Abstract
Many bacteria convert bicyclic compounds, such as indole and naphthalene, to oxidized compounds, including hydroxyindoles and naphthols. Pseudomonas aeruginosa, a ubiquitous bacterium that inhabits diverse environments, shows pathogenicity against animals, plants, and other microorganisms, and increasing evidence has shown that several bicyclic compounds alter the virulence-related phenotypes of P. aeruginosa. Here, we revealed that hydroxyindoles (4- and 5-hydroxyindoles) and naphthalene derivatives bearing hydroxyl groups specifically inhibit swarming motility but have minor effects on other motilities, including swimming and twitching, in P. aeruginosa. Further analyses using 1-naphthol showed that this effect is also associated with clinically isolated hyperswarming P. aeruginosa cells. Swarming motility is associated with the dispersion of cells from biofilms, and the addition of 1-naphthol maintained biofilm biomass without cell dispersion. We showed that this 1-naphthol-dependent swarming inhibition is independent of changes of rhamnolipid production and the intracellular level of signaling molecule cyclic-di-GMP (c-di-GMP). Transcriptome analyses revealed that 1-naphthol increases gene expression associated with multidrug efflux and represses gene expression associated with aerotaxis and with pyochelin, flagellar, and pilus synthesis. In the present study, we showed that several bicyclic compounds bearing hydroxyl groups inhibit the swarming motility of P. aeruginosa, and these results provide new insight into the chemical structures that inhibit the specific phenotypes of P. aeruginosa.
INTRODUCTION
Pseudomonas aeruginosa is a ubiquitous bacterium that colonizes various environments. This environmental bacterium can survive not only in planktonic form but also in surface-associated communities known as biofilms (1). P. aeruginosa is also an opportunistic social pathogen that affects humans, animals, and plants and that produces various virulence factors. Because these virulence factors also possess antimicrobial activities, other microbes have multiple strategies to cope with P. aeruginosa virulence, such as the production or conversion of chemicals that repress P. aeruginosa social behaviors (2). Understanding the interactions between external chemicals and P. aeruginosa would facilitate the development of virulence inhibitors that do not affect the growth of this bacterium, thereby preventing the development of drug resistance.
P. aeruginosa has an exquisite mechanism to creatively use three different types of motilities (swimming, twitching, and swarming) that potentially facilitate colonization in various environmental niches. Motilities are significantly involved in the surface attachment and maturation of biofilms (3, 4). Swimming in aqueous environments is mediated through flagella, while twitching on solid surfaces is mediated through type IV pili. Swarming is described as a social phenomenon involving the coordinated and rapid movement of bacteria across a semisolid surface using both flagella and type IV pili (5). Swarming is necessary for differentiation into specialized cells through elongation and hyperflagellation (6). To reduce surface tension, swarming P. aeruginosa cells also secrete rhamnolipids (RLs), amphiphilic glycolipids composed of l-rhamnose and 3-hydroxyalkanoic glycolipids (7). The production of RLs is regulated through the Rhl system, a cell density-dependent gene expression system based on quorum sensing (7).
Swarming motility is significantly involved in various phenotypes in P. aeruginosa. For example, swarming cells are more resistant to antibiotics than nonswarming cells and show increased gene expression for virulence factor production compared to that of swimming cells (8, 9). Thus, understanding the regulation of swarming motility is important for the development of treatment against this bacterium. Several global analyses have shown that a wide range of genes, including regulatory, metabolic, chemosensory, secretory, and hypothetical genes, affect swarming motility in P. aeruginosa (9–11). In addition, the transcriptional profiles are quite different in the areas at the center and on the edge of swarming cells, suggesting that swarming cells are a phenotypically heterogeneous population (11). Hence, swarming might be a highly organized form of motility involving multiple functions, the regulation of which is remarkably complex. It has been previously reported that P. aeruginosa swarming motility is inhibited by several compounds, including branched-chain fatty acid anteiso-C15:0, cranberry proanthocyanidins and other tannins, and several amino acids, such as arginine (12–14). However, the detailed mechanisms underlying swarming inhibition remain unknown.
It has been shown that various P. aeruginosa phenotypes are regulated through bicyclic compounds synthesized or converted by other organisms (2). P. aeruginosa possesses an acyl-homoserine lactone-independent quorum-sensing system that utilizes 2-heptyl-3-hydroxy-4(1H)-quinolone (PQS, for Pseudomonas quinolone signal) to regulate many virulence factors, including pyocyanin and exoenzymes, biofilm formation, and membrane vesicle formation (15–18). Particularly, PQS-regulated phenotypes are controlled through various bicyclic compounds. Farnesol, a cell-to-cell signal in Candida albicans, represses PQS-regulated virulence factors through direct binding with the PQS receptor PqsR (19). Indole, a signaling molecule in Escherichia coli (20), also represses PQS-regulated virulence (21, 22), but the mechanism of this activity remains unknown. Indeed, this effect is not limited to indole as many bicyclic compounds, including hydroxyindoles, isatins, and naphthalene derivatives, such as naphthols, also repress P. aeruginosa virulence (22). Hydroxyindoles and isatins are oxidized from indole in many bacteria, such as Pseudomonas spp. and Burkholderia spp. (23, 24). Naphthalene and naphthalene derivatives are widely used as useful chemicals (insecticide, dyestuff, surfactant, etc.), and several microorganisms convert naphthalene into naphthols (25, 26). Thus, there are many ubiquitous environmental bicyclic compounds that affect P. aeruginosa phenotypes.
It would be interesting to determine how chemical compounds in the environment affect bacterial social behaviors. Understanding the interactions between chemical compounds and P. aeruginosa would provide new insights into the regulation of the virulence of this pathogen in both environmental and clinical settings. Here, we investigated the effects of bicyclic compounds, including indole and naphthalene derivatives, on swarming motility, a social behavior of P. aeruginosa. The results indicated that bicyclic compounds bearing hydroxyl groups, such as 4-hydroxyindole (4HI), 5-hydroxyindole (5HI), and 1-naphthol, inhibit swarming motility in P. aeruginosa. We used 1-naphthol for further analyses and showed that this compound also inhibits biofilm dispersion. Transcriptome analyses suggested that 1-naphthol regulates the expression of various swarming-related and efflux pump-encoding genes in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Pseudomonas aeruginosa PAO1 (27) was used as a model organism in this study. PAO1 mutants, including the ΔrhlR and ΔmexAB-oprM strains, were previously constructed (28, 29). Clinical isolates of P. aeruginosa were provided from Okayama University, Japan (30). For routine culture, P. aeruginosa was cultured at 37°C in lysogeny broth (LB Lennox; Nacalai, Kyoto, Japan) medium with shaking at 200 rpm or LB plates containing 1.5% (wt/vol) agar. Bicyclic compounds were solubilized in dimethyl sulfoxide (DMSO) and added to the medium at a final concentration of 500 μM. Each liquid culture was initiated at a 660-nm optical density (OD660) of 0.01. Growth was measured according to the optical density at 660 nm. The optical density was measured using a Beckman Coulter DU640 spectrophotometer with a 2-mm gap cuvette. Escherichia coli DH5α was used for molecular techniques. When necessary, gentamicin was added at concentrations of 10 μg/ml for E. coli and 50 μg/ml for P. aeruginosa.
Motility assays.
A swarming motility assay was performed on a petri dish using a previously described method (31). Briefly, M9 minimal plates (0.2% glucose, 0.5% Casamino Acids [CAA], and 0.5% agar) containing various naphthalene derivatives were dried for 1 h. The naphthalene compounds were dissolved in dimethyl sulfoxide (DMSO) and subsequently added to each agar medium at a final concentration of 500 μM. An equal amount of DMSO was separately added to agar medium as a control. Stationary-phase cultures of P. aeruginosa were washed twice with phosphate-buffered saline (PBS) and adjusted to an OD660 of 3.0; an aliquot of 5 μl was carefully spotted onto the center of agar plates. The plates were incubated at 30°C for 12 h, and the experiments were performed in triplicate. A filter paper disc assay was conducted to determine whether 1-naphthol would repel swarming bacteria using a previously described method (14). Whatman filter paper discs (GE Healthcare Life Sciences, Pittsburgh, PA) were soaked with 10 μl of 1-naphthol at various concentrations (0 to 50 mM in DMSO). Subsequently, the discs were placed onto M9 medium containing 0.5% agar, and P. aeruginosa was spotted onto the center of the agar plates. The bacteria were grown at 30°C for 12 h.
