Abstract
The food-borne pathogenic bacterium Listeria is known for relatively low morbidity and high mortality rates, reaching up to 25 to 30%. Listeria is a hardy organism, and its control in foods represents a significant challenge. Many naturally occurring compounds, including the bacteriocin nisin and a number of plant essential oils, have been widely studied and are reported to be effective as antimicrobial agents against spoilage and pathogenic microorganisms. The aim of this study was to investigate the ability of semipurified preparations (SPP) containing either nisin A or an enhanced bioengineered derivative, nisin V, alone and in combination with low concentrations of the essential oils thymol, carvacrol, and trans-cinnamaldehyde, to control Listeria monocytogenes in both laboratory media and model food systems. Combinations of nisin V-containing SPP (25 μg/ml) with thymol (0.02%), carvacrol (0.02%), or cinnamaldehyde (0.02%) produced a significantly longer lag phase than any of the essential oil-nisin A combinations. In addition, the log reduction in cell counts achieved by the nisin V-carvacrol or nisin V-cinnamaldehyde combinations was twice that of the equivalent nisin A-essential oil treatment. Significantly, this enhanced activity was validated in model food systems against L. monocytogenes strains of food origin. We conclude that the fermentate form of nisin V in combination with carvacrol and cinnamaldehyde offers significant advantages as a novel, natural, and effective means to enhance food safety by inhibiting food-borne pathogens such as L. monocytogenes.
INTRODUCTION
The growing consumer demand for food products that are minimally processed and free of chemical preservatives presents a difficult challenge for food processors. Consequently, there has been a focus on the application of naturally produced antimicrobial compounds as a more acceptable means to control the growth of undesirable microorganisms in food (1, 2). Bacteriocins (ribosomally produced, small, heat-stable peptides that are active against other bacteria) derived from organisms generally regarded as safe provide one potential solution. However, only two bacteriocins have been made commercially available to any extent. These are nisin, produced by Lactococcus lactis, and pediocin PA-1, produced by Pediococcus acidilactici (3, 4). Of these, nisin is used in a wide variety of dairy and nondairy products, including cream and cheese products, soups, liquid egg, mayonnaises, salad dressings, tomato products, and beer (5). Nisin A exhibits antibacterial activity against a wide range of Gram-positive bacteria, including food-borne pathogens such as staphylococci, bacilli, clostridia, and Listeria (6, 7). Indeed, the success of nisin A from discovery (8) through to regulatory approval and finally to commercial application has spurred researchers to exploit its gene-encoded nature and to attempt to “bioengineer” variants with altered biological, chemical, and physical properties. Over the last decade, several studies have described the discovery of new nisin derivatives with enhanced activity against a range of food-related pathogenic microorganisms (9, 10) Of these, nisin M21V, subsequently designated nisin V, was noteworthy by virtue of its enhanced antimicrobial activity against a wide range of targets, including medically significant pathogens and food-borne pathogens such as Bacillus cereus and Listeria monocytogenes (10, 11). Significantly, this enhanced activity against L. monocytogenes was also apparent in a food setting (11). The increasing trend toward minimally processed and ready-to-eat (RTE) refrigerated foods means that more robust strategies are required to control the growth and survival of Listeria monocytogenes. Indeed, recent strategies for controlling spoilage and pathogenic microorganisms lean toward hurdle technology, whereby different preservation methods are combined to inhibit microbial growth and improve food safety. To this end, numerous studies have been carried out highlighting the potential of nisin in conjunction with other hurdle technologies, including organic acids, salt, EDTA, heat, high hydrostatic pressure, modified atmosphere packaging, and pulsed electric fields (for reviews, see references 12 and 13). Similarly, aromatic plant oils have been widely studied due to their antimicrobial activities and have found various applications, including in the preservation of raw and processed foods (14), as pharmaceuticals (15), and as natural therapies. Notably, several studies have demonstrated the synergistic activities of combinations including nisin and essential oils, including thymol (16), carvacrol (17, 18), and cinnamaldehyde (19–21), among others.
In this study, we created a stable nisin V producer of an industrial nisin production strain to enable the generation of fermentates of nisin V for comparative studies with its nisin A equivalent. We examine their solo activities as well as their activities in combination with the essential oils thymol, carvacrol, and trans-cinnamaldehyde in terms of the ability to control L. monocytogenes strains of food-borne origin in laboratory media and in model food systems. Notably, we demonstrated that the previously observed enhanced specific activity of purified nisin V over nisin A against L. monocytogenes is retained by the fermentate versions both in microbiological media and in food matrices. In addition, the essential oil and nisin V combinations display significantly increased efficacy compared to those of either compound alone or the nisin A-essential oil combinations.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. lactis strains were grown in M17 broth supplemented with 0.5% glucose (GM17) or GM17 agar at 30°C. Escherichia coli was grown in Luria-Bertani broth with vigorous shaking or agar at 37°C. Listeria strains were grown in brain heart infusion (BHI) or BHI agar at 37°C. Antibiotics were used as follows. Chloramphenicol was used at 10 and 20 μg ml−1, respectively, for L. lactis and E. coli. Erythromycin was used at 150 μg/ml and 5 μg/ml for E. coli and L. lactis, respectively. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was used at a concentration of 40 μg/ml. Stock concentrations of thymol (Sigma) at 50 mg/ml were made up in 50% ethanol and stored at −20°C. Caravcrol (Sigma) was diluted from stock (0.976 g/ml) in 100% ethanol to the desired concentration. trans-Cinnamaldehyde (Sigma) was diluted from stock (1.05 g/ml) in 100% ethanol to the desired concentration. In all experiments, the concentration of ethanol did not exceed 2% (vol/vol).
Conversion of an industrial nisin-producing strain to a nisin V producer.
