Abstract
The inability of molecular detection methods to distinguish disinfected virions from infectious ones has hampered the assessment of infectivity for enteric viruses caused by disinfection practices. In the present study, the reduction of infectivity of murine norovirus S7-PP3 and mengovirus vMC0, surrogates of human noroviruses and enteroviruses, respectively, caused by free-chlorine treatment was characterized culture independently by detecting carbonyl groups on viral capsid protein. The amount of carbonyls on viral capsid protein was evaluated by the proportion of biotinylated virions trapped by avidin-immobilized gel (percent adsorbed). This culture-independent approach demonstrated that the percent adsorbed was significantly correlated with the logarithm of the infectious titer of tested viruses. Taken together with the results of previous reports, the result obtained in this study indicates that the amount of carbonyls on viral capsid protein of four important families of waterborne pathogenic viruses, Astroviridae, Reoviridae, Caliciviridae, and Picornaviridae, is increased in proportion to the received oxidative stress of free chlorine. There was also a significant correlation between the percent adsorbed and the logarithm of the ratio of genome copy number to PFU, which enables estimation of the infectious titer of a subject virus by measuring values of the total genome copy number and the percent adsorbed. The proposed method is applicable when the validation of a 4-log reduction of viruses, a requirement in U.S. EPA guidelines for virus removal from water, is needed along with clear evidence of the oxidation of virus particles with chlorine-based disinfectants.
INTRODUCTION
Waterborne pathogenic viruses pose health risks for humans all over the world. Viruses causing waterborne infectious diseases are composed of a broad array of viral families, which include Caliciviridae and Picornaviridae (1). Infections with viruses in these families bring about a variety of symptoms, including nausea, vomiting, diarrhea, fever, and abdominal pain (2). Among these viruses, noroviruses are well documented as a cause of gastroenteritis not only in young children but also among adults, particularly the elderly (3). Norovirus has a single-stranded RNA as a genome, which is covered by capsid proteins with cubic symmetry. Its diameter is around 39 nm (4). The genus Norovirus in the family Caliciviridae is divided into five genogroups, GI, GII, GIII, GIV, and GV, and strains in each genogroup can be further divided into genotypes (5). Norovirus GI, GII, and GIV strains infect humans, and GII strains are acquired more frequently from gastroenteritis patients than the other genogroups (6). Norovirus GII.4 genotype is the most prevalent (7, 8) and is known for having a broad binding spectrum with histo-blood group antigens on susceptible intestinal epithelial cells (9). Norovirus is unusual among the enteric viruses in inducing a relatively short-lived immunity; thus, individuals remain susceptible throughout life (10). Noroviruses being shed in human feces are transported through sewage systems and may reach receiving waters (11, 12), which requires us to manage the risks of waterborne gastroenteritis caused by human noroviruses. Human enterovirus (HEV), a genus of positive-sense, single-stranded RNA virus in the family Picornaviridae, also is waterborne and is associated with a number of clinical symptoms, characterized by a brief febrile illness and vesicular lesions on the hands, feet, mouth, and buttocks of infected individuals (13). Hand-foot-and-mouth disease (HFMD) is an acute enterovirus infection, and numerous large outbreaks of HFMD have occurred in the Asia-Pacific region, including China (14), Singapore (15), and Japan (16), in recent years. Since HFMD viral pathogens appear in environmental water (17), water disinfection may be effective to control infections of these enteroviruses.
A variety of intervention measures, including the disinfection of water and environmental fomite with oxidants and UV light, have been implemented to control infectious diseases caused by enteric viruses (18). However, enteric viruses in Caliciviridae, such as human noroviruses and sapoviruses, are noncultivatable, and molecular detection methods cannot distinguish disinfected virions from infectious ones (19), which has hampered the assessment of the infectivity loss of enteric viruses by those intervention procedures. Even for cultivatable viruses, including some HEV, cell culture-based investigation of disinfection efficiency on virus inactivation is labor- and cost-intensive, and the cell tropism limits the range of viral species that can be analyzed by tissue cells. In this context, robust and reliable culture-independent approaches are required (20).
We reported previously a culture-independent approach for detecting infectious enteric viruses, in which the cumulative carbonyl groups on viral capsid protein created by oxidative stresses are detected (21). Carbonyl groups on viral capsid protein are labeled with a biotin, and damaged (biotinylated) virions are separated from intact (nonbiotinylated) ones by an avidin gel. This approach allows us to separately quantify the intact and damaged virions with real-time quantitative reverse transcription-PCR (qRT-PCR). Our previous study showed that the quantity of carbonyl groups on capsid proteins of astrovirus and rotavirus increased with a free-chlorine dose and was correlated with the infectivity loss (21, 22). This approach could be utilized as a culture-independent tool for estimating the infectious titer of enteric viruses if the correlation between the quantity of the carbonyl groups on viral capsid protein and the infectivity loss is quantitatively significant for a wide array of waterborne viruses.
In this study, murine norovirus (MNV) and mengovirus (MgV) were employed as surrogates of Caliciviridae and Picornaviridae, respectively. Each test virus was treated with low doses of free chlorine, and the infectivity loss was evaluated with a plaque assay. The reduction of genome copies of each virus with the free-chlorine treatment also was analyzed with real-time qRT-PCR. Free-chlorine-treated virions then were labeled with biotin hydrazide. The biotinylated virions were trapped by a spin column filled with an avidin-immobilized gel. The amounts of untrapped and trapped virions by the spin column were separately quantified with real-time qRT-PCR, and the relationship between the carbonylation level and the infectivity loss was investigated. Based on the results of these assays, the feasibility of the proposed approach as a promising culture-independent tool for estimating the infectious titer of enteric viruses is discussed.
