Abstract
Nucleoid-associated proteins (NAPs), which fold bacterial DNA and influence gene transcription, are considered to be global transcriptional regulators of genes on both plasmids and the host chromosome. Incompatibility P-7 group plasmid pCAR1 carries genes encoding three NAPs: H-NS family protein Pmr, NdpA-like protein Pnd, and HU-like protein Phu. In this study, the effects of single or double disruption of pmr, pnd, and phu were assessed in host Pseudomonas putida KT2440. When pmr and pnd or pmr and phu were simultaneously disrupted, both the segregational stability and the structural stability of pCAR1 were markedly decreased, suggesting that Pmr, Pnd, and Phu act as plasmid-stabilizing factors in addition to their established roles in replication and partition systems. The transfer frequency of pCAR1 was significantly decreased in these double mutants. The segregational and structural instability of pCAR1 in the double mutants was recovered by complementation of pmr, whereas no recovery of transfer deficiency was observed. Comprehensive phenotype comparisons showed that the host metabolism of carbon compounds, which was reduced by pCAR1 carriage, was restored by disruption of the NAP gene(s). Transcriptome analyses of mutants indicated that transcription of genes for energy production, conversion, inorganic ion transport, and metabolism were commonly affected; however, how their products altered the phenotypes of mutants was not clear. The findings of this study indicated that Pmr, Pnd, and Phu act synergistically to affect pCAR1 replication, maintenance, and transfer, as well as to alter the host metabolic phenotype.
INTRODUCTION
Plasmids transferred among different bacteria can confer novel phenotypes to the host, including antibiotic resistance or the ability to degrade xenobiotics (1, 2). Genes on the plasmid are regulated not only by plasmid-encoded factors but also by chromosomally encoded host factors (3). Therefore, upon transfer into a different host, regulation of genes on the plasmid may change in response to different host factors.
We examined the interaction between the plasmid and the host bacterial strain using carbazole degradation incompatibility (Inc) P-7 group conjugative plasmid pCAR1, which was originally isolated from Pseudomonas resinovorans CA10 (4). The whole nucleotide sequence of pCAR1 was reported previously (5, 6), and the transcriptomes of pCAR1 were shown to differ in six different Pseudomonas hosts (7). We also demonstrated that carriage of the plasmid affects chromosomal gene expression and the host phenotype using three different hosts: P. putida KT2440, P. aeruginosa PAO1, and P. fluorescens Pf0-1 (8). Moreover, comprehensive analysis of the host phenotype revealed that pCAR1 carriage reduces host fitness, swimming motility, and resistance to osmotic or pH stress and alters the primary metabolic capacity of the host cells (9). These effects on host phenotypes were considered to be dependent on various factors from both the plasmid and the host chromosome.
Nucleoid-associated proteins (NAPs) were suggested to be candidate effectors of the interaction between the plasmid and host chromosome (10). NAPs alter the shape of bacterial DNA to make it more compact and can influence global transcription (11). The best-studied NAPs are H-NS and HU proteins. H-NS was shown to repress genes acquired through horizontal gene transfer by recognizing sequences with low G+C content (12, 13). Several proteins (e.g., MvaT of Pseudomonas spp. or Lsr2 of Mycobacterium spp.) are included in the H-NS family of proteins as functional homologs due to their ability to silence the expression of xenogeneic DNA sequences, despite their almost negligible amino acid sequence homology with H-NS (13–15). In contrast, HU proteins bind preferentially to duplex DNA containing a nick or a gap, and they bend DNA in a sequence-independent manner (16, 17).
Notably, considerable numbers of plasmids from Gram-negative bacteria (7% of the 2,260 plasmids fully sequenced as of April 2010) contain at least a single NAP homolog gene (18). Previous studies on plasmid-encoded NAPs focused mainly on H-NS homologs, namely, H-NSR27 and Sfh, in plasmid R27 and its derivatives (19, 20). The DNA binding sites of Sfh completely overlapped those of chromosomally encoded H-NS, as shown by genome-wide chromatin immunoprecipitation with microarray technology (ChIP-chip) analysis (21). In contrast, H-NSR27, which is 98% identical to Sfh, selectively targets horizontally acquired DNA and not core genomic DNA, whereas chromosomally encoded H-NS targets both (20). H-NSR27 participates in the thermoregulation of the conjugative transfer of R27 (22).
Plasmid pCAR1 carries three genes encoding the following NAPs: Pmr (plasmid-encoded MvaT-like regulator; encoded by ORF70), Pnd (plasmid-encoded NdpA-like protein; encoded by ORF93), and Phu (plasmid-encoded HU-like protein; encoded by ORF95a) (4). One of these NAPs, Pmr, binds preferentially to horizontally acquired DNA and optimizes the transcription of genes on both the plasmid and the host P. putida KT2440 chromosome (23). Recently, two MvaT homologs encoded on the KT2440 chromosome, TurA (PP_1366) and TurB (PP_3765), were shown to play important roles in the transcriptional regulation of Pmr (24). The present study was performed to examine the functions of the other two NAPs encoded on pCAR1, Pnd and Phu, using P. putida KT2440 as the model host.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown at 37°C in lysogeny broth (LB) (25), whereas P. putida KT2440(pCAR1) and its derivative strains were grown at 30°C in LB or nitrogen plus mineral medium 4 (NMM-4) (26) containing 0.1% (wt/vol) succinate or carbazole as the sole source of carbon and energy. Ampicillin (Ap; 50 μg/ml), chloramphenicol (Cm; 30 μg/ml), gentamicin (Gm; 30 μg/ml for mating experiments and 120 μg/ml for mutant preparation), kanamycin (Km; 50 μg/ml), rifampin (Rif; 25 μg/ml), and sucrose (10% [wt/vol]) were added to the selective medium. For plate cultures, the media described above were solidified with 1.6% (wt/vol) purified agar powder (Nacalai Tesque, Kyoto, Japan).
TABLE 1.
Bacterial strains and plasmids
| Bacterial strain or plasmid | Relevant characteristic(s) | Source or reference(s) |
|---|---|---|
| Bacterial strains | ||
| E. coli K-12 DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rK− mK+) deoR thi-1 supE44 gyrA96 relA1 λ− phoA | Toyobo |
| E. coli K-12 S17-1λpir | recA thi pro hsdR; RP4-2 integrated into the chromosome (kan::Tn7 ter::Mu) λpir | 44 |
| P. putida KT2440 | Naturally Cmr | 45 |
| P. putida KT2440KR | Derivative strain of KT2440, spontaneously Rifr, with introduced Kmr gene | This study |
| P. putida KT2440(pCAR1) | KT2440 harboring pCAR1 | 35 |
| P. putida KT2440(pCAR1pmrHis::Gmr) | KT2440(pCAR1pmrHis) harboring Gmr gene cassette and FRT sites | 23 |
| P. putida KT2440(pCAR1Δpmr::Gmr) | KT2440 harboring pCAR1 carrying disrupted pmr gene by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1Δpmr)a | KT2440(pCAR1) single-deletion mutant lacking pmr gene | This study |
| P. putida KT2440(pCAR1Δpnd::Gmr) | KT2440 harboring pCAR1 carrying disrupted pnd gene by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1Δpnd) | KT2440(pCAR1) single-deletion mutant lacking pnd gene | This study |