Mutagenesis of the nisA gene was carried out as described previously (11). Briefly, to introduce the desired mutations within the hinge region of the nisA gene, plasmid pDF06 (a 774-bp product encompassing approximately 300 bp on either side of the nisA gene cloned into the vector pORI280) was amplified with the QuikChange system (Stratagene) using the primers nisinVFor (5′GAGCTCTGATGGGTTGTAACGTTAAAACAGCAACTTGTCATT3′) and nisinVRev (5′CAATGACAAGTTGCTGTTTTAACGTTACAACCCATCAGAGCT3′) (codon changes underlined). The resulting PCR products were transformed into E. coli EC101 (RepA+). To detect altered pORI280-nisA transformants, candidates were screened by PCR using a specific “check” primer (nisinV check 5′GCTCTGATGGGTTGTAACG) designed to amplify mutated plasmid template only and a reverse primer, oDF106 (5′TAGAATTCAACAGACCAGCATTA3′). Plasmids from positive candidates were sequenced (Sourcebioscience UK) using primers pORI280FOR (5′CTCGTTCATTATAACCCTC3′) and pORI280REV (5′CGCTTCCTTTCCCCCCAT3′) to verify the deliberate mutation and to confirm that no other changes had been introduced. pDF08 (pORI280-nisM21V) was then introduced into L. lactis DGCC 10042 pVE6007 by electroporation (22), and transformants were selected by growth on GM17–Ery–X-Gal plates at 30°C. Integration of pDF08 by single-crossover recombination and curing of the temperature-sensitive plasmid pVe6007 were achieved by growth at 37°C in GM17-Ery broth and plating on GM17–Ery–X-Gal agar at the same temperature. Selected colonies were checked for their inability to grow on GM17-Cm agar at 30°C and then subcultured in GM17 at 37°C. Each subculture was spread on GM17–X-Gal plates to identify candidates in which pORI280 had been excised and was lost (LacZ−) due to a second crossover event. Mutant and wild-type revertants were distinguished by deferred antagonism assays. Bac+ candidates were analyzed by mass spectrometry to verify production of the mutant nisin peptide.
Mass spectrometry.
For colony mass spectrometry (CMS), bacteria were collected with sterile plastic loops and mixed with 50 μl of 70% isopropanol adjusted to pH 2 with HCl. The suspension was vortexed, the cells were spun down in a benchtop centrifuge at 14,000 rpm for 2 min, and the supernatant was removed for analysis. Mass spectrometry in all cases was performed with an Axima CFR plus matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometer (Shimadzu Biotech, Manchester, United Kingdom). A 0.5-μl aliquot of matrix solution (alpha-cyano-4-hydroxy cinnamic acid [CHCA], 10 mg ml−1 in 50% acetonitrile–0.1% [vol/vol] trifluoroacetic acid) was placed onto the target and left for 1 to 2 min before being removed. The residual solution was then air dried, and the sample solution (resuspended lyophilized powder or CMS supernatant) was positioned onto the precoated sample spot. Matrix solution (0.5 μl) was added to the sample and allowed to air dry. The sample was subsequently analyzed in positive-ion reflectron mode.
Generation of nisin A- and nisin V-containing fermentate.
Laboratory-scale fermentations were carried out by DuPont (Beaminster, United Kingdom) using the nisin-producing strain L. lactis DGCC 10042 and the newly created nisin V-producing strain L. lactis DGCC 10042::nisV. Analysis of the resultant nisin A- and nisin V-containing SPP revealed equivalent quantities of nisin peptides by high-performance liquid chromatography (HPLC) (81.9 mg/g and 82.6 mg/g, respectively). This is in agreement with previous analysis relating to production levels of bioengineered nisin derivatives (10).
MIC assays.
MIC determinations were carried out in triplicate in 96-well microtiter plates. Ninety-six-well microtiter plates were pretreated with bovine serum albumin (BSA) prior to addition of the nisin fermentates. Briefly, to each well of the microtiter plate, 200 μl of phosphate-buffered saline (PBS) containing 1% (wt/vol) bovine serum albumin (PBS-BSA) was added and incubated at 37°C for 30 min. The wells were washed with 200 μl of PBS and allowed to dry. Target strains were grown overnight under the appropriate conditions and in the appropriate medium, subcultured into fresh broth, and allowed to grow to an optical density at 600 nm (OD600) of ∼0.5, diluted to a final concentration of 105 CFU ml−1 in a volume of 0.2 ml. The nisin SPP were resuspended in BHI broth to a stock concentration of 10 mg/ml. Wild-type nisin and nisin V peptides were adjusted to a 1.25-mg/ml starting concentration, and 2-fold serial dilutions of each peptide were made in 96-well plates for a total of 10 dilutions. The target strain was then added, and after incubation for 16 h at 37°C, the MIC was read as the lowest peptide concentration causing inhibition of visible growth. MICs were determined as described above for the three essential oils against selected Listeria strains, with some minor modifications, as the 96-well plates did not require treatment with BSA. Target strains were grown overnight, subcultured, and added at a final concentration of 105 CFU ml−1 in a volume of 0.2 ml. The various oils were diluted accordingly and added to 0.2 ml at a starting concentration of 2.5 mg/ml, 2-fold serial dilutions were subsequently carried out, and the target strain was then added. Following incubation for 16 h at 37°C, the MIC was read as the lowest peptide concentration causing inhibition of visible growth.
Growth/kill experiments.