MATERIALS AND METHODS
Test viruses and cells.
RAW264.7 macrophage cells and HeLa cells were cultured in Eagle's minimal essential medium (MEM) with Earle's salts containing 10% (vol/vol) fetal bovine serum, 0.075% NaHCO3, 2 mM l-glutamine, 10 mM nonessential amino acids, 100 mg/ml penicillin, and 100 U/ml streptomycin. Cells were grown to a confluent monolayer at 37°C with 5% CO2 in a humidifying incubator. MNV strain S7-PP3 and MgV strain vMC0 were propagated in RAW264.7 cells and HeLa cells, respectively, for 3 to 5 days at 37°C. MNV S7-PP3 (AB435515) is a plaque-purified strain of MNV S7, which was isolated from a conventional mouse by Yukinobu Tohya, Nihon University, Japan. This strain is phylogenetically distinct from MNV1 but has been used as a surrogate for human norovirus (23). MgV vMC0 (ATCC VR-1597) is a recombinant virus derived from MgV strain M, which has been used as a process control virus in the quantification of enteric viruses in food and water (24). Virus stock was prepared by the freeze-thaw method, followed by polyethylene glycol precipitation (25), and kept in 200-ml aliquots at 20°C until use.
Titration of viral infectivity and quantification of genome number.
Viral infectious titers were assayed by the plaque method using corresponding cell lines. Tissue cells were plated at a density of 106 cells/well in 6-well plates and incubated for 2 days. Tenfold serial dilutions of test viruses were inoculated into cells and incubated for 90 min at 37°C under 5% CO2. After the incubation, agar containing 0.02% MEM was added to each well, and cells were incubated for 24 h. The following day, living cells were stained for 3 h by 0.015% neutral red solution. The plaque number was counted 3 days after the virus inoculation.
For quantification of the viral genome number, viral RNA was extracted with a QIAamp viral RNA minikit (Qiagen, Valencia, CA, USA). qRT-PCR assays for quantifying viral genome number were performed according to previous studies (24, 26). A PrimeScript reagent kit (TaKaRa Bio, Otsu, Japan) using oligo(dT) primer and random hexamer was used to synthesize cDNA, in which each reaction mixture (10 μl) was composed of 2 μl of 5× PrimeScript buffer, 0.5 μl of PrimeScript RT enzyme, 0.5 μl of oligo(dT) primer, 2 μl of random hexamer, 3 μl of distilled and deionized water (DDW), and 2 μl of extracted RNA. The RT reaction was carried out by incubating at 37°C for 15 min, 42°C for 15 min, and 85°C for 5 s. The cDNA synthesis was followed by the TaqMan qPCR assays for MNV and MgV using Premix Ex Taq (Perfect real time; TaKaRa Bio, Otsu, Japan). The PCR for MNV genome amplification was carried out by heating at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min with a PCR mixture (25 μl) composed of 12.5 μl of 2× Premix Ex Taq, 0.5 μl of ROX reference dye II, 1 μl of 10 μM forward primer, 1 μl of 10 μM reverse primer, 0.8 μl of 10 μM fluorogenic probe, 5.2 μl of DDW, and 4.0 μl of template cDNA. The PCR for MgV genome amplification was carried out by heating at 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min with a PCR mixture (25 μl) composed of 12.5 μl of 2× Premix Ex Taq, 0.5 μl of ROX reference dye II, 0.5 μl of 10 μM forward primer, 0.5 μl of 10 μM reverse primer, 0.5 μl of 10 μM fluorogenic probe, 6.5 μl of DDW, and 4.0 μl of template cDNA. Primers and probes used in this study are indicated in Table S1 in the supplemental material. All PCRs were performed in MicroAmp optical 96-well reaction plates with an Applied Biosystems 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA).
Free-chlorine treatment and biotinylation of virions.
Test viruses were treated by free chlorine from sodium hypochlorite (Sigma). The initial concentration of free chlorine, measured with a chlorine and pH test kit (Macherey-Nagel), was varied between 0 and 20 ppm so as to prepare virus suspensions with various infectious titers. The contact time with free chlorine was 3 min, and then the free chlorine was neutralized immediately by the addition of 100 mM sodium thiosulfate solution. As a control, a virus suspension in the absence of free chlorine also was incubated under the same conditions for each test virus. Virus suspensions after the incubation and the neutralization were stored at −20°C. Virus particles were biotinylated as follows. First, 25 μl of 50 mM EZ-Link biotin hydrazide (Pierce) in dimethylsulfoxide (DMSO) was added to 1 ml of a virus suspension. The mixture of biotin hydrazide and virus particles next was incubated at room temperature for 2 h with moderate mixing. The mixture was purified by a Zeba desalt spin column (Thermo Scientific) to remove unreacted biotin hydrazide. Biotinylated virus particles then were stored at −20°C.
Separation of biotinylated virions with avidin-immobilized gel.
