Abstract
Nutrient germination of spores of Bacillus species occurs through germinant receptors (GRs) in spores' inner membrane (IM) in a process stimulated by sublethal heat activation. Bacillus subtilis spores maximum germination rates via different GRs required different 75°C heat activation times: 15 min for l-valine germination via the GerA GR and 4 h for germination with the l-asparagine–glucose–fructose–K+ mixture via the GerB and GerK GRs, with GerK requiring the most heat activation. In some cases, optimal heat activation decreased nutrient concentrations for half-maximal germination rates. Germination of spores via various GRs by high pressure (HP) of 150 MPa exhibited heat activation requirements similar to those of nutrient germination, and the loss of the GerD protein, required for optimal GR function, did not eliminate heat activation requirements for maximal germination rates. These results are consistent with heat activation acting primarily on GRs. However, (i) heat activation had no effects on GR or GerD protein conformation, as probed by biotinylation by an external reagent; (ii) spores prepared at low and high temperatures that affect spores' IM properties exhibited large differences in heat activation requirements for nutrient germination; and (iii) spore germination by 550 MPa of HP was also affected by heat activation, but the effects were relatively GR independent. The last results are consistent with heat activation affecting spores' IM and only indirectly affecting GRs. The 150- and 550-MPa HP germinations of Bacillus amyloliquefaciens spores, a potential surrogate for Clostridium botulinum spores in HP treatments of foods, were also stimulated by heat activation.
INTRODUCTION
Spores of Bacillus species can remain dormant for long periods in the absence of suitable growth conditions (1, 2). However, if specific nutrients are sensed, spores can rapidly become metabolically active in the process of germination followed by outgrowth. The specific nutrients that trigger spore germination are termed germinants, and these molecules are sensed by germinant receptors (GRs) located in spores' inner membrane (IM). Bacillus subtilis spores have three functional GRs: GerA, which responds to l-alanine or l-valine alone, and GerB and GerK, which together are essential for germination with a mixture of l-asparagine, d-glucose, d-fructose, and K+ (termed AGFK), with all four components of the mixture required; neither GerB nor GerK alone triggers germination with any nutrient germinant (1, 3). There is also a variant of the GerB GR, termed GerB*, that responds to l-asparagine alone, although GerB* action can be stimulated by glucose via GerK (3). All GRs in B. subtilis spores appear to be located together in a small cluster in the IM termed the germinosome, and formation of this structure is dependent on the GerD protein, which is also in the IM (2, 4). Since gerD spores do not form a germinosome and exhibit extremely slow GR-dependent germination (4), germinosome formation may be essential for rapid GR-dependent germination. GR function and germinosome assembly may also depend on the precise structure of the IM, which appears to be quite different than that of the growing cell or germinated spore plasma membrane (2, 5). In particular, despite having a lipid composition similar to that of growing cells, the lipids in the spore IM are relatively immobile. In addition, the overall IM seems to be compressed somewhat, as the IM bounded volume increases 1.5- to 2-fold early in spore germination and occurs without new membrane synthesis.
GR-dependent spore germination can be potentiated or activated by pretreatment with chemicals or sublethal heat, with the latter being most commonly used (6). The process of heat activation increases the rate and extent of germination of spores of a number of Bacillus and related species (6–15). The effect of heat activation is observed primarily as decreasing the time, defined as Tlag, between the addition of germinant to the initiation of the rapid release of most of the spore core's large depot (∼20% of core dry weight) of a 1:1 complex of Ca2+ and dipicolinic acid (DPA), with heat activation decreasing Tlag values for spores of a number of species (14, 15). However, heat activation has little or no effect on actual rates of rapid Ca-DPA release or the subsequent hydrolysis of spores' peptidoglycan cortex.
The molecular effect whereby heat activation increases rates of spore germination is not known, although there are several reports of effects accompanying heat activation such as changes in spore protein structure and the release of various spore molecules (16–18). However, heat activation appears only to stimulate nutrient germination via GRs, as heat activation does not stimulate germination by agents that act by a GR-independent mechanism, including Ca-DPA and long-chain alkylamines such as dodecylamine (1, 2). Spore germination triggered by high pressures (HPs) of ∼150 or ∼550 MPa is also reported not to be stimulated markedly by heat activation, even though an HP of 150 MPa clearly triggers spore germination by activating GRs (19, 20).
Spores of a number of Bacillus and Clostridium species are agents for food spoilage, food-borne disease, and other human diseases, and thus, there is continued interest in novel ways to kill such spores. One strategy is to first germinate spores and then kill the much less resistant germinated spores—the strategy that has been called “germinate to exterminate” for decontaminating spores of Clostridium difficile (21–23) and spores of Bacillus anthracis. Indeed, spore germination is a crucial mechanistic step in the inactivation of spores by HP processing, which uses conditions of elevated temperature (90 to 121°C) and pressure (≥600 MPa) to greatly reduce spore loads in certain foodstuffs (24–27), such as baby food purées (28). Consequently, since heat activation can be very important in determining the rates and efficiency of spore germination, the current study has analyzed the effects of heat activation on (i) nutrient germination of spores of Bacillus subtilis with various GRs and with or without GerD, (ii) germination of B. subtilis spores made at various temperatures that differ in their IM lipid compositions, and (iii) spore germination by HPs of 150 and 550 MPa, including spores of B. subtilis as well as Bacillus amyloliquefaciens spores, which have been suggested for use as a surrogate for Clostridium botulinum spores in HP treatments of foods (28, 29).
MATERIALS AND METHODS
B. subtilis strains used and spore preparation.
All B. subtilis strains used in this study are listed in Table 1 and are derivatives of strain PS832, a prototrophic laboratory 168 strain. The wild-type strain is PS533 (30), which contains plasmid pUB110, encoding resistance to kanamycin (Kmr; 10 μg/ml). B. subtilis spores of various strains were routinely prepared at 37°C on 2× Schaeffer's medium-glucose plates and in some cases at other temperatures as described previously (31, 32). Plates were incubated at sporulation temperatures until >90% of spores had been released from sporangia, generally 3 to 6 days, and were then incubated for several days at 23°C to allow lysis of growing cells and large fragments of cell debris. Spores were then scraped from plates or harvested from liquid media and purified as described previously (31, 32). All spores used in this study were free (>98%) from growing or sporulating cells, germinated spores, and cell debris as determined by phase-contrast microscopy.
TABLE 1.
Bacillus subtilis strains used in this study
| Strain | Phenotype | Antibiotic resistancea | Reference |
|---|---|---|---|
| FB10 | gerB* | None | 59 |
| FB20 | ΔgerA | Spr | 60 |
| FB61 | ΔgerA ΔgerB | Cmr Spr | 60 |
| FB62 | ΔgerD | Spr | 61 |
| FB87 | ΔgerB ΔgerK | Cmr MLSr | 60 |
| PS533 | Wild type | Kmr | 30 |
| PS3476 | PsspD::gerA | MLSr | 45 |
| PS3521 | ΔgerA gerB* | Spr | 45 |
| PS3651 | ΔgerA ΔgerK | Kmr MLSr | 3 |
| PS3665 | ΔgerA gerB* ΔgerK | MLSr Spr | 3 |
| PS4150 | ΔcotE ΔgerE | Spr Tcr | 36 |
Abbreviations for antibiotics: Cm, chloramphenicol (5 μg/ml); Km, kanamycin (10 μg/ml); MLS, erythromycin (1 μg/ml) and lincomycin (25 μg/ml); Sp, spectinomycin (100 μg/ml); Tc, tetracycline (10 μg/ml).