For growth experiments, overnight cultures were transferred (107 CFU ml−1 in a volume of 1.0 ml) into BHI supplemented with 25 μg/ml of nisin fermentates in combination with one of the essential oils being tested, i.e., thymol, carvacrol, or cinnamaldehyde, with concentrations ranging from 152 μg/ml to 304 μg/ml. A total of 0.2 ml was subsequently transferred to 96-well microtiter plates (Sarstedt). Cell growth was measured spectrophotometrically over 24-h periods by using a Spectra Max 340 spectrophotometer (Molecular Devices, Sunnyvale, CA). For kill assays, fresh overnight cultures were transferred (107 CFU ml−1 in a volume of 1.0 ml) into BHI broth containing 50 μg/ml of nisin A fermentate or nisin V fermentate alone and in combination with thymol, carvacrol, or cinnamaldehyde. The samples were incubated for 180 min at 37°C. Cell growth was measured by performing viable cell counts by dilution of cultures in one-quarter-strength Ringer solution and enumeration on BHI agar plates.
Model food trials.
A commercially produced chocolate milk drink and a fresh chilled commercially produced chicken noodle soup product were streaked on Listeria selective agar (LSA) (Oxoid) to check for the presence of Listeria. Aliquots of chocolate milk and chicken noodle soup were aseptically transferred to 1.5-ml Eppendorf tubes and inoculated with approximately 1 × 107 CFU ml−1 of Listeria monocytogenes EGDe and Listeria monocytogenes F2365, respectively. Chocolate milk samples were treated with 50 μg ml−1 of fermentates of nisin A and nisin V, alone and in combination with trans-cinnamaldehyde at a concentration of 210 μg/ml to achieve final volumes of 1 ml. Samples with trans-cinnamaldehyde alone at the same concentration served as controls. Chicken soup samples were treated with 50 μg/ml−1 of fermentates of nisin A and nisin V, alone and in combination with carvacrol at a concentration of 195.2 μg/ml to achieve final volumes of 1 ml. In both experiments, samples were incubated at 37°C for 3 h. The kill effect of nisin fermentates alone and in combination with either trans-cinnamaldehyde or carvacrol against Listeria was examined by serial dilution and plate count technique using Listeria selective agar (Oxoid Ltd.). All tests were conducted in triplicate.
Statistical analysis.
Statistical analysis was carried out using the software package Sigmastat 3.5. Groups were compared by Kruskal-Wallis one-way analysis of variance, with post hoc comparison by the Student-Neuman-Keuls method (except where group sizes were unequal; Dunn's comparison method was used in this case). A P value of <0.05 was considered to be significant in all cases.
RESULTS
Conversion of an industrial nisin A production strain to a nisin V producer.
To initiate studies that would more accurately reflect the situation in which nisin is used commercially (as a nisin preparation such as Nisaplin), it was necessary to generate a nisin V-containing SPP for experimental analysis and comparison. To that end, we created a genetically stable nisin V producer of the commercial Nisaplin production strain L. lactis DGCC 10042 through double-crossover homologous recombination. This “self-cloning” strategy means that following excision and gene replacement, no heterologous DNA is present in the final constructs. The nisV gene (generated by PCR-based mutagenesis) was inserted at the appropriate location in the chromosome of the industrial stain L. lactis DGCC 10042 via double crossover recombination to generate L. lactis DGCC 10042::nisV. Results of deferred antagonism assays with L. monocytogenes LO28 indicated that gene replacement had successfully occurred, since the bioactivity profile of the newly constructed strain was enhanced relative to that of L. lactis DGCC 10042 (Fig. 1a). Mass spectrometry analysis confirmed the production of peptides with a mass corresponding to nisin V (3,322 Da) (Fig. 1b). We also confirmed the absence of the pORI280 shuttle vector employed to facilitate the recombination process (data not shown).
FIG 1.
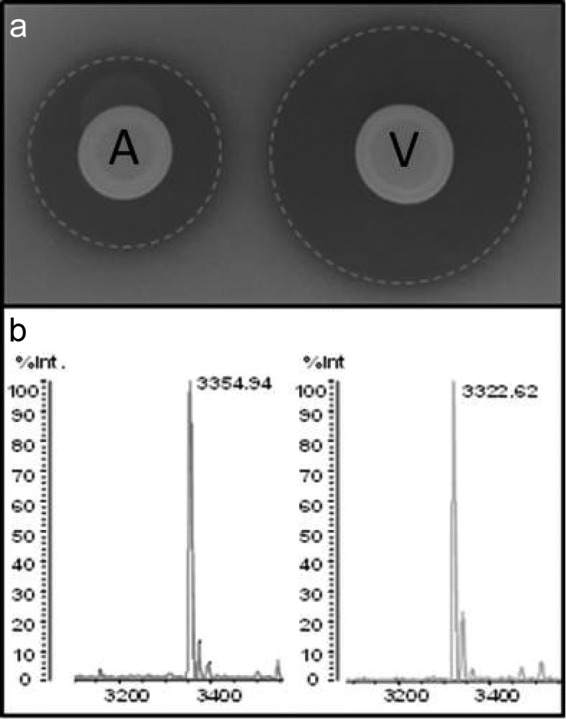
(a) Deferred antagonism assays of the nisin A-producing strain L. lactis DGCC10042 (A) and the stable nisin derivative-producing strain L. lactis DGCC 10042::nisV (V) against the indicator L. monocytogenes LO28. (b) Colony mass spectrometry analysis of the nisin A-producing (3,354 amu) and nisin V-producing (3,322 amu) strains utilized in this study.
MIC-based assessment of nisin A and V fermentates and essential oils against L. monocytogenes used in this study.