The biotinylated virions were separated as described by Tojo et al. (22), with slight modifications. Viruses were added into the spin column filled with an avidin-immobilized gel. After incubation for 10 min, the spin column was centrifuged at 2,000 × g for 1 min, and filtrate was recovered in which untrapped (nonbiotinylated) virions are included (untrapped fraction). Phosphate-buffered saline (8.00 g sodium chloride, 1.15 g disodium hydrogen phosphate, 0.20 g potassium dihydrogen phosphate, 0.20 g potassium chloride, pH 7.2) was added into the spin column for washing. After incubation for 10 min, the spin column was centrifuged at 2,000 × g for 1 min and filtrate was recovered (wash 1 fraction). This washing process was repeated (wash 2 fraction). The wash 1 and wash 2 fractions were combined to be a trapped fraction. The viral genome numbers in untrapped and trapped fractions were measured by qRT-PCR as described above. All statistical calculations were performed using Microsoft Excel (Mac 2011).
RESULTS
Free-chlorine treatment and carbonyl group formation on MNV.
The genome copy number and infectious titer were analyzed for 13 preparations of MNV suspension (Table 1). Although MNV suspensions were treated with various initial concentrations of free chlorine up to 5 ppm (this concentration was not kept during the contact time of 3 min), the genome copy number was stable at a mean (± standard deviation [SD]) of (5.02 ± 2.03) × 1010 copies/ml, which demonstrates that even the highest initial concentration of free chlorine (5.0 ppm) did not destroy the target viral genome region of qRT-PCR. Meanwhile, the infectious titer was affected by the free-chlorine treatment. The initial free-chlorine concentration of 0.2 ppm (MNV-4, MNV-7, and MNV-10) had a limited effect on the infectious titer in PFU, but the infectious titer was reduced when the initial concentration of free chlorine was 1.0 ppm or higher (we did not calculate the concentration-time [CT] value because the initial concentration of free chlorine was not controlled during the contact time). The mean (±SD) value of PFU of MNV suspensions treated with the initial free-chlorine concentration of 0.2 ppm (MNV-4, MNV-7, and MNV-10) or lower (MNV-1, MNV-3, MNV-6, and MNV-9) was (5.3 ± 4.5) × 104 PFU/ml, whereas those treated with 1.0 ppm or higher initial free-chlorine concentrations (MNV-2, MNV-5, MNV-8, MNV-11, MNV-12, and MNV-13) had an infectious titer of 5.5 × 102 PFU/ml or less. As a result, the mean (±SD) logarithmic value of the genome copy number for giving 1 PFU (copies/PFU) of MNV treated with 0.2 ppm or a lower initial free-chlorine concentration was 6.2 ± 0.4, and that of MNV treated with a 1.0 ppm or higher initial free-chlorine concentration was 7.9 or larger (Table 1).
TABLE 1.
Percentage of trapped virions of each murine norovirus preparation by avidin-immobilized gel
| Sample code | Initial free-chlorine concna (ppm) | Contact time (min) | Log (genome copies/ml) | Log (PFU/ml) | Log (genome copies/PFU) | % adsorbedb |
|---|---|---|---|---|---|---|
| MNV-1 | 10.8 | 4.9 | 5.9 | 61.1 | ||
| MNV-2 | 2.0 | 3 | 10.6 | 1.5 | 9.0 | 79.0 |
| MNV-3 | 10.9 | 4.7 | 6.2 | 54.3 | ||
| MNV-4 | 0.2 | 3 | 10.9 | 4.2 | 6.7 | 60.5 |
| MNV-5 | 2.0 | 3 | 10.6 | 2.7 | 7.9 | 70.4 |
| MNV-6 | 10.6 | 4.0 | 6.6 | 50.0 | ||
| MNV-7 | 0.2 | 3 | 10.5 | 3.8 | 6.7 | 60.0 |
| MNV-8 | 2.0 | 3 | 10.3 | 2.1 | 8.2 | 75.1 |
| MNV-9 | 10.8 | 5.1 | 5.7 | 52.5 | ||
| MNV-10 | 0.2 | 3 | 10.7 | 4.9 | 5.8 | 58.3 |
| MNV-11 | 2.0 | 3 | 10.6 | 2.7 | 7.8 | 64.9 |
| MNV-12 | 1.0 | 3 | 10.8 | 2.7 | 8.1 | 72.4 |
| MNV-13 | 5.0 | 3 | 10.4 | 1.2 | 9.3 | 92.1 |
Free-chlorine concentration was not controlled during the contact time, which is why the concentration-time (CT) value was not calculated.
Percentage of trapped virions by avidin-immobilized gel.
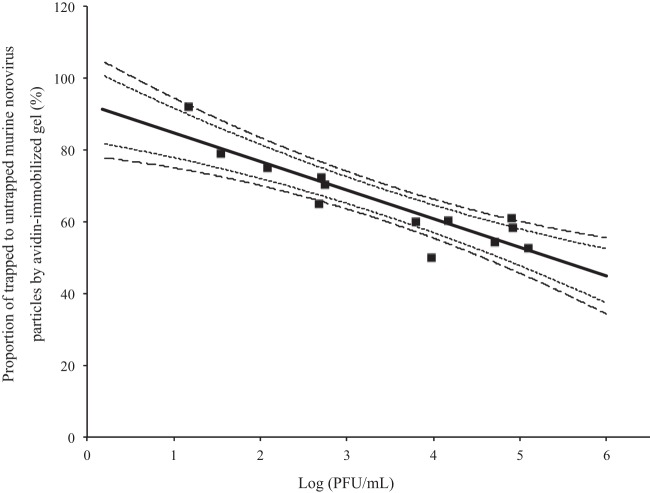
The correlation between the log (PFU/ml) of MNV and the percent adsorbed is depicted in Fig. 1. The linear correlation in Fig. 1 is statistically significant, with a coefficient of determination of 0.81 (see Table S2 in the supplemental material). The null hypothesis that the slope is not different from zero was rejected with a P value of 3.00 × 10−5 (see Table S4). This correlation analysis result indicates that percent adsorbed values, which reflect the amount of oxidative stress markers on viral capsid protein, negatively correlate with values of the log (PFU/ml) and can be used as an index of infectivity of MNV. This significant correlation between values of the log (PFU/ml) and the percent adsorbed allows us to estimate the infectious titer of a subject virus culture independently when the total genome copy number is around 1010 copies/ml, because there are only two variables (percent adsorbed and viral infectious titer), and one of them (percent adsorbed) is measurable by qRT-PCR.