Swimming assays were performed on LB plates containing 0.3% agar as previously described (32). Swimming was assessed after 24 h of growth at 30°C. The twitching assays were performed on LB plates containing 1% agar, according to a previously described method (13). The twitching assay plate was incubated at 37°C for 24 h.
Biofilm quantification, visualization, and image analysis.
For the biofilm assays, stationary-phase cultures were inoculated to a final concentration equivalent to an OD660 of 0.01 into 100 μl of LB medium in 96-well microtiter plates made of polyvinylchloride. The plates were incubated at 37°C under aerobic conditions. After 6 h, naphthalene-derivative compounds were added at 500 μM in LB liquid medium. After the planktonic cells were removed, crystal violet staining and the quantification of biofilms were performed as previously described (3). The biofilm was visualized using confocal laser scanning microscopy (CLSM) as previously described (33). The cells were grown in Nunc flasks, and the attached biofilm was observed using a Carl Zeiss Pascal laser scanning microscope equipped with a 63×/1.4-numerical aperture Plan-Apochromat objective and a 40×/0.80-numerical aperture IR Achroplan W water immersion objective (Carl Zeiss, Jena, Germany). For confocal reflection microscopy (CRM), the biofilms were illuminated with a 488- or 514-nm argon laser, and the reflected light was collected through a 470- to 500-nm or 505- to 530-nm band pass filter, respectively, to avoid the influence of auto-fluorescence (34). An NT 80/20 half mirror (Carl Zeiss) was used as a beam splitter. The confocal images were analyzed using Carl Zeiss LSM 5 Pascal software (version 3.5; Carl Zeiss). For biovolume quantification, the confocal images were analyzed using the COMSTAT computer program (35) with MATLAB (MathWorks, Natick, MA, USA).
Rhamnolipid analysis.
The cultures were initially adjusted to an OD660 of 0.01 and grown in M9 minimal medium at 30°C and 200 rpm for 12 h. The concentration of rhamnolipid (3-deoxy-hexose) in the culture supernatant was measured using an orcinol assay as previously described (36). The orcinol assay is a colorimetric test to determine the amount of hexose sugars. The culture supernatant (10 to 300 μl) was diluted with water to obtain a volume of 300 μl and extracted twice with 600 μl of diethyl ether. The pooled ether fractions were evaporated to dryness, and the pellet was dissolved in 100 μl of distilled water and mixed with 100 μl of 1.6% orcinol and 800 μl of 60% sulfuric acid. After the sample was heated to 80°C and subjected to shaking for 30 min, the absorbance was measured at 421 nm. The content of rhamnose in the samples was determined by comparing results with rhamnose standards that had a defined concentration. Rhamnolipid concentration was calculated based on the assumption that 1 μg of rhamnose corresponds to 2.5 μg of rhamnolipid (37). To harvest a large amount of rhamnolipid, the cultures were initially adjusted to an OD660 of 0.01 and grown in 100 ml of PPGAS medium (0.02 M NH4Cl2, 0.02 M KCl, 0.12 M Tris-HCl, 1.6 mM MgSO4, 0.5% [wt/vol] glucose, 1% [wt/vol] peptone, adjusted to pH 7.2) in 500-ml Erlenmeyer flasks at 37°C and 200 rpm for 24 h. The extracted rhamnolipid was added to the swarming medium at a final concentration of 0.5% (wt/vol). Values were normalized to the OD660 value.
Cyclic di-GMP reporter assay.
The intracellular cyclic-di-GMP (c-di-GMP) level was quantified using a reporter plasmid containing the promoter of cdrA (38). The DsRed-based promoter probe plasmid, pMEX-DsRed, was constructed by reference to a previous report (39). The region of the DsRed gene was amplified from pDsRed-Express (Clontech, Palo Alto, CA, USA) by PCR using the primer pair GfpF1/dsredR1 (Table 1). The amplified fragment was digested with NcoI/XhoI and subcloned into a similarly digested pET15b plasmid (Novagen). For the construction of pMEX-DsRed, the promoter region of cdrA was amplified by PCR with PAcdrAPF/PAcdrAPR primers (Table 1), digested by BamHI and EcoRI, and inserted into the multicloning site of pMEX9-DsRed. A region containing a ribosome binding site-fused DsRed gene was amplified by PCR with the primer pair pET15b-kpn/dsredR2 (Table 1), digested with KpnI/SacI, and cloned into similarly digested pMEX9 plasmid to replace the xylE reporter gene with a gene encoding DsRed. Cells grown in M9 medium containing gentamicin were collected, and fluorescence (excitation, 554 nm; emission, 586 nm) was measured at 37°C with a fluorometer (Varioskan Flash; Thermo Scientific, Waltham, MA, USA). Data are presented as arbitrary fluorescence intensity units normalized to cell growth (OD600). DsRed expression of cells harboring promoterless pMEX9-DsRed was used as a reference.
TABLE 1.
Primers used in this study
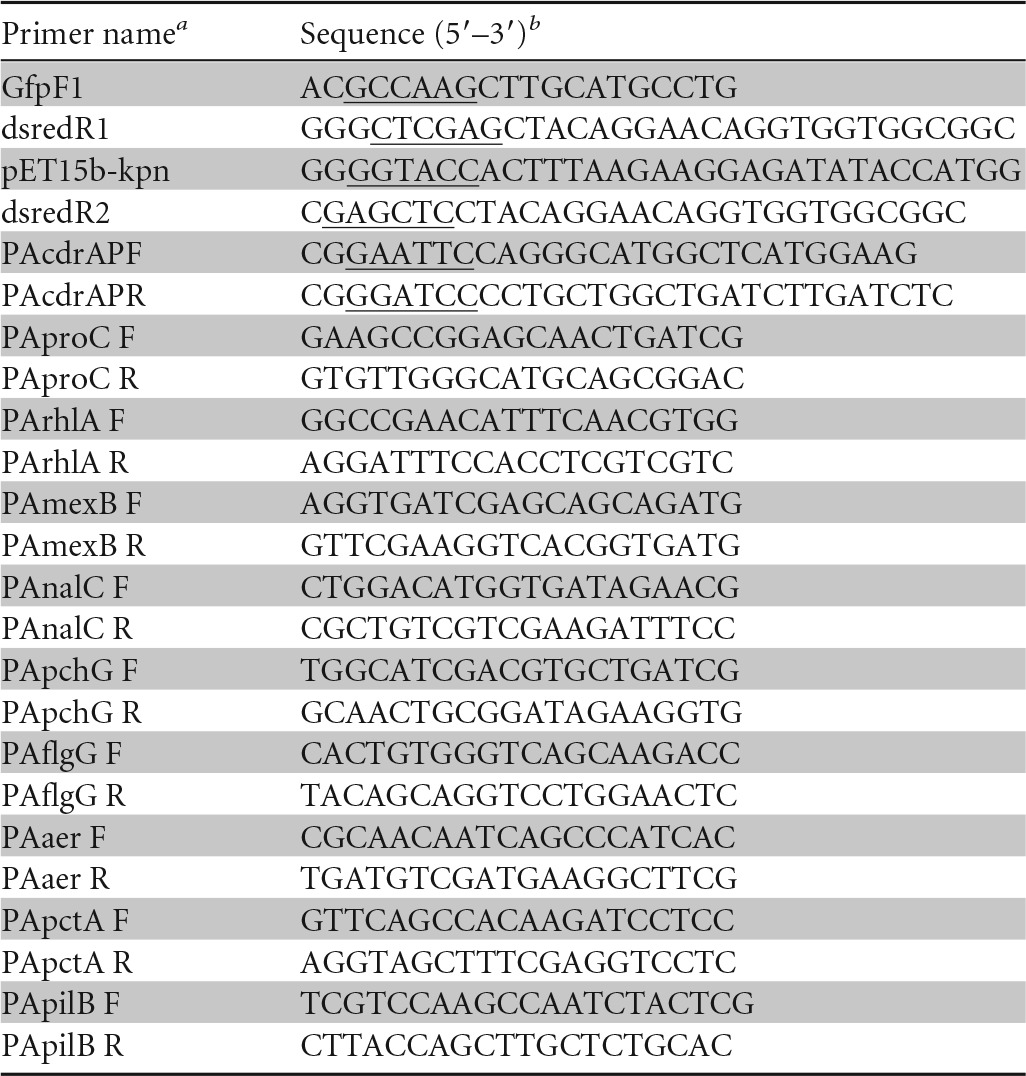
F, forward; R, reverse.