Nisin A exhibits antimicrobial activity against L. monocytogenes, but it is not very potent (23, 24). Indeed, in our previous examinations of nisin A and nisin derivatives against Gram-positive pathogens, we observed that Listeria strains exhibited the greatest natural resistance to nisin A (11). Thus, a decision was made to investigate the sensitivities of a wider range of Listeria isolates, in particular those associated with food-borne outbreaks (Table 1), to the nisin-containing fermentates for direct comparison. Additionally, the sensitivity of the strains to the essential oils thymol, carvacrol, and cinnamaldehyde was assessed to establish suitable concentrations for combinatorial studies. Previous studies have demonstrated the enhanced efficacy of nisin V to nisin A using equimolar concentrations of purified peptides (10, 11). In this study, a semipurified preparation containing nisin V obtained through fermentation was produced for the first time at laboratory scale for direct comparison with the nisin A-containing equivalent. These investigations revealed that the nisin V SPP is indeed 2-fold more active (MIC of 39 μg/ml) than its nisin A equivalent (78 μg/ml) against L. monocytogenes EGDe, L. monocytogenes LO28, and Listeria innocua FH1848 (Table 2). Similarly, nisin V was also 2-fold more active (MIC of 62.5 μg/ml) against L. monocytogenes F2365 and L. monocytogenes 33013 than nisin A (125 μg/ml).
TABLE 1.
Listeria strains utilized in this study
| Strain (equivalent name[s]) | Food vehicle | Source | Reference or source |
|---|---|---|---|
| L. monocytogenes | |||
| EGDe | NAa | Isolated from guinea pigs (Cambridge, England) | 34 |
| LO28 | NA | Clinical isolate (feces of a healthy pregnant woman) | |
| F2365 (J-119, Ts43) | Jalisco soft cheese | Human clinical (California outbreak, 1985) | 35 |
| 33413 (Ts45) | Paté | United Kingdom outbreak, 1988) | 36 |
| 33013 (Scott A) | Pasteurized milk | Human Clinical (Massachusetts outbreak, 1983) | 37 |
| L. innocua FH1848 | Food (fish paste/smoked haddock) | Food (fish paste/smoked haddock) | UCC Culture Collection |
NA, not applicable.
TABLE 2.
MIC determinations of nisin A and nisin V SPP and the essential oils thymol, carvacrol, and trans-cinnamaldehyde against a selection of Listeria strains
| Indicator organism | MIC (μg/ml) of: |
||||
|---|---|---|---|---|---|
| Nisin A | Nisin V | Thymol | Carvacrol | Cinnamaldehyde | |
| L. monocytogenes | |||||
| EGDe | 78 | 39 | 156 | 625 | 312 |
| LO28 | 78 | 39 | 312 | 625 | 156 |
| F2365 | 125 | 62.5 | 312 | 312 | 312 |
| 33413 | 62.5 | 31.25 | 156 | 156 | 312 |
| 33013 | 125 | 62.5 | 156 | 312 | 312 |
| L. innocua FH1848 | 78 | 39 | NDa | ND | ND |
ND, not determined.
The MICs for each of the essential oils under investigation were also determined for all L. monocytogenes isolates (Table 2). Thymol was found to completely inhibit growth at concentrations of 156 μg/ml (EGDe, 33013, and 33413) and 312 μg/ml (F2365 and LO28). The MICs of carvacrol were determined to be 156 μg/ml for L. monocytogenes 33413 and 625 μg/ml for both L. monocytogenes F2365 and L. monocytogenes 33013. Both L. monocytogenes EGDe and LO28 exhibited MICs of 312 μg/ml. In experiments conducted with trans-cinnamaldehyde, MICs ranged from 156 μg/ml for L. monocytogenes LO28 to 312 μg/ml for all remaining L. monocytogenes isolates (EGDe, F2365, 33013, and 33413). These values are in close agreement with previously established values (25).
Growth and kill curve-based comparisons.
Having demonstrated the increased specific activity of the nisin V SPP against all the Listeria strains utilized in this study through MIC determinations, we sought to examine (i) the impact on bacterial growth of the SPP alone and in combination with the selected essential oils through growth curve analysis and (ii) the ability of the combinations to kill the bacteria using time-kill curve analysis. Both L. monocytogenes EGDe and L. monocytogenes LO28 were selected as test strains for growth curve experiments. For growth curves, a sublethal concentration of 25 μg/ml was used (as previously employed by Field et al. [11]) for each of the nisin SPP in combination with a range of concentrations (100 to 330 μg/ml) for each essential oil. In the presence of identical concentrations of SPP (25 μg/ml), L. monocytogenes EGDe and L. monocytogenes LO28 had a longer lag time for nisin V than for nisin A in all experiments. When combined with 100 μg/ml of thymol (Fig. 2) and with carvacrol (304 μg/ml and 152 μg/ml for EGDe [Fig. 3A] and LO28 [Fig. 3B]) or trans-cinnamaldehyde (327.6 μg/ml and 327.6 μg/ml for EGDe [Fig. 4A] and LO28 [Fig. 4B]), a significantly longer delay in growth was observed with the nisin A-essential oil combination than with either compound used alone. The combination of nisin A with either carvacrol or cinnamaldehyde produces growth inhibition similar to that obtained with nisin V alone (Fig. 4 and 5). Notably, the most profound delay in growth was observed for the combination of nisin V with either thymol (Fig. 2), carvacrol (Fig. 3), or trans-cinnamaldehyde (Fig. 4) against both LO28 and EGDe.
FIG 2.
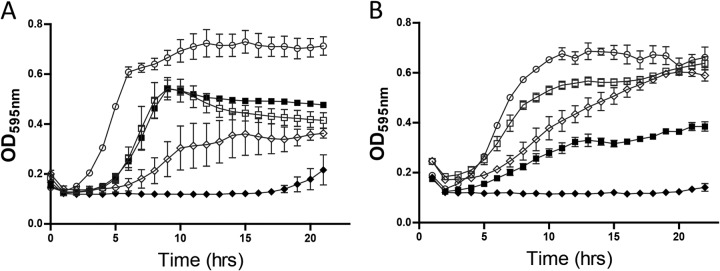
Growth curve analysis of strains L. monocytogenes EGDe (A) and Listeria monocytogenes LO28 (B) in 25 μg/ml of SPP of nisin A (open squares), nisin V (open diamonds), 100 μg/ml of thymol (open circles), and combinations of nisin A and thymol (closed squares) and nisin V and thymol (closed diamonds).