FIG 1.
Correlation between the proportion of trapped to untrapped virions by avidin-immobilized gel (percent adsorbed) and the log (PFU/ml) of murine norovirus S7-PP3. Shorter and longer dashed lines indicate 95 and 99% confidence intervals, respectively.
Free-chlorine treatment and carbonyl group formation on MgV.
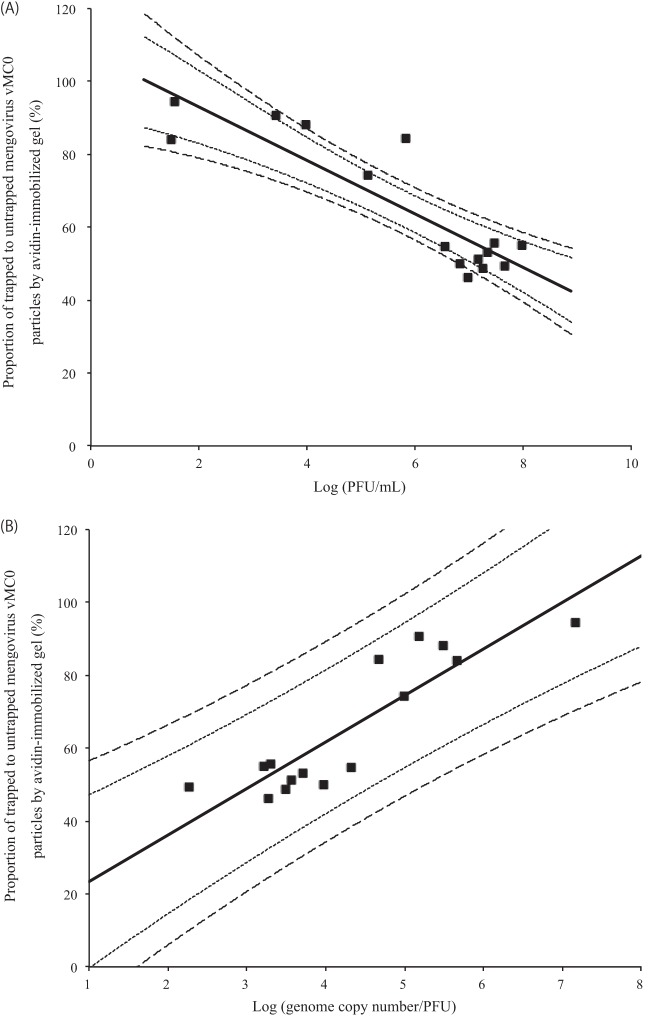
The genome copy number and infectious titer were analyzed for 15 preparations of MgV suspension (Table 2). The genome copy number of MgV (from 1.36 × 107 [MgV-3] to 1.53 × 1011 [MgV-1] copies/ml) was not stable compared to that of MNV, because MgV suspensions were treated with higher initial concentrations of free chlorine (up to 20 ppm) than MNV. Because of this stronger oxidative stress, the infectious titer (PFU/ml) was differentiated between 3.0 × 101 (MgV-3) and 9.5 × 107 (MgV-1). As a result, the logarithmic values of copies/PFU of MgV were distributed from 2.3 (MgV-9) to 7.2 (MgV-13), and the mean (±SD) was 4.3 ± 1.3. The correlation between values of the log (PFU/ml) of MgV and the percent adsorbed is shown in Fig. 2A. The linear correlation in Fig. 2A is statistically significant, with a coefficient of determination of 0.78 (see Table S5 in the supplemental material). The null hypothesis that the slope is not different from zero was rejected with a P value of 1.19 × 10−5 (see Table S7). This result of the correlation analysis indicates that values of the percent adsorbed can be used as an index of infectivity of MgV as well as MNV.
TABLE 2.
