Abstract
Pulsed light is a nonthermal processing technology recognized by the FDA for killing microorganisms on food surfaces, with cumulative fluences up to 12 J cm−2. In this study, we investigated its efficacy for inactivating murine norovirus 1 (MNV-1) as a human norovirus surrogate in phosphate-buffered saline, hard water, mineral water, turbid water, and sewage treatment effluent and on food contact surfaces, including high-density polyethylene, polyvinyl chloride, and stainless steel, free or in an alginate matrix. The pulsed-light device emitted a broadband spectrum (200 to 1,000 nm) at a fluence of 0.67 J cm−2 per pulse, with 2% UV at 8 cm beneath the lamp. Reductions in viral infectivity exceeded 3 log10 in less than 3 s (5 pulses; 3.45 J cm−2) in clear suspensions and on clean surfaces, even in the presence of alginate, and in 6 s (11 pulses; 7.60 J cm−2) on fouled surfaces except for stainless steel (2.6 log10). The presence of protein or bentonite interfered with viral inactivation. Analysis of the morphology, the viral proteins, and the RNA integrity of treated MNV-1 allowed us to elucidate the mechanisms involved in the antiviral activity of pulsed light. Pulsed light appeared to disrupt MNV-1 structure and degrade viral protein and RNA. The results suggest that pulsed-light technology could provide an effective alternative means of inactivating noroviruses in wastewaters, in clear beverages, in drinking water, or on food-handling surfaces in the presence or absence of biofilms.
INTRODUCTION
Human noroviruses are responsible for one-fifth of all cases of acute gastroenteritis worldwide (1). They spread directly via person-to-person contact (fecal-oral and vomitus-oral) or indirectly through food, water, and the environment (2). Natural biofilms in sewage treatment effluent could be responsible for many persistent waterborne outbreaks, since such biofilms have been found to harbor noroviruses (3, 4). Infection spreads quickly and widely, affecting individuals of all ages, especially where many people gather or live in close proximity, such as in long-term care residences, retirement homes, cruise ships, and the like. Infectious doses as low as 2,800 particles, the high viral loads in feces and vomit (up to 109 genomic copies per gram), persistence in the environment, prolonged duration of viral shedding even after symptoms have resolved, and inadequate long-term immunity all contribute to the high incidence of norovirus illness (5). Although the illness is usually mild and self-limiting, the large number of cases per year represents a considerable loss in productivity and constitutes a substantial burden on society (2, 6).
Among the physical disinfection processes, pulsed-light treatment appears well suited for the disinfection of food contact surfaces in industrial and health care settings and for decontaminating water and beverages. It has been proposed as a means of nonthermal pasteurization for food preservation and for decontaminating air and packaging materials (7). It has proven effective for inactivating bacteria, fungi, and yeasts in water and foods and in diagnostic laboratories. Besides its advantages of speed and cost-effectiveness, it does not require the addition of any chemical product (8). This technology is based on very short, high-intensity pulses of white light, from UV-C to near infrared. The spectrum is thus much broader than the monochromatic light (continuous-wave germicidal UV at 254 nm) emitted by low-pressure mercury lamps or the polychromatic light (200 to 300 nm) emitted by medium-pressure mercury lamps (9). Most of the photons in this broad range have enough energy to induce chemical reactions but too little to cause molecular bond breakage, as occurs in the presence of ionizing radiation (10). According to the FDA, pulsed light may be safely used for the decontamination of food and food contact surfaces by using a xenon lamp emitting wavelengths between 200 and 1,000 nm, pulse durations not exceeding 2 milliseconds, and cumulative intensity not exceeding 12 J cm−2 (11).
While the pulsed-light approach appears promising based on published results, studies of its use for inactivating viruses are scarce. Early results are nevertheless encouraging. The treatment intensity received by a sample is characterized by the fluence, which is calculated by multiplying the total radiant incident power per unit area by the exposure time. Using a PureBright device, Huffman et al. (12) obtained greater than 4-log10 reductions in the infectivity of poliovirus and rotavirus in tap water at a turbidity of 0 to 10 nephelometric turbidity units (NTU) flowing at about 15 liters min−1 with treatment intensities of 500 mJ cm−2. Roberts and Hope (13) reported similar reductions for nine enveloped and nonenveloped viruses, including poliovirus and hepatitis A virus, using an intensity of 1 J cm−2 in phosphate-buffered saline (PBS) but required 2 J cm−2 to reach a 3-log10 reduction when the buffer contained protein at a concentration of 2 mg ml−1. Focusing on the UV portion of the pulsed-light fluence, Lamont et al. (14) calculated that poliovirus in PBS buffer was completely inactivated (6 log10) after exposure to 28 mJ cm−2 UV, whereas adenovirus required 115 mJ cm−2 UV for a reduction of 3 log10. In previous work by our group (15), a 5-log10 reduction of hepatitis A virus and MNV-1 in PBS buffer was obtained after exposure to 59 and 91 mJ cm−2 calculated UV fluence, respectively. The effectiveness of the pulsed-light treatment was also decreased in the presence of dissolved protein. Results on stainless steel and polyvinyl chloride (PVC) disks were similar. Our study was the only previous study that focused on the resistance of noroviruses to pulsed light on different surfaces under various conditions of fouling. Meanwhile, the mechanisms involved in the antiviral activity of pulsed light remain unknown.
The aim of the present work was to evaluate the efficacy of pulsed light for inactivation of norovirus in sewage treatment effluent and drinking water as a function of water hardness and turbidity. We also aimed to evaluate its efficacy for decontaminating different surfaces, under clean and fouled conditions, as well as in the presence of biofilm. Finally, we sought to elucidate the mechanism involved in this antiviral activity. To achieve this, murine norovirus was used as a surrogate for human norovirus (16), which had not been cultured before the recent report of Jones et al. (17).
MATERIALS AND METHODS
Virus and cells.
Murine norovirus strain 1 and RAW 264.7 cells were obtained courtesy of Kirsten Mattison (Health Canada, Bureau of Microbial Hazards, Tunney's Pasture, Ottawa, Ontario, Canada). RAW 264.7 cell culture and MNV-1 propagation, concentration, and purification were performed as described previously (18). Viral protein was measured using the DC protein assay kit (Bio-Rad).
Virus quantification by plaque assay.
The titers of MNV-1 in stock aliquots and treated suspensions were determined by plaque assay and are expressed in PFU ml−1 as described previously (19).
Pulsed-light equipment.
Pulsed-light treatments were carried out in a benchtop Sinteron 500 system connected to an LC-915 process chamber (154 mm by 154 mm by 165 mm) fitted with an LH-910 spiral lamp (type C, 107-mm diameter; Xenon Corp., MA, USA). The system was operated at 830 J per pulse (3,800 V discharge from a 115-μF capacitor). The xenon lamp spectrum spans the 200- to 1,000-nm range. Samples in a 24-well cell culture plate that was centered on the process chamber shelf placed 80 mm beneath the lamp were exposed to one 520-μs pulse every 0.555 s for varied lengths of time.