FIG 3.
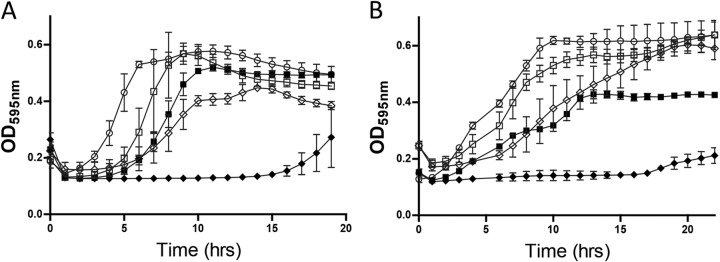
Growth curve analysis of L. monocytogenes EGDe (A) and Listeria monocytogenes LO28 (B) in 25 μg/ml of SPP of nisin A (open squares) and nisin V (open diamonds), 304 μg/ml of carvacrol (open circles), and combinations of nisin A (25 μg/ml) and 304 μg/ml of carvacrol (closed squares) and nisin V (25 μg/ml) and 304 μg/ml of carvacrol (closed diamonds).
FIG 4.
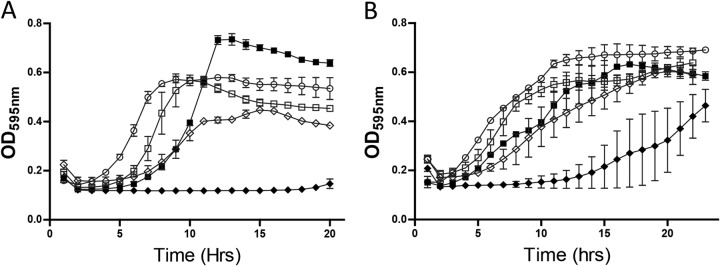
Growth curve analysis of strains L. monocytogenes EGDe (A) and Listeria monocytogenes LO28 (B) in 25 μg/ml of SPP of nisin A (open squares) or nisin V (open diamonds), 327.6 μg/ml of cinnamaldehyde (open circles), and combinations of nisin A (25 μg/ml) and 327.6 μg/ml of cinnamaldehyde (closed squares) and nisin V (25 μg/ml) and 327.6 μg/ml of cinnamaldehyde (closed diamonds).
FIG 5.
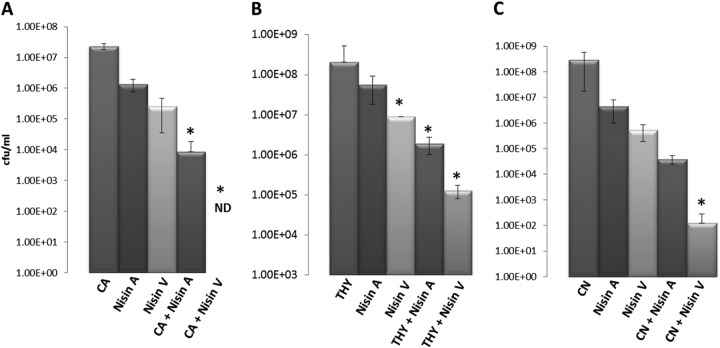
Kill curve analysis of strain L. monocytogenes EGDe (initial inoculum, 1 × 107 cells) upon exposure to 50 μg/ml of each SPP alone and in combination with carvacrol (CA) at 195.2 μg/ml (A), thymol (THY) at 100 μg/ml (B), and trans-cinnamaldehyde (CN) 210 μg/ml (C) in BHI broth for a period of 3 h at 37°C. Cell growth/killing was measured by performing viable cell counts by dilution of cultures in one-quarter-strength Ringer solution and enumeration on BHI agar plates. ND, not detected. Asterisks indicate statistically significant differences between groups (P < 0.05).
In order to compare the bactericidal activities of nisin A and nisin V fermentates over a defined period, L. monocytogenes EGDe (1 × 107 CFU/ml) was exposed to 50 μg/ml of each fermentate in combination with 195.2 μg/ml of carvacrol (Fig. 5A), 100 μg/ml of thymol (Fig. 5B), and 210 μg/ml of trans-cinnamaldehyde (Fig. 5C) in BHI broth for a period of 3 h at 37°C. In all cases and at the concentrations used, cell numbers remained static or slightly increased when the nisin A SPP, thymol, carvacrol, and trans-cinnamaldehyde were used singly (Fig. 5). In contrast, the nisin V fermentate resulted in a 1-log reduction of EGDe over the 3-h period. With respect to combinations of antimicrobials, the antimicrobial potency of nisin A was significantly enhanced when used in combination with all three essential oils, bringing about a 1-log (plus thymol, 1.8 × 106 CFU/ml) (Fig. 5B), 2-log (plus trans-cinnamaldehyde, 2.57 ×105 CFU/ml) (Fig. 5C), or 3-log (plus carvacrol, 8.53 × 103 CFU/ml) (Fig. 5A) reduction in bacterial cell counts. However, the combination of nisin V with all three essential oils proved most effective in that 2-log (plus thymol, 1.28 × 105 CFU/ml) (Fig. 5B), 4-log (plus cinnamaldehyde, 3.15 × 103 CFU/ml) (Fig. 5C), and >5-log (plus carvacrol; not detected) (Fig. 5A) reductions in L. monocytogenes EGDe cell counts were achieved.