The added restriction sites in the primers are underlined.
Whole transcriptome analysis using a tiling array.
A microarray analysis of PAO1 chromosomes was performed as previously described (40) using the database sequencing of PAO1 (GenBank accession number NC_002516) with 25-mer perfect-match probes overlapped and offset by 11 nucleotides. The synthesis, fragmentation, labeling, and detection of cDNA were performed as described previously (41). Briefly, total RNA was extracted in parallel from two independent M9 liquid cultures (the turbidity of each culture was 0.6 at 660 nm) using RNAProtect Bacteria reagent (Qiagen, Valencia, CA) and NucleoSpin RNA II (Macherey-Nagel GmbH and Co., KG, Düren, Germany). After DNA degradation with RQ1 RNase-free DNase (Promega, Madison, WI) and purification using NucleoSpin RNA II, single-stranded cDNA was synthesized using random primers (Invitrogen, Carlsbad, CA), SuperScript II (Invitrogen), and deoxynucleoside triphosphates (dNTPs), including dUTP (Roche Applied Science, Mannheim, Germany). The synthesis of cDNA was performed in the presence of actinomycin D (ActD) to prevent the generation of spurious second-strand cDNA, as previously described (42). The template RNA was degraded using NaOH, and the cDNA was purified using a QIAquick PCR purification kit (Qiagen). Fragmentation, labeling, and detection were performed as previously described (40).
Quantitative RT-PCR (qRT-PCR) analysis.
RNA was extracted from a P. aeruginosa PAO1 culture (OD660 of 0.6 in M9 medium) using an RNA extraction kit (Promega), and the DNA was removed using a Turbo DNase kit (Life Technologies). The cDNA synthesis and real-time reverse transcription-PCR (RT-PCR) analysis were performed as previously described with some modifications (43). Briefly, 1 μg of RNA was used for reverse transcription using RT SuperScript II (Invitrogen, Carlsbad, CA) with random hexamers. The cDNA was used as a template for PCR with the primers shown in Table 1. Real-time RT-PCR was conducted using a LightCycler (Roche) and a LightCycler FastStart DNA Master Plus SYBR green I kit. The proC gene was used as an internal control (44), and the data were normalized to proC expression.
Determination of MICs of antibiotics.
Comparative MIC determinations were performed with LB medium-agar containing diluted antibiotics. Cells were grown in LB medium until they reached the stationary phase and then diluted to an optical density at 660 nm (OD660) of 0.1. Five microliters of diluted cells was spotted onto LB medium-agar plates containing several concentrations of antibiotics and grown at 37°C for 24 h.
Microarray data accession number.
The two sets of the array data have been deposited in the Gene Expression Omnibus (GEO) of the NCBI (http://www.ncbi.nlm.nih.gov/geo/) under GEO series accession number GSE60343.
RESULTS
The growth of P. aeruginosa with bicyclic compounds.
To determine whether bicyclic compounds show toxicity to P. aeruginosa, bacterial growth was examined in the absence and presence of bicyclic compounds, including indole, 4HI, 5HI, naphthalene, 1-naphthol, 2-naphthol, 1,2-dihydroxynaphthalene (1,2-DHN), 2,3-dihydroxynaphthalene (2,3-DHN), and 1-aminonaphthalene. All compounds, except 1,2-DHN, showed no effect at 500 μM on growth either in liquid culture or on agar plates, and 1,2-DHN did not repress bacterial growth at 250 μM under this experimental condition. This concentration (500 μM) is similar to the extracellular indole concentration observed in the supernatant of E. coli grown in a rich medium (20).
Inhibition of swarming motility by bicyclic compounds.
The effect of bicyclic compounds on the swarming motility of PAO1 on 0.5% agar was examined, and the results showed that several bicyclic compounds, including 4HI, 5HI, 1-naphthol, 1,2-DHN, 2,3-DHN, and 1-aminonaphthalene, inhibited swarming motility (Fig. 1A). Interestingly, indole and naphthalene, which do not have a functional group, did not inhibit swarming motility. Furthermore, 2-naphthol, a structural analog of 1-naphthol, with a hydroxyl group at the neighboring carbon, showed markedly less inhibition of motility than 1-naphthol. These results suggest that the occurrence and position of a hydroxyl group largely affect the inhibition of swarming motility. Further analyses were conducted using 1-naphthol, which completely inhibited PAO1 swarming motility. Similarly, 1-naphthol inhibited the swarming motility of P. aeruginosa in a dose-dependent manner, and swarming was completely abolished at concentration of 50 μM or more (Fig. 1B). When swarming-inhibited cells on 1-naphthol swarming plates (12 h) were transferred to new swarming plates without 1-naphthol, the cells resumed active swarming motility, suggesting that 1-naphthol did not induce mutations in the genes required for swarming motility (data not shown). Furthermore, 1-naphthol and all compounds tested did not inhibit swimming motility and had minor effects on the twitching motility of PAO1 (Fig. 2), suggesting that motility inhibition by 1-naphthol is effective for swarming and that 1-naphthol does not affect the function of flagella, which are required for swimming.
FIG 1.
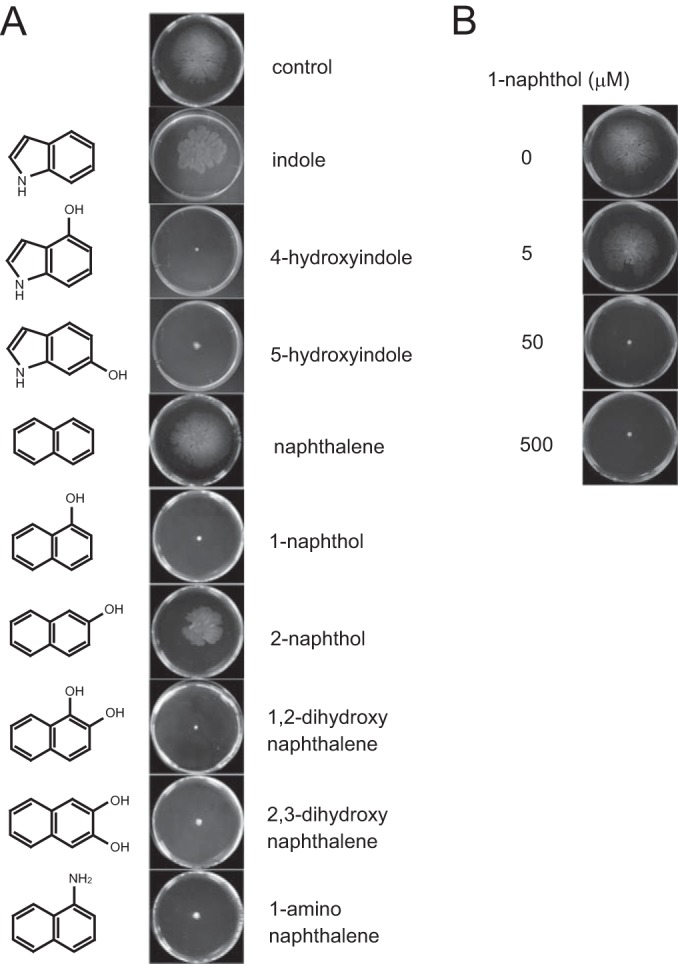
Inhibition of P. aeruginosa swarming motility by bicyclic compounds. The cells were inoculated at the center of a plate containing M9 swarming medium and 0.5% agar with or without each compound and incubated at 30°C for 12 h. (A) The effects of various compounds on swarming motility. Each compound was added at 500 μM, with the exception of 1,2-dihydroxynaphthalene, which was added to the medium at 250 μM. Three independent experiments were performed, and representative data are shown. (B) Dose-dependent effect of 1-naphthol (0, 5, 50, and 500 μM) on swarming motility. Three independent experiments were performed, and representative data are shown.
FIG 2.
