Abstract
Plant pathogen Xanthomonas campestris pv. campestris produces cis-11-methyl-2-dodecenoic acid (diffusible signal factor [DSF]) as a cell-cell communication signal to regulate biofilm dispersal and virulence factor production. Previous studies have demonstrated that DSF biosynthesis is dependent on the presence of RpfF, an enoyl-coenzyme A (CoA) hydratase, but the DSF synthetic mechanism and the influence of the host plant on DSF biosynthesis are still not clear. We show here that exogenous addition of host plant juice or ethanol extract to the growth medium of X. campestris pv. campestris could significantly boost DSF family signal production. It was subsequently revealed that X. campestris pv. campestris produces not only DSF but also BDSF (cis-2-dodecenoic acid) and another novel DSF family signal, which was designated DSF-II. BDSF was originally identified in Burkholderia cenocepacia to be involved in regulation of motility, biofilm formation, and virulence in B. cenocepacia. Functional analysis suggested that DSF-II plays a role equal to that of DSF in regulation of biofilm dispersion and virulence factor production in X. campestris pv. campestris. Furthermore, chromatographic separation led to identification of glucose as a specific molecule stimulating DSF family signal biosynthesis in X. campestris pv. campestris. 13C-labeling experiments demonstrated that glucose acts as a substrate to provide a carbon element for DSF biosynthesis. The results of this study indicate that X. campestris pv. campestris could utilize a common metabolite of the host plant to enhance DSF family signal synthesis and therefore promote virulence.
INTRODUCTION
Many microorganisms employ a cell-to-cell communication mechanism known as quorum sensing (QS) to coordinate group behavior in a cell density-dependent manner (1–3). This regulation mechanism consists of the production, release, and perception of small diffusible signal molecules, transduction of signal, and activation of target gene expression. When cell density reaches a certain threshold, the accumulated signals initiate a set of biological activities in a coordinated fashion. Since the 1980s, a range of QS signals have been identified in bacteria. Among them, the best-characterized quorum-sensing signals belong to the N-acyl homoserine lactone (AHL) family, which have been identified in many bacterial species (4, 5). The substrates for AHL synthesis are acyl-ACP or acyl-coenzyme A (CoA) and AHL S-adenosylmethionine (6–8). The biosynthetic pathways for these substrates are well known, and the catalytic mechanism of AHL synthesis has been studied in detail (8).
The diffusible signal factor (DSF) is another important type of QS signal which controls many biological functions such as biofilm formation, motility, virulence, and antibiotic resistance (1, 9, 10). DSF was originally purified and structurally characterized in Xanthomonas campestris pv. campestris (11). Subsequently, the signal and its structural analogues were found in many other bacterial pathogens such as Stenotrophomonas maltophilia (12), Xylella fastidiosa (13, 14), Burkholderia cenocepacia (15), Pseudomonas aeruginosa (16), B. cepacia complex (17), Xanthomonas oryzae pv. oryzae (18), and Streptococcus mutans (19).
Previous studies identified several rpf genes essential for DSF biosynthesis, signal perception, and transduction. The synthesis of DSF signal in X. campestris pv. campestris is dependent on rpfF, which is a dual-function enzyme that synthesizes DSF by dehydration of a 3-hydroxyacyl-acyl carrier protein fatty acid synthetic intermediate and also cleaves the thioester bond to release free DSF, whereas rpfC and rpfG encode a multidomain kinase sensor and a response regulator, which are involved in DSF signal perception and signal transduction, respectively (20–24). Interestingly, genetic and protein structure analyses unveiled that the RpfC sensor protein also plays a key role in regulation of RpfF enzyme activity via a protein-protein interaction mechanism, while RpfB plays a role as a fatty acyl-CoA ligase to counteract the RpfF thioesterase activity (21, 22, 25). The mechanism of DSF biosynthesis and signal transduction appears to be widely conserved, as the rpf gene cluster has been identified in many other bacteria, including S. maltophilia, X. axonopodis pv. citri, X. oryzae pv. oryzae, and Xylella fastidiosa (10, 18, 26–30). Recently, it was found that cis-2-dodecenoic acid (BDSF), which is an important DSF family signal originally identified in B. cenocepcia, is catalyzed by the RpfFBc possessing both dehydratase and thioesterase activity. RpfFBc could enable the direct conversion of the acyl carrier protein (ACP) thioester of 3-hydroxydodecanoic acid into cis-2-dodecenoyl-ACP and then cleavage of the thioester bond from cis-2-dodecenoyl-ACP to release the free acid molecule (31).
In addition to vital roles in regulation of bacterial pathogenesis, DSF signals also seem to play a part in interspecies signaling (15, 17). It was recently reported that the DSF system in a bacterial pathogen can provide a benefit to the host plant. DSF in S. maltophilia R551-3 could positively influence seed germination and plant growth of the host plant (32). However, little is known about the impact of host plants on the bacterial quorum sensing system. To address this issue, in this study we tested the effect of Chinese cabbage, a common X. campestris pv. campestris host plant, on transcriptional expression of the quorum sensing genes and biosynthesis of DSF signal molecules. Interestingly, we found that plant extracts had little effect on transcriptional expression of rpf genes but could significantly boost DSF production. Hence, we conducted chromatographic separation and structural characterization analyses and identified glucose as the active component that specifically stimulates DSF family signal production. Further feeding tests using isotope-labeled compound showed that the fatty acid carbon chain of DSF is derived from glucose. These results suggest that there is a complicated interaction between X. campestris pv. campestris and its host plant, as X. campestris pv. campestris can directly benefit from the presence of host plant metabolites to increase the QS signal production and therefore, of course, promote the virulence.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this work are listed in Table 1. X. campestris pv. campestris strains were described previously (22, 33), and they were maintained at 30°C in NYG (5 g peptone [Difco], 3 g yeast extract [Difco], and 20 g glycerol per liter) or YEB (10 g tryptone, 5 g yeast extract [Difco], 5 g sucrose, 5 g NaCl, and 0.5 g Mg2SO4·7H2O per liter) medium (11, 34). The following antibiotics were supplemented when necessary. Rifampin was added to the medium at 50 μg ml−1. Glucose, glycerol, mannitol, sucrose, and sodium acetate were added to the medium at a final concentration of 15 mM, unless otherwise indicated.
TABLE 1.