Investigation of the anti-Listeria activities of nisin SPP and essential oil combinations in a food matrix.
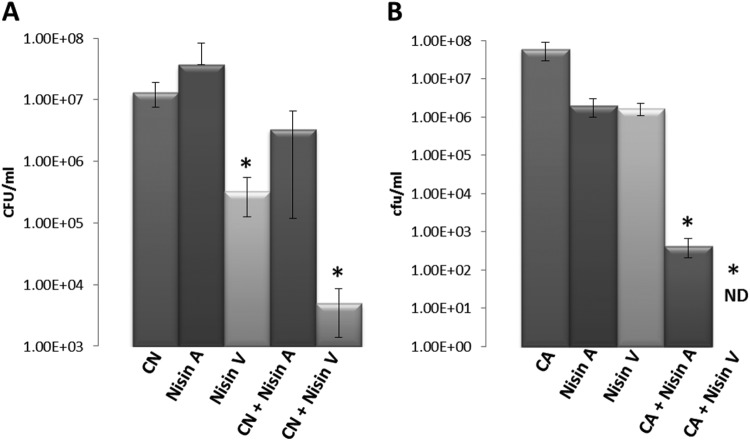
Having established the enhanced potency of nisin V alone and in combination with essential oils against a wide range of L. monocytogenes isolates using a variety of laboratory-based assays, we wished to ascertain whether this enhanced effectiveness could be translated to a food setting. We conducted two food trials, one involving a commercially produced chilled chocolate milk beverage and the other a commercially produced fresh chilled chicken noodle soup. Although L. monocytogenes is not frequently associated with soup, the organism has been isolated from rice soup with cream, lettuce, and meat products (26). The chocolate milk was spiked with L. monocytogenes EGDe to evaluate the efficacies of the nisin SPP alone and in combination with trans-cinnamaldehyde, while the soup was spiked with L. monocytogenes F2365 to evaluate nisin SPP and carvacrol combinations. For both food-based assays, chocolate milk or soup was aseptically transferred to containers to which was directly added the powdered nisin SPP A or V alone (50 μg/ml) or in combination with trans-cinnamaldehyde (210 μg/ml) or carvacrol (195.2 μg/ml). L. monocytogenes EGDe or L. monocytogenes F2365 was added at a concentration of 1 × 107 CFU/ml. Following incubation at 37°C for 3 h, bacterial growth was monitored by serial dilution and plate counts on Listeria selective agar (LSA). In chocolate milk with trans-cinnamaldehyde alone, L. monocytogenes EGDe numbers remained static relative to initial inoculum levels (1.33 × 107 CFU/ml) (Fig. 6A), while a very slight increase in cell numbers was observed for the sample containing the nisin A SPP (3.73 × 107 CFU/ml). A 1-log reduction (3.33 × 106 CFU/ml) was achieved by the nisin A and cinnamaldehyde combination. The best results were obtained for the nisin V SPP alone (a 2-log reduction to 3.33 × 105 CFU/ml) or nisin V in combination with cinnamaldehyde (a 4-log reduction to 5.0 × 103 CFU/ml) (Fig. 6A).
FIG 6.
Survival of L. monocytogenes EGDe (initial inoculum, 1 × 107 cells) in a commercial chocolate milk product in the presence of cinnamaldehyde (CN) at 210 μg/ml plus 50 μg/ml of nisin SPP A and V (A) and L. monocytogenes F2365 (initial inoculum, 1 × 107 cells)in a commercial chicken noodle soup product in the presence of carvacrol (CA) at 195.2 μg/ml plus 50 μg/ml of nisin SPP A and V (B). Samples were incubated at 37°C for 3 h prior to plate count analysis on Listeria selective agar (LSA). ND, not detected. Asterisks indicate statistically significant differences between groups (P < 0.05).
In chicken noodle soup, both the nisin A and nisin V SPP (50 μg/ml) alone resulted in a 1-log reduction (2.0 × 106 CFU/ml and 1.67 × 106 CFU/ml, respectively). The combination of either nisin A or nisin V (50 μg/ml) with carvacrol (195.2 μg/ml) demonstrated a potent effect, reducing cell numbers by 5 log (4.33 × 102 CFU/ml) and >5 log (not detected) units, respectively (Fig. 6B). While the model food results are in close agreement with the broth-based kill curve experiments, more importantly they demonstrate that the enhanced nature of nisin V when applied as a commercial-like fermentate is maintained even within the complex environment of food. Even more notable is the combined effect observed between nisin V and combinations of both trans-cinnamaldehyde and carvacrol to control L. monocytogenes in food, reducing cell numbers significantly more than either compound alone and at least 2 logs greater than nisin A or nisin A-essential oil combinations.