Fluence measurements.
The broadband energy (J) and UV fluence (J cm−2) were measured three times at four different locations 80 mm beneath the lamp. A period of at least 10 min was provided between sample treatments to prevent possible overheating of the detectors. The broadband energy was measured using a Nova display (Ophir Optronics Inc., Wilmington, MA, USA) with a stainless steel perforated plate (with a single 0.97-cm2 round hole) placed over the pyroelectric head (PE50; calibrated prior to the study) and with the pulse width and wavelength set at 1.0 ms and 254 nm, respectively. The total broadband fluence (in J cm−2) of a treatment was calculated as follows: total fluence = (energy measured after one pulse) × (number of pulses/0.97 cm2).
The UV fluence was measured using an ILT1700 display (International Light Technologies Inc., Peabody, MA, USA) connected to a SED040 photodiode covered with a W diffuser, a QNDS2 neutral density filter (to reduce intensity by 100), and an ACT5 filter that was calibrated prior to the study.
Liquid-phase inactivation of MNV-1 by pulsed light.
Viral suspensions containing approximately 105 PFU ml−1 were prepared by diluting purified MNV-1 in hard water prepared according to the methods described by the Association of Official Analytical Chemists (20), in water made turbid with bentonite (Fisher, reference B235), in PBS buffer, in bottled mineral water (Dasani brand), or in water collected at a municipal sewage treatment plant (Saint Nicolas, QC, Canada) just after the grit removal step (effluent 1) and just after activated sludge treatment (effluent 2). The sewage treatment samples were sterilized by exposure to 37 kGy of gamma irradiation in a Gammacell-220 60Co cell (Atomic Energy of Canada). The other suspension media were sterilized by autoclaving for 15 min at 121°C. Measurements of turbidity (using a model 2100AN apparatus; Hach Company, Loveland, CO, USA), conductivity (YSI 3100 conductivity meter), reduction potential (electrode VWR, reference 14002-856), dissolved oxygen (electrode VWR, reference 14002-800), and absorption and transmittance from 200 to 1,000 nm (HP 8453 spectrometer; Hewlett Packard) were carried out at 24.0 ± 1°C. One milliliter of each viral suspension was then placed in a 24-well plate (sample surface area of 2 cm2, 0.5-cm depth) for treatment. Untreated viral suspensions were included as positive controls. The viral titer was then determined as described above. Sample temperature was monitored by using a type K thermocouple (Sper Scientifics).
Inactivation of MNV-1 attached to solid materials.
One-centimeter disks of polyethylene (PET), polyvinyl chloride (PVC) type 1, grade 1 (Plastique Polyfab, Quebec, QC, Canada), and stainless steel (Den-Mar Acier Inoxydable Inc., Quebec, QC, Canada) were cleaned with 0.1% sodium dodecyl sulfate solution, rinsed with distilled water several times, and then sterilized by autoclaving for 15 min at 121°C. The viral load (105 PFU cm−2) was used under clean and fouled conditions according to the standard test method ASTM E 2197-02 (21). Inactivation experiments were conducted according to standard test method ASTM E 1053-97 (22) with some modifications. Briefly, 30 μl of virus suspension was placed on each disk and allowed to dry for 60 ± 5 min at 22 ± 2°C in a laminar flow hood, and the disks were placed in a 24-well plate and treated. Controls received no pulse-light treatment. Virions were recovered from the test materials in Earle's balanced salt solution and assayed immediately. Recoverability (R, expressed as a percentage) of infectious viral particles from the material surface was calculated as follows: R = [100 × (T60/Ti)], where Ti is the initial viral titer and T60 is the viral titer after drying for 60 min at room temperature.
Inactivation of MNV-1 entrapped in alginate.
Purified MNV-1 was suspended at 105 PFU g−1 in a sterile solution of 2% (wt/wt) low-viscosity sodium alginate (reference A2158; Sigma, Oakville, Canada). Alginate film that was 0.97 mm thick was formed as described previously (19), weighed, and then treated. Control tests received no pulsed-light treatment. Virions were recovered as described previously (19) and assayed immediately.
Experiments on mechanism of action.
Aliquots (400 μl) of purified MNV-1 suspended at a titer of approximately 5 × 109 PFU ml−1 (0.6 mg of protein ml−1) were exposed to 2.07 J cm−2 (3 pulses) or 8.98 J cm−2 (13 pulses). An unexposed viral suspension and distilled water were included as positive and negative controls. Treated aliquots were immediately separated into four tubes for visualization by transmission electron microscopy (TEM), protein analysis by SDS-PAGE under reducing conditions, RNA extraction, and plaque assay. Extracted RNA was used for integrity analysis by electrophoresis and photoproduct detection by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) after enzymatic digestion.
Transmission electron microscopy.
Five-microliter aliquots of pulsed-light-treated MNV-1 suspensions diluted 10-fold in water were placed on a glow-discharge carbon-coated EM grid and stained with 5 μl of 3% aqueous uranyl acetate for 1 min. The stained grid was then dried and examined using a JEM-1230 transmission electron microscope (JEOL) at 80 kV. Images were captured using a Gatan UltraScan 1000XP camera.
Analysis of viral proteins by SDS-PAGE.
Ten-microliter aliquots of pulsed-light-treated MNV-1 were mixed with 10 μl of Laemmli buffer (Bio-Rad) containing 5% β-mercaptoethanol. The mixture was kept on ice for 5 min, held at 90°C for 5 min, and then left again on ice for 5 min. The sample was loaded into a 10% polyacrylamide gel and electrophoresed under reducing conditions at a constant voltage of 100 V, along with a Precision Plus protein standard (Bio-Rad) as a molecular weight marker. Viral proteins were visualized by Coomassie blue staining.
RNA extraction.
Viral RNA was extracted from a 200-μl aliquot of MNV-1 (pulsed-light treated or not), diluted 10-fold in water, by using a QIAamp MinElute virus spin kit according to the manufacturer's instructions (reference 57704; Qiagen), except that carrier RNA was not added to the lysis buffer. RNA was eluted in 80 μl RNase- and DNase-free water.
Analysis of viral RNA by electrophoresis.
MNV-1 RNA in 10 μl of extract was denatured for 2 min at 70°C prior to analysis. A 1-μl aliquot was analyzed on a 6000 RNA Pico Chip apparatus (reference 5067-1513; Agilent) using a Bioanalyzer (Agilent) according to the manufacturer's instructions. RNA from untreated MNV-1 was also electrophoresed on 0.8% agarose under denaturing conditions as described previously (23).
Analysis of photochemical products by UPLC-MS/MS.