Bacterial strains used in this study
| Strain | Source or characteristic(s) | Reference |
|---|---|---|
| X. campestris pv. campestris Xc1 | Wild-type strain of Xanthomonas campestris pv. campestris | 22 |
| X. campestris pv. campestris ΔrpfF | DSF-minus mutant derived from Xc1 with rpfF being deleted | 22 |
| X. campestris pv. campestris ΔrpfC | Derived from Xc1 with rpfC being deleted | 33 |
| X. campestris pv. campestris 8004 | Wild-type strain of X. campestris pv. campestris | 22 |
| X. campestris pv. campestris 8004dF | DSF-minus mutant derived from 8004 with rpfF being deleted | 22 |
| X. campestris pv. campestris FE58 | Biosensor for DSF signals | 11 |
Extraction of Chinese cabbage with ethanol.
Chinese cabbage (1 kg) was minced using an electric juicer and incubated with 2 liters of 100% ethanol for 2 days before centrifugation and collection of supernatants. The precipitates were resuspended in 2 liters of 100% ethanol twice. The supernatants were combined and concentrated by evaporation to achieve a final volume of 100 ml.
Time course analysis of DSF activity.
Briefly, Xc1 wild-type strains were grown in NYG medium with agitation at 30°C in the presence of a 1% ethanol extract of Chinese cabbage or of an equal volume of ethanol. At different time points as indicated, 100 ml of culture supernatants was collected by centrifugation and extracted with an equal volume of ethyl acetate. The organic solvent was evaporated to dryness, and the residues were dissolved in 0.5 ml of methanol. Quantitative measurement of DSF activity was achieved using X. campestris pv. campestris biosensor strain FE58 (11). Briefly, the biosensor was grown at 30°C in 5 ml of YEB medium to an optical density at 600 nm (OD600) of 0.6 prior to the addition of 100 μl extract. Following incubation for 2.5 h at 30°C with shaking at 200 rpm, 1 ml of culture was centrifuged, the bacterial pellets were lysed, and protein concentrations were determined using a Bradford assay kit (Bio-Rad). All assays were performed with equal amounts of proteins. β-Glucuronidase (GUS) activity was determined according to methods described previously (35).
Structural analysis of DSF family signals and HPLC analysis of plant extract.
The DSF-overproducing ΔrpfC strain, which is the rpfC deletion mutant derived from wild-type strain Xc1, was grown in NYG medium overnight. The cultures were centrifuged, and the bacterial cells were resuspended in fresh NYG medium to a high initial cell density of OD600 at 2.0. Five milliliters of the plant extract was added to 500 ml of the bacterial cultures. The bacterial cells were grown at 30°C for 8 h with slow shaking at 150 rpm. Bacterial cultures were centrifuged, and the supernatants were collected and extracted with ethyl acetate (1:1 [vol/vol]) twice. Following evaporation of ethyl acetate, the residues were dissolved in 0.5 ml of methanol and subjected to high-performance liquid chromatography (HPLC) profiling analysis on a reverse-phase column (Waters Symmetry C18) (3.5-μm particle size; 150 by 4.60 mm) eluted with gradient acetonitrile in H2O at 55% to 60% at a flow rate of 1 ml min−1. Peaks were monitored using a UV detector with λ = 210 and 254 nm and were collected and assayed using DSF biosensor FE58 (11). High-resolution electrospray ionization mass spectrometry (ESI-MS) was performed on a Finnigan/MAT 95XL-T mass spectrometer using conditions described previously (11).
The 1H and 13C nuclear magnetic resonance (NMR) spectra in a CDCl3 solution were obtained using a Bruker DRX400 spectrometer operating at 400 MHz for 1H or 100 MHz for 13C. High-resolution electrospray ionization mass spectrometry was performed on a Finnigan/MAT MAT 95XL-T mass spectrometer using conditions described previously (11).
For analysis of the active component in plant extract, the extract was diluted with water and subjected to a HPLC profiling analysis on a carbohydrate column (Shodex Sugar KS-801) (8.0 by 300 mm) eluted with H2O at a flow rate of 1 ml min−1. Peaks were monitored by a refractive index detector.
DSF bioassay analysis.
The assay was performed as described previously using the FE58 biosensor strain (11, 15). Briefly, 4-mm-diameter wells were introduced on prepared bioassay plates and 20 μl concentrated culture was added to each well. The plates were incubated at 30°C overnight. DSF activity is indicated by the presence of a blue halo around the well.
Extracellular polysaccharides and biofilm analysis.
For quantification of the production of extracellular polysaccharides (EPS), 10 ml of overnight YEB cultures at an OD600 of 3.0 was centrifuged at 12,000 rpm for 20 min. The supernatants were mixed with 2.5 volumes of absolute ethanol, and the mixture was incubated at 4°C for 30 min. The precipitated EPS was isolated by centrifugation and dried overnight at 55°C before determination of dry weights was performed. The formation of biofilms was investigated as follows: cultures of the X. campestris pv. campestris 8004 strains were grown overnight in 5 ml of YEB medium. Methanol was used as a solvent control. After overnight incubation, bacterial cells were visualized by phase-contrast microscopy (Olympus BX50) and images were taken with an Olympus DP70 digital camera.
Analysis of the DSF family signals induced by carbohydrate.
Following the methods described above, the ΔrpfC strain was grown in NYG medium overnight and centrifuged to collect the cell pellet, which was inoculated in 500 ml of NYG medium from an initial cell density at an OD600 of 2.0. Glucose or another carbon source was added to each of the cultures at a final concentration of 15 mM. The bacterial cells were grown at 30°C with slow shaking at 150 rpm. Bacterial cultures were centrifuged, and the supernatants were collected and extracted with ethyl acetate (1.0 [vol/vol]) twice. Following evaporation of the organic solvent, the residues were dissolved in 0.5 ml of methanol and subjected to HPLC analysis and mass spectrometer analysis.
RESULTS
Exogenous addition of Chinese cabbage extract enhances DSF biosynthesis in X. campestris pv. campestris.
