 32.Rastegar A. 1990. Serum potassium, p 884–887. In Walker HK, Hall WD, Hurst JW (ed), Clinical methods: the history, physical, and laboratory examinations, 3rd ed Butterworths, Boston, MA. [PubMed] [Google Scholar]
- 33.Petrunkina AM, Hebel M, Waberski D, Weitze KF, Topfer-Petersen E. 2004. Requirement for an intact cytoskeleton for volume regulation in boar spermatozoa. Reproduction 127:105–115. doi: 10.1530/rep.1.00047. [DOI] [PubMed] [Google Scholar]
- 34.Hoornstra D, Andersson MA, Mikkola R, Salkinoja-Salonen MS. 2003. A new method for in vitro detection of microbially produced mitochondrial toxins. Toxicol In Vitro 17:745–751. doi: 10.1016/S0887-2333(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 35.Hoskote SS, Joshi SR, Ghosh AK. 2008. Disorders of potassium homeostasis: pathophysiology and management. J Assoc Physicians India 56:685–693. [PubMed] [Google Scholar]
- 36.Lehnhardt A, Kemper MJ. 2011. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol 26:377–384. doi: 10.1007/s00467-010-1699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F, Mayor A, Tschopp J. 2009. The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 38.Lamkanfi M, Walle LV, Kanneganti TD. 2011. Deregulated inflammasome signaling in disease. Immunol Rev 243:163–173. doi: 10.1111/j.1600-065X.2011.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters SL, Simon A, Aksentijevich I, Kastner DL. 2009. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol 27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Nardo D, De Nardo CM, Latz E. 2014. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol 184:42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heseltine E, Rosen J, World Health Organization Regional Office for Europe. 2009. WHO guidelines for indoor air quality: dampness and mould. World Health Organization, Regional Office for Europe, Copenhagen, Denmark. [Google Scholar]
- 42.Fisk WJ, Lei-Gomez Q, Mendell MJ. 2007. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air 17:284–296. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 43.Mikkola R. 2006. Ph.D. thesis. Food and indoor air isolated Bacillus non-protein toxins: structure, physico-chemical properties and mechanism of effects on eukaryotic cells. University of Helsinki, Helsinki, Finland. [Google Scholar]
- 44.Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P. 2009. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact 8:63. doi: 10.1186/1475-2859-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu HM, Rong YJ, Zhao MX, Song B, Chi ZM. 2014. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl Microbiol Biotechnol 98:127–136. doi: 10.1007/s00253-013-5291-1. [DOI] [PubMed] [Google Scholar]
- 46.Teplova VV, Mikkola R, Tonshin AA, Saris NE, Salkinoja-Salonen MS. 2006. The higher toxicity of cereulide relative to valinomycin is due to its higher affinity for potassium at physiological plasma concentration. Toxicol Appl Pharmacol 210:39–46. doi: 10.1016/j.taap.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Paananen A, Mikkola R, Sareneva T, Matikainen S, Hess M, Andersson M, Julkunen I, Salkinoja-Salonen MS, Timonen T. 2002. Inhibition of human natural killer cell activity by cereulide, an emetic toxin from Bacillus cereus. Clin Exp Immunol 129:420–428. doi: 10.1046/j.1365-2249.2002.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Code of Federal Regulations. 2012. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Part 184. Direct food substances affirmed as generally recognized as safe. 21 CFR 184.1147. [Google Scholar]
- 49.EFSA Panel on Biological Hazards. 2013. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 11:3449:1–108. doi: 10.2903/j.efsa.2013.3449. [DOI] [Google Scholar]
- 50.Mayeno AN, Gleich GJ. 1994. Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale. Trends Biotechnol 12:346–352. doi: 10.1016/0167-7799(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 51.Das YT, Bagchi M, Bagchi D, Preuss HG. 2004. Safety of 5-hydroxy-l-tryptophan. Toxicol Lett 150:111–122. doi: 10.1016/j.toxlet.2003.12.070. [DOI] [PubMed] [Google Scholar]