The B. amyloliquefaciens strain used was TMW 2.479 Fad 82 isolated from ropy bread and maintained on standard nutrient 1 (ST-1) agar (33). Spores were prepared by growing cells aerobically in ST-1 broth, plating them onto ST-1 agar plates supplemented with 10 mg/liter of MnSO4·H2O, and then incubating them for 2 to 3 days at either 30 or 37°C (33). Spores were harvested from plates by gently scraping and rinsing with water and then cleaned by repeated centrifugations and resuspension in distilled water. Remaining sporangia and vegetative cells were removed by suspending washed pellets in 200 ml of 0.05 M potassium phosphate (pH 7.0) containing 100 μg/ml of lysozyme and stirring for 1 h at 37°C, with subsequent centrifugation and washing with distilled water (34). Final spore suspensions were examined with phase-contrast microscopy and then frozen in 1-ml aliquots. Spores prepared at 30°C contained 98% phase-bright (ungerminated) and 2% germinated spores, and spores prepared at 37°C contained 87 to 90% ungerminated spores. Cells incubated at 23°C lysed and failed to sporulate.
Spore germination.
Prior to germination, spores of various strains at an optical density at 600 nm (OD600) of ∼10 were heat activated in water at 75°C for various times (0 to 6 h) and then cooled in an ice bath for ≥15 min. Spore germination with nutrient germinants was measured by monitoring the release of spore DPA by its fluorescence with Tb3+ in a multiwell fluorescence plate reader, as described previously (35). Germination took place at 37°C with spores at an OD600 of 0.5 in 200 μl of 25 mM K-HEPES buffer (pH 7.4) containing 50 μM TbCl3. Germinants used were various concentrations of either l-valine, the mixture of l-asparagine–d-glucose–d-fructose and K+ (AGFK), or l-asparagine alone, and the Tb-DPA fluorescence was read every 5 min for 100 to 150 min and expressed as relative fluorescence units (RFU). The maximum germinant concentration that was used routinely was 10 mM, because germination with 40 mM germinants in initial experiments was found to give <15% increases in germination rates. In most cases the percentage of spores that had released DPA at various germination times was determined from RFU measurements and knowing the total DPA in spores, which was determined after DPA was released from spores by boiling (35). The approximate extent of germination was also monitored at the end of all experiments by phase-contrast microscopy examining ∼100 individual spores. The rates of spore germination were determined from linear portions of plots of RFU versus time as described previously (3, 35). All experiments assessing germination by fluorescence measurements were carried out with least two replicates for each time point analyzed and in at least two separate experiments, always with very similar results.
Analysis of levels of germination proteins and germination protein biotinylation.
To determine levels of various germination proteins in unactivated or heat-activated (4 h at 75°C) spores, we used PS4150 spores with a severe coat defect that makes these spores lysozyme sensitive (36). This meant that spore lysis did not have to be preceded by a decoating step at high temperature that might also activate spores. Total lysates of unactivated or heat-activated PS4150 spores were prepared by lysozyme treatment followed by brief sonication and then incubation with SDS and 2-mercaptoethanol to extract all germination proteins as described previously (37). Levels of germination proteins in the lysates were then determined by Western blot analysis using specific antisera to germination proteins (37–40).
Biotinylation in unactivated and heat-activated (4 h at 75°C) PS4150 spores was carried out essentially as described previously for 1 h at 23°C using 2 mM EZ-Link Sulfo-NHS-SS-Biotin reagent (Pierce Chemical Co., Rockford, IL), which modifies lysyl amino groups in proteins (41). Unreacted reagent was quenched by the addition of 2 M glycine and 1 M Tris-HCl buffer (pH 7.4), followed by incubation for 30 min at 23°C and two washes with water. The IM and soluble and integument fractions from biotinylated PS4150 spores were obtained essentially as described previously (37, 41) by disruption in 0.5 ml of TEP buffer (50 mM Tris-HCl buffer [pH 7.4], 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF]) containing 1 mg of lysozyme, 1 μg each of RNase A and DNase I, and 20 μg of MgCl2 for 5 min at 37°C. After being held on ice for 20 min, the disrupted suspensions were sonicated briefly with 100 mg of glass beads (∼5 15-s bursts) and centrifuged at 4°C for 5 min in a microcentrifuge at maximum speed, and the supernatant fluid was saved. The pellet fraction was suspended in 0.5 ml of TEP buffer, sonicated for 15 s, and centrifuged; the final pellet was saved as the integument fraction, and the two supernatant fluids were pooled. The pooled supernatant fluids were centrifuged at 4°C for 1 h at 100,000 × g to give a supernatant (soluble fraction), and the pellet fractions (IM) were suspended in 160 μl of TEP buffer containing 1% Triton X-100. The integument fraction was suspended in 400 μl of TEP buffer plus 1% Triton X-100 and left to stand for 2 h at 23°C with intermittent vortexing and bath sonication, and aliquots were analyzed as described below.
Biotinylated and unbiotinylated germination proteins were separated by adsorption to NeutrAvidin agarose beads (Pierce), giving the bead eluate (E; biotinylated) and bead flowthrough (F; unbiotinylated) fractions as described previously (41). Western blot analyses as described above were performed following SDS-PAGE of equal percentages of the total biotinylated spore lysate (T fraction) and the F and E fractions, all run on the same Western blot.
HP germination of unactivated and heat-activated spores.
Spores at an OD600 of either 1 (B. subtilis spores) or 10 (B. amyloliquefaciens spores) were germinated by treating samples in 1.5 ml of 25 mM K-HEPES buffer (pH 7.4) with an HP of 150 MPa at 37°C or 550 MPa at 50°C for various periods (0 to 5 min), essentially as described previously (42). B. amyloliquefaciens spores were HP treated at a higher spore concentration because these spores did not pellet as tightly as B. subtilis spores upon centrifugation, but rather tended to form thin films of spores on the side of the centrifuge tube that significantly decreased B. amyloliquefaciens spore recovery (see below). The temperatures for HP treatments were chosen from previous work with B. subtilis spores that showed that (i) 37°C is near optimal for 150-MPa germination, with germination slower at 50°C, and (ii) 500-MPa germination is quite slow at 37°C, and while the temperature optimum for 500-MPa germination is ∼60°C, it is difficult to measure the rates of the more rapid germination at this temperature (43). Samples were frozen immediately after HP treatment and kept frozen until analyzed for germination. After thawing on ice, HP-treated B. subtilis spore samples were centrifuged in a microcentrifuge at top speed for ∼2 min, the pellet was suspended in 20 μl of water, and ∼100 spores were examined by phase-contrast microscopy to determine the percentages of spores that had become phase dark or phase gray and thus had germinated. Aliquots of HP-treated B. amyloliquefaciens spores were analyzed directly by microscopy as described above, as their concentration prior to microscopy was not needed.
HP activation of B. amyloliquefaciens spores.
Samples of sterile chicken baby food purée (Gerber baby food) were inoculated to ∼107 CFU/ml with unactivated B. amyloliquefaciens spores, sealed in sterile pouches, and either not HP treated or treated with various combinations of HP (448 to 690 MPa) and exposed to high temperature (65 to 121°C) in a PT-1 high-pressure unit with bioglycol heat transfer fluid (Dynalene, Whitehall, PA) as the heat- and pressure-transmitting medium. At the end of the come-up time (30 to 45 s), pressure was released, and samples were diluted with sterile buffer solution, mixed with a masticator, spread plated onto ST-1 agar plates, incubated at 30 or 37°C for 18 to 22 h, and enumerated using a New Brunswick colony counter.
RESULTS
Effects of heat activation on germination triggered by different GRs.