Percentage of trapped virions of each mengovirus vMC0 preparation by avidin-immobilized gel
| Sample code | Initial free-chlorine concna (ppm) | Contact time (min) | Log (genome copies/ml) | Log (PFU/ml) | Log (genome copies/PFU) | % adsorbedb |
|---|---|---|---|---|---|---|
| MgV-1 | 11.2 | 8.0 | 3.2 | 55.1 | ||
| MgV-2 | 10 | 3 | 10.5 | 5.8 | 4.7 | 84.3 |
| MgV-3 | 20 | 3 | 7.1 | 1.5 | 5.7 | 84.1 |
| MgV-4 | 10.9 | 6.6 | 4.3 | 54.6 | ||
| MgV-5 | 10 | 3 | 9.5 | 4.0 | 5.5 | 88.0 |
| MgV-6 | 10.8 | 6.8 | 4.0 | 50.0 | ||
| MgV-7 | 10 | 2 | 8.6 | 3.4 | 5.2 | 90.6 |
| MgV-8 | 10.8 | 7.5 | 3.3 | 55.7 | ||
| MgV-9 | 9.9 | 7.7 | 2.3 | 49.5 | ||
| MgV-10 | 6 | 3 | 10.7 | 7.3 | 3.5 | 48.6 |
| MgV-11 | 7 | 2 | 10.2 | 7.0 | 3.3 | 46.3 |
| MgV-12 | 11.0 | 7.3 | 3.7 | 53.0 | ||
| MgV-13 | 7 | 2 | 8.7 | 1.5 | 7.2 | 94.4 |
| MgV-14 | 3 | 3 | 10.7 | 7.2 | 3.6 | 51.4 |
| MgV-15 | 5 | 3 | 10.1 | 5.1 | 5.0 | 74.2 |
Free-chlorine concentration was not controlled during the contact time, which is why the concentration-time (CT) value was not calculated.
Percentage of trapped virions by avidin-immobilized gel.
FIG 2.
Correlation between the proportion of trapped to untrapped virions by avidin-immobilized gel (percent adsorbed) and the log (PFU/ml) (A) or the log (copies/PFU) (B) of mengovirus vMC0. Shorter dashed lines in panels A and B are 95% confidence and prediction intervals, respectively. Longer dashed lines in panels A and B are 99% confidence and prediction intervals, respectively.
However, the correlation shown in Fig. 2A is difficult to directly use for the estimation of infectious titer of MgV, in contrast to MVN in Fig. 1, because of the unstable values of the total genome copy number of MgV. For example, percent adsorbed values of MgV-2 and MgV-3 are almost the same (84.3 and 84.1%, respectively), but there is a 4-log difference between the infectious titer of these two MgV preparations (6.5 × 105 and 3.0 × 101 PFU/ml, respectively), even though there was a significant correlation between these two parameters. In this case, it is better to depict the correlation between percent adsorbed and log (copies/PFU), as shown in Fig. 2B, because the values of the genome copy number are proportional to that of PFU to some extent, which makes the values of log (copies/PFU) relatively stable at a similar level of percent adsorbed. Based on the correlation shown in Fig. 2B, it is possible to estimate the infectious titer of a subject virus using two measurable variables (total genome copy number and percent adsorbed) by qRT-PCR.
DISCUSSION
RT-PCR and its related technologies have revealed that a variety of pathogenic viruses are causing infectious diseases via water (27), foods (28), and fomites (29). It also has been shown that noncultivatable viruses, including human noroviruses, constitute a significant part of the viral disease burden (30). One of the important scientific challenges in the field of environmental and public health virology is the evaluation of health risks caused by these noncultivatable viruses in natural environments. Although the employment of surrogate viruses is a promising option for managing infection risks caused by enteric viruses (31, 32), the information related to the viability of noncultivatable viruses per se is indispensable to address the infectious risks of noncultivatable enteric viruses contaminating environments.
Culture-independent tests for assaying viral infectivity have been reviewed by several researchers (19, 20, 33). Culture-independent methods for viral infectivity test are roughly categorized into six approaches: (i) multiple-target-region PCR (34), (ii) long-target-region PCR (35, 36), (iii) affinity separation of viral antigens associated with the viral genome before PCR (37, 38), (iv) enzymatic treatment of viral particles before RT-PCR (39, 40), (v) intercalative dye treatment of viral particles before RT-PCR (41, 42), and (vi) separation of biotinylated viral particles before RT-PCR, which is our approach (21, 22). Each approach has pros and cons (20), and it is important to clarify applicable situations for each approach so that public health workers and water engineers can select the best-fit techniques.
Any culture-independent assay must give signals that have a significant correlation with the viral infectious titer in a subject sample. The proposed approach, quantifying carbonyl groups on viral capsid protein, met this requirement when the method was applied for human astrovirus (21), simian rotavirus (22), MNV, and MgV (this study). These tested viruses cover four important families of waterborne pathogenic viruses: Astroviridae, Reoviridae, Caliciviridae, and Picornaviridae. It may be possible to extend the proposed approach to other nonenveloped viruses that have not been tested, but we need to pay attention to the difference of the physicochemical properties of nonenveloped viruses, such as particle diameter and viral surface charge, which determine the movement behavior of viral particles in the column filled with an avidin-immobilized gel. If the regression line, such as those shown in Fig. 1 and 2, is reproducible within a viral family, the proposed approach is applicable to other nonenveloped viruses in each family that were not tested, although this has to be tested in a future study.
One important feature of the proposed approach is to detect carbonyl groups on the viral capsid protein formed in the early stage of the oxidation with free chlorine, because the viral genome must be associated with biotinylated capsid protein trapped by avidin so as to quantify it by qRT-PCR. The qRT-PCR for MgV is targeting the 5′ noncoding region (24), which may be vulnerable to chlorine treatment in common with the other picornaviruses (36, 43). The 4-log reduction of genome copy number of MgV at the highest oxidative stress in this study may be explained by the possible vulnerability of this target region (Table 2), although this level of the reduction in the genome copy number is unlikely to affect the applicability of the proposed approach. Approximately half of untreated stocks of MNV and MgV were shown to bind to the avidin columns (Tables 1 and 2), as were observed for untreated astrovirus (21) and rotavirus (22) in our previous studies. These observations suggest that carbonyl groups on the viral capsid protein of fresh virus stocks also are produced by the oxidative stresses during the replication processes in the host cells (44), although this is not proved in the present study.