To investigate the potential influence of host plants on the X. campestris pv. campestris quorum sensing system, we extracted small chemical molecules from Chinese cabbage, which is a common host plant of X. campestris pv. campestris, by using ethyl acetate or ethanol. We then determined their effects on the transcriptional expression of quorum sensing genes and production of DSF signal. The DSF activity was measured with the aid of the FE58 biosensor strain, in which the GUS reporter gene was driven by the promoter of the engXCA DSF-inducible endoglucanase gene (11). The results showed that the ethyl acetate extract had no effect on DSF production and expression of rpf genes (data not shown), whereas the ethanol extract could significantly boost DSF production and the DSF activity was increased by about 277% compared with the activity seen with the untreated control at 24 h postinoculation (Fig. 1A). However, the ethanol extract displayed no visible effect on the transcriptional expression of rpfF, rpfG, or rpfC (see Fig. S1 in the supplemental material). Exogenous addition of the ethanol extract increased the growth rate of Xc1 cells only slightly (Fig. 1B); therefore, such a drastic stimulating effect of the extract on DSF production appears unlikely to be due to a nutritional effect on bacterial growth.
FIG 1.
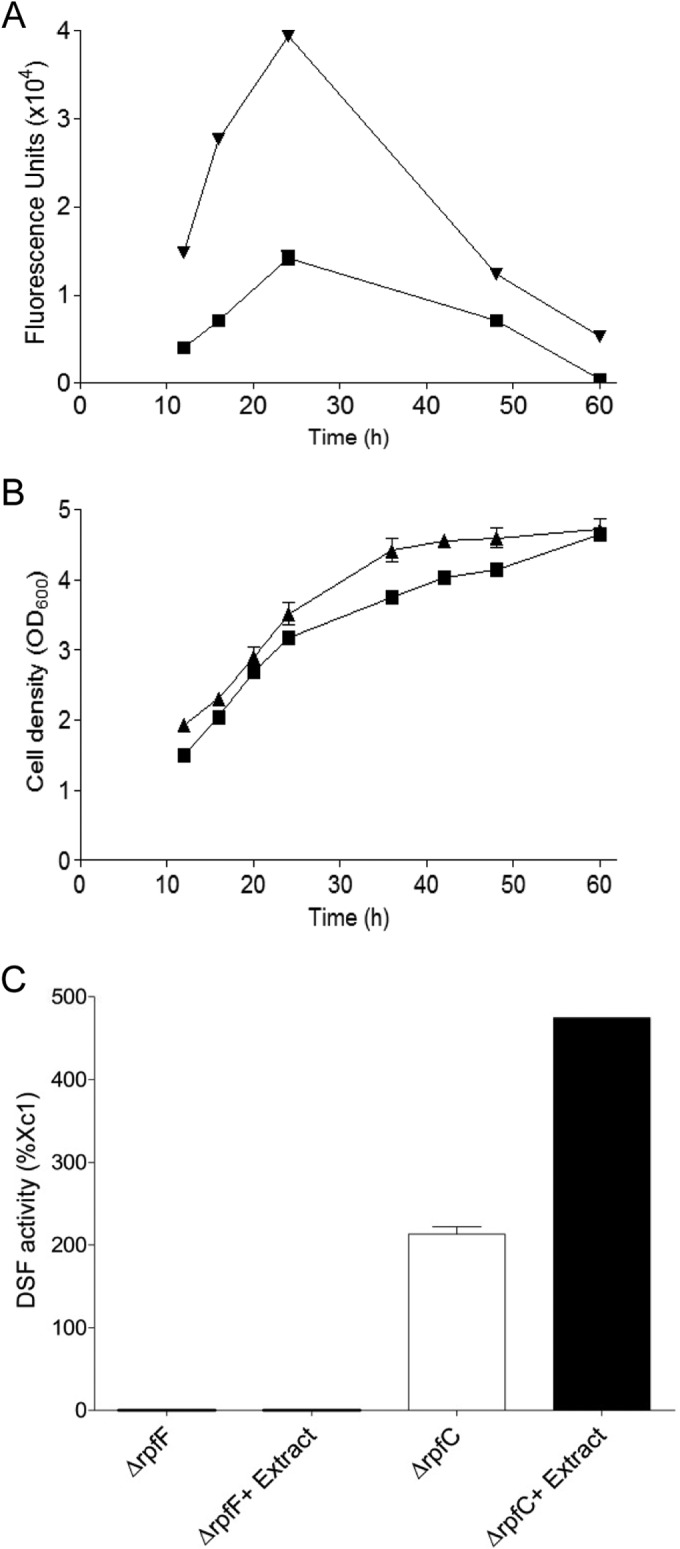
Influence of exogenous addition of Chinese cabbage ethanol extract on the DSF activity in X. campestris pv. campestris. (A) Time course analysis of the DSF activity in wild-type strain Xc1, in the absence (■) and presence (▼) of 1% extract. (B) Growth curve of Xc1, in the absence (■) and presence (▲) of 1% extract. (C) DSF activity in the ΔrpfF and ΔrpfC strains in the absence and presence of 1% extract. The data shown are the means of the results of two replicates, and error bars indicate standard deviations.
Given that DSF biosynthesis is catalyzed by RpfF and inhibited by RpfC at the posttranscriptional level (22), we then analyzed the effect of the extract on DSF biosynthesis in the null mutants RpfF and RpfC, respectively. As reported previously (22, 24), deletion of rpfC in wild-type strain Xc1 substantially increased the DSF activity, and addition of the plant extract further increased the DSF activity by 222% in the rpfC deletion mutant at 24 h postinoculation. No DSF activity was detected for the rpfF deletion mutant regardless of whether or not the extract was added (Fig. 1C). The results described above indicate that the plant extract-stimulated production of DSF is dependent on the presence of RpfF but is not associated with RpfC. Considering that the plant extract neither affects the transcription of rpf genes (see Fig. S1 in the supplemental material) nor counteracts the RpfC suppression in DSF biosynthesis (Fig. 1C), we speculate that the active component in the plant extract may actually be the substrate or precursor for DSF biosynthesis.
The extract significantly increases production of DSF family signals in X. campestris pv. campestris.
Given that RpfF enzymes in other bacterial species commonly produce two or more DSF family signals (17, 18), we tested the effect of plant extract on the production profile of DSF family molecules. The results showed that exogenous addition of the extract to the rpfC deletion mutant, which overproduces DSF signals, increased the production of at least three DSF family signals as revealed by HPLC separation coupled with DSF activity bioassay (Fig. 2A and B). Electrospray ionization mass spectrometry (ESI-MS) analysis of the two major purified signals showed m/z values of 197.30 and 211.22, respectively (Fig. 2C and D), indicating that the two signals are BDSF and DSF (11, 15).