As noted in the introduction, heat activation can increase the germination of spores of various Bacillus species, and at least one report indicates that germination via different GR-dependent germinants exhibits different requirements for heat activation (10). This suggests that different GRs may exhibit different responses to heat activation. To test this suggestion, we examined the germination of B. subtilis spores via different GRs after various heat activation times (Fig. 1 and 2). Rates of l-valine germination of wild-type spores via GerA were increased ∼40% with optimal heat activation, which required ∼15 min for spores made at 37°C, while heat activation for 4 or 6 h resulted in significantly slower germination (Fig. 1A and 2A and data not shown). Heat activation for 4 h also decreased the extent of l-valine germination after 100 min, although heat activation for ≤2.5 h had no effect (Fig. 1A and 2B). Rates of germination of wild-type spores with the mixture of l-asparagine, d-glucose, d-fructose, and K+ (AGFK) and the l-asparagine germination of gerB* spores were stimulated >20-fold and ∼5-fold, respectively, by optimal heat activation that required 4 h for the AGFK germination of wild-type spores and 2 h for l-asparagine germination of gerB* spores. Heat activation for 6 h did not further increase AGFK germination of wild-type spores or l-asparagine germination of gerB* spores (data not shown).
FIG 1.
Effects of heat activation on germination of spores via various GRs. Spores of strains PS533 (wild type) (A and B) or FB10 (gerB*) (C) were germinated with 10 mM l-valine (A), 10 mM (each) AGFK (B), or 10 mM l-asparagine (C) after various heat activation times as described in Materials and Methods. Spore germination was monitored by Tb-DPA fluorescence, with values given either in relative fluorescence units (RFU) or as percent spore germination as described in Materials and Methods. Values shown are the averages of results from measurements on duplicate germinations done simultaneously, and the individual measurements differed by ≤6% from average values. The symbols representing the heat activation times are as follows: ○, 0 min; ●, 5 min; △, 15 min; ▲, 30 min; □, 1 h; ■, 2.5 h; and ◊, 4 h. A 6-h heat activation did not increase AGFK germination further (data not shown). For the samples analyzed in panels A and B, the maximum percentages of spore germination at 100 min were 92 and 88%, respectively.
FIG 2.
Effects of heat activation times on rates and levels of spore germination. Spores of strain PS533 (wild type) or FB10 (gerB*) were prepared at 37°C and heat activated at 75°C for various times; spores were germinated in duplicate with either 10 mM l-valine, 10 mM (each) AGFK, or 10 mM l-asparagine as described in Materials and Methods. Spore germination was measured and germination rates (A) and percentages of spore germination (B) after 100 min were determined as described in Materials and Methods. Values shown are averages of duplicate determinations in two experiments with the same spore preparations and are ≤±12%. The symbols used are follows: ○, PS533 spores, l-valine germination; ●, PS533 spores, AGFK germination; and △, FB10 spores, l-asparagine germination.
GRs are located in spores' IM, and it is possible that IM composition might also alter effects of heat activation on GR-dependent spore germination. One variable that greatly alters B. subtilis spore IM fatty acid composition is sporulation temperature, which can also affect rates of spore germination (38, 44). Wild-type spores prepared at temperatures from 23 to 43°C did exhibit differences in rates of spore germination with various germinants as expected (37) (Fig. 3). However, the optimal heat activation times for l-valine or AGFK germination did not differ appreciably for the spores made at the different temperatures, although heat activation caused greater stimulations in rates of l-valine or AGFK germination of spores made at lower temperatures (Fig. 3). Heat activation for 6 h did not further increase the rates of AGFK germination of the spores made at different temperatures (data not shown).
FIG 3.
Effects of heat activation on germination of spores made at various temperatures. Spores of strain PS533 (wild type) were prepared at 23°C (○), 30°C (●), 37°C (△), or 43°C (▲) and heat activated at 75°C for various times, and spores were germinated in duplicate with either 10 mM l-valine (A) or 10 mM (each) AGFK (B) as described in Materials and Methods. Spore germination was measured and germination rates were determined as described in Materials and Methods. Values shown are averages of duplicate determinations in two experiments with the same spore preparations and were ≤±19%.
Effects of heat activation on germinant concentration dependence of spore germination.
Heat activation clearly increased the rate and sometimes the extent of nutrient germination via GRs, and one possible reason was that heat activation reduces germinant concentrations needed to trigger spore germination. To test this possibility, we determined the germinant concentration dependence of spore germination via various GRs with and without heat activation (Table 2). Notably, optimal heat activation decreased l-asparagine concentrations needed for half-maximal rates of germination via GerB plus GerK or GerB* alone 1.5- to 2-fold. However, the effect of optimal heat activation on the concentration dependence of l-valine germination was small and not significant, likely because of the significant l-valine germination even with no heat activation; this was also seen using FB87 spores, which contain only the GerA GR (data not shown).
TABLE 2.
Effect of heat activation on germinant concentrations giving half-maximal germination ratesa
| Spores | Variable germinant | Germinant concn giving half-maximal germination rate (mM) |
|
|---|---|---|---|
| No heat | Heat | ||
| PS533 (wild type) | l-Valineb | 3.2 ± 0.5 | 2.5 ± 0.4b |
| PS533 (wild type) | l-Asparaginec | 2.7 ± 0.5 | 1.1 ± 0.26d |
| PS3665 (ΔgerA gerBB* ΔgerK) | l-Asparagine | 0.63 ± 0.1 | 0.40 ± 0.1e |
| PS3521 (ΔgerA gerBB*) | l-Asparagine | 0.64 ± 0.13 | 0.48 ± 0.1b |
| PS3521 (ΔgerA gerBB*) | l-Asparaginef | 0.62 ± 0.12 | 0.41 ± 0.09b |
| PS3521 (ΔgerA gerBB*) | l-Asparaginef | 0.62 ± 0.12 | 0.31 ± 0.08e |
| PS3521 (ΔgerA gerBB*) | l-Asparaginef | 0.62 ± 0.12 | 0.18 ± 0.05d |
Spores of various strains prepared at 37°C were germinated with variable germinant concentrations from 0.05 to 10 mM, and germination rates were determined in duplicate in 2 independent experiments to allow calculation of germinant concentrations giving 50% of the maximum germination rates.
Heat activation for 30 min.
GFK were also present, each at 10 mM.
Heat activation for 4 h.
Heat activation for 2 h.
Glucose and K+ were also present, each at 10 mM.
To examine the effects of heat activation on nutrient germination requiring GerK, we examined the effects of d-glucose on l-asparagine germination of PS3521 spores (Table 2). These spores lack GerA but have GerB* and GerK, and GerK is almost certainly the GR that mediates d-glucose stimulation of l-asparagine germination via GerB* (3). d-Glucose had minimal effects on the l-asparagine concentration dependence of the germination of PS3521 spores left unactivated or heat activated for 30 min. However, with PS3521 spores heat activated for 4 h, d-glucose decreased the l-asparagine concentration needed for half-maximal rates of germination even more than heat activation alone, while with spores heat activated for 2 h, there was essentially no effect. Overall, these data are consistent with GerK having a more stringent heat activation requirement for nutrient germination than GerA, GerB, or GerB*. This was also seen when effects of heat activation on spore germination triggered by 150 MPa of HP were examined (see below).
Effect of GR overexpression on the heat activation required for spore germination.
Previous work has shown that overexpression of GerA from a strong forespore-specific promoter increases rates of spore germination with l-valine (45, 46). The levels of GerB and GerK do not decrease in spores overexpressing GerA, although these spores' germination with AGFK is significantly slowed (46). Since the inhibition of AGFK germination by elevated GerA levels might be due to altered heat activation requirements for the GRs in these spores, we examined the effects of heat activation on l-valine and AGFK germination of PS3476 spores overexpressing GerA ∼8-fold (37, 45, 46) (Fig. 4). As expected (45), l-valine germination of PS3476 spores was faster than that of wild-type spores (compare Fig. 2A and 4). However, PS3476 spores' germination rate with l-valine was highest with 4 h of heat activation, in contrast to the ∼15 min needed for spores with wild-type GerA levels, although 6 h of heat activation decreased l-valine germination of PS3476 spores markedly (data not shown). As seen previously (46), rates of AGFK germination of PS3476 spores were minimal with no or 30 min of heat activation and were below rates of AGFK germination seen with wild-type spores (compare Fig. 2B and 4). Heat activation for up to 4 h increased rates of AGFK germination of PS3476 spores to values close to those for wild-type spores (compare Fig. 2B and 4), but a 6-h heat activation caused no further increase (data not shown).