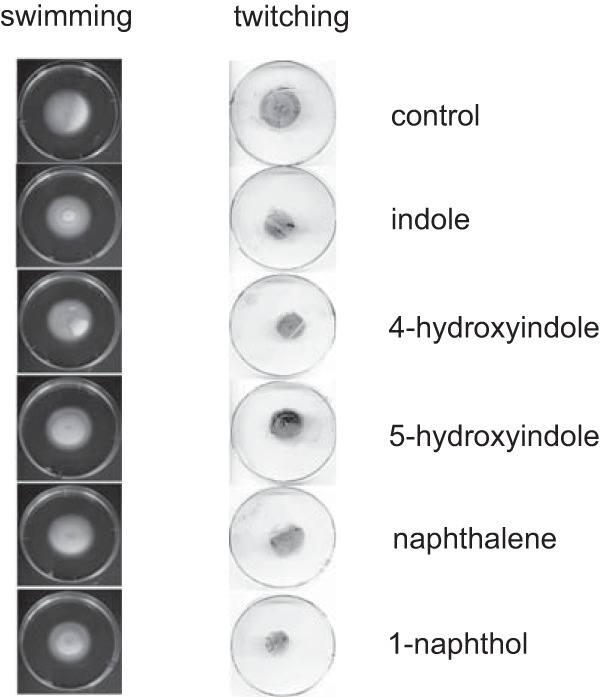
Effects of bicyclic compounds on swimming motility (left) and twitching motility (right) in P. aeruginosa PAO1. Each compound was added at a concentration of 500 μM. Three independent experiments were performed, and representative data are shown.
Inhibition of swarming motility in clinical isolates of P. aeruginosa.
Hyperswarmers, which naturally emerge inside biofilms originally developed from normal swarming cells, have been observed in P. aeruginosa (45). To determine whether the effect of 1-naphthol on swarming motility is relevant for other P. aeruginosa cells, we isolated clinical strains of P. aeruginosa, including hyperswarming strains, from patients with urinary tract infections (isolate numbers 227, 233, 287, 401, 428, and 433). The results of the swarming assay showed that both 287 and 401 exhibited hyperswarming motility compared with the motility of PAO1, and 500 μM 1-naphthol completely inhibited the swarming motility of these clinical isolates (Fig. 3). Notably, treatment with 500 μM 1-naphthol did not inhibit the growth of these clinical isolates (data not shown). These results suggest that 1-naphthol could be used as a strong inhibitor against swarming motility in P. aeruginosa strains with hyperswarming phenotypes.
FIG 3.
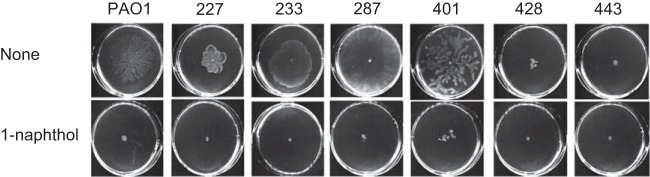
Effects of 1-naphthol on swarming motility of various clinical isolates of P. aeruginosa. The cells were inoculated at the center a plate containing M9 medium and 0.5% agar with or without 500 μM 1-naphthol and incubated for 12 h at 30°C. Three independent experiments were performed, and representative data are shown.
Repellent effect of 1-naphthol on swarming cells.
To further evaluate whether 1-naphthol affects bacterial cells exhibiting swarming motility, filter paper discs were soaked with various concentrations of 1-naphthol solution (0 to 5 mM) and subsequently placed onto 0.5% agar plates inoculated with P. aeruginosa. When swarming cells approached the discs, an altered course of direction and migration around the discs soaked with more than 50 μM 1-naphthol was observed (Fig. 4), suggesting that 1-naphthol acts as a repellent.
FIG 4.
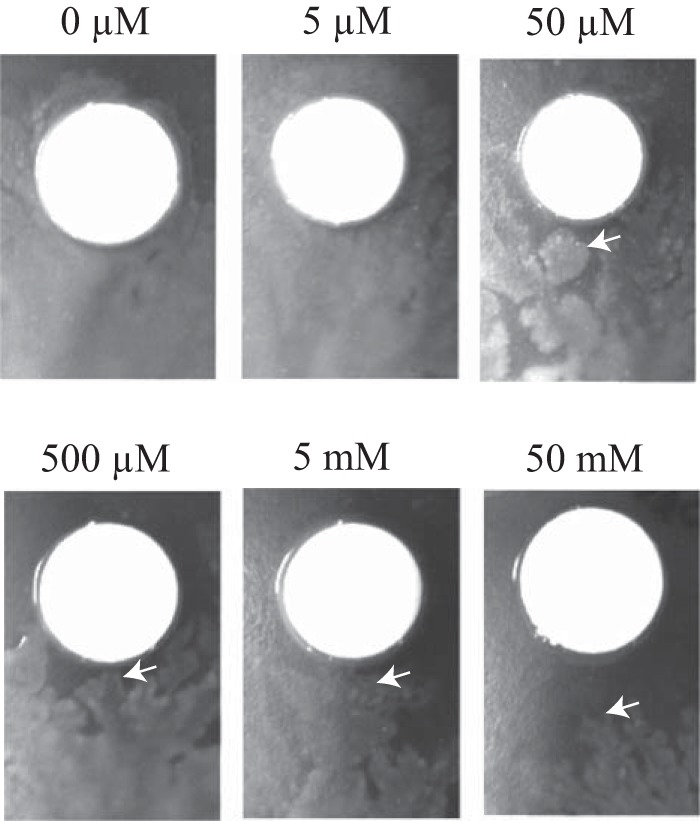
Effects of 1-naphthol on swarming cells. The filter paper discs were soaked with various concentrations of 1-naphthol (0 to 50 mM) and placed onto M9 medium containing 0.5% agar, followed by inoculation with P. aeruginosa. White arrows indicate edges of swarming cells around filter paper discs.
Inhibition of biofilm dispersion.
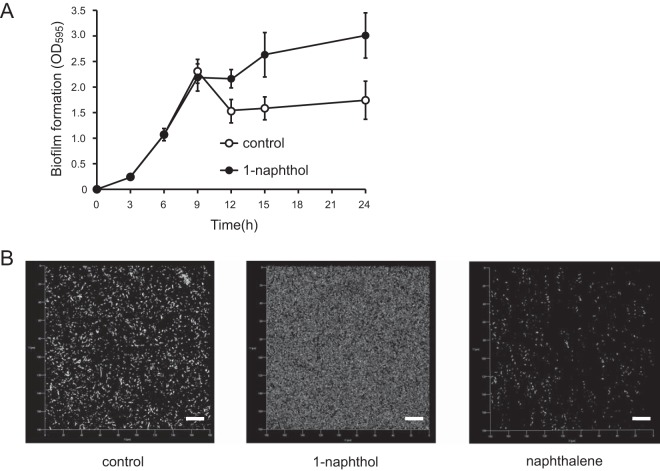
Biofilm development is a sequential process involving planktonic cell attachment, microcolony formation, biofilm maturation, and subsequent biofilm dispersion. Several studies have previously demonstrated that swarming motility is required for biofilm dispersion (45, 46), and it is assumed that 1-naphthol inhibits the dispersion of biofilm. To confirm this hypothesis, the time course of biofilm formation with and without 1-naphthol was examined using crystal violet staining. The results showed that the addition of 1-naphthol inhibited the dispersion of the matured biofilm, whereas the control biofilm dispersed after 9 h (Fig. 5A). CLSM analysis revealed that 1-naphthol inhibited the dispersal of 12-h biofilm, whereas in the absence of 1-naphthol, the control biofilm was dispersed (Fig. 5B). The biofilm quantification analysis using COMSTAT indicated that the addition of 1-naphthol increased biofilm biomass 15-fold compared with the control (0.3 ± 0.02 versus 0.02 ± 0.005 μm3/μm2). Additionally, exposure to naphthalene as a negative control (no inhibition of swarming) did not inhibit the biofilm dispersal, which was similar to that of the control. These results indicate that 1-naphthol inhibits the dispersion of biofilm in P. aeruginosa.
FIG 5.