FIG 2.
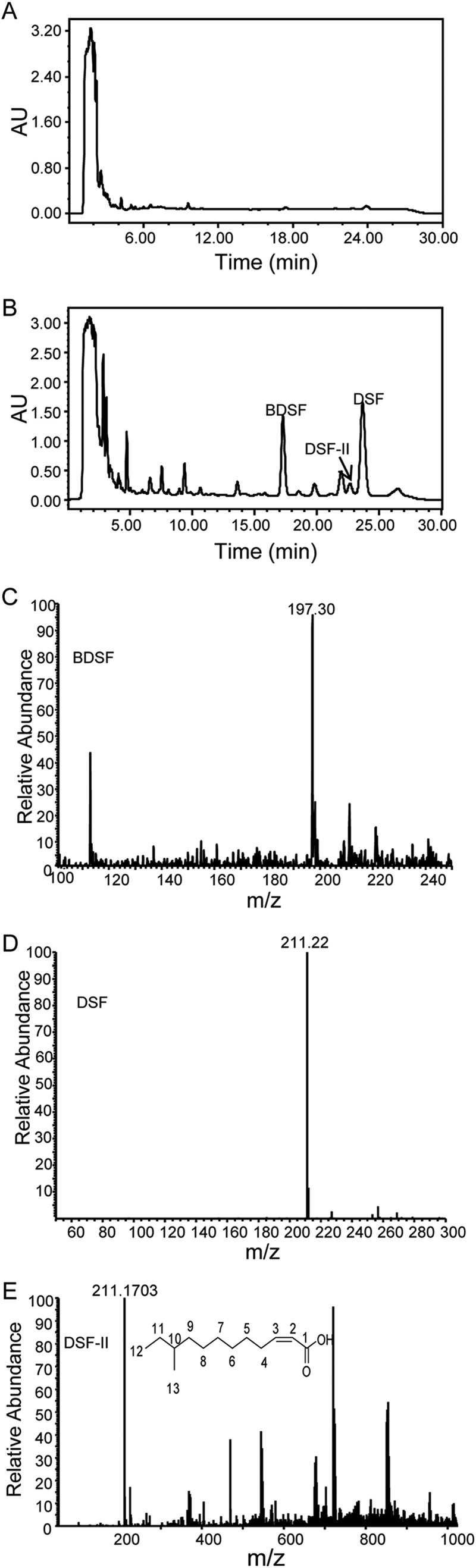
Structural analysis of DSF family signals induced by the plant extract. (A and B) HPLC analysis of the DSF family signals produced by the ΔrpfC strain cultured in NYG medium in the absence (A) and presence (B) of the extract. (C to E) ESI-MS spectra of BDSF (C), DSF (D), and DSF-II (E) produced by the ΔrpfC strain cultured in NYG medium supplemented with the extract. AU, arbitrary units.
To further characterize the remaining novel DSF family signal, the collected active fraction was analyzed using accurate electrospray ionization mass spectrometry (ESI-MS). The peak was shown at m/z 211.1703 (Fig. 2E). This m/z value is in agreement with the molecular formula C13H23O2. From the NMR analysis, in 1H spectra, there were two partially overlapped signals of methyl groups between 0.86 and 0.87 ppm; one is a triplet, while the other one is a doublet (Table 2). It is known that cis-11-methyl-2-dodecenoic acid (DSF) should have two fully overlapped doublet methyl groups at 0.86 ppm. On the basis of the 1H and 13C spectra, the structure of the novel DSF family signal was determined and was found to be cis-10-methyl-2-dodecenoic acid, which was designated DSF-II (Fig. 2E).
TABLE 2.
The NMR data of the novel DSF-II signal produced by X. campestris pv. campestrisa
| Position | Chemical shift (ppm) |
|
|---|---|---|
| δH | δC | |
| 1 | 170.8 | |
| 2 | 5.79, dt (11.6, 2.0) | 118.9 |
| 3 | 6.36, dt (11.6, 7.6) | 153.7 |
| 4 | 2.67, ddd (14.8, 7.6, 2.0) | 29.2 |
| 5 | 1.45, m | 29.4 |
| 6 | 1.20–1.40, m | 29.5 |
| 7 | 1.20–1.40, m | 30.0 |
| 8 | 1.20–1.40, m | 27.2 |
| 9 | 1.20–1.40, m | 36.8 |
| 10 | 1.20–1.40, m | 34.6 |
| 11 | 1.20–1.45, m | 29.7 |
| 12 | 0.87, t (6.8) | 11.6 |
| 13 | 0.86, d (6.8) | 19.4 |
The carbon number is based on data shown in Fig. 2E.
Furthermore, we have also tested the DSF-boosting activity of the extracts of other X. campestris pv. campestris host plants, including cauliflower and broccoli, and have found that the extracts of cauliflower and broccoli could enhance the DSF family signal production of X. campestris pv. campestris in a manner similar to that seen with the extract of cabbage (data not shown), suggesting that the DSF-boosting activity is conserved in the host plants of X. campestris pv. campestris.
DSF-II plays an important role in regulation of biofilm dispersal and EPS production.
We firstly assayed DSF-II using the DSF biosensor X. campestris pv. campestris strain FE58, which indicated the DSF-like activity of DSF-II, as there was a blue halo around the DSF-II molecule spotted in the DSF bioassay plate (Fig. 3A). In the plant pathogen X. campestris pv. campestris, DSF is required as an antiaggregation factor (36). While DSF-producing wild-type strain 8004 grows planktonically, with cells being well dispersed (Fig. 3B), the 8004dF rpfF deletion mutant forms large aggregates (15, 22) (Fig. 3B). Previous studies showed that BDSF is a functional analogue of DSF in regulation of biofilm dispersal and EPS production in X. campestris pv. campestris (15, 17). To evaluate the biological relevance of the novel DSF family signal, 5 μM DSF-II was added to an 8004 rpfF deletion mutant culture. It was found that addition of DSF-II showed an effect equal to that of DSF in that it completely dispersed the cell aggregates (Fig. 3B). We then quantitatively compared the biological activity of DSF-II with that of DSF with respect to the production of EPS in the DSF-minus 8004dF mutant. The experiment showed that addition of 5 μM DSF-II to a culture of 8004dF increased the EPS production to 96.5% of the wild-type level, which is similar to the biological activity of DSF (Fig. 3C).