FIG 4.
Effects of heat activation on germination of spores with overexpressed GerA. PS3476 (PsspD::gerA) spores were heat activated for various times and germinated in duplicate with either 10 mM l-valine (○) or 10 mM AGFK (●), spore germination was measured, and germination rates were determined as described in Materials and Methods. Values shown are averages of duplicate determinations in two experiments with the same spore preparation and were ≤±22%, with the largest variations in AGFK germinations at short heat activation times.
Effects of heat activation on synergy between GRs responding to different germinants.
One striking behavior seen in GR-dependent germination is that with low concentrations of mixtures of germinants recognized by different GRs, for example, l-valine recognized by GerA and AGFK recognized by GerB plus GerK, the germination rate is higher than the sum of the germination rates with each germinant alone (47). To examine the effects of heat activation on this apparent synergy between different GRs, we determined extents of germination of wild-type spores with various concentrations of l-valine plus AGFK and of gerB* spores with various concentrations of l-valine plus l-asparagine alone. These values were defined as actual (a) values. In addition, extents of germination were also determined for wild-type spores germinating with various concentrations of l-valine, AGFK, or l-asparagine alone (with gerB* spores), and these values allowed calculation of values predicted (p) for extents of germination by the various concentrations of the germinant mixtures if there was no synergy. The value of a/p at any particular mixture of germinant concentrations has been defined as the degree of synergy (Ds), and values of ≥1 indicate synergy between GRs (47). Ds values were invariably larger at lower germinant concentrations (Fig. 5), as seen previously (47). Notably, unactivated spores exhibited the highest Ds values, and spores heat activated for 4 h exhibited only very small changes in Ds values as a function of germinant concentrations. Thus, much of the synergy between different GRs is abolished by optimal heat activation.
FIG 5.
Effects of heat activation on the synergy between GerA and GerB plus GerK and GerA and GerB* in spore germination. Spores of strain PS533 (wild type) (A) or FB10 (gerB*) (B) were either left unheated (○), heat activated for 30 min (●), or heat activated for 4 h (△) (A) or 2 h (B). These spores were germinated in duplicate with various concentrations of l-valine or l-asparagine (plus 10 mM [each] GFK) (A) or l-valine or l-asparagine (B) as described in Materials and Methods. The extents of spore germination at various times were determined as described in Materials and Methods and added together to give the predicted extents of spore germination, p, if there was no synergy. The spores were also germinated in duplicate with various concentrations of both l-valine and l-asparagine (plus 10 mM [each] GFK) (A) or both l-valine and l-asparagine (B), and the actual extents of spore germination with the germinant mixtures, a, were also determined. The degree of synergy (Ds) in germination at various concentrations of l-valine and l-asparagine was calculated as described previously (44) as Ds = a/p, and Ds values of >1 indicate synergy. In panel A the concentrations of l-valine and l-asparagine were equal, and in panel B the l-valine concentrations were 5-fold higher than the l-asparagine concentrations. The germination times selected for calculation of Ds values were the same for all data points for a particular germinant mixture, and these germination times gave the highest Ds values throughout the germinant concentration range. Ds values shown are averages from a and p values determined from duplicate measurements of extents of spore germination in two experiments with the same spore preparations and differed by ≤±32%.
Effect of loss of GerD on heat activation required for GR germination.
One defining feature of GR germination is that loss of the GerD protein greatly decreases GR-dependent germination, perhaps because the germinosome does not assemble in gerD spores (1, 2, 4). To test if gerD spores require much greater heat activation than wild-type spores, we examined the effects of heat activation on the extents of l-valine and AGFK germination of FB62 spores, which lack GerD (Fig. 6). As expected, with gerD spores made at 23 to 43°C, rates of l-valine and AGFK germination were much lower than for germination of wild-type spores made at these temperatures (compare Fig. 3 and 6). In contrast, just as with wild-type spores, heat activation for ∼15 min or ∼4 h gave maximal rates of germination of gerD spores with l-valine and AGFK, respectively (Fig. 6), and heat activation for 6 h gave no further increase in AGFK germination (data not shown). However, optimal heat activation gave less stimulation of gerD spore germination with l-valine than of wild-type spores, and perhaps also with AGFK, although this was more difficult to quantitate because of the very low rates of AGFK germination of gerD spores given no or short heat activation treatments. Interestingly, (i) gerD spores prepared at 23°C exhibited much faster germination with either l-valine or AGFK than did spores prepared at 37°C (Fig. 6), and (ii) with spores made at 43°C in particular, long heat activation times resulted in significant decreases in the rates of l-valine germination, suggesting that the GerA GR may be quite heat labile in these spores.
FIG 6.
Effects of heat activation on the germination of gerD spores made at various temperatures. Spores of strain FB62 (gerD) made at 23°C (○), 30°C (●), 37°C (△), and 43°C (▲) were heat activated for various times and germinated in duplicate with either 10 mM l-valine (A) or 10 mM AGFK (B) as described in Materials and Methods. Rates of spore germination were also determined as described in Materials and Methods. Values shown are averages of duplicate determinations in two experiments with the same spore preparations and were ≤±22%.
Effects of heat activation on GR levels and accessibility.
The facts that germination via different GRs exhibited different requirements for heat activation and that loss of GerD did not affect heat activation requirements for germination by different GRs suggested that heat activation affects GRs. One trivial, albeit extremely unlikely, possibility is that heat activation somehow alters spores' GR levels. However, this was not the case, as levels of GerD and multiple GR subunits differed by ≤15% in total lysates from wild-type spores left unactivated or heat activated for 4 h (data not shown).
A second possibility is that heat activation alters GR conformation such that these proteins are more responsive to their nutrient ligands. To attempt to obtain evidence consistent with this possibility, the EZ-Link Sulfo-NHS-SS-Biotin biotinylation reagent used to monitor accessibility of GR subunits and GerD in decoated B. subtilis spores (41) was used to examine whether biotinylation of these proteins differed in spores left unactivated and spores heat activated for 4 h. PS4150 spores that lack most of their spore coat were used in this experiment, allowing access of the biotinylation reagent to regions of germination proteins on the outer surface of these spores' IM (36, 41). Control experiments showed that germination of PS4150 spores exhibited the same heat activation requirements as germination of wild-type spores (data not shown). The results of the biotinylation experiment showed that levels of biotinylation of GR subunits and GerD in total PS4150 spore lysates and in isolated IM, integument, and soluble fractions were essentially identical in the unactivated and heat-activated spores (see Fig. S1 in the supplemental material).
Effects of heat activation on HP germination at 150 and 550 MPa.
Previous work had indicated that in addition to nutrient germinants, HP of 150 MPa can also trigger spore germination via GRs, although heat activation of this HP germination has generally not been observed (19, 20). However, 150-MPa HP germination of unactivated or heat-activated (30 min at 70°C) B. subtilis spores is dominated by germination via GerA, with minimal contributions from GerB and GerK (20, 48). While it is possible that GerB and GerK are not especially responsive to HP of 150 MPa, it is also possible that HP germination via GerB and GerK exhibits the extreme requirement for heat activation seen with nutrient germination. Consequently, we determined rates of 150-MPa HP germination of spores of B. subtilis strains containing various GRs, alone or in combination, and examined both unactivated spores and spores heat activated at 75°C for 30 min or 4 h (Fig. 7). As found previously (20, 48), wild-type spores left unactivated or heat activated for 30 min germinated rapidly and similarly with an HP of 150 MPa, while germination of unactivated spores via GerB plus GerK or GerB, or in particular via GerK alone, was extremely slow (compare Fig. 7A and D). However, a 4-h heat activation markedly stimulated 150-MPa HP germination via GerB and/or GerK, something that has not been seen previously (19, 20, 43), but had minimal effects on wild-type spore germination. In addition, 30 min of heat activation had only small effects on the 150-MPa HP germination of spores via GerB and particularly via GerK, and this result is consistent with effects of heat activation times on nutrient germination via these GRs.