MNV-1 RNA in a 50-μl aliquot was digested enzymatically as described previously (24). The sample was injected into a Waters Acquity H-class UPLC system fitted with an ACE Excel C18 (J) column (100- by 2.1-mm internal diameter, 2-μm particle size) and UV detection (260 nm; UV trace from 210 to 800 nm). The mobile phase was a gradient of 5 mmol liter−1 ammonium formate and methanol (Fisher) at a flow rate of 200 μl min−1. The proportion of methanol reached 80% after 10 min. The Waters TQD system was operated in the positive mode. Analyses were performed in the multiple-reaction-monitoring mode. Several transitions were monitored simultaneously: 268.25 to 136.25 (adenosine), 284.15 to 152.00 (guanosine), 245.02 to 113.00 (uridine), and 244.40 to 112.00 (cytidine), as well as a scan between 200 and 600 m/z in order to detect any other molecules as dimers. The dwell time was set at 0.005 s for each signal.
Statistical analysis.
All measurements were performed in triplicate and obtained from at least two independent experiments. The reduction was expressed as the mean log10 titer ± the standard deviation and was calculated by subtracting the titer measured before exposure to pulsed light (control) from the titer measured after exposure (25). A reduction of 3 log10, which corresponds to 99.9% inactivation, was used as the criterion for declaring the treatment virucidal. One-way multiple comparisons were performed using JMP 10 statistical analysis software, a product of SAS (Cary, NC, USA). P values of <0.05 were considered statistically significant.
RESULTS
Under the conditions of the present study, the four locations of the illuminated surface were exposed to an average broadband fluence of 0.69 J cm−2 per pulse. About 2% of this energy was associated with UV (Table 1).
TABLE 1.
Broadband and UV energiesa
| No. of pulses | Fluence (J/cm2) | Energy (J) at sample surface |
||
|---|---|---|---|---|
| Suspension | Hard material | Alginate | ||
| 1 | 0.69 (0.02) | 1.38 (0.03) | 0.72 (0.02) | 3.13 (0.07) |
| 3 | 2.07 (0.05) | 4.15 (0.09) | 2.15 (0.05) | 9.38 (0.21) |
| 5 | 3.45 (0.08) | 6.91 (0.16) | 3.59 (0.08) | 15.63 (0.35) |
| 7 | 4.84 (0.11) | 9.67 (0.22) | 5.02 (0.11) | 21.89 (0.49) |
| 9 | 6.22 (0.14) | 6.46 (0.15) | ||
| 11 | 7.60 (0.17) | 7.89 (0.18) | ||
| 13 | 8.98 (0.20) | 9.33 (0.21) | ||
The broadband and UV energies were measured 80 mm below the xenon lamp using Nova and ILT1700 radiometers, respectively. The UV energy was approximately 2% of the broadband energy (200 to 1,000 nm) and appears in parentheses. Total energies received at the sample surface from one pulse were calculated by multiplying the fluence (in J/cm2) by the exposed surface area: 2.00 cm2 for suspensions, 1.04 cm2 for hard materials, and 4.54 cm2 for alginate film. Energies were averaged over 12 pulses measured at four different locations.
Activity of pulsed light against MNV-1 in aqueous suspension.
Evaluation before and after exposure to pulsed light revealed that even the most severe treatment in this study (8.98 J cm−2) generally did not result in any significant change in the liquid sample's physicochemical characteristics (Table 2) and raised the temperature by not more than 2.2 ± 0.3°C. The pH of all suspensions remained between 6.6 and 7.5.
TABLE 2.
Characteristics of sterile solutions used in this study before pulsed-light treatmenta
| Solution | Tu (NTU) | σ (μS/cm) | E (mV) | DO (mg/liter) | A254 (cm−1) | T254 (%) |
|---|---|---|---|---|---|---|
| Hard water (CaCO3 equivalents, mg/liter) | ||||||
| 50 | 0 | 166 | 338 | 3.7 | 0.0 | 100.0 |
| 100 | 0 | 215 | 330 | 3.8 | 0.0 | 100.0 |
| 150 | 0 | 286 | 325 | 3.6 | 0.0 | 100.0 |
| 200 | 0 | 362 | 320 | 4.3 | 0.0 | 100.0 |
| 400 | 0 | 686 | 337 | 2.8 | 0.0 | 100.0 |
| Turbid water (NTU) | ||||||
| 0 | 0 | 14,300 | 291 | 4.4 | 0.0 | 100.0 |
| 50 | 55 | 15,540 | 285 | 4.3 | 1.6 | 2.7 |
| 100 | 111 | 15,840 | 286 | 3.6 | 2.4 | 0.4 |
| 200 | 215 | 16,000 | 284 | 4.3 | 2.8 | 0.1 |
| 500 | 541 | 16,680 | 280 | 3.3 | 3.6 | 0.0 |
| 1,000 | 977 | 16,560 | 279 | 3.8 | 3.9 | 0.0 |
| Mineral water | 0 | 52 | 392 | 4.1 | 0.0 | 100.0 |
| Sewage treatment samples | ||||||
| Effluent 1 | 57 | 577 | 305 | 3.9 | 1.2 | 6.7 |
| Effluent 2 | 4 | 593 | 323 | 3.6 | 0.0 | 84.6 |
Characteristics measured included turbidity (Tu), conductivity (σ), reduction potential (E), dissolved O2 (DO), absorbance at 254 nm (A254), and transmittance at 254 nm (T254).
The absorbance spectra of the five hard water samples (50 to 400 mg liter−1 CaCO3 equivalent) were similar and near 0 over the 200- to 1,000-nm range, with the exception of a small peak associated with a decrease in transmittance at 974 nm due to water (26) (data not shown). The transmittance of these hard waters was 100% at the germicidal wavelength of 254 nm. Viral inactivation increased with the number of pulses. There was no statistically significant difference in inactivation among the five hard water samples (P > 0.05). Essentially total inactivation (greater than 4.0 log10 reduction) was observed with three pulses of 2.07 J cm−2 (Fig. 1A). Absorbance increased in the UV region and spread over the visible region as turbidity increased from 0 to 1,000 NTU (data not shown). Transmittance at 254 nm dropped rapidly as turbidity increased (Table 2). As shown in Fig. 1B, the treatment became significantly less effective as turbidity increased (P < 0.05). A 3-log10 reduction was achieved with a treatment of 3.45 J cm−2 (five pulses) at turbidities up to 200 NTU. Inactivation at this intensity was total only in PBS (0 NTU). Finally, the absorbance and transmittance spectra of mineral water and effluent sample 1 were very similar to those of hard waters and 50-NTU water, respectively (data not shown). Transmittance by effluent sample 2 was 84.6% at 254 nm (Table 2). There was no statistically significant difference between the three samples in terms of viral inactivation (P > 0.05). As was the case for hard waters, inactivation was total at 2.07 J cm−2 (data not shown).
FIG 1.