FIG 3.
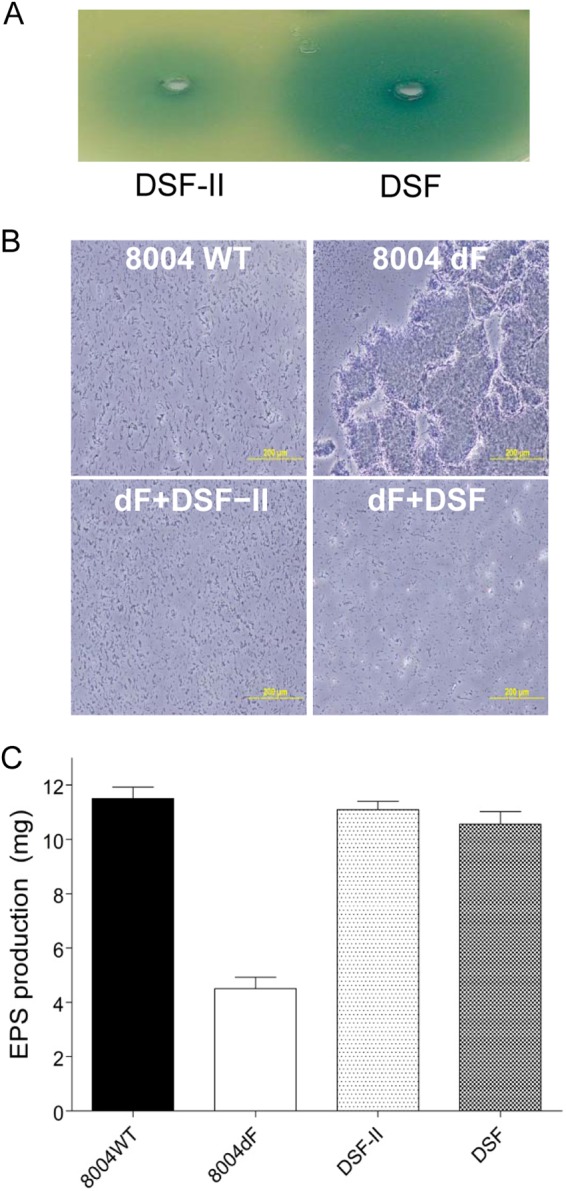
Functional analysis of the novel DSF family signal. (A) DSF bioassay analysis of DSF-II. (B) Growth characteristics of X. campestris pv. campestris wild-type (WT) strain 8004 and DSF-negative mutant 8004dF in the absence or presence of DSF signals. (C) Addition of 5 μM DSF-II or DSF to cultures of 8004dF restored EPS production. The data shown are the means of the results of three repeats, and error bars indicate standard deviations.
The active component in the plant extract is glucose.
To identify the active component that stimulates DSF family signal biosynthesis in the Chinese cabbage extract, we conducted HPLC analysis coupled with a refractive index detector and DSF activity assay. The results of the bioassay showed that the major active component(s) was eluted at around 7 to 8 min, corresponding to a peak (designated peak A) in the refractive-index spectra (Fig. 4A). Following our speculation that the active component in the plant extract could be a substrate or precursor for DSF family signal biosynthesis, we tested a range of carbohydrates in HPLC analysis, and the results showed that glucose displayed a retention time identical to that of peak A (Fig. 4B). We then weighted the extract and calculated the concentration of glucose in the extract based on the HPLC analysis result and roughly determined that the stock concentration of glucose in the extract was about 1.5 M and that the final concentration of glucose in the bacterial culture supplemented with the extract was therefore about 15 mM. Consistent with the findings described above, we found that exogenous addition of 15 mM glucose significantly enhanced the production of DSF family signals (Fig. 4C). In particular, the DSF family signal production spectrum of X. campestris pv. campestris in the presence of glucose is almost the same as that in the presence of the plant extract (Fig. 2B and 4C).
FIG 4.
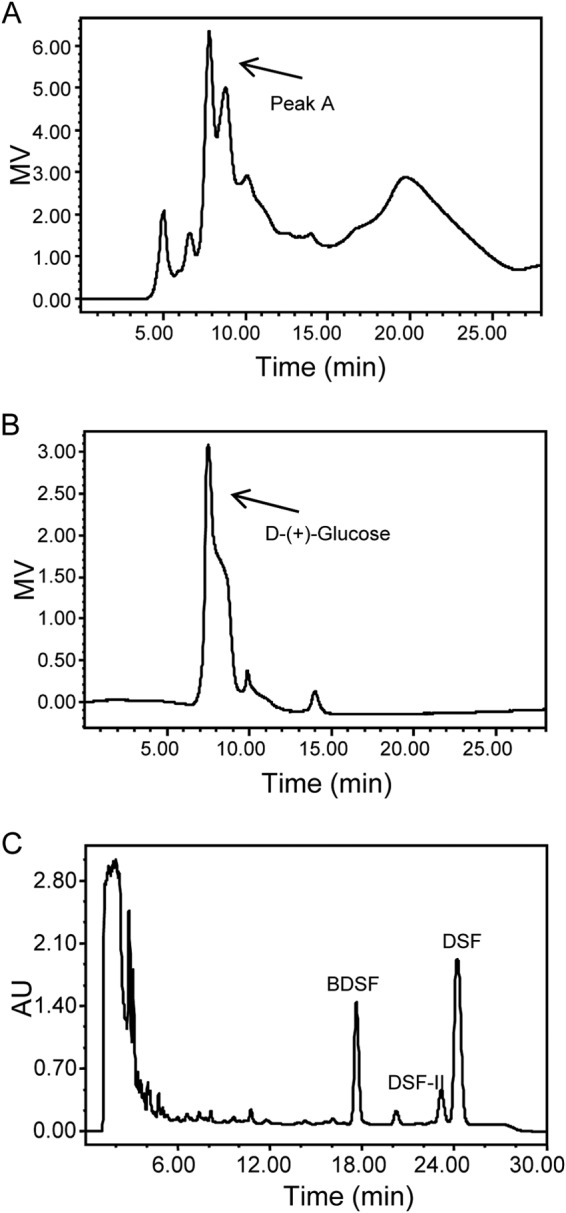
Glucose enhances the production of DSF family signals. (A) HPLC separation of the Chinese cabbage extract monitored using a refractive index detector. (B) HPLC profile of the standard glucose detected by a refractive index detector. (C) HPLC analysis of the DSF family signal production of the ΔrpfC strain in the presence of glucose. MV, millivolts.