FIG 7.
HP germination (150 MPa) of spores of various B. subtilis strains with and without heat activation. Spores of B. subtilis strains PS533 (wild type) (A), FB20 (ΔgerA) (B), FB61 (ΔgerA ΔgerB) (C), and PS3651 (ΔgerA ΔgerK) (D), without heat activation (○) or heat activated at 75°C for 30 min (●) or 4 h (△), were germinated in one experiment for various times with an HP of 150 MPa at 37°C; the extents of spore germination were measured as described in Materials and Methods.
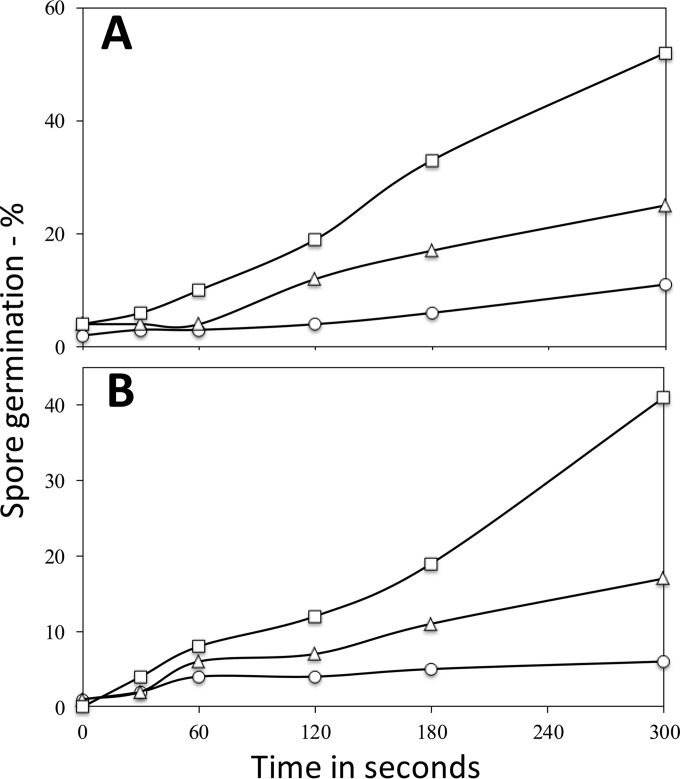
While an HP of 150 MPa gave 7 and 23% germination of unactivated B. subtilis spores via the GerK and GerB GRs, respectively (Fig. 7C and D), in 5 min, an HP of 550 MPa at 50°C gave 33 and 60% germination of unactivated spores in 5 min via GerK and GerB, respectively (Fig. 8C and D). However, while 30 min of heat activation stimulated B. subtilis spore germination by an HP of 550 MPa, even that of wild-type spores, 4 h of heat activation decreased 550-MPa HP germination during the first 3 min of HP treatment (Fig. 8), as if this long heat activation treatment had significantly damaged some essential component involved in 550-MPa HP spore germination, similar to what was seen in some instances with nutrient germination via GerA (Fig. 1A and 2A). In contrast to the kinetics observed with nutrient germination, the extent of germination observed in the 3- to 5-min region with 550-MPa HP treatment of spores heat activated for 4 h reached about 95%, a level comparable to that of spores heat activated at 75°C for 30 min and also HP treated. These effects of heat activation on B. subtilis spore germination by HP were surprising, since this has not been reported previously, as noted above.
FIG 8.
HP germination (550 MPa) of spores of various B. subtilis strains with and without heat activation. Spores of B. subtilis PS533 (wild type) (A), B. subtilis FB20 (ΔgerA) (B), B. subtilis FB61 (ΔgerA ΔgerB) (C), and B. subtilis PS3651 (ΔgerA ΔgerK) (D), without heat activation (○) or heat activated at 75°C for 30 min (●) or 4 h (△), were germinated in one experiment for various times with an HP of 550 MPa at 50°C; the extents of spore germination were measured as described in Materials and Methods.
HP treatment leading to bacterial spore germination and inactivation is used in a number of applications to reduce spore burdens in foodstuffs (24–27). As a consequence, methods to increase spore germination by HP are of significant applied interest. To determine if heat activation might affect the HP germination of spores of applied interest, we used spores of B. amyloliquefaciens, which have been suggested as a good surrogate for spores of C. botulinum in analyzing the efficacy of regimens for spore inactivation by HP (28, 29). Strikingly, heat activation for 4 h at 70°C markedly increased the germination of B. amyloliquefaciens spores at HPs of both 150 and 550 MPa (Fig. 9), although it is possible that less than 4 h at 70°C would have sufficed for maximal germination.
FIG 9.
HP germination of B. amyloliquefaciens spores with and without heat activation. Spores of B. amyloliquefaciens prepared at 37°C and without heat activation (○) or heat activated at 70°C for 30 min (△) or 4 h (□) were germinated in one experiment for various times with an HP of 150 MPa (A) or 550 MPa (B); the extents of spore germination were measured as described in Materials and Methods. Similar results were obtained with two independent spore preparations, and HP germination of spores prepared at 30°C was affected similarly by heat activation (data not shown).
HP sterilization of foods typically involves preheating to up to 90°C and then pressurizing to ≥600 MPa over some finite come-up time, during which adiabatic heating of compression causes the product temperature to increase typically ≥121°C (49). As an example, the pressure chamber containing only bioglycol oil at an initial temperature (Ti) of 75 to 95°C and HPs of 552 to 690 MPa induced adiabatic heating, giving 20 to 35°C temperature increases in the HP chamber that decayed to Ti during the ensuing hold time (see Table S1 in the supplemental material). Spores in a food matrix would experience similar treatment conditions for appreciable periods during such an HP process. The effects of temperature and HP in activating B. amyloliquefaciens spores in chicken purée were determined at various combinations of temperature and pressure. With a Ti of 75 to 95°C and pressure of 449 to 690 MPa, the viable counts increased after the come-up time (30 to 45 s) required to reach the target HP compared to the untreated samples, indicative of spore activation prior to plating and incubation (Table 3). Heating samples to 75 to 121°C at ambient pressure (1 atm ≈ 0.1 MPa) for as long as 15 min also induced activation of B. amyloliquefaciens spores, with the exception of significant inactivation by treatments at 112.5 and 121°C for 15 min. Significant inactivation was also seen with spores heated at 95°C and then exposed to 690 MPa for 3 min plus come-up time. Consequently, an HP process with these pressures and temperatures can activate, germinate, and inactivate individual spores sequentially, and these processes can occur concurrently for spore populations, given the heterogeneous distribution of resistances of spores within large populations. It is also possible that GR-dependent germination occurs during the pressurization process, although this contribution would likely be relatively minor, due to the transience of the come-up time.
TABLE 3.