| P. putida KT2440(pCAR1Δphu::Gmr) | KT2440 harboring pCAR1 carrying disrupted phu gene by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1Δphu) | KT2440(pCAR1) single-deletion mutant lacking phu gene | This study |
| P. putida KT2440(pCAR1ΔpmrΔpnd::Gmr) | KT2440(pCAR1Δpmr) in which pnd gene is disrupted by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1ΔpmrΔpnd) | KT2440(pCAR1) double-deletion mutant lacking pmr and pnd genes | This study |
| P. putida KT2440(pCAR1ΔpmrΔphu::Gmr) | KT2440(pCAR1Δpmr) in which phu gene is disrupted by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1ΔpmrΔphu) | KT2440(pCAR1) double-deletion mutant lacking pmr and phu genes | This study |
| P. putida KT2440(pCAR1ΔpndΔphu::Gmr) | KT2440(pCAR1Δpnd) in which phu gene is disrupted by Gmr gene cassette and FRT sites | This study |
| P. putida KT2440(pCAR1ΔpndΔphu) | KT2440(pCAR1) double-deletion mutant lacking pnd and phu genes | This study |
| P. putida KT2440(pCAR1PMGΔpmrΔpnd) | KT2440(pCAR1ΔpmrΔpnd) containing Gmr gene cassette with FRT sites and 0.57-kb pmr cassette inserted into 101,000–101,001 region of pCAR1 | This study |
| P. putida KT2440(pCAR1PMΔpmrΔpnd) | KT2440(pCAR1ΔpmrΔpnd) containing 0.57-kb pmr cassette inserted into 101,000–101,001 region of pCAR1 | This study |
| P. putida KT2440(pCAR1PMGΔpmrΔphu) | KT2440(pCAR1ΔpmrΔphu) containing Gmr gene cassette with FRT sites and 0.57-kb pmr cassette inserted into 101,000–101,001 region of pCAR1 | This study |
| P. putida KT2440(pCAR1PMΔpmrΔphu) | KT2440(pCAR1ΔpmrΔphu) containing 0.57-kb pmr cassette inserted into 101,000–101,001 region of pCAR1 | This study |
| Plasmids | ||
| pBBad18K | Kmr, l-arabinose-inducible vector based on the pBBR1MCS-4 replicon | 33 |
| pBBad18K-recT | pBBad18K, BamHI-XbaI fragment containing recT | This study |
| pBSLKmr | Mini-Tn5, R6K ori, Apr, Kmr | 34 |
| pFLP2Km | pFLP2, Kmr gene cassette inserted into its ScaI site | 23 |
| pK19mobsacB | Kmr, oriT (RP4), sacB, lacZα, pMB1 replicon | 32 |
| pK19mobsacBΔpmr | pK19mobsacB containing 5′- and 3′-flanking regions of pmr and Gmr gene cassette, which is flanked by FRT sites | This study |
| pK19mobsacBΔpnd | pK19mobsacB containing 5′- and 3′-flanking regions of pnd and Gmr gene cassette, which is flanked by FRT sites | This study |
| pK19mobsacBΔphu | pK19mobsacB containing 5′- and 3′-flanking regions of phu and Gmr gene cassette, which is flanked by FRT sites | This study |
| pK19mobsacBpmr | pK19mobsacB containing 100,550–101,000 and 101,001–101,382 regions of pCAR1, pmr, and Gmr gene cassette | This study |
| pPS856 | Apr, Kmr, FRT sites | 31 |
| pT7Blue T-vector | Apr, lacZα, T7 promoter, f1 origin, pUC/M13 priming sites | Novagen |
| pTpmr | pT7Blue T-vector with PCR fragment amplified from total DNA of KT2440(pCAR1) with primer set pmr_qRT_F and pmr_qRT_R | 35 |
| pTpnd | pT7Blue T-vector with PCR fragment amplified from total DNA of KT2440(pCAR1) with primer set pnd_qRT_F and pnd_qRT_R | This study |
| pTphu | pT7Blue T-vector with PCR fragment amplified from total DNA of KT2440(pCAR1) with primer set phu_qRT_F and phu_qRT_R | This study |
| pTuniv16S | pT7Blue T-vector with PCR fragment amplified from total DNA of P. resinovorans CA10 with primer set univ16S-F and univ16S-R | 35 |
| pZErO-2 | Kmr, T7 promoter, ColE1 and f1 origin, ccdB lethal gene, M13 priming sites | Invitrogen |
KT2440(pCAR1Δpmr), which was used in our previous study (23), was reconstructed in this study as described in Materials and Methods.
Standard DNA manipulation.
Standard methods were used for the extraction of plasmid DNA, digestion of DNA with restriction endonucleases, DNA ligation, and transformation of competent E. coli cells (25). The primers used in this study are listed in Table 2. Total DNA was extracted from Pseudomonas strains by the use of hexadecyltrimethylammonium bromide, as described previously (27). Electroporation of Pseudomonas was performed according to the method described by Itoh et al. (28).
TABLE 2.
Oligonucleotide primers
| Primer | Sequence (5′ → 3′)a | Reference |
|---|---|---|
| Preparation of NAP gene disruption mutants | ||
| Gm-F | CGAATTAGCTTCAAAAGCGCTCTGA | 30 |
| Gm-R | CGAATTGGGGATCTTGAAGTTCCT | 30 |
| pmr_del_up_F | GGATCCAGGAATCACTGTTCGGCAAG | This study |
| pmr_del_up_R2 | TCAGAGCGCTTTTGAAGCTAATTCGCTTGTCTCCTTGGTCTGGG | This study |
| pmr_del_down_F2 | AGGAACTTCAAGATCCCCAATTCGGTTTGCGCTACCGCGGATCT | This study |
| pmr_del_down_R | AAGCTTGTGAGCCAAGGCTTCTTCAG | This study |
| pnd_del_up_F | GGATCCCGGAGTCCCAAACCGTAATA | This study |
| pnd_del_up_R | TCAGAGCGCTTTTGAAGCTAATTCGGAGCAAAGTCCTTGTAGTTT | This study |
| pnd_del_down_F | AGGAACTTCAAGATCCCCAATTCGAAAAATGCCCCGTCCATCTC | This study |
| pnd_del_down_R | AAGCTTGGATCAATTCCAGCCTCAAA | This study |
| phu_del_up_F | GGATCCTAGACGATCGACGCAAAGTG | This study |
| phu_del_up_R2 | TCAGAGCGCTTTTGAAGCTAATTCGTCGCTTTTACTCCTTGGTTG | This study |
| phu_del_down_F2 | AGGAACTTCAAGATCCCCAATTCGGCCCCCTCCAGCCGCCCCGA | This study |
| phu_del_down_R | AAGCTTGCGATGAGAAGGGCAAATAG | This study |
| Preparation of pmr-complemented strains | ||
| Gm-F-SalI | GTCGACCGAATTAGCTTCAAAAGCGCTCTG | This study |
| Gm-R-PstI | CTGCAGCGAATTGGGGATCTTGAAGTTCCT | This study |
| ORF98_COMP_up_F | GGATCCACCTGGGTACTGGCTCATTG | This study |
| ORF98_COMP_up_R | CTGCAGGCCCGTTTTCCTACGCAGCT | This study |
| ORF99_COMP_down_F | CCCGGGCTGGTGGCACGCGTCGGCCC | This study |
| ORF99_COMP_down_R | AAGCTTCATATCGCATGGGATTTTCC | This study |
| pmr_COMP_F | CCCGGGCGCATTCTGGCCTTCCGCCG | This study |
| pmr_COMP_R | GTCGACCTCACAAAAAAGGCCGGGTT | This study |
| pCAR1 stability assay | ||
| recT-ox-F | GGATCCAAGGAGACCTTCGGGTGCGTTTTC | This study |
| recT-ox-R | TCTAGACTATCACTCGCCTTGCG | This study |
| 5′-RACE analysis | ||
| M13-F | GTAAAACGACGGCCAGT | 38 |
| M13-R | GGAAACAGCTATGACCATG | 38 |
| pnd308 | CCGGTTGAGAGGTTCGATTCTTCCAT | This study |
| pnd498 | GGACTGCTTGTTGTTCTGCCATTCG | This study |
| phu157 | TTGACTCGAAAGTACCAAAGCCCACGAG | This study |
| phu258 | CTTGAAGGACTTGCCAGGGGTGAACT | This study |
| phu_up395 | CATTACCACACTTCGGACAGAGCAATCC | This study |
| phu_up564 | GAGGTTCCCGGAGTGGTCAATGATAATG | This study |
| qRT-PCR | ||
| pmr_qRT_F | GATCCGGACTATCGAAAGCA | 35 |
| pmr_qRT_R | TTCCACTCCTTGAGCGTCTT | 35 |
| pnd_qRT_F | TGGCAGAACAACAAGCAGTC | This study |
| pnd_qRT_R | GTCTACACCCTCCTGGCAAC | This study |
| phu_qRT_F | TCGTGGGCTTTGGTACTTTC | This study |
| phu_qRT_R | GGTGAACTTTGGCTTGATCG | This study |
| univ16S-F | ACACGGTCCAGACTCCTACG | 35 |
| univ16S-R | TACTGCCCTTCCTCCCAACT | 35 |
Underlined nucleotides represent artificial restriction sites.
5′-RACE analysis.
Extraction of total RNA from P. putida KT2440(pCAR1) at the log growth phase was performed as described previously (9). cDNA synthesis, 5′-rapid amplification of cDNA ends (5′-RACE) PCR, and nested PCR were performed using a SMARTer RACE cDNA amplification kit (Clontech, Mountain View, CA) in accordance with the manufacturer's instructions. Nested PCR products were ligated into the EcoRV sites of plasmid pZErO-2 (Invitrogen, Carlsbad, CA), and the sequences of the DNA fragments were confirmed using primers M13-F and M13-R.