The other application limitation is that the proposed approach needs a high concentration of viruses, because the virions trapped by avidin have to be distinguished from untrapped virions by qRT-PCR, in which the quantification limit value is usually more than 102 copies/ml of water samples before genome extraction. An effective method of concentrating viruses present in wastewater is to be included when the proposed approach is applied to wastewater samples in the real-world setting. There are many inactivation processes along with free-chlorine treatment, such as the denaturing of the three-dimensional structure of viral capsid protein with heat, the breakage of viral capsid protein with reactive oxygen species, and the destruction of viral genome with UV light (40, 45). However, neither denaturing of the viral capsid protein with heat nor genome damage by UV light can be detected with the proposed approach. Since the denaturing of the viral capsid protein and the destruction of the genome are important processes of virus inactivation, the combination of the present approach with the genome-targeted methodologies may yield important information regarding the virus inactivation mechanisms in a variety of disinfection interventions (46). Thus, one of the most applicable situations for the proposed approach is in the efficacy tests of chlorine-based disinfection interventions using enteric viruses inoculated in water samples. Since carbonyl groups can be formed by the other oxidants, such as ozone and reactive oxygen species (47), it must be able to extend the application to the other oxidation agent-based disinfections. Critical information for the selection of culture-independent approaches is the cost of analysis, and the proposed approach has been regarded as uneconomical (20). The cost of the proposed method is about $25 per sample, 68 and 23% of which are the cost of the desalt spin column for eliminating free biotin hydrazide and the avidin gel, respectively. Alternative materials for the same purposes should be found; otherwise, this approach is not competitive with the other approaches and is applicable only when the evidence of oxidation of viral particles is critical in the disinfection efficacy evaluation.
We described in Results that it is possible to estimate the infectious titer of the subject viruses culture independently based on the correlation in Fig. 1 for MNV and Fig. 2B for MgV. Since the total genome number of MNV was stable (5.02 [±2.03] × 1010 copies/ml) in this study (Table 1), the conversion of the x axis in Fig. 1 (log [PFU/ml]) to log (genome copy number/PFU) does not give a practical significance in the estimation of the infectious titer of MNV. Figure 1 can be used for the estimation of the infectious titer of MNV only when the total genome copy number is around 1010 copies/ml. On the other hand, it is possible to estimate the infectious titer of MgV using two measurable valuables (total genome copy number and percent adsorbed) by qRT-PCR based on the correlation in Fig. 2B, but we have to pay attention to the fact that the range of 95% prediction intervals reaches almost 3 logs at an identical percent adsorbed value. Even at the observed data level, the log (genome copy number/PFU) values are in the range of a 2-log distance (from 2.3 [MgV-9] to 4.3 [MgV-4]) when the values of percent adsorbed are between 40 and 60% for MgV (Table 2). These results mean that an infectivity reduction of less than 2 logs is difficult to evaluate based on the correlation between the percent adsorbed and the ratio of the genome copy number to PFU of MgV, but at the same time, the proposed method is able to evaluate the virus inactivation efficiency of 4 logs or more in a water disinfection process. Since 4-log reduction is a critical target as a requirement in several guidelines for pathogen removal from water, such as the surface water treatment rule of the U.S. EPA, the proposed method is applicable when responsible water engineers need clear evidence that virus particles are oxidized with chlorine-based disinfectants and would like to validate the 4-log reduction of nonenveloped viruses. Alternative technologies for detecting biotinylation, such as flow cytometry (48), may help to distinguish the density of carbonyl groups on virions and validate a less than 3-log reduction, although the small size of nonenveloped virions (<100 nm) and low concentration of viruses in environmental water samples impose a limit on the application of the alternative approaches.
The fact that a number of researchers have studied enteric viruses with a variety of cell cultures leads us to speculate that enteric viruses newly discovered in the near future by whatever approach, including metagenomics (49), will be noncultivatable, at least immediately after the discovery. In this context, culture-independent approaches are indispensable for managing the virological safety of water, food, and other environmental vehicles. This study presents a culture-independent estimation process of infectious titers of enteric viruses, along with the feasibility of the application of the proposed methodology. Application examples of culture-independent approaches to viruses in real water and food samples should be accumulated in further studies to find the best combination of culture-independent approaches for specific situations.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate Yukinobu Tohya, Nihon University, Japan, for kindly providing the MNV strain. We also thank Rie Nomachi, Division of Environmental Engineering, Faculty of Engineering, Hokkaido University, for her excellent technical assistance.
This study was supported by the Japan Science and Technology Agency (JST) through Core Research for Evolutionary Science and Technology (CREST) and the Japan Society for the Promotion of Science through a Grant-in-Aid for Scientific Research (A) (24246089).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03802-14.