DISCUSSION
There is an increasing need to develop economical, natural, and effective food preservative systems to meet the public demand for convenient, safe, healthy, and nutritious food products. Such demand has opened up new opportunities for the use of natural antimicrobials derived from plant, animal, or microbial sources. Examples of investigated compounds include lactoperoxidase, lysozyme, plant essential oils, organic acids, bacteriocins, and chitosan (12, 25, 27). However, while the preservative action of these compounds alone in a food system is unlikely to ensure comprehensive protection, combinations of natural antimicrobials with other nonthermal processing technologies within the hurdle concept could prove invaluable to food manufacturers and consumers in terms of food safety, shelf life, quality, and nutritional properties. The synergistic effect of nisin in combination with the plant essential oils carvacrol and thymol to inhibit the growth of food spoilage and pathogenic organisms such as L. monocytogenes and B. cereus has been previously reported (16, 17, 19, 28). In this study, we demonstrated the ability to provide even greater protection against L. monocytogenes by combining for the first time an enhanced nisin derivative (nisin V) in the form of a fermentate with a selection of essential oils. Although the enhanced activity of the nisin V-containing SPP compared to that of nisin A against several listerial targets is in agreement with our previous study (11), the enhanced partnership observed when nisin V was combined with carvacrol or cinnamaldehyde is remarkable. In all instances, the nisin V-essential oil combination (thymol, carvacrol, and cinnamaldehyde) outperformed their nisin A equivalents as observed by the extended lag phase of several hours. In time-kill assays, a 2-log decrease in cell numbers over and above that achieved by the nisin A combination was observed (carvacrol and cinnamaldehyde) against the target L. monocytogenes EGDe. The fact that this interaction is maintained in a food setting is noteworthy and serves to highlight the potential of enhanced nisin derivatives when utilized in multihurdle systems to effectively control L. monocytogenes in food. L. monocytogenes is of particular concern to the food industry. Although listeriosis is a relatively rare disease, mortality rates associated with outbreaks are high (29). Apart from the risk to human health, food product recalls due to Listeria contamination present an enormous financial burden, estimated to cost between $1.2 and $2.5 billion per year in the United States (30). Notably, in 2003 the Food Safety and Inspection Services (FSIS) published a ruling requiring manufacturers of ready-to-eat foods to provide additional post processing control measures to kill Listeria or prevent its growth, placing increased pressure on food manufacturers with respect to food safety. Therefore, any new technologies or means to enhance the control of L. monocytogenes in foods are particularly desirable. The consistency of the nisin V and essential oil combination to control L. monocytogenes isolates is striking, especially since the level of essential oil used is bacteriostatic rather than bactericidal. Notably, despite the demonstrated efficacy of essential oils and their components in vitro, their use as food preservatives has been limited because of the high concentrations needed to achieve sufficient antimicrobial activity. Additionally, their intense aroma, even at low concentrations, can produce adverse organoleptic effects exceeding the threshold acceptable to consumers. Consequently, the observed cooperation of nisin V and the essential oils carvacrol and cinnamaldehyde at these low concentrations (equivalent to approximately 0.02%) may provide the key to implementing essential oils in food preservation without simultaneous organoleptic effects. Crucially, we have demonstrated that this relationship is still maintained in complex food matrices, an important finding given that the hydrophobic nature of essential oils can lead to reduced efficacy by interactions with food matrix components such as fat (31), starch (32), and the level of microbial contamination (33). It is worth noting that our studies employed a high initial inoculum (1 × 107 CFU ml−1), much higher than would be expected in a food processing plant (∼20 CFU/g). The enhanced cooperation of the nisin V SPP and essential oil mixture under these “abusive” conditions suggests that were it to be incorporated with good manufacturing processes and other hurdle technologies, this combination could provide very effective anti-Listeria protection in food preservation. Additionally, the use of natural food grade antimicrobials in this way could provide a more acceptable solution for consumers wishing to buy more “natural” products. In order for the food industry to fully make the change from the use of artificial sources to purely natural sources, research must address the limits of antimicrobial activity, the overall cost, the most effective concentration, optimization of the antimicrobial, and use of hurdle technology and address regulatory concerns. This study has begun the process of tackling some of those issues.
ACKNOWLEDGMENTS
We thank DuPont UK Ltd. for producing the semipurified preparations of nisin A and V. We thank Pat G. Casey for statistical analysis.
This work was supported by the Irish Government under the National Development Plan through Science Foundation Ireland Investigator awards to C.H. and R.P.R. (10/IN.1/B3027) and to C.H., R.P.R., and P.D.C. (06/IN.1/B98).
REFERENCES
- 1.Lucera A, Costa C, Conte A, Del Nobile MA. 2012. Food applications of natural antimicrobial compounds. Front Microbiol 3:287. doi: 10.3389/fmicb.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gálvez A, Abriouel H, Benomar N, Lucas R. 2010. Microbial antagonists to food-borne pathogens and biocontrol. Curr Opin Biotechnol 21:142–148. doi: 10.1016/j.copbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 4.Deegan LH, Cotter PD, Hill C, Ross P. 2006. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J 16:1058–1071. doi: 10.1016/j.idairyj.2005.10.026. [DOI] [Google Scholar]
- 5.Delves-Broughton J. 2005. Nisin as a food preservative. Food Aust 57:525–527. [DOI] [PubMed] [Google Scholar]
- 6.Bierbaum G, Sahl HG. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol 10:2–18. doi: 10.2174/138920109787048616. [DOI] [PubMed] [Google Scholar]