Glucose is used as a carbon source for DSF family signal biosynthesis.
To validate the role of glucose in DSF family signal biosynthesis, we used real-time PCR to measure the effect of glucose on the rpf gene expression in X. campestris pv. campestris. The results showed that glucose did not affect the transcriptional expression of rpfF, rpfC, and rpfG (data not shown), similarly to the effect of the Chinese cabbage extract. We then tested whether glucose is used as a carbon source in DSF biosynthesis by using 13C-labeling technology. The same final concentration of [13C]glucose (all six carbons are involved in 13C labeling) was used to replace normal glucose to feed DSF biosynthesis. HPLC analysis results showed that the DSF family signal production spectrum after addition of [13C]glucose is similar to that in the presence of the normal glucose (Fig. 5A). However, in contrast to the electrospray ionization mass spectrometry (ESI-MS) spectrum of DSF induced by normal glucose at m/z 211.22 (100%), for which the isotopic peak is at m/z 212.22 (14.1%) (Fig. 2D), ESI-MS analysis of DSF enhanced by addition of [13C]glucose showed a cluster of statistical distributions of isotopic peaks at m/z 213.42, 214.50, 215.50, 216.42, 217.58, 218.50, and 219.50 (Fig. 5B). The average mass was calculated to be 216.60 according to methods described previously (37). The 211.33 peak suggested the existence of unlabeled DSF. From the equation XH = (Ma − Mw)/[N(MH − ML)], where XH is the 13C abundance, Ma is the average mass of the cluster (216.60), Mw is the mass of unlabeled DSF (211.33) (n = 13), ML is the mass of the light isotopic form of the label element (12 atomic mass units [amu] in our case), and MH is the mass of the heavy isotopic form of label element (13 amu in our case). So XH for the labeled DSF was calculated to be 0.406 (216.60 − 211.33)/[13 × (13 − 12)]. Consistently, this result suggested that about 40% of the carbon atoms of the DSF molecule were derived from [13C]glucose.
FIG 5.
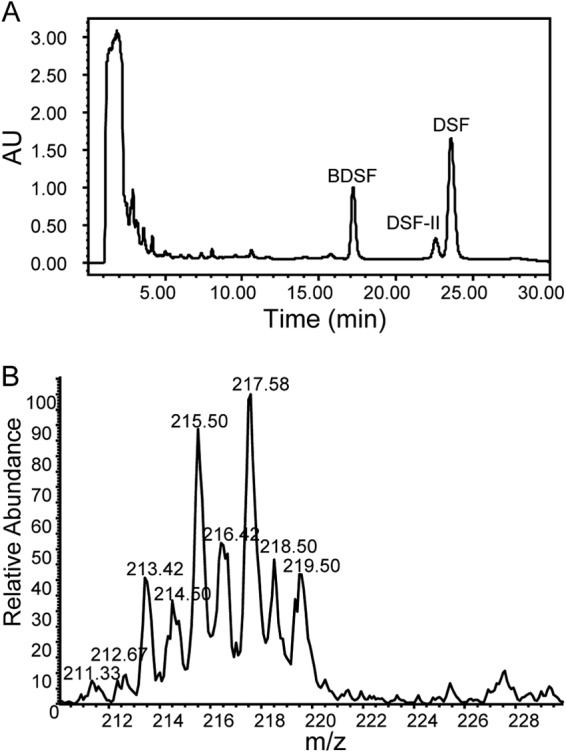
Glucose provides carbon atoms for the biosynthesis of DSF family signals. (A) HPLC analysis of the DSF family signal production of the ΔrpfC strain cultured in the NYG medium supplemented with 15 mM [13C]glucose. (B) ESI-MS spectrum of DSF produced by the ΔrpfC strain cultured in the NYG medium with addition of 15 mM [13C]glucose.
Exogenous addition of acetate also increases production of DSF family signals.
To determine whether other carbohydrates could also promote the biosynthesis of DSF family signals in X. campestris pv. campestris, we compared the DSF family signals and the BDSF, DSF-II, and DSF production profiles seen when the ΔrpfC strain was cultured in NYG medium supplemented with 15 mM concentrations of various common carbohydrates. Given the aim to identify direct precursors, a high-density (OD600 = 2.0) bacterial inoculum in NYG medium was supplemented with candidate carbohydrate and DSF family signals were extracted using ethyl acetate for HPLC analysis at 8 h after inoculation. The results showed that among the carbohydrates tested, including sodium acetate, sucrose, mannitol, and glycerol, only sodium acetate could significantly promote production of DSF family signals with a high percentage rate comparable with that of glucose (Fig. 6).
FIG 6.
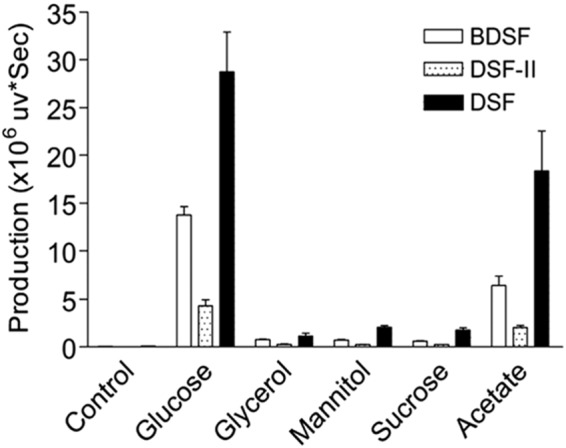
Analysis of the DSF family signal production by the ΔrpfC strain with addition of 15 mM concentrations of different carbon sources. The data shown are the means of the results of three repeats, and error bars indicate standard deviations. The relative amounts of signal molecules were calculated on the basis of their peak areas.