Quantitative reverse transcription (qRT)-PCR.
Extraction of total RNA from P. putida KT2440(pCAR1) was performed as described previously (9). The primers were designed using the Primer3 program (29), and the cDNA was quantified using an ABI 7300 real-time PCR system (Applied Biosystems, Foster City, CA) as described previously (9).
Preparation of mutants of NAP genes.
The procedures for preparation of each single-gene-disruption mutant of pmr, pnd, and phu were performed similarly using a homologous-recombination-based gene replacement system (30). Briefly, DNA fragments containing the 5′- and 3′-flanking regions of each gene and the Gm resistance cassette, which is flanked by flippase recognition target (FRT) sites on pPS856 (31), were cloned into suicide vector pK19mobsacB (32) to yield pK19mobsacBΔpmr, pK19mobsacBΔpnd, and pK19mobsacBΔphu, respectively. E. coli S17-1λpir cells carrying the resultant plasmids were conjugated with KT2440(pCAR1) on cellulose membrane filters (Advantec, Tokyo, Japan) (0.45-μm pore size) and incubated on LB agar plates at a donor-to-recipient ratio of 1:1 at 30°C for 15 h. Double-crossover recombinants were screened using sucrose counterselection, and then the Gm resistance cassette was removed by site-specific recombination of the FRT sites using Flp recombinase expressed by pFLP2Km (23). In our previous study, we used a pmr disruption mutant with a Gm resistance gene cassette without FRT sites in the pmr gene (23). In this study, we reconstructed KT2440(pCAR1Δpmr) lacking the cassette. To prepare the double-disruption pmr pnd and pmr phu mutants, pK19mobsacBΔpnd and pK19mobsacBΔphu plasmids were transferred into KT2440(pCAR1Δpmr). To prepare the double-disruption pnd phu mutant, the pK19mobsacBΔphu plasmid was transferred into KT2440(pCAR1Δpnd). The Gm resistance cassette was removed from the double-crossover recombinants as described above. To prepare pmr gene-complemented strains of KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu), pK19mobsacBpmr, which contains pmr and a Gm resistance cassette flanked by the regions corresponding to bp 100,550 to 101,000 and bp 101,001 to 101,382 of pCAR1, was transferred into the double mutants. After successful insertion of pmr and the Gm resistance cassette into pCAR1, the Gm resistance cassette was removed as described above.
pCAR1 stability assay.
P. putida KT2440(pCAR1) and its derivatives, the carbazole degradation ability of which was confirmed, were grown for 24 h in LB. Cultures were then diluted 1,000-fold with NMM-4 medium supplemented with 0.1% succinate and incubated at 30°C. After 24 h, cultures were again diluted 1,000-fold with fresh NMM-4 medium supplemented with 0.1% succinate. This procedure was repeated five times. Cultures at the first and the fifth passages were spread onto LB agar plates, and the resultant cultures were used for colony hybridization to confirm the presence of the repA and carAc genes. Probes for colony hybridization were prepared from a 1.7-kb HindIII-XbaI fragment of pCAR1 containing the repA gene and a 1.0-kb BglII-XhoI fragment of pCAR1 containing the carAc gene. Hybridization and detection was performed as described previously (9). To assess the effects of RecT overexpression on the stability of pCAR1, KT2440(pCAR1) harboring pBBad18K (33) containing the recT gene of pCAR1 was used in similar medium supplemented with 0.2% (wt/vol) l-arabinose to induce the expression of recT. To test the effects of iron ions on the stability of pCAR1, 37 or 370 μM ferric citrate was added to NMM-4 containing 0.1% succinate instead of FeCl3 (originally 37 μM).
Mating experiments.
P. putida KT2440(pCAR1) derivatives harboring a Gm resistance cassette and P. putida KT2440KR derivatives harboring a Km resistance cassette were used as donors and recipients, respectively. P. putida KT2440KR was prepared from the P. putida KT2440 rifampin resistance spontaneous mutant using pBSLKmr (34). The donor and the recipient strains were precultured in 5 ml of LB liquid medium with the appropriate antibiotics for 18 h. After washing with LB liquid medium, donors and recipients were resuspended and diluted in LB liquid medium to optical densities at 600 nm (OD600) of 0.2 and 2.0, respectively. Aliquots of 200 μl of each culture were mixed in 2-ml microtubes, and the lip was sealed using a gas-permeable adhesive seal (Nippon Genetics, Tokyo, Japan) with the lid open. After incubation at 30°C for 3 h, the cell suspensions were spread on selective media at the appropriate dilutions. The number of transconjugants was determined by counting colonies on selective medium 1 to 2 days after mating. The frequency of plasmid transfer was expressed as the number of transconjugants per number of donors.
PM analyses.
Phenotype MicroArray (PM; Biolog, Hayward, CA) analyses were performed as described previously (9) using panels PM1 through PM4, PM9, and PM10.
Transcriptome analyses of pCAR1 and the KT2440 chromosome.
Transcriptome analyses were performed with custom-made tiling arrays, which were constructed as described previously (8, 35). Total RNA extraction and cDNA syntheses in the presence of actinomycin D (Sigma-Aldrich, St. Louis, MO) were performed as described previously (9). The fragmentation and labeling of cDNA, hybridization with the tiling arrays, washing, detection, calculation of the intensities of each probe, and definition of transcriptional values for each gene were performed as described previously (8) except using 3.3 μg of cDNA. Comparisons between KT2440(pCAR1) and the mutants were performed using biologically duplicated data. We identified up- and downregulated open reading frames (ORFs) with changes of over 2-fold in all of the four data comparisons performed [between replicate 1 or 2 of KT2440(pCAR1) and replicate 1 or 2 of each mutant]. The data were visualized using the IGB software package (Affymetrix, Santa Clara, CA).
Microarray data accession number.
The array data reported in this article have been deposited in the Gene Expression Omnibus of the National Center for Biotechnology Information (NCBI) (GEO; http://www.ncbi.nlm.nih.gov/geo/) under GEO Series accession no. GSE53069.
RESULTS AND DISCUSSION
Transcription initiation start points and transcriptional profiles of pnd and phu.
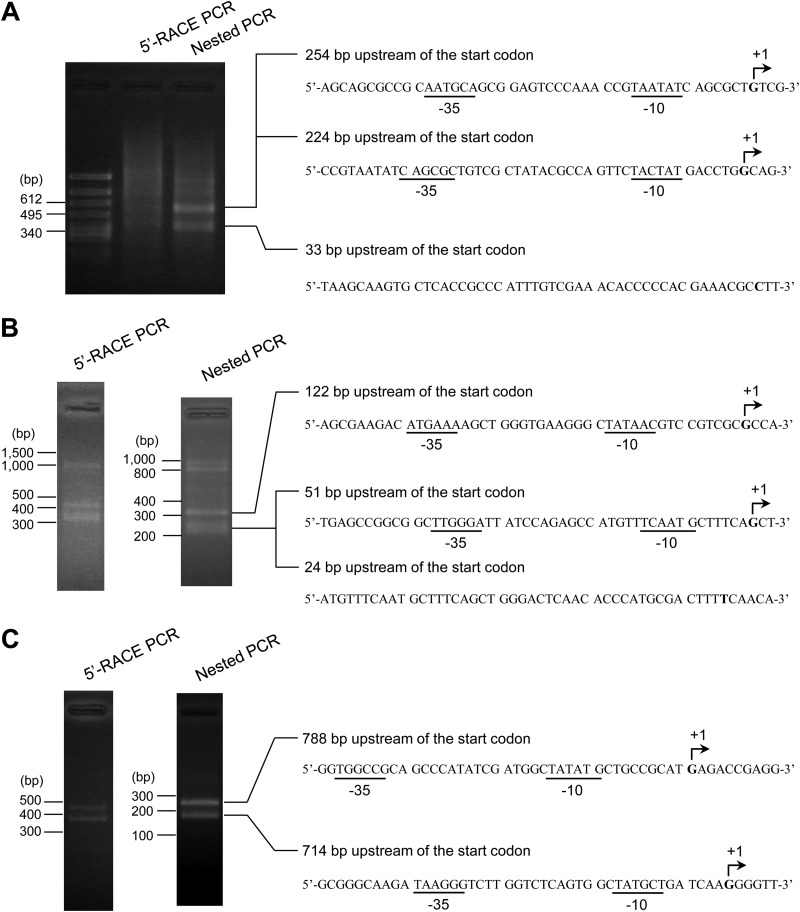
To identify the transcription initiation start points for pnd and phu, 5′-RACE analyses were performed with the total RNA of KT2440(pCAR1) cells at the log growth phase. When primers that anneal specifically to the internal region of pnd were used, the PCR products on the agarose gel electrophoresis corresponded to regions 254, 224, and 33 bp upstream of the pnd translational start site (Fig. 1A). Putative −10 and −35 elements for σ70 of pseudomonads (36) were found 254 and 224 bp upstream of the start codon, respectively, whereas no characteristic pseudomonad σ70 or σ54 binding motifs (37) were observed 33 bp upstream of the start codon. Therefore, the regions 254 and 224 bp upstream of the start codon were identified as the transcriptional start sites for pnd. Similarly, the transcription initiation start points of phu were located 788, 714, 122, and 51 bp upstream of the start codon (Fig. 1B and C). Based on the results of transcriptome analysis of KT2440(pCAR1) described below, the transcription initiation start points of pnd and phu were predicted to be ∼200 and 700 bp, respectively, upstream of the translational start points (see Fig. S1 in the supplemental material), consistent with the results of 5′-RACE analyses.