Effects of 1-naphthol on biofilm dispersion in P. aeruginosa. (A) Effect of 1-naphthol at 6 h on biofilm dispersion in PAO1. Biofilms attached to the wells were quantified at each time point. More than three independent experiments were performed, and the data are presented as means ± standard deviations of triplicate assays. (B) Visualization of biofilm at 12 h through CLSM and the quantification of biomass using the COMSTAT program. Scale bar, 20 μm. Three independent experiments were performed, and representative data are shown.
Rhamnolipid production.
Extracellular amphiphilic biosurfactant rhamnolipids promote swarming motility (47), and it is assumed that the inhibition of rhamnolipid production could be the mechanism underlying the inhibition of swarming motility through 1-naphthol. Rhamnolipid production was examined by orcinol assay, and the result showed A420/OD660 values of 0.38 ± 0.13 and 0.33 ± 0.06 in the absence and presence of 1-naphthol, respectively, suggesting that rhamnolipid production was not changed by 1-naphthol. The rhlR mutant, used as a negative control in this assay, did not produce rhamnolipid (A420/OD660, 0.02 ± 0.04). To further investigate the effect of 1-naphthol on rhamnolipid production, the expression of the rhamnolipid synthetic gene rhlA was examined by qRT-PCR. The expression of rhlA in the presence of 1-naphthol was 1.3-fold (± 0.3-fold) that of expression in its absence. These results suggest that the swarming inhibition through 1-naphthol does not result from defects in rhamnolipid production.
Intracellular level of c-di-GMP.
It has also been reported that several amino acids, such as l-arginine, inhibited swarming motility in P. aeruginosa through the enhancement of intracellular c-di-GMP (12). Intracellular levels of c-di-GMP inversely affect swarming motility and biofilm formation (48). Since 1-naphthol inhibited swarming motility and maintained the amount of biofilm formation at later stages of development in P. aeruginosa, it is assumed that the intracellular c-di-GMP level is increased by 1-naphthol. A fluorescence-based reporter plasmid was constructed by reference to a previous report (38), and the intracellular c-di-GMP levels were examined in the absence and presence of 1-naphthol. The level of c-di-GMP with 1-naphthol was 0.79-fold ± 0.54-fold that of the level without it, suggesting that the inhibitive effect of 1-naphthol on swarming is not associated with the intracellular c-di-GMP level.
Whole transcriptional analysis with 1-naphthol.
To reveal the genetic mechanism of swarming inhibition through 1-naphthol on a global basis, we performed DNA microarray analysis using exponential cells grown in minimal medium. The expression of 119 genes was significantly affected more than 2-fold upon the addition of 1-naphthol; 18 genes were upregulated (Table 2), and 101 genes were downregulated (Table 3). Notably, multidrug efflux transporter genes (mexAB and mexF) and fluorescent siderophore pyoverdine synthesis-related genes (pvdQ, pvdT, pvdN, and pvdO) were highly expressed (Table 2). The nalC gene (Table 2), a positive regulator of the efflux pump MexAB-OprM (49), showed the highest upregulation with 1-naphthol.
TABLE 2.
List of the genes upregulated with 500 μM naphthol in P. aeruginosa PAO1
| Gene function and locus no. | Gene name | Fold change | Product or function |
|---|---|---|---|
| Multidrug efflux transporter | |||
| PA0425 | mexA | 2.2 | Multidrug efflux membrane fusion protein precursor |
| PA0427 | mexB | 2.7 | Multidrug efflux transporter |
| PA2494 | mexF | 2.3 | Multidrug efflux transporter |
| PA3719 | armA | 2.7 | Antirepressor for MexR |
| Pyoverdine synthesis | |||
| PA2385 | pvdQ | 2.7 | Pyoverdine biosynthesis protein |
| PA2390 | pvdT | 2.0 | Probable ATP-binding/permease fusion ABC transporter |
| PA2391 | opmQ | 2.0 | Probable outer membrane protein precursor |
| PA2394 | pvdN | 2.3 | Pyoverdine biosynthesis protein |
| PA2395 | pvdO | 2.0 | Pyoverdine biosynthesis protein |
| Other | |||
| PA0594 | surA | 2.2 | Peptidyl-prolyl cis-trans isomerase |
| PA3006 | psrA | 2.4 | Transcriptional regulator |
| PA3014 | faoA | 2.3 | Fatty-acid oxidation complex alpha-subunit |
| PA3721 | nalC | 4.3 | Transcriptional regulator |
| PA3866 | 2.1 | Pyocin protein | |
| PA4710 | phuR | 2.0 | Heme/hemoglobin uptake outer membrane receptor precursor |
| PA5323 | argB | 2.0 | Acetylglutamate kinase |
| Hypothetical proteins | |||
| PA3229 | 3.1 | Hypothetical protein | |
| PA3720 | 4.0 | Hypothetical protein |
TABLE 3.
List of the genes downregulated with 500 μM naphthol in P. aeruginosa PAO1
| Gene function and locus no. | Gene name | Fold change | Product or functiona |
|---|---|---|---|
| Type VI protein secretion system | |||
| PA0263 | hcpC | −5.6 | Type VI protein secretion system component |
| PA1512 | hcpA | −6.3 | Type VI protein secretion system component |
| PA1656 | hsiA2 | −2.8 | Predicted component of the type VI protein secretion system |
| PA1657 | hsiB2 | −4.0 | Predicted component of the type VI protein secretion system |
| PA1658 | hsiC2 | −4.2 | Predicted component of the type VI protein secretion system |
| PA1659 | hsiC2 | −2.8 | Predicted component of the type VI protein secretion system |
| PA1660 | hsiG2 | −2.0 | Predicted component of the type VI protein secretion system |
| PA1666 | lip2 | −2.3 | Predicted component of the type VI protein secretion system |
| PA5267 | hcpB | −5.9 | Type VI protein secretion system component |
| Pyochelin synthesis | |||
| PA4224 | pchG | −3.2 | Pyocherin biosynthesis protein |
| PA4225 | pchF | −3.0 | Pyocherin biosynthesis protein |
| PA4226 | pchE | −2.7 | Dihydroaeruginoic acid synthesis |
| PA4228 | pchD | −3.0 | Pyocherin biosynthesis protein |
| PA4229 | pchC | −2.2 | Pyocherin biosynthesis protein |
| PA4230 | pchB | −2.5 | Salicylate biosynthesis protein |
| PA4231 | pchA | −2.0 | Salicylate biosynthesis isochorismate synthase |
| Metabolism | |||
| PA0895 | aruC | −2.0 | N-Succinylglutamate 5-semialdehyde dehydrogenase |
| PA1562 | acnA | −2.1 | Aconitate hydratase |
| PA1984 | exaC | −2.2 | Probable aldehyde dehydrogenase |
| PA2007 | maiA | −3.6 | Maleylacetoacetate isomerase |
| PA2008 | fahA | −3.6 | Fumarylacetoacetase |
| PA2009 | hmgA | −4.5 | Homogentisate 1,2-dioxygenase |
| PA2014 | liuB | −2.9 | Methylcrotonyl-CoA carboxylase |
| PA2015 | liuA | −5.0 | Putative isovaleryl-CoA dehydrogenase |
| PA2016 | liuR | −2.9 | Regulator of liu genes |