- 7.Field D, Hill C, Cotter PD, Ross RP. 2010. The dawning of a ‘Golden era’ in lantibiotic bioengineering. Mol Microbiol 78:1077–1087. doi: 10.1111/j.1365-2958.2010.07406.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers LA, Whittier EO. 1928. Limiting factors in the lactic fermentation. J Bacteriol 16:211–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan J, Zhang ZZ, Chen XZ, Yang W, Huan LD. 2004. Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl Microbiol Biotechnol 64:806–815. doi: 10.1007/s00253-004-1599-1. [DOI] [PubMed] [Google Scholar]
- 10.Field D, O'Connor PM, Cotter PD, Hill C, Ross RP. 2008. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol Microbiol 69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 11.Field D, Quigley L, O'Connor PM, Rea MC, Daly K, Cotter PD, Hill C, Ross RP. 2010. Studies with bioengineered nisin peptides highlight the broad-spectrum potency of nisin V. Microb Biotechnol 3:473–486. doi: 10.1111/j.1751-7915.2010.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gálvez A, Abriouel H, Lopez RL, Omar NB. 2007. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120:51–70. doi: 10.1016/j.ijfoodmicro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Sobrino-Lopez A, Martin-Belloso O. 2008. Use of nisin and other bacteriocins for preservation of dairy products. Int Dairy J 18:329–343. doi: 10.1016/j.idairyj.2007.11.009. [DOI] [Google Scholar]
- 14.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Edris AE. 2007. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res 21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 16.Ettayebi K, El Yamani J, Rossi-Hassani B. 2000. Synergistic effects of nisin and thymol on antimicrobial activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol Lett 183:191–195. doi: 10.1111/j.1574-6968.2000.tb08956.x. [DOI] [PubMed] [Google Scholar]
- 17.Pol IE, Smid EJ. 1999. Combined action of nisin and carvacrol on Bacillus cereus and Listeria monocytogenes. Lett Appl Microbiol 29:166–170. doi: 10.1046/j.1365-2672.1999.00606.x. [DOI] [PubMed] [Google Scholar]
- 18.Periago PM, Moezelaar R. 2001. Combined effect of nisin and carvacrol at different pH and temperature levels on the viability of different strains of Bacillus cereus. Int J Food Microbiol 68:141–148. doi: 10.1016/S0168-1605(01)00461-5. [DOI] [PubMed] [Google Scholar]
- 19.Valero M, Frances E. 2006. Synergistic bactericidal effect of carvacrol, cinnamaldehyde or thymol and refrigeration to inhibit Bacillus cereus in carrot broth. Food Microbiol 23:68–73. doi: 10.1016/j.fm.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Gill AO, Holley RA. 2004. Mechanisms of bactericidal action of cinnamaldehyde against Listeria monocytogenes and of eugenol against L. monocytogenes and Lactobacillus sakei. Appl Environ Microbiol 70:5750–5755. doi: 10.1128/AEM.70.10.5750-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baskaran SA, Amalaradjou MA, Hoagland T, Venkitanarayanan K. 2010. Inactivation of Escherichia coli O157:H7 in apple juice and apple cider by trans-cinnamaldehyde. Int J Food Microbiol 141:126–129. doi: 10.1016/j.ijfoodmicro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Papagianni M, Avramidis N, Filioussis G. 2007. High efficiency electrotransformation of Lactococcus lactis spp. lactis cells pretreated with lithium acetate and dithiothreitol. BMC Biotechnol 7:15. doi: 10.1186/1472-6750-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignolo G, Palacios J, Farias ME, Sesma F, Schillinger U, Holzapfel W, Oliver G. 2000. Combined effect of bacteriocins on the survival of various Listeria species in broth and meat system. Curr Microbiol 41:410–416. doi: 10.1007/s002840010159. [DOI] [PubMed] [Google Scholar]
- 24.Chi-Zhang Y, Yam KL, Chikindas ML. 2004. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. Int J Food Microbiol 90:15–22. doi: 10.1016/S0168-1605(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 25.Hyldgaard M, Mygind T, Meyer RL. 2012. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol 3:12. doi: 10.3389/fmicb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conner DE, Brackett RE, Beuchat LR. 1986. Effect of temperature, sodium chloride, and pH on growth of Listeria monocytogenes in cabbage juice. Appl Environ Microbiol 52:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari BK, Valdramidis VP, O'Donnell CP, Muthukumarappan K, Bourke P, Cullen PJ. 2009. Application of natural antimicrobials for food preservation. J Agric Food Chem 57:5987–6000. doi: 10.1021/jf900668n. [DOI] [PubMed] [Google Scholar]
- 28.Esteban MD, Palop A. 2011. Nisin, carvacrol and their combinations against the growth of heat-treated Listeria monocytogenes cells. Food Technol Biotechnol 49:89–95. [Google Scholar]
- 29.Schlech WF., III 2000. Foodborne listeriosis. Clin Infect Dis 31:770–775. doi: 10.1086/314008. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen MR, McKenzie AM. 2001. Market incentives for safe foods: an examination of shareholder losses from meat and poultry recalls. Am J Agric Econ 83:526–538. doi: 10.1111/0002-9092.00175. [DOI] [Google Scholar]
- 31.Cava-Roda R, Taboada-Rodríguez A, Valverde-Franco M, Marín-Iniesta F. 2012. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157:H7 in milk. Food Bioprocess Technol 5:2120–2131. doi: 10.1007/s11947-010-0484-4. [DOI] [Google Scholar]
- 32.Gutierrez J, Barry-Ryan C, Bourke P. 2008. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microbiol 124:91–97. doi: 10.1016/j.ijfoodmicro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Somolinos M, Garcia D, Condon S, Mackey B, Pagan R. 2010. Inactivation of Escherichia coli by citral. J Appl Microbiol 108:1928–1939. [DOI] [PubMed] [Google Scholar]
- 34.Murray EGD, Webb RA, Swann MBR. 1926. A disease of rabbits characterised by a large mononuclear leucocytosis, caused by a hitherto undescribed bacillus Bacterium monocytogenes (n.sp.). J Pathol Bacteriol 29:407–439. doi: 10.1002/path.1700290409. [DOI] [Google Scholar]
- 35.Linnan MJ, Mascola L, Lou XD, Goulet V, May S, Salminen C, Hird DW, Yonekura ML, Hayes P, Weaver R, Audurier A, Plikaytis BD, Fannin SL, Kleks A, Broome CV. 1988. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med 319:823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 36.McLauchlin J, Hall SM, Velani SK, Gilbert RJ. 1991. Human listeriosis and paté: a possible association. BMJ 303:773–775. doi: 10.1136/bmj.303.6805.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming DW, Cochi SL, MacDonald KL, Brondum J, Hayes PS, Plikaytis BD, Holmes MB, Audurier A, Broome CV, Reingold AL. 1985. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med 312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]