Inactivation of MNV-1 in experimentally contaminated hard waters (A) and turbid waters (B) after pulsed-light treatments at 80 mm from a xenon lamp. White bars, 0.69 J cm−; white bars with black dots, 2.07 J cm−2; checkered bars, 3.45 J cm−2; black bars, 4.84 J cm−2, provided in 1, 3, 5, and 7 pulses, respectively. Water hardness was adjusted with calcium carbonate. Turbidity was adjusted with bentonite suspended in PBS. Values are the mean log10 reduction per ml of three replicates from at least two independent experiments. The + symbol indicates that inactivation of the virus was total. The error bars represent standard errors.
Activity of pulsed light against immobilized MNV-1.
A critical step in the study of norovirus inactivation on hard surfaces is recovery of attached virus. Using the protocol described in Materials and Methods, recoveries of infectious MNV-1 particles from PET, PVC, and stainless steel surfaces (1.04 cm2) were similar and ranged from 95% to 98% (data not shown). The reduction in infectivity again increased with the number of pulses (P < 0.001) (Fig. 2). Under clean conditions, there was no significant difference between inactivation of MNV-1 on the different materials (P > 0.05). However, the presence of protein residue (204 μg per disk) significantly decreased the effectiveness of the treatment (P < 0.01), regardless of the material. A reduction of at least 3 log10 occurred on clean disks when the applied fluence was 3.45 J cm−2. Twice as much energy (7.60 J cm−2) was required to achieve the same reduction on fouled plastic disks (Fig. 2A and B), while the greatest reduction obtained on fouled stainless steel was 2.6 log10 with 8.98 J cm−2 (Fig. 2C). Using the protocol described above, the recovery of MNV-1 from alginate films was 96.4 ± 0.6%. These films (4.52 cm2; 0.39 ± 0.01 g) retained 5.8 log10 PFU g−1. The infectivity of entrapped MNV-1 was reduced by 3.58 ± 0.1 log10 after application of 0.69 J cm−2 and eliminated after exposure to 2.07 J cm−2 (data not shown).
FIG 2.
Inactivation of MNV-1 on experimentally contaminated polyethylene (A), polyvinyl chloride (B), and stainless steel (C) disks (1.04 cm2) under clean (bars with light cross-hatching) and fouled (bars with dark cross-hatching) conditions after treatment with pulsed light at 80 mm from a xenon lamp. Fluence increased from 0.69 to 8.98 J cm−2 as the number of pulses increased from 1 to 13. Values are mean log10 reductions of infectious particles per cm2 based on three replicates and at least two independent experiments. The + symbol indicates that inactivation of the virus was total. The error bars represent standard errors.
Mechanism of action.
To gain insight into the mechanism involved in viral inactivation by pulsed light, we compared treatments at 2.07 (3 pulses) and 8.98 J cm−2 (13 pulses). The moderate treatment left a mixture of infectious and noninfectious viral particles (3.88 ± 0.03 log10 PFU ml−1), while the intensive treatment produced total inactivation.
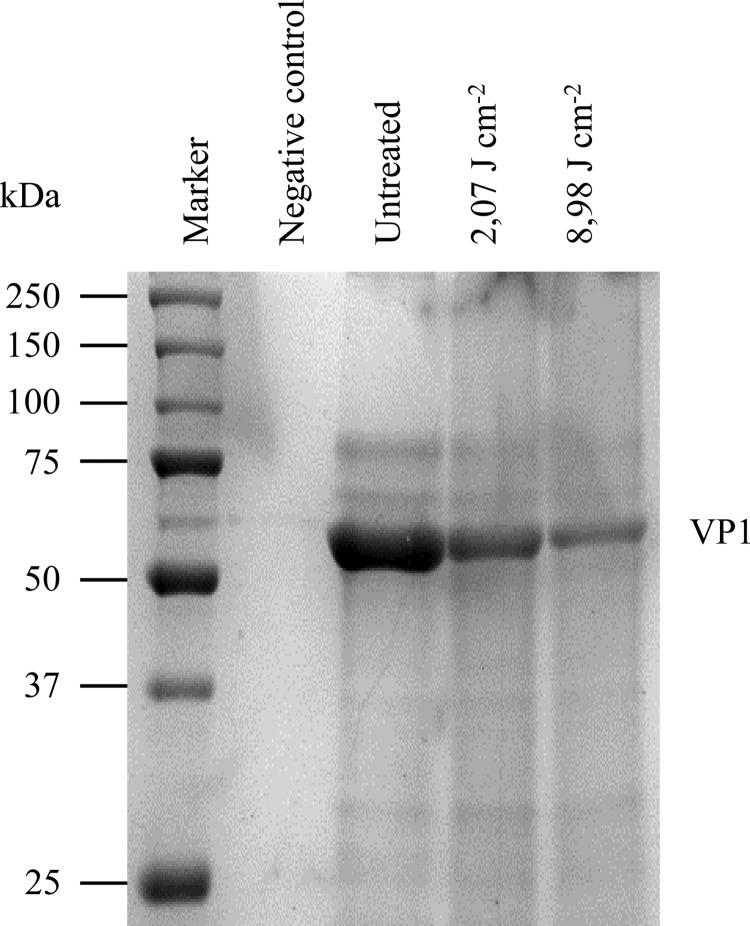
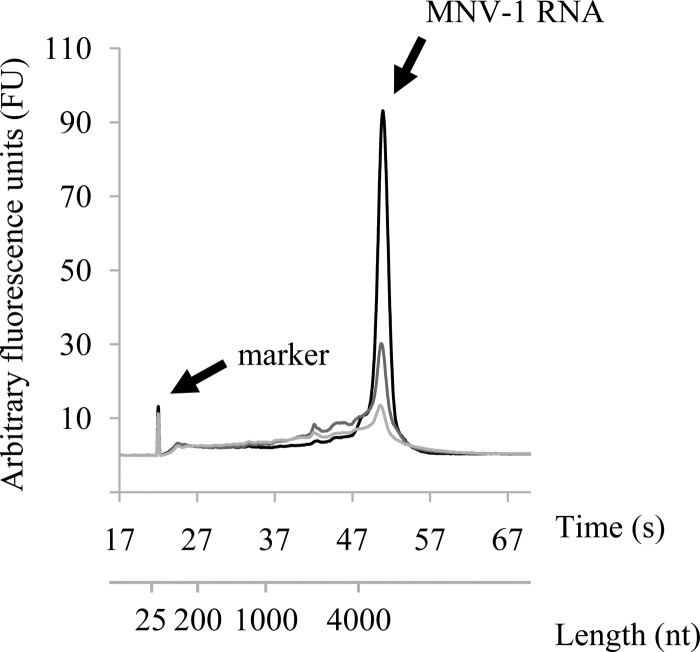
As shown in Fig. 3A, untreated MNV-1 particles were round and 35 to 40 nm in diameter. After the moderate treatment, the appearance was significantly different from the control, featuring a mixture of debris, apparently intact particles, distorted particles, and some empty particles (Fig. 3B and C). After the intensive treatment, a larger amount of debris was observed plus a few distorted particles and apparently intact particles (Fig. 3D and E). SDS-PAGE analysis of the capsid of untreated MNV-1 confirmed the presence of major and minor proteins VP1 and VP2, respectively (27). At a molecular mass of approximately 56 kDa, VP1 was clearly visible (Fig. 4). The abundance of VP1 (analyzed using Quantity One software on images obtained with a Bio-Rad ChemiDoc XRS system) decreased by 53.5 ± 3.6% relative to the untreated control after the moderate treatment. The intensive treatment did not increase this value significantly. These results suggest that pulsed light caused random breakage in the VP1 primary structure, leading to a reduction of VP1 and then a reduction of VP1 length (the assay was performed under denaturing conditions). Finally, RNA size (around 7,000 bases) and integrity were analyzed on an 0.8% agarose gel under denaturing conditions and with a Bioanalyzer, respectively. All samples were mixed with a 25-nucleotide marker as an internal control, as shown in Fig. 5. RNA from untreated MNV-1 produced a well-resolved peak (light gray curve). RNA from treated MNV-1 also produced this peak but at a lower intensity, meaning that the quantity of intact RNA decreased due to the treatment. This observation was corroborated by the decrease in the total quantity of extracted RNA detected after the moderate (2.0 ± 0.2 ng μl−1) and intensive (1.6 ± 0.0 ng μl−1) treatments. Pulsed light thus appeared to cause breaking of RNA into fragments smaller than 200 nucleotides, which would not be retained during purification and therefore did not appear on the electropherogram. Moreover, UPLC profiles were similar for the three samples enzymatically digested. No photoproducts were detected on the chromatogram or in the mass spectrum. However, it is possible that photoproducts too small to be detected were produced.