| PA2247 | bkdA1 | −2.1 | 2-Oxoisovalerate dehydrogenase |
| PA2248 | bkdA2 | −2.4 | 2-Oxoisovalerate dehydrogenase |
| PA2249 | bkdB | −3.1 | Branched-chain alpha-keto acid dehydrogenase |
| PA2250 | lpdV | −2.6 | Lipoamide dehydrogenase |
| PA2321 | −2.1 | Gluconokinase | |
| PA2554 | −2.5 | Probable short-chain dehydrogenase | |
| PA2555 | −4.0 | Probable AMP-binding enzyme | |
| PA3183 | zwf | −3.8 | Glucose-6-phosphate 1-dehydrogenase |
| PA3194 | edd | −2.4 | Phosphogluconate dehydratase |
| PA3195 | gapA | −2.9 | Glyceraldehyde 3-phosphate dehydrogenase |
| PA3559 | −2.3 | Probable nucleotide sugar dehydrogenase | |
| PA3584 | glpD | −3.6 | Glycerol-3-phosphate dehydrogenase |
| PA3723 | yqjM | −2.1 | Probable FMN oxidoreductase |
| PA3924 | −3.4 | Probable medium-chain acyl-CoA ligase | |
| PA4022 | hdhA | −2.2 | Hydrazone dehydrogenase |
| PA5056 | phaC1 | −2.3 | Poly(3-hydroxyalkanoic acid) synthase |
| PA5435 | −3.4 | Probable transcarboxylase subunit | |
| PA5436 | −2.9 | Probable biotin carboxylase subunit of a transcarboxylase | |
| Cell motility and secretion | |||
| PA0176 | aer2 | −2.0 | Aerotaxis transducer |
| PA0178 | −2.4 | Probable two-component sensor | |
| PA0179 | −2.1 | Probable two-component response regulator | |
| PA1078 | flgC | −2.0 | Flagellar basal-body rod protein |
| PA1079 | flgD | −2.4 | Flagellar basal-body rod modification protein |
| PA1082 | flgG | −2.3 | Flagellar basal-body rod protein |
| PA1561 | aer | −2.7 | Aerotaxis receptor |
| PA2573 | −2.1 | Probable chemotaxis transducer | |
| PA2788 | −4.1 | Probable chemotaxis transducer | |
| PA2867 | −2.2 | Chemotaxis transducer | |
| PA4309 | pctA | −2.9 | Chemotaxis transducer |
| PA4310 | pctB | −1.8 | Chemotaxis transducer |
| PA4525 | pilA | −2.0 | Type 4 fimbrial precursor |
| PA4526 | pilB | −3.0 | Type 4 fimbrial biogenesis protein |
| PA4663 | −2.3 | Probable chemotaxis transducer | |
| Transport of small molecules | |||
| PA1183 | dctA | −5.3 | C4-dicarboxylate transport protein |
| PA1342 | −2.3 | Probable binding protein component of ABC transporter | |
| PA1875 | −2.7 | Probable outer membrane protein precursor | |
| PA1876 | −2.1 | Probable ATP-binding permeate fusion ABC transporter | |
| PA4221 | fptA | −2.6 | Fe(III)-pyochelin outer membrane receptor precursor |
| PA4222 | −2.5 | Probable ATP-binding component of ABC transporter | |
| PA4223 | −2.9 | Probable ATP-binding component of ABC transporter | |
| PA4359 | −5.6 | Probable ferrous iron transport protein | |
| PA5152 | −2.5 | Probable ATP-binding component of ABC transporter | |
| PA5167 | dctP | −2.1 | Probable C4-dicarboxylate-binding protein |
| Other | |||
| PA0865 | hpd | −10.0 | 4-Hydroxyphenylpyruvate dioxygenase |
| PA1546 | hemN | −2.1 | Oxygen-independent coproporphyrinogen III oxidase |
| PA2570 | lecA | −2.6 | Lectin |
| PA2897 | −2.6 | Probable transcriptional regulator | |
| PA3225 | −2.3 | Probable transcriptional regulator | |
| PA3552 | arnB | −2.6 | ArnB |
| PA4776 | pmrA | −2.1 | Two-component regulator system response regulator |
| PA5100 | hutA | −4.3 | Urocanase |
| Hypothetical proteins | |||
| PA1095 | −2.1 | Hypothetical protein | |
| PA1096 | −2.3 | Hypothetical protein | |
| PA1404 | −2.0 | Hypothetical protein | |
| PA1559 | −2.3 | Hypothetical protein | |
| PA1679 | −2.3 | Hypothetical protein | |
| PA1874 | −2.6 | Hypothetical protein | |
| PA1914 | −3.4 | Conserved hypothetical protein | |
| PA3369 | −3.3 | Hypothetical protein | |
| PA3370 | −3.0 | Hypothetical protein | |
| PA3371 | −2.9 | Hypothetical protein | |
| PA3488 | −3.6 | Hypothetical protein | |
| PA3520 | −2.4 | Hypothetical protein | |
| PA3601 | −2.9 | Conserved hypothetical protein | |
| PA3919 | −2.5 | Conserved hypothetical protein | |
| PA3923 | −4.0 | Hypothetical protein | |
| PA3969 | −2.0 | Conserved hypothetical protein | |
| PA4063 | −3.8 | Hypothetical protein | |
| PA4115 | −2.3 | Conserved hypothetical protein | |
| PA4155 | −3.6 | Hypothetical protein | |
| PA4220 | −2.0 | Hypothetical protein | |
| PA4648 | −2.1 | Hypothetical protein | |
| PA4773 | −4.2 | Hypothetical protein | |
| PA4774 | −2.8 | Hypothetical protein | |
| PA4916 | −2.0 | Hypothetical protein | |
| PA5460 | −2.9 | Hypothetical protein | |
| PA5481 | −2.6 | Hypothetical protein |
CoA, coenzyme A; FMN, flavin mononucleotide.
The expression of various genes was repressed through 1-naphthol (Table 3). Nine genes related to the type VI protein secretion system were repressed more than 2-fold. Particularly, hcpA, hcpB, and hcpC were significantly repressed with 1-naphthol, showing 6.3-, 5.9-, and 5.6-fold repression, respectively. The pyochelin synthesis operon (pch) was repressed through 1-naphthol. Although 1-naphthol represses PQS synthesis in rich medium (22), the PQS synthesis operon (pqs) and PQS-regulated virulence-coding genes, including the pyocyanin synthesis operon (pch), were not repressed in minimal medium, with the exception of pyochelin. Flagellar-related genes (flgC, flgD, and flgG), aerotaxis-related genes (aer and aer2), type IV pilus-related genes (pilA and pilB), and several chemotaxis transducer-coding genes were repressed (2- to 4-fold). Many of the genes associated with the metabolism and transport of small molecules were repressed.
Transcriptional analysis through qRT-PCR with 1-naphthol or 4-HI.
To verify the results of the microarray and investigate the effects of 4HI on gene expression, qRT-PCR analysis was conducted (Fig. 6). The expression of both mexB and nalC was increased in the presence of not only 1-naphthol but also 4HI. The expression of the pyochelin synthesis gene pchG was significantly inhibited with either 1-naphthol or 4HI (Fig. 6). Swarming motility requires both flagella and pili, and the qRT-PCR analysis showed that the expression of the related genes was partially repressed through 1-naphthol and 4HI: 0.58-fold (flgG) and 0.56-fold (pilB) with 1-naphthol and 0.61-fold (flgG) and 0.76-fold (pilB) with 4HI. The expression of aer was also repressed with 1-naphthol and 4HI (0.68- and 0.65-fold, respectively).
FIG 6.
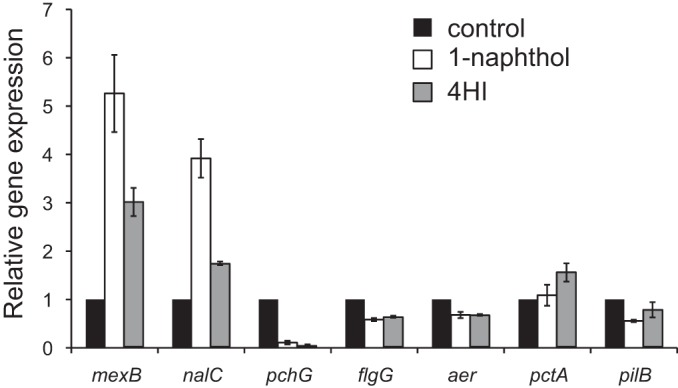
Gene expression of mexB, nalC, pchG, flgG, aer, pctA, and pilB with or without 1-naphthol and 4-hydroxyindole. Quantitative real-time RT-PCR analysis of the transcript levels in cells at the exponential phase was performed. The values are compared to the control (0.5% DMSO). The data are expressed as means ± standard deviations (error bars) from triplicate samples.
The effect of 1-naphthol on resistance.