Activation of B. amyloliquefaciens spores by heat with or without HPa
| Inoculum (CFU/ml, ×10−7) | Treatment |
Time (s) | No. of survivors (CFU/ml) | % change | |
|---|---|---|---|---|---|
| Temp (°C) | P (MPa) | ||||
| Come-up | |||||
| 3.51 ± 1.03 | 65 | 552 | 36 | (3.41 ± 0.32) × 107 | −2.8 |
| 5.21 ± 1.20 | 75 | 448 | 29 | (8.27 ± 4.28) × 107 | +58.7 |
| 2.78 ± 0.61 | 75 | 552 | 36 | (7.09 ± 1.55) × 107 | +155 |
| 3.04 ± 1.59 | 75 | 690 | 45 | (7.07 ± 0.95) × 107 | +132.6 |
| 1.87 ± 0.46 | 95 | 690 | 45 | (2.72 ± 0.06) × 107 | +45.5 |
| Hold | |||||
| 1.87 ± 0.46 | 95 | 0 | 180 | (2.96 ± 0.71) × 107 | +58.3 |
| 2.72 ± 0.06 | 95 | 690 | 180 | (1.08 ± 0.13) × 104 | −99.9b |
| 2.50 ± 0.54 | 112.5 | 0 | 180 | (9.30 ± 0.99) × 107 | +372 |
| 1.56 ± 0.47 | 121 | 0 | 180 | (4.50 ± 0.42) × 107 | +188.5 |
| 3.40 ± 0.28 | 75 | 0 | 420 | (5.50 ± 0.85) × 107 | +61.8 |
| 3.40 ± 0.28 | 85 | 0 | 420 | (1.14 ± 0.24) × 108 | +235.3 |
| 5.35 ± 0.92 | 95 | 0 | 420 | (1.21 ± 0.18) × 108 | +126.2 |
| 2.50 ± 0.54 | 105 | 0 | 420 | (1.39 ± 0.08) × 108 | +456 |
| 2.50 ± 0.54 | 112.5 | 0 | 420 | (1.59 ± 0.04) × 108 | +536 |
| 3.45 ± 0.07 | 121 | 0 | 420 | (4.95 ± 0.21) × 107 | +43.4 |
| 2.50 ± 0.54 | 95 | 0 | 900 | (1.18 ± 0.13) × 108 | +372 |
| 2.50 ± 0.54 | 105 | 0 | 900 | (1.06 ± 0.26) × 108 | +324 |
| 2.50 ± 0.54 | 112.5 | 0 | 900 | (1.12 ± 0.05) × 107 | −55.2 |
| 3.45 ± 0.07 | 121 | 0 | 900 | (1.45 ± 0.08) × 107 | −58.0 |
Activation of spores was measured after heat treatment with or without subsequent HP treatments, as increases in viable counts before (inoculum) and after (no. of survivors) heat and HP treatments. All viability measurements are mean plate counts and standard deviations from 2 to 12 replicates.
Representative data indicating inactivation by HP treatment perhaps due to adiabatic heating.
DISCUSSION
Heat activation of germination of spores of Bacillus species was described more than 45 years ago (6, 11), although it has been studied relatively little recently. Overall, heat activation has been shown to be (i) temperature dependent, as extended incubation at relatively low temperatures also activates spores, although activation is faster at high temperatures, and (ii) reversible to some degree by incubation at low temperatures. These observations, as well as thermodynamic studies, are consistent with heat activation causing a reversible conformational change in one or more proteins (6, 11, 17, 50). However, such proteins have not been identified, although a recent study found reversible changes in global protein structure accompanying spore heat activation (17).
Since heat activation affects only GR-dependent germination and not germination by GR-independent germinants (1, 2), GRs are attractive as heat activation targets, perhaps via temperature-dependent conformational changes. However, other proteins, notably GerD, are also involved in GR-dependent germination. The current study with B. subtilis spores provided evidence consistent with GRs being the major heat activation target as follows. (i) Heat activation times to get maximal germination via different GRs varied between 15 min and 4 h, with GerA exhibiting the lowest requirement, GerK the highest, and GerB* an intermediate requirement. It is reasonable that high temperatures, and thus heat activation, could have different effects on different GRs, as the same subunits in different GRs from the same species exhibit <35% amino acid sequence identity (51, 52) and thus could have different temperature requirements for structural changes. However, what such a conformational change might do is not clear. (ii) The different heat activation times for optimal stimulation of nutrient germination via different GRs were generally similar to the effects of heat activation times on 150-MPa HP germination of spores containing various GRs. Thus, HP germination via GerK was simulated most by heat activation, and germination predominantly by GerA was stimulated least. One difference between effects of heat activation on germination by an HP of 150 MPa and nutrients is that there was essentially no effect of heat activation on 150-MPa germination by GerA, in contrast to an ∼40% stimulation in l-valine germination rate via GerA by heat activation. However, since HP can cause conformational changes in proteins (53–55) in addition to triggering GR-dependent germination, an HP of 150 MPa could also activate GRs, with GerA being most responsive to activation by this HP. (iii) While the loss of GerD greatly reduces rates of GR-dependent germination, gerD spores required heat activation times similar to those of wild-type spores for maximal germination rates. This rules out heat activation as affecting GerD or germinosome assembly or function rather than GRs directly. Indeed, the germinosome appearance in spores carrying functional GerA-mCherry or GerD-green fluorescent protein fusions (4) was not altered by 30 min or 4 h of heat activation (A. J. Troiano and P. Setlow, unpublished data). Interestingly, the gerD mutation had a much smaller effect on the germination of spores prepared at 23°C, something that has not been seen previously. Perhaps this is due to the likely greater IM fluidity in spores made at 23°C (44), which may allow GRs not in the germinosome in gerD spores to more readily move in the IM and interact. (iv) Heat activation decreased the nutrient germinant concentrations for 50% of the maximum germination rate, in particular in germination via GerB plus GerK or GerB* with or without GerK. Previous work showed that elevated GR levels decrease germinant concentrations needed for the same rates of spore germination of wild-type spores (3, 45), and heat activation may make more GRs functional or responsive to their nutrient germinants, thus decreasing germinant concentrations needed for a given spore germination rate.
While the results noted above suggest that GRs are the target for heat activation, heat activation may affect GRs only indirectly and directly affect the state of the spore IM, which then alters GR structure and responsiveness. It is difficult to rule out the latter mechanism of heat activation, and the lack of effect of heat activation on the biotinylation of GR proteins seen in this study was inconsistent with heat activation directly affecting GRs. However, several results described in this communication as well as one from the literature are less consistent with the IM as the heat activation target compared with GRs, as follows. (i) As shown in the current work, long heat activation times reduced GerA germination, while GerB and GerK function was increased, and this may reflect lower GerA thermal stability. (ii) The IM fatty acid compositions differ greatly in spores made at temperatures from 23 to 43°C (44). Such changes in IM lipid composition, and thus presumably in the degree of lipid mobility in the IM, could well influence the behavior of proteins embedded in the IM, as the A and B subunits of GRs are. This altered IM lipid mobility, in turn, could also modify the effects of heat activation on these proteins. However, as shown in the current work, different sporulation temperatures did not alter optimal heat activation times for germination via GerA or GerB plus GerK. (iii) In contrast to spores of Bacillus and Clostridium species which exhibit heat activation of GR-dependent germination, with C. difficile spores that have no GRs in the IM but germinate in response to specific bile salts that activate a protease that activates a cortex peptidoglycan lytic enzyme, heat activation has no effect on bile salt germination (56, 57).