FIG 3.
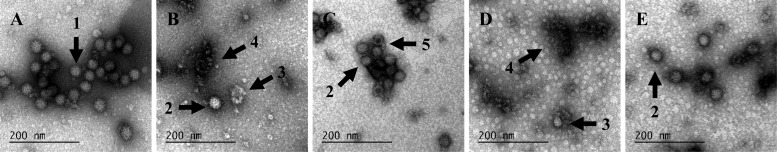
Viral particles disrupted by pulsed light. Infectivity of purified MNV-1 dropped by 5.15 ± 0.18 log10 after treatment with 2.07 J cm−2 (3 pulses) and was completely eliminated after treatment with 8.98 J cm−2 (13 pulses). Treated and untreated viral particles were stained with 3% aqueous uranyl acetate and visualized by transmission electron microscopy. (A) Untreated MNV-1; (B and C) MNV-1 treated with 3 pulses; (D and E) MNV-1 treated with 13 pulses. Arrows: 1, intact particle; 2, apparently intact particles; 3, distorted particle; 4, debris; 5, empty particle.
FIG 4.
SDS-PAGE analysis of purified MNV-1 untreated, treated with 2.07 J cm−2 (3 pulses), or treated with 8.98 J cm−2 (13 pulses). Viral proteins were analyzed via 10% SDS-PAGE followed by Coomassie staining. VP1, major capsid protein.
FIG 5.
Viral genomic RNA degraded by pulsed light. Viral genomic RNA extracted from treated or untreated purified MNV-1 was loaded onto a 6000 RNA Pico chip and analyzed on a Bioanalyzer with a UV detector. Results are presented in electropherogram form. Light gray line, untreated MNV-1; dark gray line, 2.07 J cm−2 (3 pulses); black line, 8.98 J cm−2 (13 pulses). Electropherograms show averages of triplicate analyses.
DISCUSSION
Pulsed-light technology has been found effective for eliminating bacteria and fungi on eggshells (28), on fruits (29), in fruit juices (30, 31), on packaged chicken (32), and on food packaging materials (33). The FDA has recommended a total fluence up to 12 J cm−2 for decontamination of food and food contact surfaces (11). However, studies on virus inactivation by pulsed light remain rare and difficult to compare because of differences in the equipment used and the type of data reported. In the present study, inactivation of a surrogate of human norovirus by pulsed light was evaluated in an aqueous suspension, on food contact surfaces, and in artificial biofilms. The mechanism of viral inhibition was also investigated.
Several parameters may influence the efficacy of pulsed-light treatment, including the composition and turbidity of the sample, the pulse energy, the number of pulses, and treatment time. According to our results, pulsed-light treatment was effective at inactivating MNV-1 in clear liquids up to 0.5 cm in depth. The viral titer in PBS was reduced by 2.0 ± 0.2 and 3.3 ± 0.5 log10 after treatments with broadband fluences of 0.69 and 2.07 J cm−2, respectively, of which UV fluences were 16 and 47 mJ cm−2. These values are consistent with previous results obtained for hepatitis A virus and poliovirus type 1 treated with pulsed light (13, 14) and for MNV-1 treated with continuous UV light (34, 35). As observed in the case of bacteria and fungi (36), inactivation of MNV-1 appeared equivalent whether the light was continuous or pulsed but was faster with pulsed light (232 s [34] versus 1.5 s).
In this study, we also investigated the influence of medium composition on the efficacy of pulsed light. Our results showed that water hardness up to 400 mg liter−1 CaCO3 equivalent (686 μS cm−1), mineral water, or with conductivities up to 14.3 mS cm−1 did not interfere with viral inactivation with pulsed light, suggesting a high potential of the technology for treating municipal drinking water, which has been a vehicle for the spread of norovirus infection (37, 38). In contrast, turbidity did have a negative impact on treatment efficacy. While infectivity was reduced by at least 3.9 ± 0.5 log10 when we used a broadband fluence of 4.84 J cm−2 (109 mJ cm−2 UV fluence) at up to 200 NTU, reductions were only 2.06 ± 0.3 log10 at 1,000 NTU. Fruits and vegetables as well as their juices are often involved in norovirus outbreaks (39–42). Since the turbidity of clarified fruit juices is under 200 NTU (43), at least these products could be treated without major modification of the pulsed-light treatment system. Pulsed light appears not to alter food composition (44, 45) or nutritional properties (7, 46). Finally, our results suggest that pulsed light would be effective for decontaminating effluent from primary sewage treatment. Because of the speed with which it acts, this technology offers several advantages over continuous UV treatment. It could be used to deal with contamination issues, including contamination of shellfish by municipal sewage released into marine product growing areas (47) and contamination of vegetables by irrigation (48, 49).