REFERENCES
- 1.Bosch A, Guix S, Sano D, Pintó RM. 2008. New tools for the study and direct surveillance of viral pathogens in water. Curr Opin Biotechnol 19:295–301. doi: 10.1016/j.copbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasickova P, Dvorska L, Lorencova A, Pavlik I. 2005. Viruses as a cause of foodborne diseases: a review of the literature. Vet Med 50:89–104. [Google Scholar]
- 3.Harris JP, Edmunds WJ, Pebody R, Brown DW, Lopman BA. 2008. Deaths from norovirus among the elderly, England and Wales. Emerg Infect Dis 14:1546–1552. doi: 10.3201/eid1410.080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad BVV, Hardy ME, Dokland T, Bella J, Rossman MG, Estes MK. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 5.Kageyama T, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Kojima S, Takai R, Oka T, Takeda N, Katayama K. 2004. Coexisting of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to Norovirus in Japan. J Clin Microbiol 42:2988–2995. doi: 10.1128/JCM.42.7.2988-2995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svraka S, van der Veer B, Duizer E, Dekkers J, Koopmans M, Vennema H. 2009. Novel approach for detection of enteric viruses to enable syndrome surveillance of acute viral gastroenteritis. J Clin Microbiol 47:1674–1679. doi: 10.1128/JCM.00307-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebenga JJ, Vennema H, Duizer E, Koopmans MP. 2007. Gastroenteritis caused by norovirus GGII.4, The Netherlands, 1994-2005. Emerg Infect Dis 13:144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motomura K, Oka T, Yokoyama M, Nakamura H, Mori H, Ode H, Hansman GS, Katayama K, Kanda T, Tanaka T, Takeda N, Sato H. 2008. Identification of monomorphic and divergent haplotypes in the 2006-2007 norovirus GII/4 epidemic population by genomewide tracing of evolutionary history. J Virol 82:11247–11262. doi: 10.1128/JVI.00897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirato H, Ogawa S, Ito H, Sato T, Kameyama A, Narimatsu H, Xiaofan Z, Miyamura T, Wakita T, Ishii K, Takeda N. 2008. Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding. J Virol 82:10756–10767. doi: 10.1128/JVI.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green K, Chanock M, Kapikian A. 2001. Human caliciviruses, p 841–874. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Wolters Kluwer Business, Philadelphia, PA. [Google Scholar]
- 11.Lodder WJ, de Roda Husman AM. 2005. Presence of norovirus and other enteric viruses in sewage and surface waters in The Netherlands. Appl Environ Microbiol 71:1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueki Y, Sano D, Watanabe T, Akiyama K, Omura T. 2005. Norovirus pathway in water environment estimated by genetic analysis of strains from patients of gastroenteritis, sewage, treated wastewater, river water and oysters. Water Res 39:4271–4280. doi: 10.1016/j.watres.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC, How OM, Cardosa MJ, McMinn PC. 2007. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam. Emerg Infect Dis 13:1733–1741. doi: 10.3201/eid1311.070632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang D, Yan D, Zhu S, Liu J, Wang H, Zhao S, Yu D, Nan L, An J, Chan L, An H, Xu A, Xu W. 2010. Molecular evidence of persistent epidemic and evolution of subgenotype B1 coxsackie virus A16-associated hand, foot, and mouth disease in China. J Clin Microbiol 48:619–622. doi: 10.1128/JCM.02338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, Chow VTK. 2010. The largest outbreak of hand, foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis 14:e1076–1081. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Hosoya M, Kawasaki Y, Sato M, Honzumi K, Hayashi A, Hiroshima T, Ishiko H, Kato K, Suzuki H. 2007. Genetic diversity of coxsackievirus A16 associated with hand, foot, and mouth disease epidemics in Japan from 1983 to 2003. J Clin Microbiol 45:112–120. doi: 10.1128/JCM.00718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Z, Wang XC, Xu L, Zhang C, Funamizu N, Okabe S, Sano D. 2014. Estimation of contamination sources of human enteroviruses in a wastewater treatment and reclamation system by PCR-DGGE. Food Environ Virol 6:99–109. doi: 10.1007/s12560-014-9140-x. [DOI] [PubMed] [Google Scholar]
- 18.Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM. 2008. Science and technology for water purification in the coming decades. Science 452:301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez RA, Pepper IL, Gerba CP. 2009. Application of PCR-based methods to assess the infectivity of enteric viruses in environmental samples. Appl Environ Microbiol 75:297–307. doi: 10.1128/AEM.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza IA, Jurzik L, Uberla K, Wilhelm M. 2011. Methods to detect infectious human enteric viruses in environmental water samples. Int J Hyg Environ Health 214:424–436. doi: 10.1016/j.ijheh.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano D, Pintó RM, Omura T, Bosch A. 2010. Detection of oxidative damages on viral capsid protein for evaluating structural integrity and infectivity of human norovirus. Environ Sci Technol 44:808–812. doi: 10.1021/es9018964. [DOI] [PubMed] [Google Scholar]
- 22.Tojo K, Sano D, Miura T, Nakagomi T, Nakagomi O, Okabe S. 2013. A new approach for evaluating the infectivity of noncultivatable enteric viruses without cell culture. Water Sci Technol 67:2236–2240. doi: 10.2166/wst.2013.114. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Tohya Y, Matsubara K, Haramoto E, Utagawa E, Katayama H. 2010. Chlorine inactivation of human norovirus, murine norovirus and poliovirus in drinking water. Lett Appl Microbiol 51:119–121. doi: 10.1111/j.1472-765X.2010.02869.x. [DOI] [PubMed] [Google Scholar]