In addition to the major findings noted above, other observations on effects of heat activation on spore germination are as follows. (i) Spores made at different temperatures had the same optimal heat activation times for maximum rates of nutrient germination, but germination of spores made at lower temperatures, in particular via GerA, was stimulated more by heat activation. The latter effect could be due either to less heat activation, in particular of GerA, during sporulation at lower temperatures, or to differences in the IM of spores made at different temperatures. (ii) Unactivated spores exhibited much greater synergy between germinants that act via different GRs than heat-activated spores. This suggests that GR-GR interaction is essential for this synergy, whether direct or indirect, and this interaction can be stimulated by heat activation. (iii) Overexpressing GerA from a moderately strong forespore-specific promoter gives spores with 8-fold-increased GerA levels, although with no changes in GerB and GerK levels (46). When these spores were not heat activated, rates of l-valine and AGFK germination were higher and lower, respectively, than rates with wild-type spores. Heat activation of these spores increased GerA-dependent germination ≥3-fold but required 4 h for this effect instead of the 15 min for wild-type spores, for reasons that are not clear. However, even 4 h of heat activation did not restore AGFK germination rates of spores overexpressing GerA to those in wild-type spores. Thus, inhibition of AGFK germination by elevated GerA levels affects GR function directly, as suggested previously (46). (iv) Germination of B. subtilis spores by an HP of 550 MPa was stimulated by 30 min of heat activation, something that has not been seen previously. This result was surprising since spore germination at 550 MPa is thought to be GR independent (19, 20, 48). However, effects of heat activation on 550-MPa germination of B. subtilis spores differed considerably from effects on GR-dependent 150-MPa HP germination as follows. (a) Heat activation affected 550-MPa HP germination of wild-type spores and spores containing only GerB plus GerK, only GerB, or only GerK relatively similarly, although germination of unactivated spores containing only GerK was a bit slower than that of other spores; (b) a 4-h heat activation decreased 550-MPa HP germination of all spores markedly over that following a 30-min activation. Thus, heat activation may have effects on spores' germination by 550 MPa of HP that are largely, but perhaps not completely, GR independent, as suggested previously (43). The target modified by heat activation leading to increased 550-MPa HP germination is not clear, but two possibilities are (a) the spore IM, where many germination proteins, including the SpoVA proteins comprising the Ca-DPA channel thought to be activated by HPs of ≥500 MPa, are located (1, 2, 19, 24, 43), and (b) one or more of the SpoVA proteins themselves. (v) Finally, the observation that heat activation also markedly stimulated HP germination of B. amyloliquefaciens spores may have applied implications, since HP germination and subsequent heat inactivation of less resistant germinated spores can minimize spore loads in shelf-stable foods (24–27), and B. amyloliquefaciens spores have been proposed for use as a surrogate for C. botulinum spores in analysis of regimens for spore inactivation by HP (28, 29). HP treatment for the commercial sterilization of foodstuffs is almost always carried out at temperatures much higher than the 50°C used in our study, and even with short processing times, temperatures and HPs may be sufficient to cause significant spore heat activation during the HP treatment. However, it is also possible that a pretreatment step at high temperature might increase the efficacy of subsequent HP treatments in food processing regimens; this possibility certainly seems to merit further study.
Supplementary Material
ACKNOWLEDGMENTS
This communication is based upon work supported by a Department of Defense Multi-Disciplinary Research Initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911NF-09-1-0286.
We thank Haiqing Chen and Jonathan Huang for use of high-pressure equipment at the University of Delaware.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00193-15.
REFERENCES
- 1.Setlow P. 2013. When the sleepers wake: the germination of spores of Bacillus species. J Appl Microbiol 115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- 2.Setlow P. 2014. The germination of spores of Bacillus species: what we know and don't know. J Bacteriol 196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Co-operativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this co-operativity by alterations in the GerB receptor. J Bacteriol 188:28–36. doi: 10.1128/JB.188.1.28-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths KK, Zhang J, Cowan AE, Yu J, Setlow P. 2011. Germination proteins in the inner membrane of dormant Bacillus subtilis spores colocalize in a discrete cluster. Mol Microbiol 81:1061–1077. doi: 10.1111/j.1365-2958.2011.07753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan AE, Olivastro EM, Koppel DE, Loshon CA, Setlow B, Setlow P. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are largely immobile. Proc Natl Acad Sci U S A 101:7733–7738. doi: 10.1073/pnas.0306859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keynan A, Evenchick Z. 1969. Activation, p 359–396. In Gould GW, Hurst A (ed), The bacterial spore. Academic Press, New York, NY. [Google Scholar]
- 7.Aoki H, Slepecky RA. 1973. Inducement of a heat-shock requirement for germination and production of increased heat resistance in Bacillus fastidiosus spores by manganous ions. J Bacteriol 114:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Foegeding PM. 1990. Effects of heat-, CaCl2- and ethanol-treatments on activation of Bacillus spores. J Appl Bacteriol 69:414–420. doi: 10.1111/j.1365-2672.1990.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull PCB, Frawley DA, Bull RL. 2007. Heat activation/shock temperatures for Bacillus anthracis spores and the issue of spore plate counts versus true numbers of spores. J Microbiol Methods 68:353–357. doi: 10.1016/j.mimet.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.van der Voort M, Garcia D, Moezelaar R, Abee T. 2010. Germinant receptor diversity and germination responses of four strains of the Bacillus cereus group. Int J Food Microbiol 139:108–115. doi: 10.1016/j.ijfoodmicro.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Levinson HS, Hyatt MT. 1969. Heat activation kinetics of Bacillus megaterium spores. Biochem Biophys Res Commun 37:909–916. doi: 10.1016/0006-291X(69)90217-4. [DOI] [PubMed] [Google Scholar]
- 12.Huo Z, Yang X, Raza W, Huang Q, Xu Y, Shen Q. 2010. Investigation of factors influencing spore germination of Paenibacillus polymyxa ACCC10252 and SQR-21. Appl Microbiol Biotechnol 87:527–536. doi: 10.1007/s00253-010-2520-8. [DOI] [PubMed] [Google Scholar]
- 13.Løvdal IS, Hovda MB, Granum PE, Rosnes JT. 2011. Promoting Bacillus cereus spore germination for subsequent inactivation by mild heat treatment. J Food Prot 74:2079–2089. doi: 10.4315/0362-028X.JFP-11-292. [DOI] [PubMed] [Google Scholar]
- 14.Zhou T, Dong Z, Setlow P, Li YQ. 2013. Kinetics of germination of individual spores of Geobacillus stearothermophilus as measured by Raman spectroscopy and differential interference contrast microscopy. PLoS One 8:e74987. doi: 10.1371/journal.pone.0074987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Garner W, Yi X, Yu J, Li YQ, Setlow P. 2010. Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. doi: 10.1128/JB.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alimova A, Katz A, Gottlieb P, Alfano RR. 2006. Proteins and dipicolinic acid released during heat shock activation of Bacillus subtilis spores probed by optical spectroscopy. Appl Opt 45:445–450. doi: 10.1364/AO.45.000445. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Setlow P, Li YQ. 2009. Characterization of single heat-activated Bacillus spores using laser tweezers Raman spectroscopy. Optics Express 17:16480–16491. doi: 10.1364/OE.17.016480. [DOI] [PubMed] [Google Scholar]
- 18.Beaman TC, Pankratz HS, Gerhardt P. 1988. Heat shock affects permeability and resistance of Bacillus stearothermophilus spores. Appl Environ Microbiol 54:2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setlow P. 2007. Germination of spores of Bacillus subtilis by high pressure, p 15–40. In Doona CJ, Feeherry FE (ed), High pressure processing of foods. Blackwell Publishing, London, United Kingdom. [Google Scholar]