MexAB-OprM contributes to the intrinsic resistance of bacteria to a number of antibiotics, including fluoroquinolones, β-lactams, tetracycline, macrolides, chloramphenicol, novobiocin, trimethoprim, and sulfonamides (50). As 1-naphthol increased the expression of mexA and mexB (Table 3), we hypothesized that 1-naphthol would also affect the resistance of bacteria to antibiotics. When MICs were examined, the addition of 1-naphthol increased resistance against tetracycline and the fluoroquinolone ofloxacin. MICs for tetracycline in the absence and presence of 500 μM 1-naphthol were 64 and 128 μg/ml, respectively, and these values for ofloxacin were 8 and 16 μg/ml, respectively. The resistance to the aminoglycoside gentamicin was not affected by MexAB-OprM, and the MIC (64 μg/ml) was not changed after the addition of 500 μM 1-naphthol. These results suggest that the presence of 500 μM 1-naphthol increased MexAB-OprM-related antibiotic resistance. Furthermore, to determine whether MexAB-OprM is associated with 1-naphthol efflux, the growth of wild-type (WT) and ΔmexAB-oprM bacteria was compared under several concentrations of 1-naphthol. The WT bacteria grew well, but the ΔmexAB-oprM strain did not grow in the presence of 1,250 μM 1-naphthol, suggesting that MexAB-OprM is associated with resistance to 1-naphthol.
DISCUSSION
Swarming is significantly associated with various phenotypes of P. aeruginosa; swarming cells are more resistant to antibiotics, and virulence factor expression is higher than that in planktonic cells (8, 9). Therefore, the inhibition of swarming motility is significantly important to control the behavior of P. aeruginosa. In the present study, we revealed that hydroxyindoles and hydroxynaphthalenes drastically inhibit the swarming motility of P. aeruginosa without affecting growth. Particularly, 1-naphthol exhibits a strong inhibitory effect against swarming motility in various strains, such as hyperswarming clinical isolates.
We previously reported that various bicyclic compounds (indole, naphthalene, and their derivatives) repress PQS synthesis and PQS-regulated phenotypes, including membrane vesicle production and pyocyanin synthesis (22). The detailed mechanism underlying this effect remains unknown, but the inhibition through indole does not involve the binding of receptor PqsR to PQS. Here, we showed that some bicyclic compounds repress swarming motility in P. aeruginosa. Interestingly, indole and naphthalene did not affect swarming motility, while the hydric compounds of these molecules successfully repressed this activity (Fig. 1A). Lee et al. reported that indole repressed swarming motility (21), and this discrepancy might reflect the use of different media. These authors also showed that 7-hydroxyindole abolished swarming motility, and this result is consistent with the results of the present study showing that the hydric groups of bicyclic compounds strengthen inhibitory activity against P. aeruginosa swarming. Notably, the potency of swarming inhibition is different between the naphthalene derivatives 1-naphthol and 2-naphthol (Fig. 1A), suggesting that the presence and structural location of the hydroxyl groups are important to inhibit P. aeruginosa swarming motility.
Swarming motility is controlled through Rhl (32, 51), and Rhl-regulated rhamnolipid synthesis is required for swarming motility in P. aeruginosa (45, 48). In this study, we showed that rhamnolipid production was equivalent with or without 1-naphthol, using colorimetric analysis and qRT-PCR analysis, suggesting that the inhibitive effect of 1-naphthol on swarming does not result from defects in rhamnolipid production. The decreased intracellular level of c-di-GMP is also an important factor in swarming motility behavior in P. aeruginosa (52). A plasmid-based reporter analysis showed that intracellular level of c-di-GMP was not altered by 1-naphthol. The synthesis of c-di-GMP from two molecules of GTP is catalyzed by diguanylate cyclases (DGCs), and the breakdown of c-di-GMP is catalyzed by phosphodiesterases (PDEs) (53). We also confirmed that expression of genes encoding neither DGCs nor PDEs was altered by 1-naphthol in microarray analysis. Hence, it is suggested that swarming inhibition by 1-naphthol is independent of changes in the intracellular level of c-di-GMP. Furthermore, it has been reported that PQS and phenazine-1-carboxylic acid (PCA), a precursor of pyocyanin whose synthesis is positively regulated by PQS, repress swarming motility via a pathway different from that of c-di-GMP (51). 1-Naphthol repressed PQS and pyocyanin production in LB medium (22), whereas genes coding for the synthesis of PQS, PCA, and pyocyanin were not altered, based on microarray analysis in minimal medium, suggesting that swarming inhibition by 1-naphthol does not alter PCA synthesis. However, little is known about the downstream mechanism to repress swarming motility by PCA as well as 1-naphthol. Thus, our data indicated that the inhibitive effect of 1-naphthol on swarming motility in minimal medium is via a yet known mechanism independent of rhamnolipid production and c-di-GMP level; whether the inhibitory effect of 1-naphthol is linked with the mechanism of PCA-dependent swarming repression remains unknown.
Microarray analysis showed that the expression of flagellar-related protein synthesis genes, including flgC, flgD, and flgG, was repressed with 1-naphthol (Table 2). Although both swimming and swarming motility require flagellum-driven motility, 1-naphthol significantly inhibited swarming motility but did not affect swimming motility in P. aeruginosa, suggesting that something other than the flagella greatly contributes to the inhibition of swarming through 1-naphthol. Expression levels of pilA and pilB, required for both swarming and twitching motility, were repressed through 1-naphthol, and twitching motility was slightly repressed by 1-naphthol. The repression of pilus synthesis by 1-naphthol is considered to be one of the means by which it represses swarming motility, but this cannot be the complete explanation of the mechanism that represses swarming motility. The synergetic effect of pilus synthesis repression with a yet unknown mechanism probably causes the inhibition of swarming by 1-naphthol in P. aeruginosa.
Interestingly, the resistance to tetracycline and ofloxacin increases the response to 1-naphthol, probably due to upregulation of mexAB expression. Although it has been reported that swarming cells develop antibiotic resistance (8, 9), little is understood about the effect of efflux pump overexpression on swarming motility. Therefore, it remains unknown whether the inhibitory effect of 1-naphthol on swarming motility is due to upregulation of mexAB. Because the resistance to 1-naphthol decreased in the ΔmexAB-oprM strain compared to that in the WT strain, MexAB-OprM may be associated with the efflux of 1-naphthol in response to 1-naphthol in the milieu. Hence, the resistance to tetracycline and ofloxacin may be the side effect of eliminating 1-naphthol from cells.
In conclusion, the results of the present study revealed that hydroxyindoles and naphthalene derivatives influence the social behavior and swarming motility of P. aeruginosa. While naphthalene derivatives are ubiquitous in natural environments, information about the effects of these compounds on bacteria is scarce. Thus, the results of the present study could provide a better understanding of the bacterial response to these compounds and contribute useful information for the design of novel agents to control the phenotypes of the opportunistic pathogen P. aeruginosa.
ACKNOWLEDGMENTS
We thank Arne Heydorn for kindly providing the COMSTAT program and Reiko Kariyama and Hiromi Kumon for kindly providing clinical isolates.
This study was supported in part through funding from Grant-in-aid for Scientific Research (25241020) to N.N. from the Ministry of Education, Culture, Sports, and Technology of Japan. This research was also funded through the Japan Science and Technology Agency, CREST, and ALCA.