- 24.Costafreda MI, Bosch A, Pinto RM. 2006. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl Environ Microbiol 72:3846–3855. doi: 10.1128/AEM.02660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis GD, Metcalf TG. 1989. Polyethylene glycol precipitation for recovery of pathogenic viruses including hepatitis A and human rotavirus from oyster, water and sediments. Appl Environ Microbiol 54:1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitajima M, Oka T, Tohya Y, Katayama H, Takeda N, Katayama K. 2010. Development and application of a broadly reactive real-time reverse transcription-PCR assay to detect murine noroviruses. J Virol Methods 169:269–273. doi: 10.1016/j.jviromet.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Hewitt J, Bell D, Simmons DC, Rivera-Aban M, Wolf S, Greening GE. 2007. Gastroenteritis outbreak caused by waterborne norovirus at a New Zealand ski resort. Appl Environ Microbiol 73:7853–7857. doi: 10.1128/AEM.00718-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Guyader FS, Bon F, DeMedici D, Parnaudeau S, Bertone A, Crudeli S, Doyle A, Zidane M, Suffredini E, Kohli E, Maddalo F, Monini M, Gallay A, Pommepuy M, Pothier P, Ruggeri FM. 2006. Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption. J Clin Microbiol 44:3878–3882. doi: 10.1128/JCM.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boone SA, Gerba CP. 2007. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol 73:1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae J, Schwab KJ. 2008. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol 74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinclair RG, Rose JB, Hashsham SA, Gerba CP, Haas CN. 2012. Criteria for selection of surrogates used to study the fate and control of pathogens in the environment. Appl Environ Microbiol 78:1969–1977. doi: 10.1128/AEM.06582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cliver DO. 2009. Capsid and infectivity in virus detection. Food Environ Virol 1:123–128. doi: 10.1007/s12560-009-9020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pecson BM, Ackermann M, Kohn T. 2011. Framework for using quantitative PCR as a nonculture based method to estimate virus infectivity. Environ Sci Technol 45:2257–2263. doi: 10.1021/es103488e. [DOI] [PubMed] [Google Scholar]
- 35.Shin GA, Sobsey MD. 2003. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl Environ Microbiol 69:3975–3978. doi: 10.1128/AEM.69.7.3975-3978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonet J, Gantzer C. 2006. Inactivation of poliovirus 1 and F-specific RNA phages and degradation of their genomes by UV irradiation at 254 nanometers. Appl Environ Microbiol 72:7671–7677. doi: 10.1128/AEM.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang YC, Leong OM, Chen W, Yates MV. 2007. Comparison of a reporter assay and immunomagnetic separation real-time reverse transcription-PCR for the detection of enteroviruses in seeded environmental water samples. Appl Environ Microbiol 73:2338–2340. doi: 10.1128/AEM.01758-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Baert L, Coillie EV, Uyttendaele M. 2011. Critical studies on binding-based RT-PCR detection of infectious noroviruses. J Virol Methods 177:153–159. doi: 10.1016/j.jviromet.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Nuanualsuwan S, Cliver DO. 2002. Pretreatment to avoid positive RT-PCR results with inactivated viruses. J Virol Methods 104:217–225. doi: 10.1016/S0166-0934(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 40.Pecson BM, Martin LV, Kohn T. 2009. Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UV-B, radiation, and singlet oxygen: advantages and limitations of an enzymatic treatment to reduce false-positive results. Appl Environ Microbiol 75:5544–5554. doi: 10.1128/AEM.00425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parshionikar S, Laseke I, Fout GS. 2010. Use of propidium monoazide in reverse transcriptase PCR to distinguish between infectious and noninfectious enteric viruses in water samples. Appl Environ Microbiol 76:4318–4326. doi: 10.1128/AEM.02800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim K, Katayama H, Kitajima M, Tohya Y, Ohgaki S. 2011. Development of a real-time RT-PCR assay combined with ethidium monoazide treatment for RNA viruses and its application to detect viral RNA after heat exposure. Water Sci Technol 63:502–507. doi: 10.2166/wst.2011.249. [DOI] [PubMed] [Google Scholar]
- 43.Li JW, Xin ZT, Wang XW, Zheng JL, Chao FH. 2002. Mechanisms of inactivation of hepatitis A virus by chlorine. Appl Environ Microbiol 68:4951–4955. doi: 10.1128/AEM.68.10.4951-4955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGuire KA, Barlan AU, Griffin TM, Wiethoff CM. 2011. Adenovirus type 5 rupture of lysosomes leads to cathepsin b-dependent mitochondrial stress and production of reactive oxygen species. J Virol 85:10806–10813. doi: 10.1128/JVI.00675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieto-Juarez JI, Pierzchla K, Sienkiewicz A, Kohn T. 2010. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: role of transition metals, hydrogen peroxide and sunlight. Environ Sci Technol 44:3351–3356. doi: 10.1021/es903739f. [DOI] [PubMed] [Google Scholar]
- 46.Kingsley DH, Vincent EM, Meade GK, Watson CL, Fan X. 2014. Inactivation of human norovirus using chemical sanitizers. Int J Food Microbiol 171:94–99. doi: 10.1016/j.ijfoodmicro.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. 2003. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 48.Inghirami G, Nakamura M, Balow JE, Notkins AL, Casali P. 1988. Model for studying virus attachment: identification and quantification of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J Virol 62:2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aw TG, Howe A, Rose JB. 2014. Metagenomic approaches for direct and cell culture evaluation of the virological safety of wastewater. J Virol Methods 210:15–21. doi: 10.1016/j.jviromet.2014.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.