DISCUSSION
The findings from this study showed that DSF is not the sole DSF family signal produced by X. campestris pv. campestris. We showed here that strain Xc1 produces at least two other DSF family signals, BDSF and DSF-II (Fig. 2). Among them, BDSF was originally identified in B. cenocepacia (15). It was reported previously that exogenous addition of BDSF to the DSF-deficient mutant of X. campestris pv. campestris could restore biofilm dispersion and extracellular polysaccharide (EPS) production to the wild-type levels. We also confirmed that the activity of DSF-II is similar to that of DSF with respect to restoring biofilm dispersion and EPS production in the X. campestris pv. campestris DSF-deficient mutant in this study (Fig. 3B), demonstrating that, similarly to the previously characterized DSF (11), BDSF and DSF-II should also play an important role in X. campestris pv. campestris cell-cell communication (15, 17). Nevertheless, the yield of DSF was substantially higher than that of BDSF or DSF-II in the presence of Chinese cabbage extract or glucose, suggesting that DSF is the prominent signal in the X. campestris pv. campestris QS system. Several other bacterial species, such as S. maltophilia (12, 28), some B. cepacia species (17), and X. oryzae pv. oryzae (18), also produce multiple DSF family signals. Given that RpfF homologues are highly conserved in different bacterial species (33), it would not be surprising if more bacterial pathogens were to be found to produce multiple DSF family signals.
Fatty acids are synthesized from acetyl-CoA and malonyl-CoA precursors by fatty acid synthases. As DSF family signals are either saturated or unsaturated fatty acid derivatives (1), it is believed that DSF signals are synthesized through the fatty acid biosynthesis pathway. It was reported recently that BDSF is synthesized from a fatty acid synthetic intermediate, i.e., an acyl carrier protein (ACP) thioester of 3-hydroxydodecanoic acid. It was revealed that this intermediate was converted to cis-2-dodecenoyl-ACP by RpfFBc and that the thioester bond of cis-2-dodecenoyl-ACP was subsequently cleaved by the same enzyme to release the free acid molecule of BDSF (31). However, 3-hydrododecanoic acid could be derived from either a fatty acid synthetic pathway or a degradation pathway. The findings from this study provide solid evidence that DSF family signals can be generated through the fatty acid synthetic pathway, in particular, under in planta conditions. The biosynthesis of DSF family signals could be drastically and quickly enhanced by addition of glucose or sodium acetate to X. campestris pv. campestris growth media (Fig. 6), suggesting that DSF family molecules are possibly synthesized from acetyl-CoA through a fatty acid biosynthesis pathway. Consistently, it was observed that among the members of the cluster, the odd m/z values are prevalent over the even m/z values (Fig. 5B). As the m/z of the DSF molecule is an odd value, this MS spectrum indicated that most of the DSF molecules were incorporated with an even number of 13C carbon atoms, which were possibly derived directly from acetyl-CoA converted from [13C]glucose.
The isotope-labeling feeding test further confirmed that many carbon atoms in DSF were derived from glucose. Fatty acid synthesis represents the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors; this means that DSF is derived from both acetyl-CoA and malonyl-CoA precursors. In our experiments, under the culturing conditions that included slow shaking, we assumed that some acetyl-CoA and malonyl-CoA precursors for the DSF family signal biosynthesis were derived from glucose; the remaining precursors were derived from other forms of fatty acid biosynthesis/degradation metabolism. This speculation was further confirmed by our observation that the 13C abundance of DSF molecules was 40.6% when the bacterial cells were fed with the isotope labeling glucose (Fig. 5B). Interestingly, under the conditions used in this study, addition of sucrose, mannitol, or glycerol was not able to enhance the production of DSF family signals. The likely reason behind this observation is that, under the culture conditions of high bacterial cell density (OD600 = 2.0) and slow shaking (150 rpm) used in this study, bacterial growth is poor, and sucrose, mannitol, and glycerol may be more slowly metabolized than glucose to produce acetyl-CoA and with greater difficulty. In contrast, the enzymes associated with fatty acid biosynthesis seem to remain active at the stationary phase, as maximal production of DSF occurs at the late stationary phase (11). This speculation was supported by the result showing that addition of sodium acetate could also boost the production of DSF family signals, as acetate is quickly converted to acetyl-CoA by the enzyme acetyl-CoA synthetase (Fig. 6). These findings indicate that glucose is first converted to acetyl-CoA and then used as the carbon source for the DSF family signal biosynthesis.
The role of DSF in the interaction between bacterial pathogens and host plants is an emerging area of interest. It was recently shown that DSF in S. maltophilia could increase the germination rate of oilseed rape seeds (32). This demonstrates that DSF can provide a benefit to the host plant. However, the relationship between the bacterial pathogen and the host plant is competitive or even antagonistic in the most cases. As X. campestris pv. campestris employs DSF to positively control virulence factor production and pathogenicity, enhancement of this weapon is definitely important for X. campestris pv. campestris cells with respect to infection of the host plant. Because glucose is the direct product of plant photosynthesis reaction, utilization of glucose from the host plant as an energy source and DSF family signal precursor is a dual benefit to X. campestris pv. campestris for the infection. Interestingly, recent reports showed that there are sugar efflux transporters which translocate the sugar produced in mesophyll cells into phloem cells throughout the plant. Bacterial pathogens may exploit these transporters by inducing the expression of transporter genes and then enhancing their activities for nutritional gain (38, 39). Consistently with our results, infection of Arabidopsis thaliana by Xc1 wild-type cells caused an increase of the expression level of some transporter genes (unpublished data), which may enhance the translocation of glucose into phloem cells and finally benefit the X. campestris pv. campestris cells by enabling them to obtain the glucose as an energy source and a precursor of DSF family signals.
Supplementary Material
ACKNOWLEDGMENTS
The funding for this work was provided by the Biomedical Research Council, the Agency of Science, Technology and Research (A*Star), Singapore, and by the Introduction of Innovative R&D Team Program of Guangdong Province (no. 2013S034), China.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03813-14.