Food contact surfaces are frequently involved in the contamination of foods during processing. Food spoilage and pathogenic microorganisms, including viruses, may adhere to these surfaces and persist for a long period of time. Recent studies have clearly demonstrated the persistence of norovirus on inert surfaces. In this study, we have shown that inactivation of MNV-1 on clean surfaces increased with fluence, reaching reductions of 3.8 to 4.3 log10 when a broadband fluence of 4.84 J cm−2 (UV fluence of 109 mJ cm−2) was applied. These results are consistent with a previous report for hepatitis A virus (15). The presence of protein reduced the efficacy of pulsed light by up to 100 times. Under the fouled conditions used in the present study, each disk (1 cm2) was loaded with 204 μg of protein, which was 240 times higher than the amount (0.9 μg cm−2) used previously and found to decrease viral destruction by 10 and 1,000 times, respectively, on PVC and stainless steel (15). It therefore appears that protein in quantities as small as 1 μg cm−2 can reduce the effectiveness of pulsed light, but increasing the protein amount did not result in any further inhibition. Since biofilms in drinking water reservoirs and distribution systems have been shown to harbor norovirus (3), we assessed the efficacy of pulsed light at inactivating MNV-1 entrapped in alginate. One-milliliter-thick alginate film did not seem to protect against pulsed light, since MNV-1 was inactivated at the same level as the virus alone. This result is consistent with the finding of Bialka (50), who reported that penetration of the UV portion to depths up to 1 cm occurs in opaque solid materials, such as whey protein gels. In addition, several authors have noted that the alginate slime of mucoid strains of Pseudomonas aeruginosa does not provide any protection against the lethal action of pulsed light (51, 52). The viability of P. aeruginosa in biofilms was reduced by 5.8 ± 0.2 log10 when up to 21.6 μJ cm−2 UV fluence was used (52). It is obvious that our preliminary result obtained with an artificial biofilm should be validated with natural bacterial biofilms.
Attempts to elucidate the mechanism by which pulsed light kills bacteria and yeasts have been published (10, 53–57). In the present study, multiple assays were performed to provide insight into the mechanism of virus inactivation by pulsed light. To our knowledge, this is the first time that effects of pulsed light on viral structure have been investigated. Modifications at the levels of both nucleic acids and capsids were observed. Our analyses indicated single-strand breakage of MNV-1 RNA as well as damage to the virion structure (distorted and empty particles) and breakage of viral proteins. These physical alterations rendered, with no doubt, the virions unable to recognize their binding site and enter into the host cells or to replicate. UV wavelengths included in pulsed light are known to induce strand breakage and/or photoproducts such as cyclobutane pyrimidine dimers (10, 53, 54). Although no photoproducts were detected by UPLC-MS/MS analysis, their formation in small amounts cannot be excluded. However, this was not likely a major factor in the inactivation of MNV-1. While UV fluence received by samples is capable of altering viral nucleic acids (58, 59), it is probably not capable of altering the viral capsid directly (60–62). This rupture of viral capsid could be explained by the model proposed by Wekhof et al. (63), who observed the same phenomenon on bacterial and fungal cells. Those authors supposed that it arose from instantaneous overheating, caused by absorbance of UV light, which is transformed into heat and transferred to the water contained in microbial cells. This idea is consistent with previous work on phages T4 and T7, in which long-pass 295- and 400-nm filters reduced pulsed-light efficiency (64). The focus has been on UV, since the other wavelengths emitted are poorly, or not at all, absorbed by nucleic acids, proteins, and water (10).
In conclusion, this study shows that pulsed light under the specific conditions allowed by the FDA is effective at inactivating MNV-1. Since the process is rapid, environmentally friendly, and active against a broad range of microorganisms, including bacteria (65), yeasts (66), and enteric viruses (67), its application to the disinfection of drinking water, clear beverages, food contact surfaces, and even biofilms and sewage treatment effluent should be possible, provided that agents that interfere with light transmission, particularly proteins, are taken into account. We have also provided insight into the mechanism by which pulsed light inactivates viruses. Our results suggest that this technology has a dual impact on viral particles, damaging both the genetic material and the structure, thereby leaving little chance of recovery. The development of this alternative is timely, because the incidence of norovirus illness remains unacceptably high and its prevention constitutes a superior approach compared to the costs and consequences of treating infections.
ACKNOWLEDGMENTS
This work was supported by grants from Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ), and Agriculture and Agri-Food Canada (810154) and from the Natural Sciences and Engineering Research Council of Canada (NSERC; 262956).
We thank Kirsten Mattison for providing the virus and RAW 264.7 cell line, XenonCorp for complementary information about lamp spectrum and irradiance measurements, Ali Demirci for the generous loan of the Nova meter and for technical advice, and Richard Janvier for help with electron microscopy.
REFERENCES
- 1.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. 2014. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 14:725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barclay L, Park GW, Vega E, Hall A, Parashar U, Vinjé J, Lopman B. 2014. Infection control for norovirus. Clin Microbiol Infect 20:731–740. doi: 10.1111/1469-0691.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skraber S, Ogorzaly L, Helmi K, Maul A, Hoffmann L, Cauchie H-M, Gantzer C. 2009. Occurrence and persistence of enteroviruses, noroviruses and F-specific RNA phages in natural wastewater biofilms. Water Res 43:4780–4789. doi: 10.1016/j.watres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Vasickova P, Pavlik I, Verani M, Carducci A. 2010. Issues concerning survival of viruses on surfaces. Food Environ Virol 2:24–34. doi: 10.1007/s12560-010-9025-6. [DOI] [Google Scholar]
- 5.Robilotti E, Deresinski S, Pinsky BA. 2015. Norovirus. Clin Microbiol Rev 28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn J, Ott T, Clark W. 1995. Pulsed-light treatment of food and packaging. Food Technol 49:95–98. [Google Scholar]
- 8.Elmnasser N, Guillou S, Leroi F, Orange N, Bakhrouf A, Federighi M. 2007. Pulsed-light system as a novel food decontamination technology: a review. Can J Microbiol 53:813–821. doi: 10.1139/W07-042. [DOI] [PubMed] [Google Scholar]