FIG 1.
Identification of the transcription initiation start points of pnd and phu. 5′-RACE PCR followed by nested PCR was performed using primers designed to anneal specifically to the internal regions of pnd (A) and phu (B) and to the upstream region of phu (C). As templates, cDNA synthesized from the total RNA of KT2440(pCAR1) was used for 5′-RACE PCR and the 5′-RACE PCR products were used for a nested PCR. The 5′-end nucleotides of the nested PCR products are shown in bold type. The nucleotide sequences up- and downstream of the 5′-end nucleotide are also shown in the panels. The arrow indicates the transcription initiation start point (+1); the −35 and −10 hexamers are underlined.
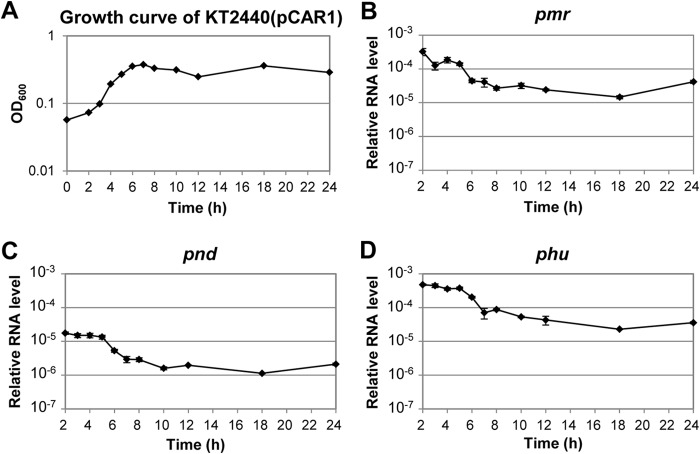
qRT-PCR analyses of pmr, pnd, and phu along with the growth curve of KT2440(pCAR1) indicated that their transcription levels were similarly higher during the log growth phase (2 to 5 h) than in stationary phase (6 to 24 h; Fig. 2). This was consistent with the results of our previous microarray analyses (9) and the presence of putative σ70-dependent promoters on pnd and phu as described above. The putative promoter of pmr was also predicted to be dependent on σ70 in our previous study (23). Whereas the levels of pmr and phu transcription were higher than those of pnd, the growth phase-dependent transcription profiles of pmr, pnd, and phu were similar to each other.
FIG 2.
Transcriptional profiles of the pCAR1 genes encoding NAPs. (A) Growth curve of KT2440(pCAR1). (B to D) The mRNA levels of pmr (B), pnd (C), and phu (D) were measured by qRT-PCR along the growth curve. The data were normalized using the average of the 16S rRNA data as the internal standard. Means and standard deviations (error bars) of triplicate data are shown.
Effects of NAP gene disruption on host cell function.
To assess the effects of NAP gene(s) disruption on P. putida KT2440(pCAR1), we prepared single or double mutants of pmr, pnd, and phu with and without the Gm resistance gene cassette.
Stability of pCAR1.
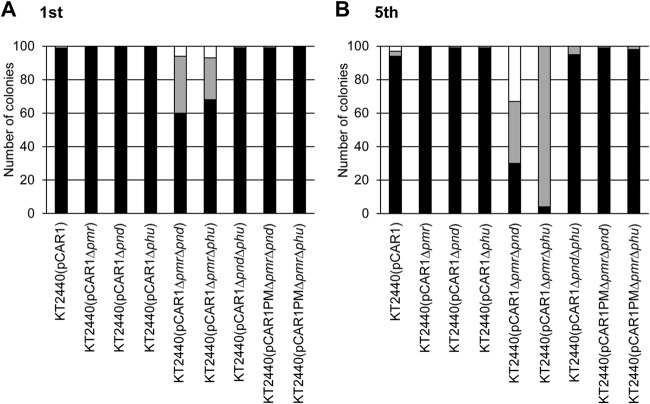
First, the stability of pCAR1 was assessed in the wild-type (WT) strain and six mutants without the Gm resistance gene cassette, which were cultured for 24 h in NMM-4 (26) supplemented with succinate as the sole source of carbon. As shown in Fig. 3, pCAR1 was maintained stably in the WT, three single mutants, and one double mutant. In contrast, some colonies from the cultures of two double mutants, KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu), did not contain the repA gene (i.e., they did not have pCAR1). In addition, many pCAR1-containing colonies of the two double mutants, particularly in the fifth passage in culture, did not have the carAc gene. DNA rearrangements likely due to homologous recombination between two copies of ISPre1 may have occurred in these strains, as described previously (38). Neither segregational instability nor structural instability of pCAR1 was detected in these two double mutants when pmr and its promoter region were supplied (Fig. 3). Our previous study showed that a mini-replicon of pCAR1 [containing only DNA regions involved in replication (repA and oriV) and partition (parWASB)] is not stably maintained in KT2440, whereas the full-length pCAR1 is stable, suggesting that pCAR1 encodes stabilizing factors outside the rep and par regions (39). The results of this study suggested that the stabilizing factors may be Pmr, Pnd, and Phu and also that the disruption of either a phu gene or a pnd gene with a pmr gene on pCAR1 could promote DNA rearrangement on pCAR1 in the host cell.
FIG 3.
pCAR1 stability assay using P. putida KT2440(pCAR1), NAP gene disruption mutants, and pmr-complemented double mutants. The strains were grown on succinate as the sole source of carbon for 24 h, and then the resultant cultures were diluted into fresh media and cultured again. This dilution-incubation procedure was repeated five times, and the carriage of pCAR1 in colonies from the first (A) and fifth (B) passages in culture was examined using the repA and carAc genes. Black, gray, and white bars indicate the numbers of colonies containing intact pCAR1, pCAR1 without the carAc gene, and no pCAR1, respectively. Representative data from more than five independent experiments are shown.
Transferability of pCAR1.
To assess the effects of NAPs on the transferability of pCAR1, mating assays were performed using NAP gene disruption mutants as donors and the KT2440-derivative strain as the recipient. To select donor cells carrying each plasmid and to distinguish donors from recipients, each mutant with a Gm resistance cassette was used as a donor. As a control, we used the KT2440(pCAR1pmrHis::Gmr) strain harboring a Gm resistance cassette downstream of the pmr gene (23). KT2440(pCAR1Δpmr::Gmr) and KT2440(pCAR1Δpnd::Gmr) showed transfer frequencies similar to those seen with the control strain, whereas KT2440(pCAR1Δphu::Gmr) exhibited an about 10-fold decreased frequency (Fig. 4A). Larger differences were obtained when the double mutants were used as donors; the transfer frequencies of KT2440(pCAR1ΔpmrΔpnd::Gmr) and KT2440(pCAR1ΔpmrΔphu::Gmr) decreased markedly to below the limit of detection (≤10−7 per donor cell), whereas that of KT2440(pCAR1ΔpndΔphu::Gmr) was similar to the control (Fig. 4B). Notably, the transfer deficiencies of the double mutants were not recovered by complementation of pmr (i.e., they were below the limit of detection) (≤10−6 per donor cell). As instability of pCAR1 in the double mutants was recovered by complementation of pmr (Fig. 3), the transfer deficiency may not have been due to plasmid replication and stability.