REFERENCES
- 1.Deng Y, Wu J, Tao F, Zhang LH. 2011. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev 111:160–173. doi: 10.1021/cr100354f. [DOI] [PubMed] [Google Scholar]
- 2.Fuqua C, Greenberg EP. 2002. Listening in on bacteria: acyl-homoserine lactone signaling. Nat Rev Mol Cell Biol 3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 3.Zhang LH, Dong YH. 2004. Quorum sensing and signal interference: diverse implications. Mol Microbiol 53:1563–1571. doi: 10.1111/j.1365-2958.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams P, Winzer K, Chan WC, Cámara M. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci 362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moré MI, Finger LD, Stryker JL, Fuqua C, Eberhard A, Winans SC. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer AL, Val DL, Hanzelka BL, Cronan JE Jr, Greenberg EP. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci U S A 93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LH. 2003. Quorum quenching and proactive host defense. Trends Plant Sci 8:238–244. doi: 10.1016/S1360-1385(03)00063-3. [DOI] [PubMed] [Google Scholar]
- 9.Deng Y, Boon C, Eberl L, Zhang LH. 2009. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by quorum sensing signal BDSF and its synthase. J Bacteriol 191:7270–7278. doi: 10.1128/JB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YW, Zhang LH. 2008. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol Rev 32:842–857. doi: 10.1111/j.1574-6976.2008.00120.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912. [DOI] [PubMed] [Google Scholar]
- 12.Huang TP, Lee Wong AC. 2007. Extracellular fatty acids facilitate flagella-independent translocation by Stenotrophomonas maltophilia. Res Microbiol 158:702–711. doi: 10.1016/j.resmic.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Almeida RP, Killiny N, Newman KL, Chatterjee S, Ionescu M, Lindow SE. 2012. Contribution of rpfB to cell-to-cell signal synthesis, virulence, and vector transmission of Xylella fastidiosa. Mol Plant Microbe Interact 25:453–462. doi: 10.1094/MPMI-03-11-0074. [DOI] [PubMed] [Google Scholar]
- 14.Colnaghi Simionato AV, da Silva DS, Lambais MR, Carrilho E. 2007. Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J Mass Spectrom 42:490–496. doi: 10.1002/jms.1181. [DOI] [PubMed] [Google Scholar]
- 15.Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J 2:27–36. doi: 10.1038/ismej.2007.76. [DOI] [PubMed] [Google Scholar]
- 16.Davies DG, Marques CN. 2009. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Wu JE, Eberl L, Zhang LH. 2010. Structural and functional characterization of diffusible signal factor family quorum-sensing signals produced by members of the Burkholderia cepacia complex. Appl Environ Microbiol 76:4675–4683. doi: 10.1128/AEM.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He YW, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10:187. doi: 10.1186/1471-2180-10-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, Sztajer H, Wagner-Döbler I. 2010. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem 11:1552–1562. doi: 10.1002/cbic.201000086. [DOI] [PubMed] [Google Scholar]
- 20.Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJ, Slater H, Dow JM, Williams P, Daniels MJ. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24:555–566. doi: 10.1046/j.1365-2958.1997.3721736.x. [DOI] [PubMed] [Google Scholar]
- 21.Bi H, Yu Y, Dong H, Wang H, Cronan JE. 2014. Xanthomonas campestris RpfB is a fatty Acyl-CoA required to counteract the thioesterase activity of the RpfF diffusible signal factor (DSF) synthase. Mol Microbiol 93:262–275. doi: 10.1111/mmi.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He YW, Wang C, Zhou L, Song H, Dow JM, Zhang LH. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J Biol Chem 281:33414–33421. doi: 10.1074/jbc.M606571200. [DOI] [PubMed] [Google Scholar]
- 23.Ryan RP, Fouhy Y, Lucey JF, Crossman LC, Spiro S, He YW, Zhang LH, Heeb S, Cámara M, Williams P, Dow JM. 2006. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci U S A 103:6712–6717. doi: 10.1073/pnas.0600345103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. 2000. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol Microbiol 38:986–1003. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Z, He YW, Lim SC, Qamra R, Walsh MA, Zhang LH, Song H. 2010. Structural basis of the sensor-synthase interaction in autoinduction of the quorum sensing signal DSF biosynthesis. Structure 18:1199–1209. doi: 10.1016/j.str.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Andrade MO, Alegria MC, Guzzo CR, Docena C, Rosa MC, Ramos CH, Farah CS. 2006. The HD-GYP domain of RpfG mediates a direct linkage between the Rpf quorum-sensing pathway and a subset of diguanylate cyclase proteins in the phytopathogen Xanthomonas axonopodis pv. citri. Mol Microbiol 62:537–551. doi: 10.1111/j.1365-2958.2006.05386.x. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee S, Wistrom C, Lindow SE. 2008. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc Natl Acad Sci U S A 105:2670–2675. doi: 10.1073/pnas.0712236105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouhy Y, Scanlon K, Schouest K, Spillane C, Crossman L, Avison MB, Ryan RP, Dow JM. 2007. Diffusible signal factor-dependent cell-cell signaling and virulence in the nosocomial pathogen Stenotrophomonas maltophilia. J Bacteriol 189:4964–4968. doi: 10.1128/JB.00310-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Newman KL, Almeida RP, Purcell AH, Lindow SE. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci U S A 101:1737–1742. doi: 10.1073/pnas.0308399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarpari LM, Lambais MR, Silva DS, Carraro DM, Carrer H. 2003. Expression of putative pathogenicity-related genes in Xylella fastidiosa grown at low and high cell density conditions in vitro. FEMS Microbiol Lett 222:83–92. doi: 10.1016/S0378-1097(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 31.Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. 2012. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol Microbiol 83:840–855. doi: 10.1111/j.1365-2958.2012.07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alavi P, Muller H, Cardinale M, Zachow C, Sanchez MB, Martinez JL, Berg G. 2013. The DSF quorum sensing system controls the positive influence of Stenotrophomonas maltophilia on plants. PLoS One 8:e67103. doi: 10.1371/journal.pone.0067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YW, Ng AY, Xu M, Lin K, Wang LH, Dong YH, Zhang LH. 2007. Xanthomonas campestris cell-cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signalling network. Mol Microbiol 64:281–292. doi: 10.1111/j.1365-2958.2007.05670.x. [DOI] [PubMed] [Google Scholar]
- 34.Daniels MJ, Barber CE, Turner PC, Cleary WG, Sawczyc MK. 1984. Isolation of mutants of Xanthomonas campestris pathovar campestris showing altered pathogenicity. J Gen Microbiol 130:2447–2455. [Google Scholar]
- 35.Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL. 2003. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci U S A 100:10995–11000. doi: 10.1073/pnas.1833360100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blom K, Dybowski C, Munson B. 1987. Mass spectral analysis of isotopically labeled compounds: average mass approach. Anal Chem 59:1372–1374. doi: 10.1021/ac00136a025. [DOI] [Google Scholar]
- 38.Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB. 2010. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–534. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB. 2012. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