- 9.Bolton J, Linden G. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng 129:209–215. doi: 10.1061/(ASCE)0733-9372(2003)129:3(209). [DOI] [Google Scholar]
- 10.Bolton JR, Cotton CA. 2008. Fundamentals of UV light and photochemistry, p 11–23. In Bolton JR, Cotton CA (ed), Ultraviolet disinfection handbook, 1st ed American Water Works Association, Denver, CO. [Google Scholar]
- 11.Code of Federal Regulations. 2013. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter B. Food for human consumption. Part 179.41. Pulsed light for the treatment of food. 21 CFR 179.41. [Google Scholar]
- 12.Huffman DE, Slifko TR, Salisbury K, Rose JB. 2000. Inactivation of bacteria, virus and Cryptosporidium by a point-of-use device using pulsed broad spectrum white light. Water Res 34:2491–2498. doi: 10.1016/S0043-1354(00)00014-2. [DOI] [Google Scholar]
- 13.Roberts P, Hope A. 2003. Virus inactivation by high intensity broad spectrum pulsed light. J Virol Methods 110:61–65. doi: 10.1016/S0166-0934(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 14.Lamont Y, Rzeżutka A, Anderson JG, MacGregor SJ, Given MJ, Deppe C, Cook N. 2007. Pulsed UV-light inactivation of poliovirus and adenovirus. Lett Appl Microbiol 45:564–567. doi: 10.1111/j.1472-765X.2007.02261.x. [DOI] [PubMed] [Google Scholar]
- 15.Jean J, Morales-Rayas R, Anoman M-N, Lamhoujeb S. 2011. Inactivation of hepatitis A virus and norovirus surrogate in suspension and on food-contact surfaces using pulsed UV light (pulsed light inactivation of food-borne viruses). Food Microbiol 28:568–572. doi: 10.1016/j.fm.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Hirneisen KA, Kniel KE. 2013. Comparing human norovirus surrogates: murine norovirus and tulane virus. J Food Prot 76:139–143. doi: 10.4315/0362-028X.JFP-12-216. [DOI] [PubMed] [Google Scholar]
- 17.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. 2014. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng K, Divers E, Ma Y, Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vimont A, Fliss I, Jean J. 22 November 2014, posting date Study of the virucidal potential of organic peroxyacids against norovirus on food-contact surfaces. Food Environ Virol 7:49–57. doi: 10.1007/s12560-014-9174-0. [DOI] [PubMed] [Google Scholar]
- 20.Association of Official Analytical Chemists. 1990. Disinfectants, section 960.09A, p 139–140. Official methods of analysis, 15th ed Association of Official Analytical Chemists, Washington, DC. [Google Scholar]
- 21.ASTM. 2002. ASTM E 2197-02: standard quantitative disk carrier test method for determining the bactericidal, virucidal, fungicidal, mycobactericidal and sporicidal activities of liquid chemical germicides, ASTM International, West Conshohocken, PA. [Google Scholar]
- 22.ASTM. 1997. ASTM E 1053-97: standard test method for efficacy of virucidal agents intended for inanimate environmental surfaces. ASTM International, West Conshohocken, PA. [Google Scholar]
- 23.Masek T, Vopalensky V, Suchomelova P, Pospisek M. 2005. Denaturing RNA electrophoresis in TAE agarose gels. Anal Biochem 336:46–50. doi: 10.1016/j.ab.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Douki T, Court M, Sauvaigo S, Odin F, Cadet J. 2000. Formation of the main UV-induced thymine dimeric lesions within isolated and cellular DNA as measured by high performance liquid chromatography-tandem mass spectrometry. J Biol Chem 275:11678–11685. doi: 10.1074/jbc.275.16.11678. [DOI] [PubMed] [Google Scholar]
- 25.De Vries T, Hamilton M. 1999. Estimating the antimicrobial log reduction. Part 1: quantitative assays. Quant Microbiol 1:29–45. [Google Scholar]
- 26.Curcio JA, Petty CC. 1951. The near infrared absorption spectrum of liquid water. J Opt Soc Am 41:302. doi: 10.1364/JOSA.41.000302. [DOI] [Google Scholar]
- 27.Wobus CE, Thackray LB, Virgin HW IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol 80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasagabaster A, Arboleya JC, de Marañón IM. 2011. Pulsed light technology for surface decontamination of eggs: impact on Salmonella inactivation and egg quality. Innov Food Sci Emerg Technol 12:124–128. doi: 10.1016/j.ifset.2011.01.007. [DOI] [Google Scholar]
- 29.Huang Y, Chen H. 2014. A novel water-assisted pulsed light processing for decontamination of blueberries. Food Microbiol 40:1–8. doi: 10.1016/j.fm.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Maftei NA, Ramos-Villarroel AY, Nicolau AI, Martín-Belloso O, Soliva-Fortuny R. 2014. Influence of processing parameters on the pulsed-light inactivation of Penicillium expansum in apple juice. Food Control 41:27–31. doi: 10.1016/j.foodcont.2013.12.023. [DOI] [Google Scholar]
- 31.Pataro G, Muñoz A, Palgan I, Noci F, Ferrari G, Lyng JG. 2011. Bacterial inactivation in fruit juices using a continuous flow pulsed light (PL) system. Food Res Int 44:1642–1648. doi: 10.1016/j.foodres.2011.04.048. [DOI] [Google Scholar]
- 32.Haughton PN, Lyng JG, Morgan DJ, Cronin DA, Fanning S, Whyte P. 2011. Efficacy of high-intensity pulsed light for the microbiological decontamination of chicken, associated packaging, and contact surfaces. Foodborne Pathog Dis 8:109–117. doi: 10.1089/fpd.2010.0640. [DOI] [PubMed] [Google Scholar]
- 33.Ringus DL, Moraru CI. 2013. Pulsed light inactivation of Listeria innocua on food packaging materials of different surface roughness and reflectivity. J Food Eng 114:331–337. doi: 10.1016/j.jfoodeng.2012.08.022. [DOI] [Google Scholar]
- 34.Lee J, Zoh K, Ko G. 2008. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol 74:2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park GW, Linden KG, Sobsey MD. 2011. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett Appl Microbiol 52:162–167. doi: 10.1111/j.1472-765X.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 36.Levy C, Aubert X, Lacour B, Carlin F. 2012. Relevant factors affecting microbial surface decontamination by pulsed light. Int J Food Microbiol 152:168–174. doi: 10.1016/j.ijfoodmicro.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Werber D, Lausevic D, Mugosa B, Vratnica Z, Ivanovic-Nikolic L, Zizic L, Alexandre-Bird A, Fiore L, Ruggeri F, Di Bartolo I, Battistone A, Gassilloud B, Perelle S, Nitzan Kaluski D, Kivi M, Andraghetti R, Pollock K. 2009. Massive outbreak of viral gastroenteritis associated with consumption of municipal drinking water in a European capital city. Epidemiol Infect 137:1713–1720. doi: 10.1017/S095026880999015X. [DOI] [PubMed] [Google Scholar]
- 38.Scarcella C, Carasi S, Cadoria F, Macchi L, Pavan A, Salamana M, Alborali G, Losio M, Boni P, Lavazza A, Seyler T. 2009. An outbreak of viral gastroenteritis linked to municipal water supply, Lombardy, Italy, June 2009. Euro Surveill 14:pii=19274 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19274. [DOI] [PubMed] [Google Scholar]
- 39.Maunula L, Roivainen M, Keränen M, Mäkelä S, Söderberg K, Summa M, von Bonsdorff C, Lappalainen M, Korhonen T, Kuusi M, Niskanen T. 2009. Detection of human norovirus from frozen raspberries in a cluster of gastroenteritis outbreaks. Euro Surveill 14:pii=19345 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19345. [PubMed] [Google Scholar]