FIG 4.
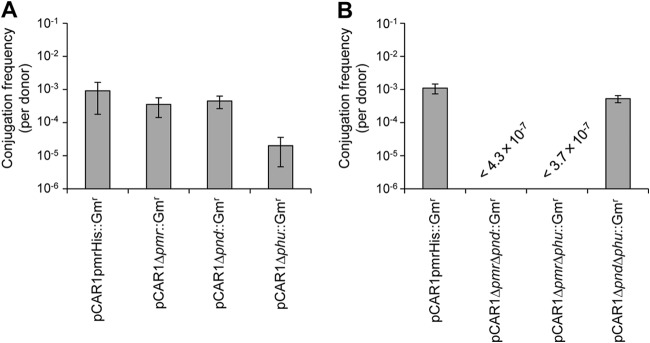
Conjugation frequency of NAP gene disruption plasmid pCAR1. Single mutants (A) and double mutants (B) of KT2440 harboring a Gm resistance cassette were used as the donors, and KT2440KR harboring the Km resistance cassette was the recipient. As a control, the KT2440(pCAR1pmrHis::Gmr) strain harboring a Gm resistance cassette downstream of pmr was used. The donor and recipient were mixed in LB liquid medium to OD600 of 0.2 and 2.0, respectively, and the conjugation frequency was calculated as the number of transconjugants per number of donors. Data are expressed as means ± standard deviations of the conjugation frequencies, which were obtained in five experiments performed at the same time. Representative data from four (A) and two (B) independent experiments are shown.
Taken together, these results suggest that Pmr, Pnd, and Phu are key factors in both the maintenance and the conjugation of pCAR1. As the three single mutants and KT2440(pCAR1ΔpndΔphu) did not show the phenotypes described above, the pCAR1-encoded NAPs likely have synergistic functions, and Pmr may play the most important role in these processes.
Phenotypic screening of NAP gene disruption mutants.
To assess the global effects of NAP gene disruption(s) in greater detail, we performed comprehensive phenotypic comparisons of the metabolic capacities and levels of stress resistance of the WT and NAP gene disruption mutants by assessing cellular respiration using Biolog Phenotype MicroArrays (PMs). This experiment was performed on the WT strain, three single mutants, and the two double mutants showing significant phenotypic differences as described above. Mutants without the Gm resistance cassette were used. The utilization of 23 [KT2440(pCAR1Δpmr)], 22 [KT2440(pCAR1Δpnd)], 15 [KT2440(pCAR1Δphu)], 18 [KT2440(pCAR1ΔpmrΔpnd)], and 20 [KT2440(pCAR1ΔpmrΔphu)] compounds that were supplemented as the sole source of carbon, nitrogen, phosphate, or sulfur was affected by the single or double disruption of NAP genes (Table 3; see also Tables S1 to S4 in the supplemental material). Previous analyses comparing KT2440 and KT2440(pCAR1) showed that the utilization of 66 compounds was affected by pCAR1 carriage and that respiration activities for 57 of these 66 compounds had decreased (9). Notably, the utilization of intermediate compounds in the tricarboxylic acid (TCA) cycle (α-ketoglutaric acid, d,l-malic acid, succinic acid, and fumaric acid) and those several steps away from the TCA cycle (acetic acid and l-lactic acid) were affected, and the cell respiration activities for these compounds decreased (9) (see Table S1). In contrast, the disruption of NAP genes increased the cell respiration activities for these compounds, and the levels of activity for these compounds were restored to levels similar to that of pCAR1-free KT2440 (see Table S1), although a portion of double mutants lost the plasmid during the assay. No marked alterations were observed in resistance to osmotic or pH stresses by NAP gene disruption(s) (see Tables S5 and S6 in the supplemental material).
TABLE 3.
The numbers of compounds supplemented as the sole source of carbon, nitrogen, phosphate, or sulfur differentially metabolized between KT2440(pCAR1) and NAP gene disruptantsa
| Strain | No. of C sources (PM1 and PM2; 190 compounds) | No. of N sources (PM3; 95 compounds) | No. of P sources (PM4; 59 compounds) | No. of S sources (PM4; 35 compounds) | Total no. of sources |
|---|---|---|---|---|---|
| KT2440(pCAR1)b | 22 (3, 19) | 18 (1, 17) | 22 (5, 17) | 4 (0, 4) | 66 (9, 57) |
| KT2440 (pCAR1Δpmr)c | 13 (13, 0) | 4 (4, 0) | 5 (2, 3) | 1 (1, 0) | 23 (20, 3) |
| KT2440 (pCAR1Δpnd)c | 10 (8, 2) | 4 (4, 0) | 8 (5, 3) | 0 | 22 (17, 5) |
| KT2440 (pCAR1Δphu)c | 10 (9, 1) | 1 (0, 1) | 4 (0, 4) | 0 | 15 (9, 6) |
| KT2440 (pCAR1ΔpmrΔpnd)c | 11 (9, 2) | 1 (0, 1) | 6 (0, 6) | 0 | 18 (9, 9) |
| KT2440 (pCAR1ΔpmrΔphu)c | 10 (7, 3) | 2 (0, 2) | 7 (5, 2) | 1 (1, 0) | 20 (13, 7) |
Two independent analyses were performed with each strain for each compound, and cellular respiration activity was compared among the four combinations. The numbers in parentheses in columns 2 to 6 indicate the numbers of compounds with which cell respiration increased (left) or decreased (right).
The results from KT2440(pCAR1) were compared with those from KT2440 (9).
The results from NAP gene disruptants were compared with those from KT2440(pCAR1).
Alteration of the transcriptome by NAP gene(s) disruption.
To elucidate the effects of NAP gene disruption on transcriptional networks in the host cells, transcriptome comparisons of three single mutants and two double mutants (without the Gm resistance cassette) were performed at the log growth phase using a tiling array. Among the transcribed genes in each strain, genes that showed an over-2-fold change between the WT and the mutants were defined as differentially transcribed genes caused by the disruption of a NAP gene(s). The ORFs of differentially transcribed genes on the KT2440 chromosome were classified into 23 groups based on their putative functions by Clusters of Orthologous Groups (COG) protein analysis.
Overview.
The numbers of up- and downregulated genes on the KT2440 chromosome (with COG categories) and pCAR1 are shown in Tables 4 and 5, respectively, and the differentially transcribed genes are presented in Data Sets S1 and S2 in the supplemental material, respectively. Of the 226 genes (199 on the chromosome and 27 on pCAR1) differentially transcribed in KT2440(pCAR1Δpmr), 224 were upregulated and only 2 were downregulated, suggesting that Pmr functions predominantly as a transcriptional repressor. In contrast, all 174 genes (165 on the chromosome and 9 on pCAR1) differentially transcribed in KT2440(pCAR1Δpnd) were downregulated, suggesting that Pnd functions predominantly as a transcriptional activator. In KT2440(pCAR1Δphu), only 46 genes (42 on the chromosome and 4 on pCAR1) were up- or downregulated, which was less than in KT2440(pCAR1Δpmr) or KT2440(pCAR1Δpnd). The upregulated genes in KT2440(pCAR1Δphu) on both the chromosome and pCAR1 were also upregulated in KT2440(pCAR1Δpmr), and 26 of the 33 downregulated genes on the chromosome in KT2440(pCAR1Δphu) were also downregulated in KT2440(pCAR1Δpnd) (see Data Sets S1 and S2 in the supplemental material), suggesting that Phu has regulons similar to the Pmr and Pnd regulons and not unique regulons. Fisher's exact test showed that the number of genes categorized in COG code C, “energy production and conversion,” (19 genes) among the upregulated genes of KT2440(pCAR1Δpmr) was significantly greater than that of genes categorized in COG code C (274 genes) in the whole genome in KT2440 (5,408 genes; P<0.05, Fisher's exact test) (Table 4), which may have been related to the increase in the respiration activity of the KT2440(pCAR1Δpmr) mutant in the phenotypic scanning analysis described above. In contrast, the numbers of COG code S and uncategorized (—) genes in KT2440(pCAR1Δpnd) and KT2440(pCAR1Δphu) mutants were significantly greater than the number of genes in the whole genome of KT2440 (P<0.05, Fisher's exact test) (Table 4), indicating that disruption of pnd or phu affected the transcription of unknown genes and suggesting that these NAP genes may have unknown regulons in the host KT2440. We focused on the putative genes involved in phenotype alterations via the disruption of NAP genes (see below).