- 20.Black EP, Koziol-Dube K, Guan D, Wei J, Setlow B, Cortezzo DE, Hoover DG, Setlow P. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl Environ Microbiol 71:5879–5887. doi: 10.1128/AEM.71.10.5879-5887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nerandzic MM, Donskey CJ. 2013. Activate to eradicate: inhibition of Clostridium difficile spore outgrowth by the synergistic effects of osmotic activation and nisin. PLoS One 8:e54740. doi: 10.1371/journal.pone.0054740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowden CJ, Wheeldon LJ, Lambert PA, Rathbone DL, Worthington T. 2009. Abstr 19th Eur Cong Clin Microbiol Infect Dis, abstr P1260. [Google Scholar]
- 23.Nerandzic MM, Donskey CJ. 2010. Triggering germination represents a novel strategy to enhance killing of Clostridium difficile spores. PLoS One 5:e12285. doi: 10.1371/journal.pone.0012285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reineke K, Mathys A, Heinz V, Knorr D. 2013. Mechanisms of endospore inactivation under high pressure. Trends Microbiol 21:296–304. doi: 10.1016/j.tim.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Knorr D, Froehling A, Jaeger H, Reineke K, Schlueter O, Schoessler K. 2011. Emerging technologies in food processing. Annu Rev Food Sci Technol 2:203–235. doi: 10.1146/annurev.food.102308.124129. [DOI] [PubMed] [Google Scholar]
- 26.Considine KM, Kelly AL, Fitzgerald GF, Hill C, Sleator RD. 2008. High-pressure processing—effects on microbial safety and food quality. FEMS Microbiol Lett 281:1–9. doi: 10.1111/j.1574-6968.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- 27.Rastogi NK, Raghavarao KS, Balasubramaniam VM, Niranjan K, Knorr D. 2007. Opportunities and challenges in high pressure processing of foods. Crit Rev Food Sci Nutr 47:69–112. doi: 10.1080/10408390600626420. [DOI] [PubMed] [Google Scholar]
- 28.Sevenich R, Kleinstueck E, Crews C, Anderson W, Pye C, Riddellova K, Hradecky J, Moravcova E, Reineke K, Knorr D. 2014. High-pressure thermal sterilization: food safety and food quality of baby food puree. J Food Sci 79:M230–M237. doi: 10.1111/1750-3841.12345. [DOI] [PubMed] [Google Scholar]
- 29.Margosch D, Ehrmann MA, Gänzle MG, Vogel RF. 2004. Comparison of pressure and heat resistance of Clostridium botulinum and other endospores in mashed carrots. J Food Prot 67:2530–2537. [DOI] [PubMed] [Google Scholar]
- 30.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood CR, Cutting SM (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom. [Google Scholar]
- 32.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margosch D, Ehrmann MA, Buckow R, Heinz V, Vogel RF, Gänzle MG. 2006. High-pressure-mediated survival of Clostridium botulinum and Bacillus amyloliquefaciens endospores at high temperature. Appl Environ Microbiol 72:3476–3481. doi: 10.1128/AEM.72.5.3476-3481.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feeherry FE, Munsey DT, Rowley DB. 1987. Thermal inactivation and injury of Bacillus stearothermophilus spores. Appl Environ Microbiol 53:365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol 192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S, Setlow B, Wahome PG, Cowan AE, Plomp M, Malkin AJ, Setlow P. 2008. Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190:6741–6748. doi: 10.1128/JB.00896-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart K-AV, Setlow P. 2013. Numbers of individual nutrient germinant receptors and other germination proteins in spores of Bacillus subtilis. J Bacteriol 195:3575–3582. doi: 10.1128/JB.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez-Peralta A, Zhang P, Li YQ, Setlow P. 2012. Effects of sporulation conditions on the germination and germination protein levels of spores of Bacillus subtilis. Appl Environ Microbiol 78:2689–2697. doi: 10.1128/AEM.07908-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramirez-Peralta A, Stewart K-AV, Thomas SK, Setlow B, Chen Z, Li YQ, Setlow P. 2012. Effects of the SpoVT regulatory protein on the germination and germination protein levels of spores of Bacillus subtilis. J Bacteriol 194:3417–3425. doi: 10.1128/JB.00504-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez-Peralta A, Gupta S, Butzin XY, Setlow B, Korza G, Leyva-Vazquez MA, Christie G, Setlow P. 2013. Identification of new proteins that modulate the germination of spores of Bacillus species. J Bacteriol 195:3009–3021. doi: 10.1128/JB.00257-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korza G, Setlow P. 2013. Topology and accessibility of germination proteins in the Bacillus subtilis spore inner membrane. J Bacteriol 195:1484–1491. doi: 10.1128/JB.02262-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doona CJ, Ghosh S, Feeherry FF, Ramirez-Peralta A, Huang Y, Chen H, Setlow P. 2014. High pressure germination of Bacillus subtilis spores with alterations in levels and types of germination proteins. J Appl Microbiol 117:711–720. doi: 10.1111/jam.12557. [DOI] [PubMed] [Google Scholar]
- 43.Black EP, Wei J, Atluri S, Cortezzo DE, Koziol-Dube K, Hoover DG, Setlow P. 2007. Analysis of factors influencing the rate of germination of spores of Bacillus subtilis by very high pressure. J Appl Microbiol 102:65–76. doi: 10.1111/j.1365-2672.2006.03062.x. [DOI] [PubMed] [Google Scholar]
- 44.Cortezzo DE, Setlow P. 2005. Analysis of factors that influence the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J Appl Microbiol 98:606–617. doi: 10.1111/j.1365-2672.2004.02495.x. [DOI] [PubMed] [Google Scholar]
- 45.Cabrera-Martinez R-M, Tovar-Rojo F, Vepachedu VR, Setlow P. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J Bacteriol 185:2457–2464. doi: 10.1128/JB.185.8.2457-2464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart K-AV, Yi X, Ghosh S, Setlow P. 2012. Germination protein levels and rates of germination of spores of Bacillus subtilis with overexpressed or deleted genes encoding germination proteins. J Bacteriol 194:3156–3164. doi: 10.1128/JB.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi X, Liu J, Faeder JR, Setlow P. 2011. Synergism between different germinant receptors in the germination of Bacillus subtilis spores. J Bacteriol 193:4664–4671. doi: 10.1128/JB.05343-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paidhungat M, Setlow B, Daniels WB, Hoover D, Papafragkou E, Setlow P. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl Environ Microbiol 68:3172–3175. doi: 10.1128/AEM.68.6.3172-3175.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sevenich R, Bark F, Crews C, Anderson W, Riddellova K, Hradecky J, Moravcova E, Reineke K, Knorr D. 2013. Effects of high pressure thermal sterilization on the formation of food processing contaminants. Innov Food Sci Emerg Technol 20:42–50. doi: 10.1016/j.ifset.2013.07.006. [DOI] [Google Scholar]
- 50.Lødval IS, Granum PE, Rosnes JT, Lodval T. 2013. Activation of Bacillus spores at moderately elevated temperatures. Antonie Van Leeuwenhoek 103:693–700. doi: 10.1007/s10482-012-9839-3. [DOI] [PubMed] [Google Scholar]
- 51.Ross CA, Abel-Santos E. 2010. Guidelines for nomenclature assignment of Ger receptors. Res Microbiol 10:830–837. doi: 10.1016/j.resmic.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Catta P, Stewart KA, Dufner M, Setlow P, Hao B. 2011. Structure-based functional studies of the effects of amino acid substitutions in GerBC, the C subunit of the Bacillus subtilis GerB spore germinant receptor. J Bacteriol 193:4143–4152. doi: 10.1128/JB.05247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouget J-B, Aksel T, Roche J, Saldana J-L, Garcia AE, Barrick D, Royer CA. 2011. Size and sequence and the volume change of protein folding. J Am Chem Soc 133:6020–6027. doi: 10.1021/ja200228w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cioni P, Gabellieri E. 2011. Protein dynamics and pressure: what can high pressure tell us about protein structural flexibility. Biochim Biophys Acta 1814:934–941. doi: 10.1016/j.bbapap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Meersman F, Dobson CM, Heremans K. 2006. Protein unfolding, amyloid fibril formation and conformational energy landscapes under high pressure. Chem Soc Rev 35:908–917. doi: 10.1039/b517761h. [DOI] [PubMed] [Google Scholar]
- 56.Dembek M, Stabler RA, Witney AA, Wren BW, Fairweather NF. 2013. Transcriptional analysis of temporal gene expression in germinating Clostridium difficile 630 endospores. PLoS One 8:e64011. doi: 10.1371/journal.pone.0064011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Francis MB, Allen CA, Shrestha R, Sorg JA. 2013. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog 9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Paidhungat M, Setlow P. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant D-alanine. J Bacteriol 181:3341–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paidhungat M, Setlow P. 2000. Role of Ger proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. doi: 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Igarashi T, Setlow B, Paidhungat M, Setlow P. 2004. Analysis of the effects of a gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J Bacteriol 186:2984–2991. doi: 10.1128/JB.186.10.2984-2991.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.