- 40.Bowen A, Fry A, Richards G, Beauchat L. 2006. Infections associated with cantaloupe consumption: a public health concern. Epidemiol Infect 134:675–685. doi: 10.1017/S0950268805005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleet GH, Heiskanen P, Reid I, Buckle KA. 2000. Foodborne viral illness: status in Australia. Int J Food Microbiol 59:127–136. doi: 10.1016/S0168-1605(00)00249-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Visser H, Verhoef L, Schop W, Götz H. 2010. Outbreak investigation in two groups of coach passengers with gastroenteritis returning from Germany to the Netherlands in February 2009. Euro Surveill 15:pii=19615 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19615. [PubMed] [Google Scholar]
- 43.Dhiman SS, Garg G, Sharma J, Mahajan R. 2011. Characterization of statistically produced xylanase for enrichment of fruit juice clarification process. New Biotechnol 28:746–755. doi: 10.1016/j.nbt.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Lammertyn J, De Ketelaere B, Marquenie D, Molenberghs G, Nicolaï BM. 2003. Mixed models for multicategorical repeated response: modelling the time effect of physical treatments on strawberry sepal quality. Postharvest Biol Technol 30:195–207. doi: 10.1016/S0925-5214(03)00100-5. [DOI] [Google Scholar]
- 45.Palgan I, Caminiti IM, Muñoz A, Noci F, Whyte P, Morgan DJ, Cronin DA, Lyng JG. 2011. Effectiveness of high intensity light pulses (HILP) treatments for the control of Escherichia coli and Listeria innocua in apple juice, orange juice and milk. Food Microbiol 28:14–20. doi: 10.1016/j.fm.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 46.Palmieri L, Cacace D. 2005. High intensity pulsed light technology, p 279–306. In Sun D-W. (ed), Emerging technologies for food processing. Academic Press, London, United Kingdom. [Google Scholar]
- 47.Campos C, Avant J, Lowther J, Till D, Lees D. 2013. Levels of norovirus and E. coli in untreated, biologically treated and UV-disinfected sewage effluent discharged to a shellfish water. J Water Resour Prot 5:978–982. doi: 10.4236/jwarp.2013.510101. [DOI] [Google Scholar]
- 48.Esseili MA, Wang Q, Zhang Z, Saif LJ. 2012. Internalization of sapovirus, a surrogate for norovirus, in romaine lettuce and the effect of lettuce latex on virus infectivity. Appl Environ Microbiol 78:6271–6279. doi: 10.1128/AEM.01295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiCaprio E, Ma Y, Purgianto A, Hughes J, Li J. 2012. Internalization and dissemination of human norovirus and animal caliciviruses in hydroponically grown romaine lettuce. Appl Environ Microbiol 78:6143–6152. doi: 10.1128/AEM.01081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bialka K. 2006. Decontamination of berries using ozone and pulsed UV-light. Ph.D. thesis. Pennsylvania State University, University Park, PA. [Google Scholar]
- 51.Farrell HP, Garvey M, Cormican M, Laffey JG, Rowan NJ. 2010. Investigation of critical inter-related factors affecting the efficacy of pulsed light for inactivating clinically relevant bacterial pathogens. J Appl Microbiol 108:1494–1508. doi: 10.1111/j.1365-2672.2009.04545.x. [DOI] [PubMed] [Google Scholar]
- 52.Garvey M, Rabbitt D, Stocca A, Rowan N. 18 April 2014, posting date Pulsed ultraviolet light inactivation of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Water Environ J 29:36–42. doi: 10.1111/wej.12088. [DOI] [Google Scholar]
- 53.Wekhof A. 1991. Treatment of contaminated water, air and soil with UV flashlamps. Environ Prog 10:241–247. doi: 10.1002/ep.670100408. [DOI] [Google Scholar]
- 54.Keklik N, Demirci A. 2014. Applications and modeling aspects of UV and pulsed UV-light for food decontamination, p 67–101. In Boziaris IS. (ed), Novel food preservation and microbial assessment techniques. CRC Press, Boca Raton, FL. [Google Scholar]
- 55.Cheigh C-I, Park M-H, Chung M-S, Shin J-K, Park Y-S. 2012. Comparison of intense pulsed light- and ultraviolet (UVC)-induced cell damage in Listeria monocytogenes and Escherichia coli O157:H7. Food Control 25:654–659. doi: 10.1016/j.foodcont.2011.11.032. [DOI] [Google Scholar]
- 56.Takeshita K, Shibato J, Sameshima T, Fukunaga S, Isobe S, Arihara K, Itoh M. 2003. Damage of yeast cells induced by pulsed light irradiation. Int J Food Microbiol 85:151–158. doi: 10.1016/S0168-1605(02)00509-3. [DOI] [PubMed] [Google Scholar]
- 57.Krishnamurthy K, Tewari J, Irudayaraj J, Demirci A. 2010. Microscopic and spectroscopic evaluation of inactivation of Staphylococcus aureus by pulsed UV light and infrared heating. Food Bioprocess Technol 3:93–104. doi: 10.1007/s11947-008-0084-8. [DOI] [Google Scholar]
- 58.Simonet J, Gantzer C. 2006. Inactivation of poliovirus 1 and F-specific RNA phages and degradation of their genomes by UV irradiation at 254 nanometers. Appl Environ Microbiol 72:7671–7677. doi: 10.1128/AEM.01106-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nuanualsuwan S, Cliver DO. 2003. Infectivity of RNA from inactivated poliovirus. Appl Environ Microbiol 69:1629–1632. doi: 10.1128/AEM.69.3.1629-1632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller RL, Plagemann PGW. 1974. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol 13:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuanualsuwan S, Cliver DO. 2003. Capsid functions of inactivated human picornaviruses and feline calicivirus. Appl Environ Microbiol 69:350–357. doi: 10.1128/AEM.69.1.350-357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolton JR, Cotton CA. 2008. Mechanism of UV disinfection, p 25–40. In Bolton JR, Cotton CA (ed), Ultraviolet disinfection handbook, 1st ed American Water Works Association, Denver, CO. [Google Scholar]
- 63.Wekhof A, Trompeter F, Franken O. 2001. Pulsed UV-disintegration (PUVD): a new sterilisation mechanism for packaging and broad medical-hospital applications. First International Conference on Ultraviolet Technologies, 14 to 16 June 2001, Washington, DC. [Google Scholar]
- 64.Bohrerova Z, Shemer H, Lantis R, Impellitteri CA, Linden KG. 2008. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Res 42:2975–2982. doi: 10.1016/j.watres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Rowan NJ, MacGregor SJ, Anderson JG, Fouracre RA, McIlvaney L, Farish O. 1999. Pulsed-light inactivation of food-related microorganisms. Appl Environ Microbiol 65:1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jun S, Irudayaraj J, Demirci A, Geiser D. 2003. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int J Food Microbiol 38:883–888. doi: 10.1046/j.0950-5423.2003.00752.x. [DOI] [Google Scholar]
- 67.Vimont A, Fliss I, Jean J. 2014. Inactivation of foodborne viruses: recent findings applicable to food-processing technologies, p 471–495. In Bhat R, Gomez-Lopez VM (ed), Practical food safety: contemporary issues and future directions. Wiley and Sons, Hoboken, NJ. [Google Scholar]