TABLE 4.
Numbers of up- and downregulated genes on KT2440 chromosome in NAP gene disruptants and their COG categories
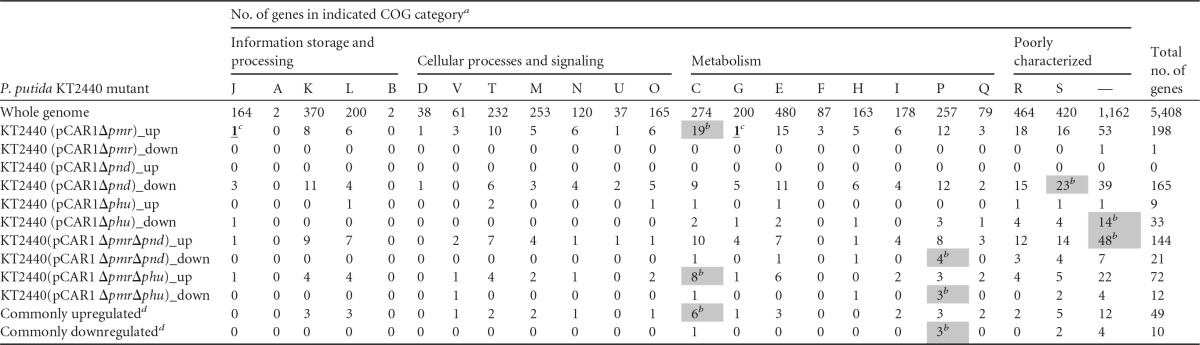
The descriptions for each COG code are as follows: J, translation, ribosomal structure, and biogenesis; A, RNA processing and modification; K, transcription; L, replication, recombination, and repair; B, chromatin structure and dynamics; D, cell cycle control, cell division; V, defense mechanisms; T, signal transduction mechanisms; M, cell wall/membrane/envelope biogenesis; N, cell motility; U, intracellular trafficking, secretion, and vesicular transport; O, posttranslational modification, protein turnover, chaperones; C, energy production and conversion; G, carbohydrate transport and metabolism; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; H, coenzyme transport and metabolism, chromosome partitioning; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, biosynthesis of secondary metabolites, transport, and catabolism; R, general function prediction only; S, function unknown; —, uncategorized.
The number of ORFs categorized in the COG code in the differentially transcribed genes was significantly larger than that of ORFs categorized in the same COG code in the whole-genome ORFs (P < 0.05, Fisher's exact test). These cells are shown in gray.
The number of ORFs categorized in the COG code in the differentially transcribed genes was significantly smaller than that of ORFs categorized in the same COG code in the whole-genome ORFs (P < 0.05, Fisher's exact test). These numbers are underlined and shown in boldface.
“Commonly upregulated” and “Commonly downregulated” indicate the numbers of upregulated or downregulated genes in both KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu) mutants.
TABLE 5.
Numbers of up- and downregulated genes on pCAR1 in NAP gene disruptants
| Mutant | No. of upregulated genes | No. of downregulated genes | Total no. of genes |
|---|---|---|---|
| KT2440(pCAR1Δpmr) | 26 | 1 | 27 |
| KT2440(pCAR1Δpnd) | 0 | 9 | 9 |
| KT2440(pCAR1Δphu) | 3 | 1 | 4 |
| KT2440(pCAR1ΔpmrΔpnd) | 51 | 2 | 53 |
| KT2440(pCAR1ΔpmrΔphu) | 27 | 2 | 29 |
Transcriptome alterations of genes related to pCAR1 stability.
As noted above, pCAR1 itself was lost in a portion of double mutants KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu) (Fig. 3). The DNA regions on pCAR1 containing carAc were deleted in several double mutants (Fig. 3). These observations indicated that the maintenance system(s) of pCAR1 itself was disrupted or that the DNA rearrangements in the host cell were promoted. In our previous study, repA and parWAB genes of pCAR1 were shown to be involved in replication and stable maintenance of pCAR1 (40), while the parI (PP_3700) product, an orphan ParA family protein, is a negative host factor for the maintenance system of pCAR1 (39). The transcriptional levels of these genes were not changed in the double mutants, suggesting that the instability of pCAR1 itself in the double mutants may not have been due to a deficiency of partitions. Therefore, we attempted to find candidate genes involved in stable maintenance of pCAR1 or its DNA regions on both the chromosome and pCAR1. As the two double mutants showed similar levels of instability, commonly up- or downregulated genes in both strains were considered candidates; 49 and 10 genes on the chromosome were commonly upregulated and downregulated, respectively, in the double mutants (Table 4). Among these genes, the numbers of differentially transcribed genes classified as COG categories C (“energy production and conversion”) (6 genes, upregulated) and P (“inorganic ion transport and metabolism”) (3 genes, downregulated) were significantly greater than those of genes in the “C” (274 genes) and “P” (257 genes) categories among the whole genes (5,408 genes; P<0.05, Fisher's exact test) (Table 4). Notably, the upregulated genes in the “C” category were those encoding dehydrogenase (PP_1073, PP_4401, and PP_4667), ferredoxin (PP_1625), azurin (PP_4870), and cytochrome c-type protein (PP_3823), and the downregulated genes in the “P” category were putative TonB-dependent siderophore receptors (PP_3330 and PP_3340) and a copper receptor (PP_4838). Previously, we showed that pCAR1 carriage resulted in transient iron deficiency in the host and that one of the reasons for this deficiency was the constitutive expression of carbazole-degrading (Car) enzymes (8). Indeed, the deletion of car genes partially ameliorated the deficiency (8). In another study, DNA rearrangements were shown to occur in pCAR1 in host cells because the carriage of car genes would be disadvantageous to the host cells (38, 41). As pCAR1 in the double mutants also showed deletion of the car genes, some disadvantageous effects in the presence of pCAR1 with NAP gene deletion may be related to the DNA rearrangements of pCAR1 and instability of the plasmid. Commonly downregulated genes related to the iron acquisition system suggested that pCAR1 instability and DNA rearrangements in the double mutants of NAP genes may be due to iron deficiency. Then, pCAR1 stability assays were performed under conditions of iron abundance (10-fold concentration of ferric citrate, 370 μM). However, the segregational instability and structural instability of pCAR1 did not change under these conditions (see Fig. S2 in the supplemental material). Therefore, the instability of pCAR1 itself and the DNA rearrangements in the double mutants of NAP genes may not have been caused solely by iron deficiency. In total, 27 genes on pCAR1 were commonly upregulated in KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu), but no genes were commonly downregulated except for pmr (Table 6; see also Data Set S2 in the supplemental material). Four of the 27 upregulated genes (ORF108b, ORF108c, ORF155, and recT) were upregulated only in the double mutants, whereas the other 23 genes were also upregulated in at least one of the three single mutants. One of these genes, recT, which has a translated sequence with 31% identity to RecT of E. coli DH10B, is a RecA-independent homologous recombination factor, and a RecT homolog of P. syringae was shown to promote efficient homologous recombination between genomic loci and linear DNA substrates (42). Considering our previous result (38), the elevated expression of RecT may have increased the frequency of homologous recombination between two nearly identical copies of ISPre1 to generate strains harboring pCAR1 without car operons. However, the DNA region containing carAc on pCAR1 was not deleted in the RecT-overexpressing strain (see Fig. S3 in the supplemental material), which suggested that upregulation of the recT gene did not affect the structural stability of pCAR1.
TABLE 6.