REFERENCES
- 1.Costerton J, Stewart P, Greenberg E. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Tashiro Y, Yawata Y, Toyofuku M, Uchiyama H, Nomura N. 2013. Interspecies interaction between Pseudomonas aeruginosa and other microorganisms. Microbes Environ 28:13–24. doi: 10.1264/jsme2.ME12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Toole G, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 4.Shrout JD, Chopp DL, Just CL, Hentzer M, Givskov M, Parsek MR. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol Microbiol 62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 5.Fraser GM, Hughes C. 1999. Swarming motility. Curr Opin Microbiol 2:630–635. doi: 10.1016/S1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 6.Harshey RM. 2003. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol 57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 7.Daniels R, Vanderleyden J, Michiels J. 2004. Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev 28:261–289. doi: 10.1016/j.femsre.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Lai S, Tremblay J, Déziel E. 2009. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ Microbiol 11:126–136. doi: 10.1111/j.1462-2920.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 9.Overhage J, Bains M, Brazas MD, Hancock RE. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol 190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Overhage J, Lewenza S, Marr AK, Hancock RE. 2007. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J Bacteriol 189:2164–2169. doi: 10.1128/JB.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tremblay J, Deziel E. 2010. Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics 11:587. doi: 10.1186/1471-2164-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier SP, Ha D-G, Khan W, Merritt JH, O'Toole GA. 2011. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol 162:680–688. doi: 10.1016/j.resmic.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Shingaki R, Fukui K. 2008. Inhibition of swarming motility of Pseudomonas aeruginosa by branched-chain fatty acids. FEMS Microbiol Lett 281:81–86. doi: 10.1111/j.1574-6968.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 14.O'May C, Tufenkji N. 2011. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol 77:3061–3067. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesci E, Milbank J, Pearson J, McKnight S, Kende A, Greenberg E, Iglewski B. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 17.Tashiro Y, Uchiyama H, Nomura N. 2012. Multifunctional membrane vesicles in Pseudomonas aeruginosa. Environ Microbiol 14:1349–1362. doi: 10.1111/j.1462-2920.2011.02632.x. [DOI] [PubMed] [Google Scholar]
- 18.Dubern JF, Diggle SP. 2008. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst 4:882–888. doi: 10.1039/b803796p. [DOI] [PubMed] [Google Scholar]
- 19.Cugini C, Calfee MW, Farrow JM III, Morales DK, Pesci EC, Hogan DA. 2007. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa. Mol Microbiol 65:896–906. doi: 10.1111/j.1365-2958.2007.05840.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Ding X, Rather P. 2001. Indole can act as an extracellular signal in Escherichia coli. J Bacteriol 183:4210–4216. doi: 10.1128/JB.183.14.4210-4216.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Attila C, Cirillo S, Cirillo J, Wood T. 2009. Indole and 7-hydroxyindole diminish Pseudomonas aeruginosa virulence. Microbial Biotechnol 2:75–90. doi: 10.1111/j.1751-7915.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tashiro Y, Toyofuku M, Nakajima-Kambe T, Uchiyama H, Nomura N. 2010. Bicyclic compounds repress membrane vesicle production and Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. FEMS Microbiol Lett 304:123–130. doi: 10.1111/j.1574-6968.2010.01897.x. [DOI] [PubMed] [Google Scholar]
- 23.Ensley B, Ratzkin B, Osslund T, Simon M, Wackett L, Gibson D. 1983. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 222:167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- 24.Rui L, Reardon K, Wood T. 2005. Protein engineering of toluene ortho-monooxygenase of Burkholderia cepacia G4 for regiospecific hydroxylation of indole to form various indigoid compounds. Appl Microbiol Biotechnol 66:422–429. doi: 10.1007/s00253-004-1698-z. [DOI] [PubMed] [Google Scholar]
- 25.Samanta SK, Chakraborti AK, Jain RK. 1999. Degradation of phenanthrene by different bacteria: evidence for novel transformation sequences involving the formation of 1-naphthol. Appl Microbiol Biotechnol 53:98–107. doi: 10.1007/s002530051621. [DOI] [PubMed] [Google Scholar]
- 26.Garikipati SVBJ, McIver AM, Peeples TL. 2009. Whole-cell biocatalysis for 1-naphthol production in liquid-liquid biphasic systems. Appl Environ Microbiol 75:6545–6552. doi: 10.1128/AEM.00434-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holloway BW, Krishnapillai V, Morgan AF. 1979. Chromosomal genetics of Pseudomonas. Microbiol Rev 43:73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. 2004. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob Agents Chemother 48:1320–1328. doi: 10.1128/AAC.48.4.1320-1328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyofuku M, Nomura N, Fujii T, Takaya N, Maseda H, Sawada I, Nakajima T, Uchiyama H. 2007. Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J Bacteriol 189:4969–4972. doi: 10.1128/JB.00289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tashiro Y, Nomura N, Nakao R, Senpuku H, Kariyama R, Kumon H, Kosono S, Watanabe H, Nakajima T, Uchiyama H. 2008. Opr86 is essential for viability and is a potential candidate for a protective antigen against biofilm formation by Pseudomonas aeruginosa. J Bacteriol 190:3969–3978. doi: 10.1128/JB.02004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay J, Richardson A-P, Lépine F, Déziel E. 2007. Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environ Microbiol 9:2622–2630. doi: 10.1111/j.1462-2920.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 32.Köhler T, Curty LK, Barja F, van Delden C, Pechère J-C. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182:5990–5996. doi: 10.1128/JB.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tashiro Y, Inagaki A, Ono K, Inaba T, Yawata Y, Uchiyama H, Nomura N. 2014. Low concentrations of ethanol stimulate biofilm and pellicle formation in Pseudomonas aeruginosa. Biosci Biotechnol Biochem 78:178–181. doi: 10.1080/09168451.2014.877828. [DOI] [PubMed] [Google Scholar]
- 34.Yawata Y, Nomura N, Uchiyama H. 2008. Development of a novel biofilm continuous culture method for simultaneous assessment of architecture and gaseous metabolite production. Appl Environ Microbiol 74:5429–5435. doi: 10.1128/AEM.00801-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407. [DOI] [PubMed] [Google Scholar]
- 36.Koch AK, Käppeli O, Fiechter A, Reiser J. 1991. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J Bacteriol 173:4212–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ochsner UA, Fiechter A, Reiser J. 1994. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem 269:19787–19795. [PubMed] [Google Scholar]
- 38.Rybtke MT, Borlee BR, Murakami K, Irie Y, Hentzer M, Nielsen TE, Givskov M, Parsek MR, Tolker-Nielsen T. 2012. Fluorescence-based reporter for gauging cyclic di-GMP levels in Pseudomonas aeruginosa. Appl Environ Microbiol 78:5060–5069. doi: 10.1128/AEM.00414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyofuku M, Zhou S, Sawada I, Takaya N, Uchiyama H, Nomura N. 2014. Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environ Microbiol 16:2927–2938. doi: 10.1111/1462-2920.12260. [DOI] [PubMed] [Google Scholar]
- 40.Shintani M, Takahashi Y, Tokumaru H, Kadota K, Hara H, Miyakoshi M, Naito K, Yamane H, Nishida H, Nojiri H. 2010. Response of the Pseudomonas host chromosomal transcriptome to carriage of the IncP-7 plasmid pCAR1. Environ Microbiol 12:1413–1426. doi: 10.1111/j.1462-2920.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 41.Miyakoshi M, Nishida H, Shintani M, Yamane H, Nojiri H. 2009. High-resolution mapping of plasmid transcriptomes in different host bacteria. BMC Genomics 10:12. doi: 10.1186/1471-2164-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi Y, Shintani M, Takase N, Kazo Y, Kawamura F, Hara H, Nishida H, Okada K, Yamane H, Nojiri H. 2015. Modulation of primary cell function of host Pseudomonas bacteria by the conjugative plasmid pCAR1. Environ Microbiol 17:134–155. doi: 10.1111/1462-2920.12515. [DOI] [PubMed] [Google Scholar]
- 43.Tashiro Y, Sakai R, Toyofuku M, Sawada I, Nakajima-Kambe T, Uchiyama H, Nomura N. 2009. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J Bacteriol 191:7509–7519. doi: 10.1128/JB.00722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savli H, Karadenizli A, Kolayli F, Gundes S, Ozbek U, Vahaboglu H. 2003. Expression stability of six housekeeping genes: a proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J Med Microbiol 52:403–408. doi: 10.1099/jmm.0.05132-0. [DOI] [PubMed] [Google Scholar]
- 45.Boles BR, Thoendel M, Singh PK. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57:1210–1223. doi: 10.1111/j.1365-2958.2005.04743.x. [DOI] [PubMed] [Google Scholar]
- 46.Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S. 2009. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol 191:7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Déziel E, Lépine F, Milot S, Villemur R. 2003. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- 48.Caiazza NC, Merritt JH, Brothers KM, O'Toole GA. 2007. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao L, Srikumar R, Poole K. 2004. MexAB-OprM hyperexpression in NalC-type multidrug-resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol Microbiol 53:1423–1436. doi: 10.1111/j.1365-2958.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- 50.Schweizer H. 2003. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet Mol Res 2:48–62. [PubMed] [Google Scholar]
- 51.Ha D-G, Merritt JH, Hampton TH, Hodgkinson JT, Janecek M, Spring DR, Welch M, O'Toole GA. 2011. 2-Heptyl-4-quinolone, a precursor of the Pseudomonas quinolone signal molecule, modulates swarming motility in Pseudomonas aeruginosa. J Bacteriol 193:6770–6780. doi: 10.1128/JB.05929-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, O'Toole GA. 2007. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]