Commonly upregulated genes in KT2440(pCAR1ΔpmrΔpnd) and KT2440(pCAR1ΔpmrΔphu)
| Designation or gene name | Putative function of the gene product | Regulation categorya |
||
|---|---|---|---|---|
| pCAR1Δpmr | pCAR1Δpnd | pCAR1Δphu | ||
| ORF40 | Hypothetical protein | Up | NC | NC |
| ORF100 | Hypothetical protein | Up | NC | NC |
| ORF101 | Cobalamin biosynthesis protein | Up | NC | Up |
| ORF102 | Cobalamin biosynthesis protein | Up | NC | NC |
| ORF103 | Hypothetical protein | Up | NC | NC |
| ORF104 | Hypothetical protein | Up | NC | Up |
| ORF105 | Hypothetical protein | Up | NC | NC |
| ORF106 | Hypothetical protein | Up | NC | NC |
| ORF107 | Hypothetical protein | Up | NC | Up |
| ORF108 | Hypothetical protein | Up | NC | NC |
| ORF108a | Hypothetical protein | Up | NC | NC |
| ORF108b | Hypothetical protein | NC | NC | NC |
| ORF108c | Hypothetical protein | NC | NC | NC |
| ORF109 | Hypothetical protein | Up | NC | NC |
| ORF114 | Hypothetical protein | Up | NC | NC |
| ORF115 | Hypothetical protein | Up | NC | NC |
| ssb | Single-strand DNA binding protein | Up | NC | NC |
| recT | DNA recombination protein | NC | NC | NC |
| trhK | Putative transfer protein | Up | NC | NC |
| trhV | Putative pilus assembly protein | Up | NC | NC |
| trhA | Putative transfer protein | Up | NC | NC |
| dsbC | Putative disulfide bond isomerase | Up | NC | NC |
| ORF144 | Hypothetical protein | Up | NC | NC |
| ORF145a | Hypothetical protein | Up | NC | NC |
| ORF145 | Putative DNA primase | Up | NC | NC |
| ORF146 | Putative DNA primase | Up | NC | NC |
| ORF155 | Hypothetical protein | NC | NC | NC |
Transcription levels of genes in each single mutant in comparison with those in KT2440(pCAR1). “Up” indicates that the gene is upregulated in the mutant, and “NC” indicates that the transcription level did not change.
Transcriptome alterations of genes related to pCAR1 transferability.
Transcriptome analyses showed that several tra and trh genes on pCAR1 were differentially transcribed in the complemented double mutants (Table 6; see also Data Set S2 in the supplemental material), which are considered to be essential for the conjugative transfer of pCAR1 (40, 43). In KT2440(pCAR1ΔpmrΔpnd), trhK, trhB, trhV, trhA, trhC, trhF, trhH, and trhG were upregulated. Of these, trhK, trhV, and trhA were also upregulated in both KT2440(pCAR1Δpmr) and KT2440(pCAR1ΔpmrΔphu). Other than the tra and trh regions, ORF145 and ORF146 were upregulated commonly in KT2440(pCAR1Δpmr), KT2440(pCAR1ΔpmrΔpnd), and KT2440(pCAR1ΔpmrΔphu). Although ORF145 and ORF146 were not essential for the transfer of pCAR1, the transfer frequency of pCAR1 decreased when these genes were disrupted (see the text in the supplemental material and Fig. S4 in the supplemental material). In the case of the other plasmid, R27, which also carries tra and trh genes and hnsR27 (corresponding to pmr on pCAR1), transcription of the tra and trh genes of R27 was upregulated and the transfer frequency of R27 was derepressed when hnsR27 was disrupted (22). The pCAR1 in the double mutants showed significantly low transfer frequencies in the mating experiments (Fig. 4B), whereas the transcriptional level of the tra and trh genes increased, perhaps due to the different media (LB or NMM-4 supplemented with succinate), growth phases (stationary or log phase), or plasmids (with or without the Gm resistance cassette in the pnd or phu gene) used in the mating experiments and transcriptome analyses. The mechanism underlying the loss of the plasmid transfer ability in the double mutants was unclear.
Conclusion.
In this study, we assessed the roles of plasmid-encoded HU (Phu) and NdpA (Pnd) proteins. The function of NdpA-like proteins, which constitute a well-conserved protein family in Gram-negative bacteria, is not known regardless of their location (i.e., plasmid or chromosome). The findings of this study showed that Phu and Pnd could have alternative functions that compensate for the lack of Pmr functions in cases of pmr disruption. The double disruption of pmr (encoding H-NS family protein) and one of the other two NAP genes on pCAR1 had a significant effect on the host phenotype, especially for the stable maintenance of pCAR1. The NAPs may be stabilizing factors on the full-length pCAR1 in KT2440 which were not identified in our previous studies (39, 40). Recently, we found that these NAPs also have effects on biofilm formation of KT2440(pCAR1) (S. Lee, Y. Takahashi, H. Oura, K. Okada, H. Yamane, N. Nomura, and H. Nojiri, unpublished data). However, the molecular mechanisms by which the NAPs affected the phenotypes in the host cell remain unclear. Considering that heterologous protein-protein interactions of Pmr, Pnd, and Phu were not detected in vitro (see the text in the supplemental material and Fig. S5 in the supplemental material), investigation of in vivo DNA binding sites of these proteins will help us to understand how they regulate the phenotypes observed in this study. In addition, as the TurA and TurB chromosomally encoded H-NS family proteins have many important effects on the function of Pmr (23, 24), characterization of chromosomally encoded NdpA and HU homologs is necessary to clarify the molecular mechanisms behind the functions of Pnd and Phu. Disruption of pnd or phu did not affect transcription of the genes encoding NdpA or HU homologs on the chromosome, except for hupA in KT2440(pCAR1ΔpmrΔphu) (see Data Set S1 in the supplemental material), suggesting that Pnd and Phu do not simply function to provide a molecular “backup” for their chromosomally encoded homologs. Future analyses of protein-protein interactions, DNA binding motifs, and DNA compaction using both plasmid-encoded and chromosomally encoded NAPs will clarify how these proteins function in bacterial cells, and such studies will shed light on the significance of plasmid-encoded NAPs.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hiromi Nishida of Biotechnology Research Center and Department of Biotechnology, Toyama Prefectural University, for technical advice in transcriptome analysis and Central Scientific Commerce, Inc. (Tokyo, Japan), for the use of their OmniLog system and technical advice. We also thank Bryan Swingle of the Department of Plant Pathology and Plant-Microbe Biology, Cornell University, for generously providing the plasmids.
C.S.-M. and Y.T. were supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists. The Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) and JSPS KAKENHI grant 24380043 (to H.N.) also supported this work.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00023-15.
REFERENCES
- 1.Top EM, Springael D. 2003. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr Opin Biotechnol 14:262–269. doi: 10.1016/S0958-1669(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 2.Frost LS, Leplae R, Summers AO, Toussaint A. 2005. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 3.Ramos JL, Marqués S, Timmis KN. 1997. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid-encoded regulators. Annu Rev Microbiol 51:341–373. doi: 10.1146/annurev.micro.51.1.341. [DOI] [PubMed] [Google Scholar]
- 4.Nojiri H. 2012. Structural and molecular genetic analyses of the bacterial carbazole degradation system. Biosci Biotechnol Biochem 76:1–18. doi: 10.1271/bbb.110620. [DOI] [PubMed] [Google Scholar]
- 5.Maeda K, Nojiri H, Shintani M, Yoshida T, Habe H, Omori T. 2003. Complete nucleotide sequence of carbazole/dioxin-degrading plasmid pCAR1 in Pseudomonas resinovorans strain CA10 indicates its mosaicity and the presence of large catabolic transposon Tn4676. J Mol Biol 326:21–33. doi: 10.1016/S0022-2836(02)01400-6. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Shintani M, Yamane H, Nojiri H. 2009. The complete nucleotide sequence of pCAR2: pCAR2 and pCAR1 were structurally identical IncP-7 carbazole degradative plasmids. Biosci Biotechnol Biochem 73:744–746. doi: 10.1271/bbb.80665. [DOI] [PubMed] [Google Scholar]
- 7.Shintani M, Tokumaru H, Takahashi Y, Miyakoshi M, Yamane H, Nishida H, Nojiri H. 2011. Alterations of RNA maps of IncP-7 plasmid pCAR1 in various Pseudomonas bacteria. Plasmid 66:85–92. doi: 10.1016/j.plasmid.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Shintani M, Takahashi Y, Tokumaru H, Kadota K, Hara H, Miyakoshi M, Naito K, Yamane H, Nishida H, Nojiri H. 2010. Response of the Pseudomonas host chromosomal transcriptome to carriage of the IncP-7 plasmid pCAR1. Environ Microbiol 12:1413–1426. doi: 10.1111/j.1462-2920.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Shintani M, Takase N, Kazo Y, Kawamura F, Hara H, Nishida H, Okada K, Yamane H, Nojiri H. 2015. Modulation of primary cell function of host Pseudomonas bacteria by the conjugative plasmid pCAR1. Environ Microbiol 17:134–155. doi: 10.1111/1462-2920.12515. [DOI] [PubMed] [Google Scholar]
- 10.Nojiri H. 2013. Impact of catabolic plasmids on host cell physiology. Curr Opin Biotechnol 24:423–430. doi: 10.1016/j.copbio.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Dillon SC, Dorman CJ. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 12.Dorman CJ, Kane KA. 2009. DNA bridging and antibridging: a role for bacterial nucleoid-associated proteins in regulating the expression of laterally acquired genes. FEMS Microbiol Rev 33:587–592. doi: 10.1111/j.1574-6976.2008.00155.x. [DOI] [PubMed] [Google Scholar]
- 13.Ali SS, Xia B, Liu J, Navarre WW. 2012. Silencing of foreign DNA in bacteria. Curr Opin Microbiol 15:175–181. doi: 10.1016/j.mib.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Tendeng C, Bertin PN. 2003. H-NS in Gram-negative bacteria: a family of multifaceted proteins. Trends Microbiol 11:511–518. doi: 10.1016/j.tim.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Tendeng C, Soutourina OA, Danchin A, Bertin PN. 2003. MvaT proteins in Pseudomonas spp.: a novel class of H-NS-like proteins. Microbiology 149:3047–3050. doi: 10.1099/mic.0.C0125-0. [DOI] [PubMed] [Google Scholar]
- 16.Castaing B, Zelwer C, Laval J, Boiteux S. 1995. HU protein of Escherichia coli binds specifically to DNA that contains single-strand breaks or gaps. J Biol Chem 270:10291–10296. doi: 10.1074/jbc.270.17.10291. [DOI] [PubMed] [Google Scholar]
- 17.Pinson V, Takahashi M, Rouviere-Yaniv J. 1999. Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J Mol Biol 287:485–497. doi: 10.1006/jmbi.1999.2631. [DOI] [PubMed] [Google Scholar]
- 18.Takeda T, Yun CS, Shintani M, Yamane H, Nojiri H. 2011. Distribution of genes encoding nucleoid-associated protein homologs in plasmids. Int J Evol Biol 2011:685015. doi: 10.4061/2011/685015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle M, Fookes M, Ivens A, Mangan MW, Wain J, Dorman CJ. 2007. An H-NS-like stealth protein aids horizontal DNA transmission in bacteria. Science 315:251–252. doi: 10.1126/science.1137550. [DOI] [PubMed] [Google Scholar]
- 20.Baños RC, Vivero A, Aznar S, García J, Pons M, Madrid C, Juárez A. 2009. Differential regulation of horizontally acquired and core genome genes by the bacterial modulator H-NS. PLoS Genet 5:e1000513. doi: 10.1371/journal.pgen.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dillon SC, Cameron AD, Hokamp K, Lucchini S, Hinton JC, Dorman CJ. 2010. Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Mol Microbiol 76:1250–1265. doi: 10.1111/j.1365-2958.2010.07173.x. [DOI] [PubMed] [Google Scholar]
- 22.Forns N, Baños RC, Balsalobre C, Juárez A, Madrid C. 2005. Temperature-dependent conjugative transfer of R27: role of chromosome- and plasmid-encoded Hha and H-NS proteins. J Bacteriol 187:3950–3959. doi: 10.1128/JB.187.12.3950-3959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yun CS, Suzuki C, Naito K, Takeda T, Takahashi Y, Sai F, Terabayashi T, Miyakoshi M, Shintani M, Nishida H, Yamane H, Nojiri H. 2010. Pmr, a histone-like protein H1 (H-NS) family protein encoded by the IncP-7 plasmid pCAR1, is a key global regulator that alters host function. J Bacteriol 192:4720–4731. doi: 10.1128/JB.00591-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki C, Kawazuma K, Horita S, Terada T, Tanokura M, Okada K, Yamane H, Nojiri H. 2014. Oligomerization mechanisms of an H-NS family protein, Pmr, encoded on the plasmid pCAR1 provide a molecular basis for functions of H-NS family members. PLoS One 9:e105656. doi: 10.1371/journal.pone.0105656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 26.Shintani M, Yoshida T, Habe H, Omori T, Nojiri H. 2005. Large plasmid pCAR2 and class II transposon Tn4676 are functional mobile genetic elements to distribute the carbazole/dioxin-degradative car gene cluster in different bacteria. Appl Microbiol Biotechnol 67:370–382. doi: 10.1007/s00253-004-1778-0. [DOI] [PubMed] [Google Scholar]
- 27.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1990. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY. [Google Scholar]
- 28.Itoh N, Kouzai T, Koide Y. 1994. Efficient transformation of Pseudomonas strains with pNI vectors by electroporation. Biosci Biotechnol Biochem 58:1306–1308. doi: 10.1271/bbb.58.1306. [DOI] [PubMed] [Google Scholar]
- 29.Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386. [DOI] [PubMed] [Google Scholar]
- 30.Choi KH, Schweizer HP. 2005. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol 5:30. doi: 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 32.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 33.Sukchawalit R, Vattanaviboon P, Sallabhan R, Mongkolsuk S. 1999. Construction and characterization of regulated l-arabinose-inducible broad host range expression vectors in Xanthomonas. FEMS Microbiol Lett 181:217–223. doi: 10.1111/j.1574-6968.1999.tb08847.x. [DOI] [PubMed] [Google Scholar]
- 34.Kouzuma A, Endoh T, Omori T, Nojiri H, Yamane H, Habe H. 2007. The ptsP gene encoding the PTS family protein EI(Ntr) is essential for dimethyl sulfone utilization by Pseudomonas putida. FEMS Microbiol Lett 275:175–181. doi: 10.1111/j.1574-6968.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 35.Miyakoshi M, Shintani M, Terabayashi T, Kai S, Yamane H, Nojiri H. 2007. Transcriptome analysis of Pseudomonas putida KT2440 harboring the completely sequenced IncP-7 plasmid pCAR1. J Bacteriol 189:6849–6860. doi: 10.1128/JB.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domínguez-Cuevas P, Marqués S. 2004. Compiling sigma-70-dependent promoters, p 319–343. In Ramos JL. (ed), The Pseudomonas, vol 2 Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 37.Valls M, Cases I, de Lorenzo V. 2004. Transcription mediated by rpoN-dependent promoters, p 289–317. In Ramos JL. (ed), The Pseudomonas, vol 2 Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 38.Takahashi Y, Shintani M, Li L, Yamane H, Nojiri H. 2009. Carbazole-degradative IncP-7 plasmid pCAR1.2 is structurally unstable in Pseudomonas fluorescens Pf0-1, which accumulates catechol, the intermediate of the carbazole degradation pathway. Appl Environ Microbiol 75:3920–3929. doi: 10.1128/AEM.02373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyakoshi M, Shintani M, Inoue K, Terabayashi T, Sai F, Ohkuma M, Nojiri H, Nagata Y, Tsuda M. 2012. ParI, an orphan ParA family protein from Pseudomonas putida KT2440-specific genomic island, interferes with the partition system of IncP-7 plasmids. Environ Microbiol 14:2946–2959. doi: 10.1111/j.1462-2920.2012.02861.x. [DOI] [PubMed] [Google Scholar]
- 40.Shintani M, Yano H, Habe H, Omori T, Yamane H, Tsuda M, Nojiri H. 2006. Characterization of the replication, maintenance, and transfer features of the IncP-7 plasmid pCAR1, which carries genes involved in carbazole and dioxin degradation. Appl Environ Microbiol 72:3206–3216. doi: 10.1128/AEM.72.5.3206-3216.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shintani M, Matsumoto T, Yoshikawa H, Yamane H, Ohkuma M, Nojiri H. 2011. DNA rearrangement has occurred in the carbazole-degradative plasmid pCAR1 and the chromosome of its unsuitable host, Pseudomonas fluorescens Pf0-1. Microbiology 157:3405–3416. doi: 10.1099/mic.0.053280-0. [DOI] [PubMed] [Google Scholar]
- 42.Swingle B, Bao Z, Markel E, Chambers A, Cartinhour S. 2010. Recombineering using RecTE from Pseudomonas syringae. Appl Environ Microbiol 76:4960–4968. doi: 10.1128/AEM.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shintani M, Habe H, Tsuda M, Omori T, Yamane H, Nojiri H. 2005. Recipient range of IncP-7 conjugative plasmid pCAR2 from Pseudomonas putida HS01 is broader than from other Pseudomonas strains. Biotechnol Lett 27:1847–1853. doi: 10.1007/s10529-005-3892-1. [DOI] [PubMed] [Google Scholar]
- 44.de Lorenzo V, Timmis KN. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol 235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 45.Bagdasarian M, Lurz R, Rückert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.